Potential Impact of Human Cytomegalovirus Infection on Immunity to Ovarian Tumours and Cancer Progression
Abstract
:1. Background
2. Human Cytomegalovirus (HCMV)
3. Effect of HCMV Infection on Innate and Adaptive Immune Response
4. Anti-Tumor Immunity in Ovarian Cancer
5. Immune Homeostasis
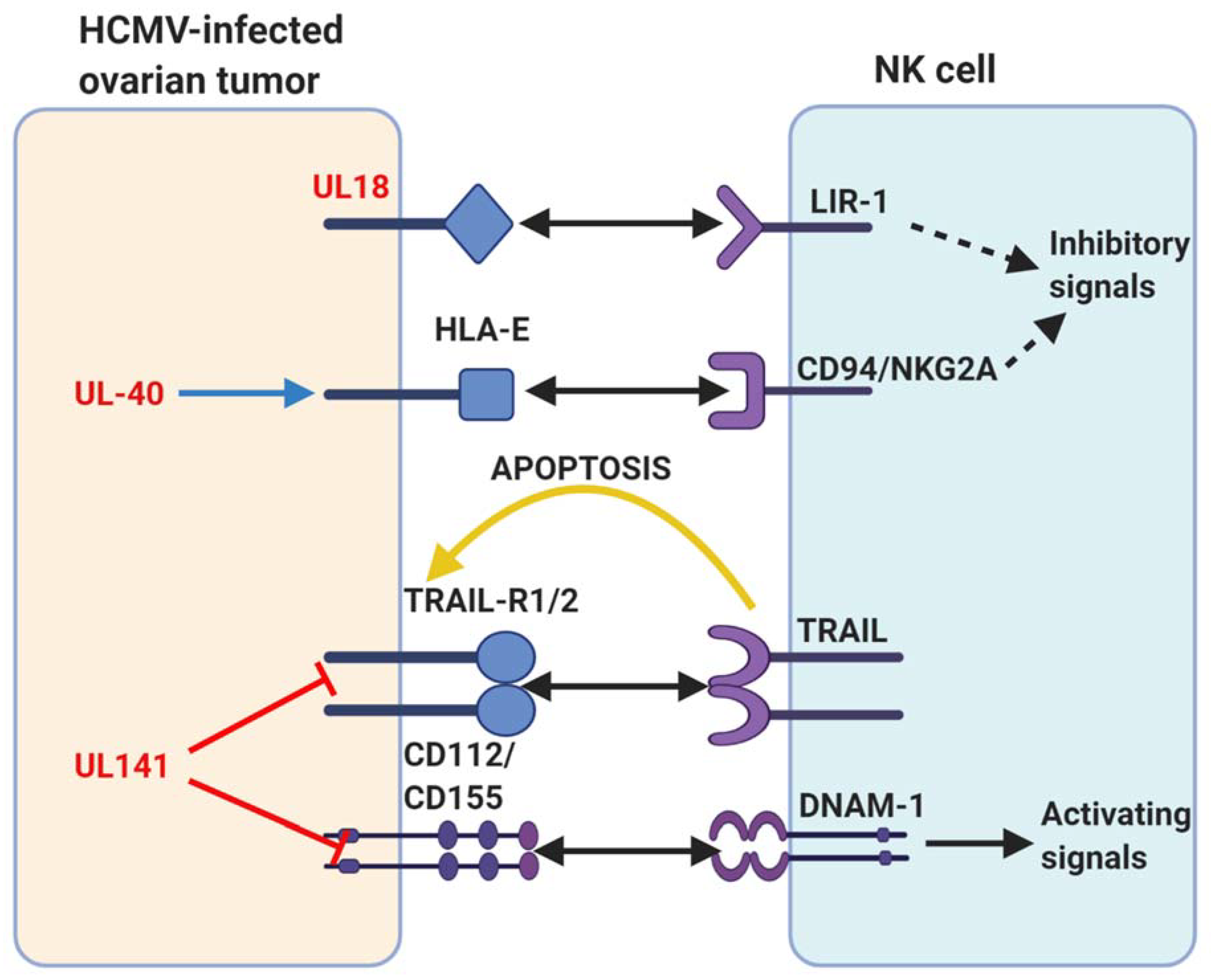
6. HCMV Infection in Ovarian Cancer
7. Significance of HCMV Infection in the OC TME
8. Association between Human Leucocyte Antigens, γ Markers and Killer Immuno-Globulin-Like Receptors with Human Cytomegalovirus Infection
9. Potential Modulation of Intrinsic Inhibitory Receptors by HCMV
10. HCMV and Inflammation, a Possible Link for Ovarian Cancer Progression
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sankaranarayanan, R.; Ferlay, J. Worldwide burden of gynaecological cancer: The size of the problem. Best Pr. Res. Clin. Obstet. Gynaecol. 2006, 20, 207–225. [Google Scholar] [CrossRef]
- Reid, B.M.; Permuth, J.B.; Sellers, T.A. Epidemiology of ovarian cancer: A review. Cancer Biol. Med. 2017, 14, 9–32. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.; Yang, M.; Ding, Y.; Chen, J. Microbial infection, inflammation and epithelial ovarian cancer. Oncol. Lett. 2017, 14, 1911–1919. [Google Scholar] [CrossRef]
- Kampan, N.C.; Madondo, M.T.; McNally, O.M.; A Quinn, M.; Plebanski, M. Paclitaxel and Its Evolving Role in the Management of Ovarian Cancer. BioMed. Res. Int. 2015, 2015, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Aust, S.; Seebacher-Shariat, V. Screening for ovarian cancer: Is there still hope? MEMO Mag. Eur. Med. Oncol. 2020, 13, 189–192. [Google Scholar] [CrossRef] [Green Version]
- Kampan, N.C.; Madondo, M.T.; Reynolds, J.; Hallo, J.; McNally, O.M.; Jobling, T.W.; Stephens, A.N.; Quinn, M.A.; Plebanski, M. Pre-operative sera interleukin-6 in the diagnosis of high-grade serous ovarian cancer. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kuchenbaecker, K.B.; Hopper, J.L.; Barnes, D.R.; Phillips, K.-A.; Mooij, T.M.; Roos-Blom, M.-J.; Jervis, S.; Van Leeuwen, F.E.; Milne, R.L.; Andrieu, N.; et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA 2017, 317, 2402–2416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neff, R.T.; Senter, L.; Salani, R. BRCA mutation in ovarian cancer: Testing, implications and treatment considerations. Ther. Adv. Med. Oncol. 2017, 9, 519–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsop, K.; Fereday, S.; Meldrum, C.; DeFazio, A.; Emmanuel, C.; George, J.; Dobrovic, A.; Birrer, M.J.; Webb, P.M.; Stewart, C.; et al. BRCA Mutation Frequency and Patterns of Treatment Response in BRCA Mutation–Positive Women With Ovarian Cancer: A Report From the Australian Ovarian Cancer Study Group. J. Clin. Oncol. 2012, 30, 2654–2663. [Google Scholar] [CrossRef] [Green Version]
- Roy, R.; Chun, J.; Powell, S.N. BRCA1 and BRCA2: Different roles in a common pathway of genome protection. Nat. Rev. Cancer 2011, 12, 68–78. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Lu, L.-Y.; Yu, X. The role of BRCA1 in DNA damage response. Protein Cell 2010, 1, 117–123. [Google Scholar] [CrossRef] [Green Version]
- Risques, R.A.; Kennedy, S.R. Aging and the rise of somatic cancer-associated mutations in normal tissues. PLoS Genet. 2018, 14, e1007108. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.-H.; Meng, Q.; Rao, M.; Liu, Z.; Paraschoudi, G.; Dodoo, E.; Maeurer, M. The impact of inflationary cytomegalovirus-specific memory T cells on anti-tumour immune responses in patients with cancer. Immunology 2018, 155, 294–308. [Google Scholar] [CrossRef]
- Taher, C.; Frisk, G.; Fuentes, S.; Religa, P.; Costa, H.; Assinger, A.; Vetvik, K.K.; Bukholm, I.R.; Yaiw, K.-C.; Smedby, K.E.; et al. High Prevalence of Human Cytomegalovirus in Brain Metastases of Patients with Primary Breast and Colorectal Cancers. Transl. Oncol. 2014, 7, 732–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlson, J.W.; Rådestad, A.F.; Söderberg-Naucler, C.; Rahbar, A. Human cytomegalovirus in high grade serous ovarian cancer possible implications for patients survival. Medicine 2018, 97, e9685. [Google Scholar] [CrossRef] [PubMed]
- Rådestad, A.F.; Estekizadeh, A.; Cui, H.L.; Kostopoulou, O.N.; Davoudi, B.; Hirschberg, A.L.; Carlson, J.; Rahbar, A.; Söderberg-Naucler, C. Impact of Human Cytomegalovirus Infection and its Immune Response on Survival of Patients with Ovarian Cancer. Transl. Oncol. 2018, 11, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.; Adetifa, J.U.; Noho-Konteh, F.; Njie-Jobe, J.; Sanyang, L.C.; Drammeh, A.; Plebanski, M.; Whittle, H.C.; Rowland-Jones, S.L.; Robertson, I.; et al. Limited Impact of Human Cytomegalovirus Infection in African Infants on Vaccine-Specific Responses Following Diphtheria-Tetanus-Pertussis and Measles Vaccination. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Geisler, J.; Touma, J.; Rahbar, A.; Söderberg-Nauclér, C.; Vetvik, K. A Review of the Potential Role of Human Cytomegalovirus (HCMV) Infections in Breast Cancer Carcinogenesis and Abnormal Immunity. Cancers 2019, 11, 1842. [Google Scholar] [CrossRef] [Green Version]
- Manandhar, T.; Hò, G.-G.T.; Pump, W.C.; Blasczyk, R.; Bade-Doeding, C. Battle between Host Immune Cellular Responses and HCMV Immune Evasion. Int. J. Mol. Sci. 2019, 20, 3626. [Google Scholar] [CrossRef] [Green Version]
- Rossini, G.; Cerboni, C.; Santoni, A.; Landini, M.P.; Landolfo, S.; Gatti, D.; Gribaudo, G.; Varani, S. Interplay between Human Cytomegalovirus and Intrinsic/Innate Host Responses: A Complex Bidirectional Relationship. Mediat. Inflamm. 2012, 2012, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkinson, G.W.; Tomasec, P.; Stanton, R.J.; Armstrong, M.; Prod’Homme, V.; Aicheler, R.; McSharry, B.P.; Rickards, C.R.; Cochrane, D.; Llewellyn-Lacey, S.; et al. Modulation of natural killer cells by human cytomegalovirus. J. Clin. Virol. 2008, 41, 206–212. [Google Scholar] [CrossRef] [Green Version]
- Hanley, P.J.; Bollard, C.M. Controlling Cytomegalovirus: Helping the Immune System Take the Lead. Viruses 2014, 6, 2242–2258. [Google Scholar] [CrossRef]
- Avdic, S.; McSharry, B.P.; Steain, M.; Poole, E.; Sinclair, J.; Abendroth, A.; Slobedman, B. Human Cytomegalovirus-Encoded Human Interleukin-10 (IL-10) Homolog Amplifies Its Immunomodulatory Potential by Upregulating Human IL-10 in Monocytes. J. Virol. 2016, 90, 3819–3827. [Google Scholar] [CrossRef] [Green Version]
- Wagner, C.S.; Walther-Jallow, L.; Buentke, E.; Ljunggren, H.-G.; Achour, A.; Chambers, B.J. Human cytomegalovirus-derived protein UL18 alters the phenotype and function of monocyte-derived dendritic cells. J. Leukoc. Biol. 2007, 83, 56–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fulop, T.; Larbi, A.; Pawelec, G. Human T Cell Aging and the Impact of Persistent Viral Infections. Front. Immunol. 2013, 4, 271. [Google Scholar] [CrossRef] [Green Version]
- Bennett, J.M.; Glaser, R.; Malarkey, W.B.; Beversdorf, D.Q.; Peng, J.; Kiecolt-Glaser, J.K. Inflammation and reactivation of latent herpesviruses in older adults. Brain Behav. Immun. 2012, 26, 739–746. [Google Scholar] [CrossRef] [Green Version]
- Goodier, M.R.; White, M.J.; Darboe, A.; Nielsen, C.M.; Goncalves, A.; Bottomley, C.; Moore, S.E.; Riley, E.M. Rapid NK cell differentiation in a population with near-universal human cytomegalovirus infection is attenuated by NKG2C deletions. Blood 2014, 124, 2213–2222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gumá, M.; Budt, M.; Sáez, A.; Brckalo, T.; Hengel, H.; Angulo, A.; López-Botet, M. Expansion of CD94/NKG2C+ NK cells in response to human cytomegalovirus-infected fibroblasts. Blood 2006, 107, 3624–3631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, P.T.; Ngui, J.; Farmer, M.W.; Hutchinson, P.; Holmes, P.W.; Holdsworth, S.R. Cytotoxic T lymphocyte and natural killer cell responses to non-typeable Haemophilus influenzae. Clin. Exp. Immunol. 2008, 152, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Viswanathan, C. Natural killer cells: In health and disease. Hematol. Stem Cell Ther. 2015, 8, 47–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anikeeva, N.; Sykulev, Y. Mechanisms controlling granule-mediated cytolytic activity of cytotoxic T lymphocytes. Immunol. Res. 2011, 51, 183–194. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Lostao, L.; Anel, A.; Pardo, J. How Do Cytotoxic Lymphocytes Kill Cancer Cells? Clin. Cancer Res. 2015, 21, 5047–5056. [Google Scholar] [CrossRef] [Green Version]
- Osińska, I.; Popko, K.; Demkow, U. Perforin: An important player in immune response. Central Eur. J. Immunol. 2014, 1, 109–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben Safta, T.; Ziani, L.; Favre, L.; Lamendour, L.; Gros, G.; Mami-Chouaib, F.; Martinvalet, D.; Chouaib, S.; Thiery, J. Granzyme B–Activated p53 Interacts with Bcl-2 To Promote Cytotoxic Lymphocyte–Mediated Apoptosis. J. Immunol. 2014, 194, 418–428. [Google Scholar] [CrossRef] [Green Version]
- Curiel, T.J.; Coukos, G.; Zou, L.; Alvarez, X.; Cheng, P.; Mottram, P.; Evdemon-Hogan, M.; Conejo-Garcia, J.R.; Zhang, L.; Burow, M.; et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004, 10, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Yigit, R.; Figdor, C.G.; Zusterzeel, P.L.; Pots, J.M.; Torensma, R.; Massuger, L.F. Cytokine analysis as a tool to understand tumour–host interaction in ovarian cancer. Eur. J. Cancer 2011, 47, 1883–1889. [Google Scholar] [CrossRef] [PubMed]
- Barnett, J.C.; Bean, S.M.; Whitaker, R.S.; Kondoh, E.; Baba, T.; Fujii, S.; Marks, J.R.; Dressman, H.K.; Murphy, S.K.; Berchuck, A. Ovarian cancer tumor infiltrating T-regulatory (Treg) cells are associated with a metastatic phenotype. Gynecol. Oncol. 2010, 116, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Preston, C.C.; Maurer, M.J.; Oberg, A.L.; Visscher, D.W.; Kalli, K.R.; Hartmann, L.C.; Goode, E.L.; Knutson, K.L. The Ratios of CD8+ T Cells to CD4+CD25+ FOXP3+ and FOXP3- T Cells Correlate with Poor Clinical Outcome in Human Serous Ovarian Cancer. PLoS ONE 2013, 8, e80063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Freud, A.G.; Caligiuri, M.A. Location and cellular stages of natural killer cell development. Trends Immunol. 2013, 34, 573–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korbel, D.S.; Finney, O.C.; Riley, E.M. Natural killer cells and innate immunity to protozoan pathogens. Int. J. Parasitol. 2004, 34, 1517–1528. [Google Scholar] [CrossRef] [PubMed]
- Pegram, H.J.; Andrews, D.M.; Smyth, M.J.; Darcy, P.K.; Kershaw, M.H. Activating and inhibitory receptors of natural killer cells. Immunol. Cell Biol. 2010, 89, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef]
- Webb, J.R.; Milne, K.; Watson, P.; DeLeeuw, R.J.; Nelson, B.H. Tumor-Infiltrating Lymphocytes Expressing the Tissue Resident Memory Marker CD103 Are Associated with Increased Survival in High-Grade Serous Ovarian Cancer. Clin. Cancer Res. 2014, 20, 434–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moody, R.; Wilson, K.; Jaworowski, A.; Plebanski, M. Natural Compounds with Potential to Modulate Cancer Therapies and Self-Reactive Immune Cells. Cancers 2020, 12, 673. [Google Scholar] [CrossRef] [Green Version]
- Linhares, A.D.S.; Leitner, J.; Grabmeier-Pfistershammer, K.; Steinberger, P. Not All Immune Checkpoints Are Created Equal. Front. Immunol. 2018, 9, 1909. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Sun, Q.; Zhang, X. PD-1 and its ligands are important immune checkpoints in cancer. Oncotarget 2017, 8, 2171–2186. [Google Scholar] [CrossRef] [Green Version]
- Francisco, L.M.; Sage, P.T.; Sharpe, A.H. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 2010, 236, 219–242. [Google Scholar] [CrossRef]
- Fife, B.T.; Bluestone, J.A. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol. Rev. 2008, 224, 166–182. [Google Scholar] [CrossRef]
- Francisco, L.M.; Salinas, V.H.; Brown, K.E.; Vanguri, V.K.; Freeman, G.J.; Kuchroo, V.K.; Sharpe, A.H. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 2009, 206, 3015–3029. [Google Scholar] [CrossRef]
- E Latchman, Y.; Wood, C.R.; Chernova, T.; Chaudhary, D.; Borde, M.; Chernova, I.; Iwai, Y.; Long, A.J.; A Brown, J.; Nunes, R.; et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2001, 2, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [Green Version]
- Dyck, L.; Mills, K.H. Immune checkpoints and their inhibition in cancer and infectious diseases. Eur. J. Immunol. 2017, 47, 765–779. [Google Scholar] [CrossRef] [PubMed]
- Naran, K.; Nundalall, T.; Chetty, S.; Barth, S. Principles of Immunotherapy: Implications for Treatment Strategies in Cancer and Infectious Diseases. Front. Microbiol. 2018, 9, 3158. [Google Scholar] [CrossRef] [Green Version]
- Shanmughapriya, S.; Senthilkumar, G.; Vinodhini, K.; Das, B.C.; Vasanthi, N.; Natarajaseenivasan, K. Viral and bacterial aetiologies of epithelial ovarian cancer. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 2311–2317. [Google Scholar] [CrossRef]
- Ugel, S.; De Sanctis, F.; Mandruzzato, S.; Bronte, V. Tumor-induced myeloid deviation: When myeloid-derived suppressor cells meet tumor-associated macrophages. J. Clin. Investig. 2015, 125, 3365–3376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, E.; Olson, S.H.; Ahn, J.; Bundy, B.; Nishikawa, H.; Qian, F.; Jungbluth, A.A.; Frosina, D.; Gnjatic, S.; Ambrosone, C.; et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 18538–18543. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Tripathy, M.K.; Pasquereau, S.; Al Moussawi, F.; Abbas, W.; Coquard, L.; Khan, K.A.; Russo, L.; Algros, M.-P.; Valmary-Degano, S.; et al. The Human Cytomegalovirus Strain DB Activates Oncogenic Pathways in Mammary Epithelial Cells. EBioMedicine 2018, 30, 167–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dziurzynski, K.; Wei, J.; Qiao, W.; Hatiboglu, M.A.; Kong, L.-Y.; Wu, A.; Wang, Y.; Cahill, D.; Levine, N.; Prabhu, S.; et al. Glioma-Associated Cytomegalovirus Mediates Subversion of the Monocyte Lineage to a Tumor Propagating Phenotype. Clin. Cancer Res. 2011, 17, 4642–4649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bishop, R.K.; Oseguera, C.A.V.; Spencer, J.V. Human Cytomegalovirus interleukin-10 promotes proliferation and migration of MCF-7 breast cancer cells. Cancer Cell Microenviron. 2015, 2. [Google Scholar] [CrossRef] [Green Version]
- Golubovskaya, V.; Wu, L. Different Subsets of T Cells, Memory, Effector Functions, and CAR-T Immunotherapy. Cancers 2016, 8, 36. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Wu, M.; Wang, F. Immune regulation by CD8+ Treg cells: Novel possibilities for anticancer immunotherapy. Cell. Mol. Immunol. 2018, 15, 805–807. [Google Scholar] [CrossRef]
- Zhang, S.; Ke, X.; Zeng, S.; Wu, M.; Lou, J.; Wu, L.; Huang, P.; Huang, L.; Wang, F.; Pan, S. Analysis of CD8+ Treg cells in patients with ovarian cancer: A possible mechanism for immune impairment. Cell. Mol. Immunol. 2015, 12, 580–591. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Bjorkman, P.J. Structure of UL18, a peptide-binding viral MHC mimic, bound to a host inhibitory receptor. Proc. Natl. Acad. Sci. USA 2008, 105, 10095–10100. [Google Scholar] [CrossRef] [Green Version]
- Smith, W.; Tomasec, P.; Aicheler, R.; Loewendorf, A.; Nemčovičová, I.; Wang, E.C.; Stanton, R.J.; Macauley, M.; Norris, P.; Willen, L.; et al. Human Cytomegalovirus Glycoprotein UL141 Targets the TRAIL Death Receptors to Thwart Host Innate Antiviral Defenses. Cell Host Microbe 2013, 13, 324–335. [Google Scholar] [CrossRef] [Green Version]
- Prod’Homme, V.; Sugrue, D.M.; Stanton, R.J.; Nomoto, A.; Davies, J.; Rickards, C.R.; Cochrane, D.; Moore, M.; Wilkinson, G.W.G.; Tomasec, P. Human cytomegalovirus UL141 promotes efficient downregulation of the natural killer cell activating ligand CD112. J. Gen. Virol. 2010, 91, 2034–2039. [Google Scholar] [CrossRef]
- Aiello, A.; Accardi, G.; Candore, G.; Caruso, C.; Colomba, C.; Di Bona, D.; Duro, G.; Gambino, C.M.; Ligotti, M.E.; Pandey, J.P. Role of Immunogenetics in the Outcome of HCMV Infection: Implications for Ageing. Int. J. Mol. Sci. 2019, 20, 685. [Google Scholar] [CrossRef] [Green Version]
- Di Bona, D.; Accardi, G.; Aiello, A.; Bilancia, M.; Candore, G.; Colomba, C.; Caruso, C.; Duro, G.; Gambino, C.M.; Macchia, L.; et al. Association between γ marker, human leucocyte antigens and killer immunoglobulin-like receptors and the natural course of human cytomegalovirus infection: A pilot study performed in a Sicilian population. Immunology 2017, 153, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Pandey, J.P.; Kistner-Griffin, E.; Radwan, F.F.; Kaur, N.; Namboodiri, A.M.; Black, L.; Butler, M.A.; Carreón, T.; Ruder, A.M. Immunoglobulin genes influence the magnitude of humoral immunity to cytomegalovirus glycoprotein B. J. Infect. Dis. 2014, 210, 1823–1826. [Google Scholar] [CrossRef] [Green Version]
- Di Bona, D.; Scafidi, V.; Plaia, A.; Colomba, C.; Nuzzo, D.; Occhino, C.; Tuttolomondo, A.; Giammanco, G.; De Grazia, S.; Montalto, G.; et al. HLA and Killer Cell Immunoglobulin-like Receptors Influence the Natural Course of CMV Infection. J. Infect. Dis. 2014, 210, 1083–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirano, F.; Kaneko, K.; Tamura, H.; Dong, H.; Wang, S.; Ichikawa, M.; Rietz, C.; Flies, D.B.; Lau, J.S.; Zhu, G.; et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005, 65, 1089–1096. [Google Scholar] [PubMed]
- Currie, A.J.; Prosser, A.; McDonnell, A.; Cleaver, A.L.; Robinson, B.W.S.; Freeman, G.J.; Van Der Most, R.G. Dual Control of Antitumor CD8 T Cells through the Programmed Death-1/Programmed Death-Ligand 1 Pathway and Immunosuppressive CD4 T Cells: Regulation and Counterregulation. J. Immunol. 2009, 183, 7898–7908. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Gu, Z.; Chen, Y.; Chen, B.; Chen, W.; Weng, L.; Liu, X. Application of PD-1 Blockade in Cancer Immunotherapy. Comput. Struct. Biotechnol. J. 2019, 17, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Lang, J. Programmed death-1 pathway blockade produces a synergistic antitumor effect: Combined application in ovarian cancer. J. Gynecol. Oncol. 2017, 28, e64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmer, A.S.; Nichols, E.; Cimino-Mathews, A.; Peer, C.; Cao, L.; Lee, M.-J.; Kohn, E.C.; Annunziata, C.M.; Lipkowitz, S.; Trepel, J.B.; et al. A phase I study of the PD-L1 inhibitor, durvalumab, in combination with a PARP inhibitor, olaparib, and a VEGFR1–3 inhibitor, cediranib, in recurrent women’s cancers with biomarker analyses. J. Immunother. Cancer 2019, 7, 197. [Google Scholar] [CrossRef] [PubMed]
- Gallez-Hawkins, G.M.; Thao, L.; Palmer, J.; Dagis, A.; Li, X.; Franck, A.E.; Tegtmeier, B.; Lacey, S.F.; Diamond, D.J.; Forman, S.J.; et al. Increased Programmed Death-1 Molecule Expression in Cytomegalovirus Disease and Acute Graft-versus-Host Disease after Allogeneic Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2009, 15, 872–880. [Google Scholar] [CrossRef] [Green Version]
- Sester, U.; Presser, D.; Dirks, J.; Gärtner, B.C.; Köhler, H.; Sester, M. PD-1 Expression and IL-2 Loss of Cytomegalovirus- Specific T Cells Correlates with Viremia and Reversible Functional Anergy. Arab. Archaeol. Epigr. 2008, 8, 1486–1497. [Google Scholar] [CrossRef]
- A Gutman, J.; Schmidt, C.; Freed, B.; Palmer, B. PD-1 Expression On Total and CMV-Specific T Cells In Early Post Transplant Is Associated With Donor Source, T Cell Maturation Profile, and Effectiveness Of CMV Control. Blood 2013, 122, 2062. [Google Scholar] [CrossRef]
- Kato, T.; Nishida, T.; Murase, M.; Murata, M.; Naoe, T. Exhaustion of CMV Specific T Cells with Enhanced PD-1 Expression In Persistent Cytomegalovirus Infection After Allogeneic Stem Cell Transplantation. Blood 2010, 116, 3912. [Google Scholar] [CrossRef]
- Pesce, S.; Greppi, M.; Tabellini, G.; Rampinelli, F.; Parolini, S.; Olive, D.; Moretta, L.; Moretta, A.; Marcenaro, E. Identification of a subset of human natural killer cells expressing high levels of programmed death 1: A phenotypic and functional characterization. J. Allergy Clin. Immunol. 2017, 139, 335–346.e3. [Google Scholar] [CrossRef] [Green Version]
- Nduom, E.K.; Wei, J.; Yaghi, N.K.; Huang, N.; Kong, L.-Y.; Gabrusiewicz, K.; Ling, X.; Zhou, S.; Ivan, C.; Chen, J.Q.; et al. PD-L1 expression and prognostic impact in glioblastoma. Neuro-Oncology 2016, 18, 195–205. [Google Scholar] [CrossRef] [Green Version]
- Qin, Z.; Zhang, L.; Xu, Y.; Zhang, X.; Fang, X.; Qian, D.; Liu, X.; Liu, T.; Li, L.; Yu, H.; et al. TLR3 regulates PD-L1 expression in human cytomegalovirus infected glioblastoma. Int. J. Clin. Exp. Pathol. 2018, 11, 5318–5326. [Google Scholar]
- Herbein, G. The Human Cytomegalovirus, from Oncomodulation to Oncogenesis. Viruses 2018, 10, 408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, G.; Bivins-Smith, E.R.; Smith, M.S.; Yurochko, A.D. NF-κB and phosphatidylinositol 3-kinase activity mediates the HCMV-induced atypical M1/M2 polarization of monocytes. Virus Res. 2009, 144, 329–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Compton, T.; Kurt-Jones, E.A.; Boehme, K.W.; Belko, J.; Latz, E.; Golenbock, D.T.; Finberg, R.W. Human Cytomegalovirus Activates Inflammatory Cytokine Responses via CD14 and Toll-Like Receptor 2. J. Virol. 2003, 77, 4588–4596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varani, S.; Landini, M.P. Cytomegalovirus-induced immunopathology and its clinical consequences. Herpesviridae 2011, 2, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voigt, V.; Andoniou, C.E.; Schuster, I.S.; Oszmiana, A.; Ong, M.L.; Fleming, P.; Forrester, J.V.; Degli-Esposti, M.A. Cytomegalovirus establishes a latent reservoir and triggers long-lasting inflammation in the eye. PLOS Pathog. 2018, 14, e1007040. [Google Scholar] [CrossRef] [Green Version]
- Van De Berg, P.J.; Heutinck, K.M.; Raabe, R.; Minnee, R.C.; La Young, S.; Pant, K.A.V.D.-V.D.; Bemelman, F.J.; Van Lier, R.A.; Berge, I.J.T. Human Cytomegalovirus Induces Systemic Immune Activation Characterized by a Type 1 Cytokine Signature. J. Infect. Dis. 2010, 202, 690–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forte, E.; Zhang, Z.; Thorp, E.B.; Hummel, M. Cytomegalovirus Latency and Reactivation: An Intricate Interplay With the Host Immune Response. Front. Cell. Infect. Microbiol. 2020, 10, 130. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, Inflammation, and Cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [Green Version]
- Quinn, K.M.; Kartikasari, A.E.R.; Cooke, R.E.; Koldej, R.M.; Ritchie, D.S.; Plebanski, M. Impact of age-, cancer-, and treatment-driven inflammation on T cell function and immunotherapy. J. Leukoc. Biol. 2020, 108, 953–965. [Google Scholar] [CrossRef]
- Rasmussen, C.B.; Kjaer, S.K.; Albieri, V.; Bandera, E.V.; Doherty, J.A.; Høgdall, E.; Webb, P.M.; Jordan, S.J.; Rossing, M.A.; Wicklund, K.G.; et al. Pelvic Inflammatory Disease and the Risk of Ovarian Cancer and Borderline Ovarian Tumors: A Pooled Analysis of 13 Case-Control Studies. Am. J. Epidemiol. 2016, 185, 8–20. [Google Scholar] [CrossRef] [Green Version]
- Risch, H.A.; Howe, G.R. Pelvic inflammatory disease and the risk of epithelial ovarian cancer. Cancer Epidemiol. Biomark. Prev. 1995, 4, 447. [Google Scholar]
- Shu, X.O.; Brinton, L.A.; Gao, Y.T.; Yuan, J.M. Population-based case-control study of ovarian cancer in Shanghai. Cancer Res. 1989, 49, 3670. [Google Scholar]
- Ahmed, N.; Stenvers, K.L. Getting to Know Ovarian Cancer Ascites: Opportunities for Targeted Therapy-Based Translational Research. Front. Oncol. 2013, 3, 256. [Google Scholar] [CrossRef] [Green Version]
- Kipps, E.; Tan, D.S.P.; Kaye, S.B. Meeting the challenge of ascites in ovarian cancer: New avenues for therapy and research. Nat. Rev. Cancer 2013, 13, 273–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Kim, B.; Song, Y.S. Ascites modulates cancer cell behavior, contributing to tumor heterogeneity in ovarian cancer. Cancer Sci. 2016, 107, 1173–1178. [Google Scholar] [CrossRef] [Green Version]
- Kampan, N.C.; Madondo, M.T.; McNally, O.M.; Stephens, A.N.; Quinn, M.A.; Plebanski, M. Interleukin 6 Present in Inflammatory Ascites from Advanced Epithelial Ovarian Cancer Patients Promotes Tumor Necrosis Factor Receptor 2-Expressing Regulatory T Cells. Front. Immunol. 2017, 8, 1482. [Google Scholar] [CrossRef]
- Govindaraj, C.; Scalzo-Inguanti, K.; Madondo, M.; Hallo, J.; Flanagan, K.; Quinn, M.; Plebanski, M. Impaired Th1 immunity in ovarian cancer patients is mediated by TNFR2+ Tregs within the tumor microenvironment. Clin. Immunol. 2013, 149, 97–110. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cox, M.; Kartikasari, A.E.R.; Gorry, P.R.; Flanagan, K.L.; Plebanski, M. Potential Impact of Human Cytomegalovirus Infection on Immunity to Ovarian Tumours and Cancer Progression. Biomedicines 2021, 9, 351. https://doi.org/10.3390/biomedicines9040351
Cox M, Kartikasari AER, Gorry PR, Flanagan KL, Plebanski M. Potential Impact of Human Cytomegalovirus Infection on Immunity to Ovarian Tumours and Cancer Progression. Biomedicines. 2021; 9(4):351. https://doi.org/10.3390/biomedicines9040351
Chicago/Turabian StyleCox, Momodou, Apriliana E. R. Kartikasari, Paul R. Gorry, Katie L. Flanagan, and Magdalena Plebanski. 2021. "Potential Impact of Human Cytomegalovirus Infection on Immunity to Ovarian Tumours and Cancer Progression" Biomedicines 9, no. 4: 351. https://doi.org/10.3390/biomedicines9040351






