Ultra-Small Superparamagnetic Iron-Oxide Nanoparticles Exert Different Effects on Erythrocytes in Normotensive and Hypertensive Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Experiment 1 (Single Administration)
2.3. Experiment 2 (Repeated Administration)
2.4. RBC Deformability
2.5. NO Production by RBCs
2.6. Osmotic Resistance of RBC
2.7. Determination of the USPION-Originated Iron Content in RBCs
2.8. Biochemical Parameters
2.9. Statistical Analyses
3. Results
3.1. Experiment 1
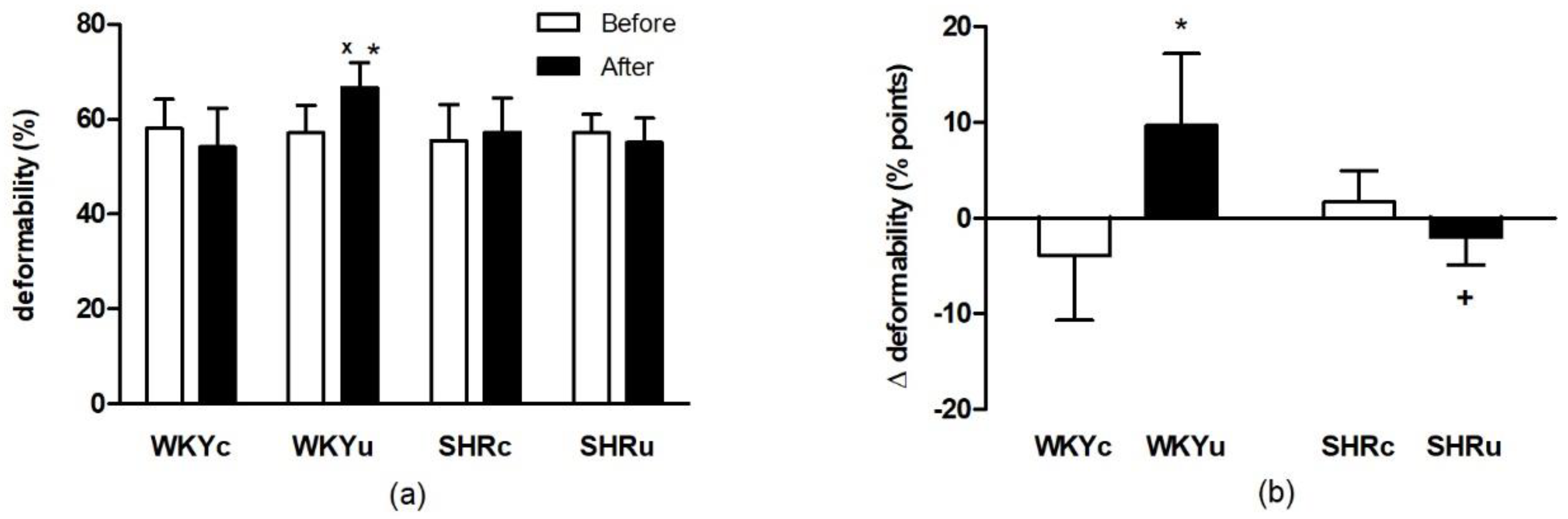
3.1.1. RBC Deformability
3.1.2. NO Production in Red Blood Cells
3.1.3. Total Iron Content in Plasma and USPION-Originated Iron Content in RBCs
3.1.4. Biochemical Analyses
3.2. Experiment 2
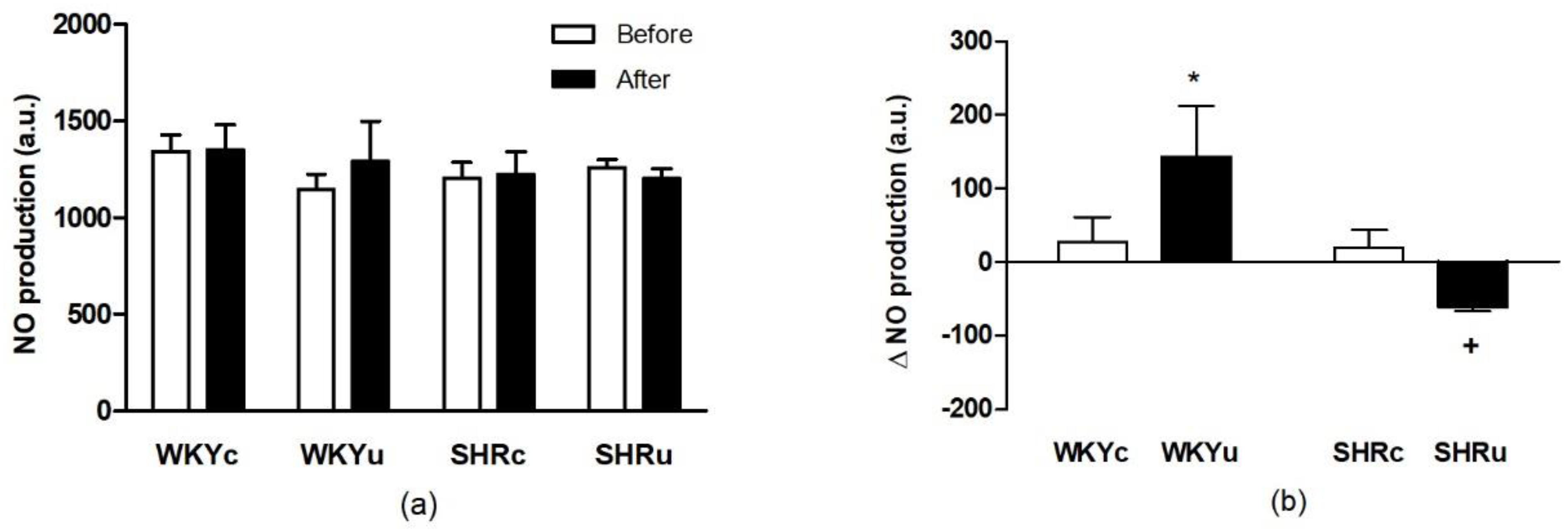
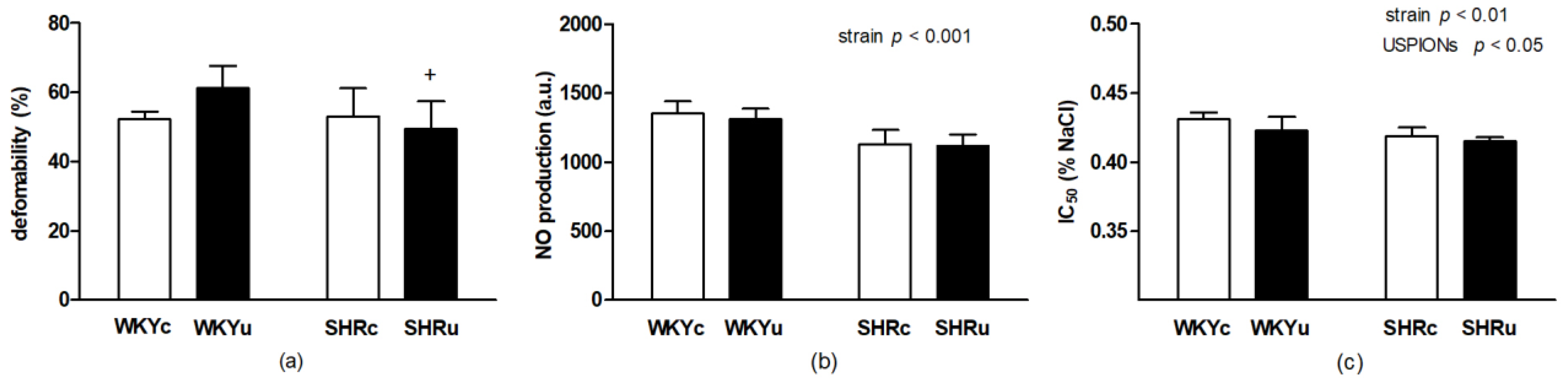
3.2.1. RBC Deformability
3.2.2. RBC NO Production
3.2.3. RBC Osmotic Resistance
3.2.4. Total Iron Content in Plasma and USPION-Originated Iron Content in RBCs
3.2.5. Biochemical Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pernow, J.; Mahdi, A.; Yang, J.; Zhou, Z. Red Blood Cell Dysfunction: A New Player in Cardiovascular Disease. Cardiovasc. Res. 2019, 115, 1596–1605. [Google Scholar] [CrossRef] [Green Version]
- Weisel, J.W.; Litvinov, R.I. Red Blood Cells: The Forgotten Player in Hemostasis and Thrombosis. J. Thromb. Haemost. 2019, 17, 271–282. [Google Scholar] [CrossRef] [Green Version]
- Radosinska, J.; Vrbjar, N. The Role of Red Blood Cell Deformability and Na, K-ATPase Function in Selected Risk Factors of Cardiovascular Diseases in Humans: Focus on Hypertension, Diabetes Mellitus and Hypercholesterolemia. Physiol. Res. 2016, S43–S54. [Google Scholar] [CrossRef]
- Viallat, A.; Abkarian, M. Red Blood Cell: From Its Mechanics to Its Motion in Shear Flow. Int. J. Lab. Hematol. 2014, 36, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Baskurt, O.K.; Meiselman, H.J. Blood Rheology and Hemodynamics. Semin. Thromb. Hemost. 2003, 29, 435–450. [Google Scholar] [CrossRef] [Green Version]
- Bor-Kucukatay, M.; Wenby, R.B.; Meiselman, H.J.; Baskurt, O.K. Effects of Nitric Oxide on Red Blood Cell Deformability. Am. J. Physiol. Circ. Physiol. 2003, 284, H1577–H1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amaiden, M.R.; Santander, V.S.; Monesterolo, N.E.; Nigra, A.D.; Rivelli, J.F.; Campetelli, A.N.; Pie, J.; Casale, C.H. Effects of Detyrosinated Tubulin on Na+, K+-ATPase Activity and Erythrocyte Function in Hypertensive Subjects. FEBS Lett. 2015, 589, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Radosinska, J.; Jasenovec, T.; Puzserova, A.; Krajcir, J.; Lacekova, J.; Kucerova, K.; Kalnovicova, T.; Tothova, L.; Kovacicova, I.; Vrbjar, N. Promotion of Whole Blood Rheology after Vitamin C Supplementation: Focus on Red Blood Cells 1. Can. J. Physiol. Pharmacol. 2019, 97, 837–843. [Google Scholar] [CrossRef]
- Cortese-Krott, M.M.; Kelm, M. Endothelial Nitric Oxide Synthase in Red Blood Cells: Key to a New Erythrocrine Function? Redox Biol. 2014, 2, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, S.; Tsuchikura, S.; Iida, H. Age-Related Changes in Blood Pressure, Hematological Values, Concentrations of Serum Biochemical Constituents and Weights of Organs in the SHR/Izm, SHRSP/Izm and WKY/Izm. Exp. Anim. 2004, 53, 67–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lominadze, D.; Joshua, I.G.; Schuschke, D.A. Increased Erythrocyte Aggregation in Spontaneously Hypertensive Rats. Am. J. Hypertens. 1998, 11, 784–789. [Google Scholar] [CrossRef] [Green Version]
- Ariyoshi, K.; Maruyama, T.; Odashiro, K.; Akashi, K.; Fujino, T.; Uyesaka, N. Impaired Erythrocyte Filterability of Spontaneously Hypertensive Rats: Investigation by Nickel Filtration Technique. Circ. J. 2010, 74, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Amaiden, M.R.; Monesterolo, N.E.; Santander, V.S.; Campetelli, A.N.; Arce, C.A.; Pie, J.; Hope, S.I.; Vatta, M.S.; Casale, C.H. Involvement of Membrane Tubulin in Erythrocyte Deformability and Blood Pressure. J. Hypertens. 2012, 30, 1414–1422. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.V.; Kitts, D.D.; Godin, D.V. Heart and Red Blood Cell Antioxidant Status and Plasma Lipid Levels in the Spontaneously Hypertensive and Normotensive Wistar-Kyoto Rat. Can. J. Physiol. Pharmacol. 1996, 74, 290–297. [Google Scholar] [CrossRef]
- Chan, T.C.; Godin, D.V.; Sutter, M.C. Erythrocyte Membrane Abnormalities in Hypertension: A Comparison between Two Animal Models. Clin. Exp. Hypertens. A 1983, 5, 691–719. [Google Scholar] [CrossRef]
- Postnov, Y.U.; Orlov, S.; Gulak, P.; Shevchenko, A. Altered Permeability of the Erythrocyte Membrane for Sodium and Potassium Ions in Spontaneously Hypertensive Rats. Pflugers Arch. 1976, 365, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Huang, S.; Yu, K.J.; Clyne, A.M. Dextran and Polymer Polyethylene Glycol (PEG) Coating Reduce Both 5 and 30 Nm Iron Oxide Nanoparticle Cytotoxicity in 2D and 3D Cell Culture. Int. J. Mol. Sci. 2012, 13, 5554–5570. [Google Scholar] [CrossRef] [Green Version]
- Estelrich, J.; Busquets, M.A. Iron Oxide Nanoparticles in Photothermal Therapy. Molecules 2018, 23, 1567. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.; Jenkins, G.J.S.; Asadi, R.; Doak, S.H. Potential Toxicity of Superparamagnetic Iron Oxide Nanoparticles (SPION). Nano Rev. 2010, 1, 5358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoudi, M.; Simchi, A.; Imani, M.; Häfeli, U.O. Superparamagnetic Iron Oxide Nanoparticles with Rigid Cross-Linked Polyethylene Glycol Fumarate Coating for Application in Imaging and Drug Delivery. J. Phys. Chem. C 2009, 113, 8124–8131. [Google Scholar] [CrossRef]
- Ruiz, A.; Ali, L.M.A.; Cáceres-Vélez, P.R.; Cornudella, R.; Gutiérrez, M.; Moreno, J.A.; Piñol, R.; Palacio, F.; Fascineli, M.L.; de Azevedo, R.B.; et al. Hematotoxicity of Magnetite Nanoparticles Coated with Polyethylene Glycol: In Vitro and in Vivo Studies. Toxicol. Res. 2015, 4, 1555–1564. [Google Scholar] [CrossRef] [Green Version]
- Karabasz, A.; Szczepanowicz, K.; Cierniak, A.; Bereta, J.; Bzowska, M. In Vitro Toxicity Studies of Biodegradable, Polyelectrolyte Nanocapsules. Int. J. Nanomed. 2018, 13, 5159–5172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roacho-Pérez, J.A.; Ruiz-Hernandez, F.G.; Chapa-Gonzalez, C.; Martínez-Rodríguez, H.G.; Flores-Urquizo, I.A.; Pedroza-Montoya, F.E.; Garza-Treviño, E.N.; Bautista-Villareal, M.; García-Casillas, P.E.; Sánchez-Domínguez, C.N. Magnetite Nanoparticles Coated with PEG 3350-Tween 80: In Vitro Characterization Using Primary Cell Cultures. Polymers 2020, 12, 300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.X. Superparamagnetic iron oxide based MRI contrast agents: Current status of clinical application. Quant. Imaging Med. Surg. 2011, 1, 35–40. [Google Scholar]
- Líšková, S.; Bališ, P.; Mičurová, A.; Kluknavský, M.; Okuliarová, M.; Puzserová, A.; Škrátek, M.; Sekaj, I.; Maňka, J.; Valovič, P.; et al. Effect of Iron Oxide Nanoparticles on Vascular Function and Nitric Oxide Production in Acute Stress-Exposed Rats. Physiol. Res. 2020, 69, 1067–1083. [Google Scholar] [CrossRef] [PubMed]
- Škrátek, M.; Dvurečenskij, A.; Kluknavský, M.; Barta, A.; Bališ, P.; Mičurová, A.; Cigáň, A.; Eckstein-Andicsová, A.; Maňka, J.; Bernátová, I. Sensitive SQUID Bio-Magnetometry for Determination and Differentiation of Biogenic Iron and Iron Oxide Nanoparticles in the Biological Samples. Nanomaterials 2020, 10, 1993. [Google Scholar] [CrossRef]
- Radosinska, J.; Mezesova, L.; Okruhlicova, L.; Frimmel, K.; Breierova, E.; Bartekova, M.; Vrbjar, N. Effect of Yeast Biomass with High Content of Carotenoids on Erythrocyte Deformability, NO Production and Na,K-ATPase Activity in Healthy and LPS Treated Rats. Clin. Hemorheol. Microcirc. 2016, 64, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Kluknavsky, M.; Balis, P.; Skratek, M.; Manka, J.; Bernatova, I. (-)-Epicatechin Reduces the Blood Pressure of Young Borderline Hypertensive Rats During the Post-Treatment Period. Antioxidants 2020, 9, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonelli, A.; Szwargulski, P.; Scarpa, E.-S.; Thieben, F.; Cordula, G.; Ambrosi, G.; Guidi, L.; Ludewig, P.; Knopp, T.; Magnani, M. Development of Long Circulating Magnetic Particle Imaging Tracers: Use of Novel Magnetic Nanoparticles and Entrapment into Human Erythrocytes. Nanomedicine 2020, 15, 739–753. [Google Scholar] [CrossRef]
- Nikitin, M.P.; Zelepukin, I.V.; Shipunova, V.O.; Sokolov, I.L.; Deyev, S.M.; Nikitin, P.I. Enhancement of the Blood-Circulation Time and Performance of Nanomedicines via the Forced Clearance of Erythrocytes. Nat. Biomed. Eng. 2020, 4, 717–731. [Google Scholar] [CrossRef]
- Neun, B.W.; Ilinskaya, A.N.; Dobrovolskaia, M.A. Updated Method for In Vitro Analysis of Nanoparticle Hemolytic Properties. In Characterization of Nanoparticles Intended for Drug Delivery; Methods in Molecular Biology; McNeil, S.E., Ed.; Springer: New York, NY, USA, 2018; pp. 91–102. [Google Scholar] [CrossRef]
- Schlenk, F.; Werner, S.; Rabel, M.; Jacobs, F.; Bergemann, C.; Clement, J.H.; Fischer, D. Comprehensive Analysis of the in Vitro and Ex Ovo Hemocompatibility of Surface Engineered Iron Oxide Nanoparticles for Biomedical Applications. Arch. Toxicol. 2017, 91, 3271–3286. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Poon, W.; Tavares, A.J.; McGilvray, I.D.; Chan, W.C.W. Nanoparticle-Liver Interactions: Cellular Uptake and Hepatobiliary Elimination. J. Control. Release 2016, 240, 332–348. [Google Scholar] [CrossRef]
- Simmonds, M.J.; Detterich, J.A.; Connes, P. Nitric Oxide, Vasodilation and the Red Blood Cell. Biorheology 2014, 51, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Zalba, G.; Beaumont, F.J.; San José, G.; Fortuño, A.; Fortuño, M.A.; Díez, J. Is the Balance between Nitric Oxide and Superoxide Altered in Spontaneously Hypertensive Rats with Endothelial Dysfunction? Nephrol. Dial. Transplant. 2001, 16 (Suppl. S1), 2–5. [Google Scholar] [CrossRef]
- Moss, M.B.; Brunini, T.M.C.; Soares De Moura, R.; Novaes Malagris, L.E.; Roberts, N.B.; Ellory, J.C.; Mann, G.E.; Mendes Ribeiro, A.C. Diminished L-Arginine Bioavailability in Hypertension. Clin. Sci. 2004, 107, 391–397. [Google Scholar] [CrossRef] [Green Version]
- Tsuda, K.; Minatogawa, Y.; Nishio, I.; Masuyama, Y. Increased Osmotic Fragility of Erythrocytes in Essential Hypertension. Clin. Exp. Hypertens. A 1984, 6, 2235–2247. [Google Scholar] [CrossRef]
- Fasanmade, A.A. Erythrocyte Osmotic Fragility in Hypertension and during Diuretic Therapy. West. Afr. J. Med. 1999, 18, 183–186. [Google Scholar]
- Kitts, D.D.; Yuan, Y.V.; Godin, D.V. Plasma and Lipoprotein Lipid Composition and Hepatic Antioxidant Status in Spontaneously Hypertensive (SHR) and Normotensive (WKY) Rats. Can. J. Physiol. Pharmacol. 1998, 76, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Cartland, S.P.; Tamer, N.; Patil, M.S.; Di Bartolo, B.A.; Kavurma, M.M. A “Western Diet” Promotes Symptoms of Hepatic Steatosis in Spontaneously Hypertensive Rats. Int. J. Exp. Path. 2020, 101, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Brookes, M.J.; Cooper, B.T. Hypertension and Fatty Liver: Guilty by Association? J. Hum. Hypertens. 2007, 21, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Frith, J.; Day, C.P.; Henderson, E.; Burt, A.D.; Newton, J.L. Non-Alcoholic Fatty Liver Disease in Older People. Gerontology 2009, 55, 607–613. [Google Scholar] [CrossRef]
- Rahman, S.; Islam, S.; Haque, T.; Kathak, R.R.; Ali, N. Association between Serum Liver Enzymes and Hypertension: A Cross-Sectional Study in Bangladeshi Adults. BMC Cardiovasc. Disord. 2020, 20, 128. [Google Scholar] [CrossRef] [PubMed]
- Miguel-Carrasco, J.L.; Zambrano, S.; Blanca, A.J.; Mate, A.; Vázquez, C.M. Captopril reduces cardiac inflammatory markers in spontaneously hypertensive rats by inactivation of NF-kB. J. Inflamm. 2010, 7, 21. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Gao, Y.H.; Tian, D.K.; Zheng, J.P.; Zhu, C.Y.; Ke, Y.; Bian, K. Inflammation of different tissues in spontaneously hypertensive rats. Sheng Li Xue Bao 2006, 58, 318–323. [Google Scholar] [PubMed]
- Heijnen, B.F.; Van Essen, H.; Schalkwijk, C.G.; Janssen, B.J.; Struijker-Boudier, H.A. Renal inflammatory markers during the onset of hypertension in spontaneously hypertensive rats. Hypertens. Res. 2014, 37, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, E.; Kell, D.B. Diagnostic morphology: Biophysical indicators for iron-driven inflammatory diseases. Integr. Biol. 2014, 6, 486–510. [Google Scholar] [CrossRef] [Green Version]


| Experiment 1 | |||||||
|---|---|---|---|---|---|---|---|
| Parameter | WKY | SHR | Two-Way ANOVA | ||||
| Control | USPIONs | Control | USPIONs | Strain | Intervention | Interaction | |
| Calcium (mmol/L) | 2.5 ± 0.1 | 2.4 ± 0.1 | 2.3 ± 0.2 | 2.4 ± 0.1 | ns | ns | ns |
| Potassium (mmol/L) | 6.2 ± 1.6 | 5.8 ± 1.0 | 5.8 ± 0.4 | 5.9 ± 0.5 | ns | ns | ns |
| Magnesium (mmol/L) | 0.93 ± 0.08 | 0.96 ± 0.14 | 0.88 ± 0.04 | 0.87 ± 0.12 | ns | ns | ns |
| Sodium (mmol/L) | 143.3 ± 1.8 | 143.6 ± 2.5 | 140.8 ± 2.5 | 140.0 ± 2.3 | F(1,20) = 10.4 p < 0.005 | ns | ns |
| AP (U/L) | 106.0 ± 7.4 | 94.3 ± 12.5 | 98.2 ± 16.7 | 110.9 ± 10.8 | ns | ns | F(1,22) = 4.47 p < 0.05 |
| ALT (U/L) | 29.1 ± 7.6 | 29.3 ± 4.5 | 31.0 ± 4.7 | 29.1 ± 5.6 | ns | ns | ns |
| LDH (U/L) | 1092 ± 743 | 1124 ± 662 | 1338 ± 434 | 1124 ± 328 | ns | ns | ns |
| AST (U/L) | 90.2 ± 53.6 | 69.1 ± 19.6 | 116.7 ± 35.1 | 99.85 ± 21.6 | ns | ns | ns |
| Cholesterol (mmol/L) | 2.7 ± 0.4 | 2.8 ± 0.4 | 1.3 ± 0.1 * | 1.5 ± 0.2 + | F(1,21) = 10.75 p < 0.0001 | ns | ns |
| Triglycerides (mmol/L) | 0.54 ± 0.15 | 0.91 ± 0.45 | 0.44 ± 0.11 | 0.67 ± 0.31 | ns | F(1.21) = 5.39 p < 0.05 | ns |
| Uric acid (µmol/L) | 37.8 ± 4.4 | 47.0 ± 20.1 | 61.5 ± 14.8 | 46.6 ± 14.5 | ns | ns | ns |
| Creatinine (µmol/L) | 31.5 ± 3.0 | 32.0 ± 5.1 | 29.3 ± 2.4 | 28.3 ± 0.5 | ns | ns | ns |
| Inorg. P (mmol/L) | 2.40 ± 0.14 | 2.36 ± 0.20 | 2.35 ± 0.33 | 2.14 ± 0.27 | ns | ns | ns |
| Total proteins (g/L) | 58.8 ± 5.6 | 57.7 ± 4.6 | 55.5 ± 5.8 | 55.7 ± 4.3 | ns | ns | ns |
| Albumins (g/L) | 39.3 ± 3.3 | 38.7 ± 3.2 | 37.4 ± 3.6 | 39.4 ± 3.5 | ns | ns | ns |
| Urea (mmol/L) | 6.2 ± 0.5 | 6.6 ± 1.2 | 6.1 ± 1.1 | 5.8 ± 0.5 | ns | ns | ns |
| Experiment 2 | |||||||
|---|---|---|---|---|---|---|---|
| Parameter | WKY | SHR | Two-Way ANOVA | ||||
| Control | USPIONs | Control | USPIONs | Strain | Intervention | Interaction | |
| Calcium (mmol/L) | 2.6 ± 0.04 | 2.6 ± 0.1 | 2.6 ± 0.1 | 2.6 ± 0.1 | ns | ns | ns |
| Potassium (mmol/L) | 5.6 ± 0.4 | 5.2 ± 0.3 | 5.6 ± 0.4 | 5.8 ± 0.6 | ns | ns | ns |
| Magnesium (mmol/L) | 1.03 ± 0.13 | 0.94 ± 0.05 | 0.97 ± 0.04 | 0.95 ± 0.05 | ns | ns | ns |
| Sodium (mmol/L) | 141.3 ± 1.1 | 140.3 ± 1.1 | 140.4 ± 1.1 | 140.6 ± 1.3 | ns | ns | ns |
| AP (U/L) | 94.0 ± 14.0 | 93.3 ± 7.8 | 113.0 ± 4.2 * | 113.9 ± 12.4 + | F(1,24) = 24.48 p < 0.0001 | ns | ns |
| ALT (U/L) | 34.76 ± 5.5 | 44.7 ± 23.0 | 35.9 ± 9.7 | 30.8 ± 7.9 | ns | ns | ns |
| LDH (U/L) | 926 ± 143 | 960 ± 133 | 970 ± 142 | 1036 ± 232 | ns | ns | ns |
| AST (U/L) | 93.3 ± 15.3 | 100.5 ± 36.2 | 89.7 ± 11.3 | 85.8 ± 16.6 | ns | ns | ns |
| Cholesterol (mmol/L) | 2.9 ± 0.5 | 2.8 ± 0.2 | 1.8 ± 0.1 *** | 1.7 ± 0.2 +++ | F(1,26) = 88.69 p < 0.0001 | ns | ns |
| Triglycerides (mmol/l) | 1.27 ± 0.16 | 1.42 ± 0.36 | 1.14 ± 0.13 | 1.11 ± 0.21 | F(1,26) = 7.025 p < 0.05 | ns | ns |
| Uric acid (µmol/L) | 38.9 ± 7.0 | 32.4 ± 6.2 | 87.5 ± 22 *** | 47.6 ± 3.5 ### | F(1,26) = 49.14 p < 0.0001 | F(1,26) = 25.85 p < 0.0001 | F(1,26) = 13.48 p < 0.0001 |
| Creatinine (µmol/L) | 31.4 ± 4.4 | 27 ± 1.3 * | 31.6 ± 1.3 | 27.0 ± 1.6 # | ns | F(1,25) = 23.57 p < 0.0001 | ns |
| Inorganic P (mmol/L) | 2.37 ± 0.21 | 2.37 ± 0.05 | 2.16 ± 0.20 | 2.03 ± 0.15 ++ | F(1,25) = 19.27 p < 0.0005 | ns | ns |
| Total proteins (g/L) | 63.3 ± 3.8 | 59.6 ± 5.8 | 62.2 ± 1.9 | 62.8 ± 2.5 | ns | ns | ns |
| Albumins (g/L) | 40.7 ± 1.6 | 37.6 ± 3.7 | 41.6 ± 1.6 | 40.7 ± 1.7 | F(1,26) = 5.657 p < 0.05 | F(1,26) = 5.947 p < 0.05 | ns |
| Urea (mmol/L) | 7.0 ± 1.2 | 7.3 ± 1.6 | 8.4 ± 1.2 | 7.7 ± 0.7 | ns | ns | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radosinska, J.; Jasenovec, T.; Radosinska, D.; Balis, P.; Puzserova, A.; Skratek, M.; Manka, J.; Bernatova, I. Ultra-Small Superparamagnetic Iron-Oxide Nanoparticles Exert Different Effects on Erythrocytes in Normotensive and Hypertensive Rats. Biomedicines 2021, 9, 377. https://doi.org/10.3390/biomedicines9040377
Radosinska J, Jasenovec T, Radosinska D, Balis P, Puzserova A, Skratek M, Manka J, Bernatova I. Ultra-Small Superparamagnetic Iron-Oxide Nanoparticles Exert Different Effects on Erythrocytes in Normotensive and Hypertensive Rats. Biomedicines. 2021; 9(4):377. https://doi.org/10.3390/biomedicines9040377
Chicago/Turabian StyleRadosinska, Jana, Tomas Jasenovec, Dominika Radosinska, Peter Balis, Angelika Puzserova, Martin Skratek, Jan Manka, and Iveta Bernatova. 2021. "Ultra-Small Superparamagnetic Iron-Oxide Nanoparticles Exert Different Effects on Erythrocytes in Normotensive and Hypertensive Rats" Biomedicines 9, no. 4: 377. https://doi.org/10.3390/biomedicines9040377






