Long COVID-19 Syndrome: A Comprehensive Review of Its Effect on Various Organ Systems and Recommendation on Rehabilitation Plans
Abstract
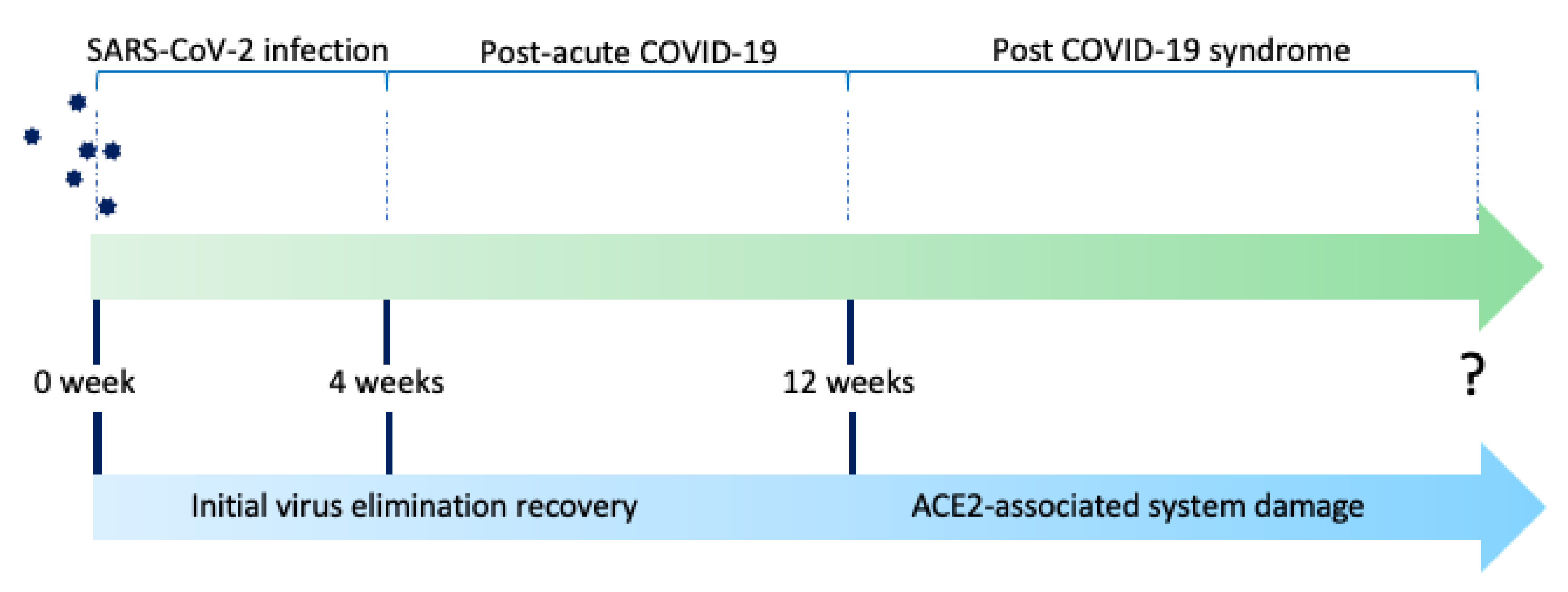
:1. Introduction
2. The Effects of Long COVID-19 Syndrome on Various Systems
2.1. Respiratory System
2.2. Cardiovascular System
2.3. Haematological System
2.4. Renal System
2.5. Digestive System
2.6. Neurological System
2.7. Metabolic System
3. Recommendation of Follow-Up
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Weekly Epidemiological Update on COVID-19—11 May 2021. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---11-may-2021 (accessed on 20 June 2021).
- World Health Organization. COVID-19 Weekly Epidemiological Update. 2021. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---22-june-2021 (accessed on 22 June 2021).
- Dennis, A.; Wamil, M.; Alberts, J.; Oben, J.; Cuthbertson, D.J.; Wootton, D.; Crooks, M.; Gabbay, M.; Brady, M.; Hishmeh, L.; et al. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: A prospective, community-based study. BMJ Open 2021, 11, e048391. [Google Scholar] [PubMed]
- Rubin, R. As Their Numbers Grow, COVID-19 “Long Haulers” Stump Experts. JAMA 2020, 324, 1381–1383. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, T.; Knight, M.; A’Court, C.; Buxton, M.; Husain, L. Management of post-acute covid-19 in primary care. BMJ 2020, 370, m3026. [Google Scholar]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and predictors of long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, P. NICE guideline on long COVID. Lancet Respir. Med. 2021, 9, 129. [Google Scholar] [CrossRef]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.; Cuapio, A.; Villapol, S. More than 50 Long-Term Effects of COVID-19: A Systematic Review and Meta-Analysis. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Petersen, M.S.; Kristiansen, M.F.; Hanusson, K.D.; Danielsen, M.E.; Steig, B.; Gaini, S.; Strøm, M.; Weihe, P. Long COVID in the Faroe Islands—A longitudinal study among non-hospitalized patients. Clin. Infect. Dis. 2020, ciaa1792. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.P.; Yang, M.; Lai, C.L. COVID-19 Vaccines: A Review of the Safety and Efficacy of Current Clinical Trials. Pharmaceuticals 2021, 14, 406. [Google Scholar] [CrossRef]
- Mori, J.; Oudit, G.Y.; Lopaschuk, G.D. SARS-CoV-2 perturbs the renin-angiotensin system and energy metabolism. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E43–E47. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Lai, C.L. SARS-CoV-2 infection: Can ferroptosis be a potential treatment target for multiple organ involvement? Cell Death Discov. 2020, 6, 130. [Google Scholar] [CrossRef]
- Gheblawi, M.; Wang, K.; Viveiros, A.; Nguyen, Q.; Zhong, J.C.; Turner, A.J.; Raizada, M.K.; Grant, M.B.; Oudit, G.Y. Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System: Celebrating the 20th Anniversary of the Discovery of ACE2. Circ. Res. 2020, 126, 1456–1474. [Google Scholar] [CrossRef]
- World Health Organization. Laboratory Testing of Human Suspected Cases of Novel Coronavirus (nCOV) Infection (Interim Guidance). 2020. Available online: https://apps.who.int/iris/bitstream/handle/10665/330374/WHO-2019-nCoV-laboratory-2020.1-eng.pdf (accessed on 29 June 2021).
- Ahmed, H.; Patel, K.; Greenwood, D.C.; Halpin, S.; Lewthwaite, P.; Salawu, A.; Eyre, L.; Breen, A.; O’Connor, R.; Jones, A.; et al. Long-term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: A systematic review and meta-analysis. J. Rehabil. Med. 2020, 52, jrm00063. [Google Scholar]
- Hui, D.S.; Joynt, G.M.; Wong, K.T.; Gomersall, C.D.; Li, T.S.; Antonio, G.; Ko, F.W.; Chan, M.C.; Chan, D.P.; Tong, M.W.; et al. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax 2005, 60, 401–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, K.C.; Ng, A.W.; Lee, L.S.; Kaw, G.; Kwek, S.K.; Leow, M.K.; Earnest, A. Pulmonary function and exercise capacity in survivors of severe acute respiratory syndrome. Eur. Respir. J. 2004, 24, 436–442. [Google Scholar] [CrossRef] [Green Version]
- Daugherty, S.E.; Guo, Y.; Heath, K.; Dasmarinas, M.C.; Jubilo, K.G.; Samranvedhya, J.; Lipsitch, M.; Cohen, K. Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: Retrospective cohort study. BMJ 2021, 373, n1098. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Mo, X.; Jian, W.; Su, Z.; Chen, M.; Peng, H.; Peng, P.; Lei, C.; Chen, R.; Zhong, N.; Li, S. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur. Respir. J. 2020, 55, 2001217. [Google Scholar] [CrossRef] [PubMed]
- Froidure, A.; Mahsouli, A.; Liistro, G.; De Greef, J.; Belkhir, L.; Gerard, L.; Bertrand, A.; Koenig, S.; Pothen, L.; Yildiz, H.; et al. Integrative respiratory follow-up of severe COVID-19 reveals common functional and lung imaging sequelae. Respir. Med. 2021, 181, 106383. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Sonnweber, T.; Sahanic, S.; Pizzini, A.; Luger, A.; Schwabl, C.; Sonnweber, B.; Kurz, K.; Koppelstatter, S.; Haschka, D.; Petzer, V.; et al. Cardiopulmonary recovery after COVID-19: An observational prospective multicentre trial. Eur. Respir. J. 2021, 57, 2003481. [Google Scholar] [CrossRef]
- Ambardar, S.R.; Hightower, S.L.; Huprikar, N.A.; Chung, K.K.; Singhal, A. Post-COVID-19 Pulmonary Fibrosis: Novel Sequelae of the Current Pandemic. J. Clin. Med. 2021, 10, 2452. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, C.; Hu, Y.; Li, C.; Ren, Q.; Zhang, X.; Shi, H.; Zhou, M. Temporal Changes of CT Findings in 90 Patients with COVID-19 Pneumonia: A Longitudinal Study. Radiology 2020, 296, E55–E64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, L.; Khan, A.; Zhou, W.; Dai, Y.; Md, E.; Chen, R.; Cheng, G. Follow-up study of clinical and chest CT scans in confirmed COVID-19 patients. Radiol. Infect. Dis. 2020, 7, 106–113. [Google Scholar] [CrossRef]
- Barisione, E.; Grillo, F.; Ball, L.; Bianchi, R.; Grosso, M.; Morbini, P.; Pelosi, P.; Patroniti, N.A.; De Lucia, A.; Orengo, G.; et al. Fibrotic progression and radiologic correlation in matched lung samples from COVID-19 post-mortems. Virchows Arch. 2021, 478, 471–485. [Google Scholar] [CrossRef]
- Huang, W.; Wu, Q.; Chen, Z.; Xiong, Z.; Wang, K.; Tian, J.; Zhang, S. The potential indicators for pulmonary fibrosis in survivors of severe COVID-19. J. Infect. 2021, 82, e5–e7. [Google Scholar] [CrossRef]
- Chen, J.Y.; Qiao, K.; Liu, F.; Wu, B.; Xu, X.; Jiao, G.Q.; Lu, R.G.; Li, H.X.; Zhao, J.; Huang, J.; et al. Lung transplantation as therapeutic option in acute respiratory distress syndrome for coronavirus disease 2019-related pulmonary fibrosis. Chin. Med. J. 2020, 133, 1390–1396. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, J.; Wang, S.; Li, X.; Zhou, J.; Huang, B.; Luo, D.; Cao, Q.; Chen, Y.; Chen, S.; et al. Progression to fibrosing diffuse alveolar damage in a series of 30 minimally invasive autopsies with COVID-19 pneumonia in Wuhan, China. Histopathology 2021, 78, 542–555. [Google Scholar] [CrossRef] [PubMed]
- Ostergaard, L. SARS CoV-2 related microvascular damage and symptoms during and after COVID-19: Consequences of capillary transit-time changes, tissue hypoxia and inflammation. Physiol. Rep. 2021, 9, e14726. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.P.; Blet, A.; Smyth, D.; Li, H. The Science Underlying COVID-19: Implications for the Cardiovascular System. Circulation 2020, 142, 68–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giamarellos-Bourboulis, E.J.; Netea, M.G.; Rovina, N.; Akinosoglou, K.; Antoniadou, A.; Antonakos, N.; Damoraki, G.; Gkavogianni, T.; Adami, M.E.; Katsaounou, P.; et al. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe 2020, 27, 992–1000.e3. [Google Scholar] [CrossRef] [PubMed]
- Mitrani, R.D.; Dabas, N.; Goldberger, J.J. COVID-19 cardiac injury: Implications for long-term surveillance and outcomes in survivors. Heart Rhythm 2020, 17, 1984–1990. [Google Scholar] [CrossRef] [PubMed]
- Townsend, L.; Fogarty, H.; Dyer, A.; Martin-Loeches, I.; Bannan, C.; Nadarajan, P.; Bergin, C.; O’Farrelly, C.; Conlon, N.; Bourke, N.M.; et al. Prolonged elevation of D-dimer levels in convalescent COVID-19 patients is independent of the acute phase response. J. Thromb. Haemost. 2021, 19, 1064–1070. [Google Scholar] [CrossRef]
- Roncon, L.; Zuin, M.; Zonzin, P. Age-adjusted D-dimer cut-off levels to rule out venous thromboembolism in COVID-19 patients. Thromb. Res. 2020, 190, 102. [Google Scholar] [CrossRef]
- Henry, B.M.; de Oliveira, M.H.S.; Benoit, S.; Plebani, M.; Lippi, G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): A meta-analysis. Clin. Chem. Lab. Med. 2020, 58, 1021–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Jin, Y.; Li, R.; Zhang, Z.; Sun, R.; Chen, D. Prevalence and impact of acute renal impairment on COVID-19: A systematic review and meta-analysis. Crit. Care 2020, 24, 356. [Google Scholar] [CrossRef]
- Ng, J.H.; Hirsch, J.S.; Hazzan, A.; Wanchoo, R.; Shah, H.H.; Malieckal, D.A.; Ross, D.W.; Sharma, P.; Sakhiya, V.; Fishbane, S.; et al. Outcomes among Patients Hospitalized with COVID-19 and Acute Kidney Injury. Am. J. Kidney Dis. 2021, 77, 204–215.e1. [Google Scholar] [CrossRef] [PubMed]
- Post, A.; den Deurwaarder, E.S.G.; Bakker, S.J.L.; de Haas, R.J.; van Meurs, M.; Gansevoort, R.T.; Berger, S.P. Kidney Infarction in Patients with COVID-19. Am. J. Kidney Dis. 2020, 76, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Pei, G.; Zhang, Z.; Peng, J.; Liu, L.; Zhang, C.; Yu, C.; Ma, Z.; Huang, Y.; Liu, W.; Yao, Y.; et al. Renal Involvement and Early Prognosis in Patients with COVID-19 Pneumonia. J. Am. Soc. Nephrol. 2020, 31, 1157–1165. [Google Scholar] [CrossRef]
- Monkemuller, K.; Fry, L.; Rickes, S. COVID-19, coronavirus, SARS-CoV-2 and the small bowel. Rev. Esp. Enferm. Dig. 2020, 112, 383–388. [Google Scholar] [PubMed]
- Wu, Y.; Guo, C.; Tang, L.; Hong, Z.; Zhou, J.; Dong, X.; Yin, H.; Xiao, Q.; Tang, Y.; Qu, X.; et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. Hepatol. 2020, 5, 434–435. [Google Scholar] [CrossRef]
- Xu, Y.; Li, X.; Zhu, B.; Liang, H.; Fang, C.; Gong, Y.; Guo, Q.; Sun, X.; Zhao, D.; Shen, J.; et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat. Med. 2020, 26, 502–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Effenberger, M.; Grabherr, F.; Mayr, L.; Schwaerzler, J.; Nairz, M.; Seifert, M.; Hilbe, R.; Seiwald, S.; Scholl-Buergi, S.; Fritsche, G.; et al. Faecal calprotectin indicates intestinal inflammation in COVID-19. Gut 2020, 69, 1543–1544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbara, G.; Feinle-Bisset, C.; Ghoshal, U.C.; Quigley, E.M.; Santos, J.; Vanner, S.; Vergnolle, N.; Zoetendal, E.G. The Intestinal Microenvironment and Functional Gastrointestinal Disorders. Gastroenterology 2016, 150, 1305–1318.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De-Madaria, E.; Siau, K.; Cardenas-Jaen, K. Increased Amylase and Lipase in Patients with COVID-19 Pneumonia: Don’t Blame the Pancreas Just Yet! Gastroenterology 2021, 160, 1871. [Google Scholar] [CrossRef] [PubMed]
- Pribadi, R.R.; Simadibrata, M. Increased serum amylase and/or lipase in coronavirus disease 2019 (COVID-19) patients: Is it really pancreatic injury? JGH Open 2021, 5, 190–192. [Google Scholar] [CrossRef] [PubMed]
- Nordvig, A.S.; Fong, K.T.; Willey, J.Z.; Thakur, K.T.; Boehme, A.K.; Vargas, W.S.; Smith, C.J.; Elkind, M.S.V. Potential Neurologic Manifestations of COVID-19. Neurol. Clin. Pract. 2021, 11, e135–e146. [Google Scholar] [CrossRef] [PubMed]
- Fotuhi, M.; Mian, A.; Meysami, S.; Raji, C.A. Neurobiology of COVID-19. J. Alzheimer’s Dis. 2020, 76, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Garrigues, E.; Janvier, P.; Kherabi, Y.; Le Bot, A.; Hamon, A.; Gouze, H.; Doucet, L.; Berkani, S.; Oliosi, E.; Mallart, E.; et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J. Infect. 2020, 81, e4–e6. [Google Scholar] [CrossRef]
- Miners, S.; Kehoe, P.G.; Love, S. Cognitive impact of COVID-19: Looking beyond the short term. Alzheimer’s Res. Ther. 2020, 12, 170. [Google Scholar] [CrossRef]
- Lee, M.H.; Perl, D.P.; Nair, G.; Li, W.; Maric, D.; Murray, H.; Dodd, S.J.; Koretsky, A.P.; Watts, J.A.; Cheung, V.; et al. Microvascular Injury in the Brains of Patients with Covid-19. N. Engl. J. Med. 2021, 384, 481–483. [Google Scholar] [CrossRef]
- Kaseda, E.T.; Levine, A.J. Post-traumatic stress disorder: A differential diagnostic consideration for COVID-19 survivors. Clin. Neuropsychol. 2020, 34, 1498–1514. [Google Scholar] [CrossRef]
- Chee, Y.J.; Ng, S.J.H.; Yeoh, E. Diabetic ketoacidosis precipitated by Covid-19 in a patient with newly diagnosed diabetes mellitus. Diabetes Res. Clin. Pract. 2020, 164, 108166. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Chen, J.; Zuo, X.; Zhang, H.; Deng, A. COVID-19 infection may cause ketosis and ketoacidosis. Diabetes Obes. Metab. 2020, 22, 1935–1941. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Yang, Y.; Wang, F.; Yan, Y.; Shi, X.; Dong, K.; Yu, X.; Zhang, S. Association of the insulin resistance marker TyG index with the severity and mortality of COVID-19. Cardiovasc. Diabetol. 2020, 19, 58. [Google Scholar] [CrossRef]
- Suwanwongse, K.; Shabarek, N. Newly diagnosed diabetes mellitus, DKA, and COVID-19: Causality or coincidence? A report of three cases. J. Med. Virol. 2021, 93, 1150–1153. [Google Scholar] [CrossRef]
- Tzotzos, S.J.; Fischer, B.; Fischer, H.; Zeitlinger, M. Incidence of ARDS and outcomes in hospitalized patients with COVID-19: A global literature survey. Crit. Care 2020, 24, 516. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C.; et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef] [Green Version]
- Frija-Masson, J.; Debray, M.P.; Gilbert, M.; Lescure, F.X.; Travert, F.; Borie, R.; Khalil, A.; Crestani, B.; d’Ortho, M.P.; Bancal, C. Functional characteristics of patients with SARS-CoV-2 pneumonia at 30 days post-infection. Eur. Respir. J. 2020, 56, 2001754. [Google Scholar] [CrossRef]
- Mandal, S.; Barnett, J.; Brill, S.E.; Brown, J.S.; Denneny, E.K.; Hare, S.S.; Heightman, M.; Hillman, T.E.; Jacob, J.; Jarvis, H.C.; et al. Long-COVID: A cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax 2021, 76, 396–398. [Google Scholar] [CrossRef]
- Daher, A.; Balfanz, P.; Cornelissen, C.; Muller, A.; Bergs, I.; Marx, N.; Muller-Wieland, D.; Hartmann, B.; Dreher, M.; Muller, T. Follow up of patients with severe coronavirus disease 2019 (COVID-19): Pulmonary and extrapulmonary disease sequelae. Respir. Med. 2020, 174, 106197. [Google Scholar] [CrossRef] [PubMed]
- Lund, L.C.; Hallas, J.; Nielsen, H.; Koch, A.; Mogensen, S.H.; Brun, N.C.; Christiansen, C.F.; Thomsen, R.W.; Pottegard, A. Post-acute effects of SARS-CoV-2 infection in individuals not requiring hospital admission: A Danish population-based cohort study. Lancet Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Klok, F.A.; Kruip, M.; van der Meer, N.J.M.; Arbous, M.S.; Gommers, D.; Kant, K.M.; Kaptein, F.H.J.; van Paassen, J.; Stals, M.A.M.; Huisman, M.V.; et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020, 191, 145–147. [Google Scholar] [CrossRef]
- Helms, J.; Tacquard, C.; Severac, F.; Leonard-Lorant, I.; Ohana, M.; Delabranche, X.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Fagot Gandet, F.; et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Med. 2020, 46, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, L.F.; Kroft, L.J.M.; van der Wal, L.I.; Cannegieter, S.C.; Eikenboom, J.; de Jonge, E.; Huisman, M.V.; Klok, F.A. Clinical and computed tomography characteristics of COVID-19 associated acute pulmonary embolism: A different phenotype of thrombotic disease? Thromb. Res. 2020, 193, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, R.T.; Gopalan, D.; Howard, L.; Vicente, A.; Park, M.; Manalan, K.; Wallner, I.; Marsden, P.; Dave, S.; Branley, H.; et al. Beyond the clot: Perfusion imaging of the pulmonary vasculature after COVID-19. Lancet Respir. Med. 2021, 9, 107–116. [Google Scholar] [CrossRef]
- Gasecka, A.; Borovac, J.A.; Guerreiro, R.A.; Giustozzi, M.; Parker, W.; Caldeira, D.; Chiva-Blanch, G. Thrombotic Complications in Patients with COVID-19: Pathophysiological Mechanisms, Diagnosis, and Treatment. Cardiovasc. Drugs Ther. 2021, 35, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.V.; Arachchillage, D.J.; Ridge, C.A.; Bianchi, P.; Doyle, J.F.; Garfield, B.; Ledot, S.; Morgan, C.; Passariello, M.; Price, S.; et al. Pulmonary Angiopathy in Severe COVID-19: Physiologic, Imaging, and Hematologic Observations. Am. J. Respir. Crit. Care Med. 2020, 202, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Heiss, R.; Grodzki, D.M.; Horger, W.; Uder, M.; Nagel, A.M.; Bickelhaupt, S. High-performance low field MRI enables visualization of persistent pulmonary damage after COVID-19. Magn. Reson. Imaging 2021, 76, 49–51. [Google Scholar] [CrossRef] [PubMed]
- University of Oxford. New National Study of Long-Term Impacts of Debilitating Lung Damage from COVID-19. 2021. Available online: https://www.manchester.ac.uk/discover/news/new-national-study-of-long-term-impacts-of-debilitating-lung-damage-from-covid-19/ (accessed on 22 June 2021).
- Diao, B.; Wang, C.; Tan, Y.; Chen, X.; Liu, Y.; Ning, L.; Chen, L.; Li, M.; Liu, Y.; Wang, G.; et al. Reduction and Functional Exhaustion of T Cells in Patients with Coronavirus Disease 2019 (COVID-19). Front. Immunol. 2020, 11, 827. [Google Scholar] [CrossRef]
- Sollini, M.; Ciccarelli, M.; Cecconi, M.; Aghemo, A.; Morelli, P.; Gelardi, F.; Chiti, A. Vasculitis changes in COVID-19 survivors with persistent symptoms: An [18F]FDG-PET/CT study. Eur. J. Nucl. Med. Mol. Imaging 2020, 48, 1460–1466. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.S.; Lee, A.G.; Renz, J.; DeSantis, K.; Liang, J.; Powell, C.A.; Ventetuolo, C.E.; Poor, H.D. Reply to Cherian et al.: Positive Bubble Study in Severe COVID-19 Indicates the Development of Anatomical Intrapulmonary Shunts in Response to Microvascular Occlusion. Am. J. Respir. Crit. Care Med. 2021, 203, 265–266. [Google Scholar] [CrossRef] [PubMed]
- Gerotziafas, G.T.; Catalano, M.; Colgan, M.P.; Pecsvarady, Z.; Wautrecht, J.C.; Fazeli, B.; Olinic, D.M.; Farkas, K.; Elalamy, I.; Falanga, A.; et al. Guidance for the Management of Patients with Vascular Disease or Cardiovascular Risk Factors and COVID-19: Position Paper from VAS-European Independent Foundation in Angiology/Vascular Medicine. Thromb. Haemost. 2020, 120, 1597–1628. [Google Scholar] [CrossRef]
- Del Rio, C.; Collins, L.F.; Malani, P. Long-term Health Consequences of COVID-19. JAMA 2020, 324, 1723–1724. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, R.; Sinclair, H.C.; Patel, K.; Low, B.; Pericao, A.; Manisty, C.; Guttmann, O.; Zemrak, F.; Miller, O.; Longhi, P.; et al. Delayed-onset myocarditis following COVID-19. Lancet Respir. Med. 2021, 9, e32–e34. [Google Scholar] [CrossRef]
- Eiros, R.; Barreiro-Perez, M.; Martin-Garcia, A.; Almeida, J.; Villacorta, E.; Perez-Pons, A.; Merchan, S.; Torres-Valle, A.; Pablo, C.S.; González-Calle, D.; et al. Pericarditis and myocarditis long after SARS-CoV-2 infection: A cross-sectional descriptive study in health-care workers. medRxiv 2020. [Google Scholar] [CrossRef]
- Wang, D.; Li, S.; Jiang, J.; Yan, J.; Zhao, C.; Wang, Y.; Ma, Y.; Zeng, H.; Guo, X.; Wang, H.; et al. Chinese society of cardiology expert consensus statement on the diagnosis and treatment of adult fulminant myocarditis. Sci. China Life Sci. 2019, 62, 187–202. [Google Scholar] [CrossRef] [Green Version]
- Sawalha, K.; Abozenah, M.; Kadado, A.J.; Battisha, A.; Al-Akchar, M.; Salerno, C.; Hernandez-Montfort, J.; Islam, A.M. Systematic Review of COVID-19 Related Myocarditis: Insights on Management and Outcome. Cardiovasc. Revasc. Med. 2021, 23, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.D.; Boursiquot, B.C.; Melki, L.; Wan, E.Y. Management of Arrhythmias Associated with COVID-19. Curr. Cardiol. Rep. 2020, 23, 2. [Google Scholar] [CrossRef]
- Gasior, M.; Jaroszewicz, J.; Wita, K.; Ciesla, D.; Hudzik, B. High post-discharge mortality in hospitalized COVID-19 patients with cardiovascular comorbidities. Pol. Arch. Intern. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Lavery, A.M.; Preston, L.E.; Ko, J.Y.; Chevinsky, J.R.; DeSisto, C.L.; Pennington, A.F.; Kompaniyets, L.; Datta, S.D.; Click, E.S.; Golden, T.; et al. Characteristics of Hospitalized COVID-19 Patients Discharged and Experiencing Same-Hospital Readmission—United States, March-August 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1695–1699. [Google Scholar] [CrossRef]
- Chopra, V.; Flanders, S.A.; O’Malley, M.; Malani, A.N.; Prescott, H.C. Sixty-Day Outcomes among Patients Hospitalized with COVID-19. Ann. Intern. Med. 2021, 174, 576–578. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Tang, N.; Peluso, M.J.; Iyer, N.S.; Torres, L.; Donatelli, J.L.; Munter, S.E.; Nixon, C.C.; Rutishauser, R.L.; Rodriguez-Barraquer, I.; et al. Characterization and Biomarker Analyses of Post-COVID-19 Complications and Neurological Manifestations. Cells 2021, 10, 386. [Google Scholar] [CrossRef]
- Fogarty, H.; Townsend, L.; Ni Cheallaigh, C.; Bergin, C.; Martin-Loeches, I.; Browne, P.; Bacon, C.L.; Gaule, R.; Gillett, A.; Byrne, M.; et al. COVID19 coagulopathy in Caucasian patients. Br. J. Haematol. 2020, 189, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Maksimowski, N.; Williams, V.R.; Scholey, J.W. Kidney ACE2 expression: Implications for chronic kidney disease. PLoS ONE 2020, 15, e0241534. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.; Abo Omirah, M.; Hussein, A.; Saeed, H. Assessment and characterisation of post-COVID-19 manifestations. Int. J. Clin. Pract. 2020, 75, e13746. [Google Scholar] [CrossRef] [PubMed]
- Zarebska-Michaluk, D.; Jaroszewicz, J.; Rogalska, M.; Lorenc, B.; Rorat, M.; Szymanek-Pasternak, A.; Piekarska, A.; Berkan-Kawinska, A.; Sikorska, K.; Tudrujek-Zdunek, M.; et al. Impact of Kidney Failure on the Severity of COVID-19. J. Clin. Med. 2021, 10, 2042. [Google Scholar] [CrossRef] [PubMed]
- Nugent, J.; Aklilu, A.; Yamamoto, Y.; Simonov, M.; Li, F.; Biswas, A.; Ghazi, L.; Greenberg, J.; Mansour, S.; Moledina, D.; et al. Assessment of Acute Kidney Injury and Longitudinal Kidney Function after Hospital Discharge among Patients with and without COVID-19. JAMA Netw. Open 2021, 4, e211095. [Google Scholar] [CrossRef]
- Gupta, S.; Coca, S.G.; Chan, L.; Melamed, M.L.; Brenner, S.K.; Hayek, S.S.; Sutherland, A.; Puri, S.; Srivastava, A.; Leon-berg-Yoo, A.; et al. AKI Treated with Renal Replacement Therapy in Critically Ill Patients with COVID-19. J. Am. Soc. Nephrol. 2021, 32, 161–176. [Google Scholar] [CrossRef]
- Stockmann, H.; Hardenberg, J.B.; Aigner, A.; Hinze, C.; Gotthardt, I.; Stier, B.; Eckardt, K.U.; Schmidt-Ott, K.M.; Enghard, P. High rates of long-term renal recovery in survivors of coronavirus disease 2019-associated acute kidney injury requiring kidney replacement therapy. Kidney Int. 2021, 99, 1021–1022. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Reis, T.; Husain-Syed, F. Management of acute kidney injury in patients with COVID-19. Lancet Respir. Med. 2020, 8, 738–742. [Google Scholar] [CrossRef]
- Minami, T.; Iwata, Y.; Wada, T. Renal complications in coronavirus disease 2019: A systematic review. Inflamm. Regen. 2020, 40, 31. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Hirsch, J.S.; Ng, J.H.; Ross, D.W.; Sharma, P.; Shah, H.H.; Barnett, R.L.; Hazzan, A.D.; Fishbane, S.; Jhaveri, K.D.; Northwell COVID-19 Research Consortium; et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020, 98, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Rong, L.; Nian, W.; He, Y. Review article: Gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol. Ther. 2020, 51, 843–851. [Google Scholar] [CrossRef]
- Schmulson, M.; Davalos, M.F.; Berumen, J. Beware: Gastrointestinal symptoms can be a manifestation of COVID-19. Rev. Gastroenterol. Mex. 2020, 85, 282–287. [Google Scholar] [CrossRef]
- Matthai, J.; Shanmugam, N.; Sobhan, P.; Indian Society of Pediatric Gastroenterology, Hepatology and Nutrition; Pediatric Gastroenterology Chapter of Indian Academy of Pediatrics. Coronavirus Disease (COVID-19) and the Gastrointestinal System in Children. Indian Pediatr. 2020, 57, 533–535. [Google Scholar] [CrossRef]
- Xing, Y.H.; Ni, W.; Wu, Q.; Li, W.J.; Li, G.J.; Wang, W.D.; Tong, J.N.; Song, X.F.; Wing-Kin Wong, G.; Xing, Q.S. Prolonged viral shedding in feces of pediatric patients with coronavirus disease 2019. J. Microbiol. Immunol. Infect. 2020, 53, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, V.; Motwani, R.; Kumar, A.; Kumari, C.; Raza, K. Histopathological observations in COVID-19: A systematic review. J. Clin. Pathol. 2021, 74, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Zhang, F.; Lui, G.C.Y.; Yeoh, Y.K.; Li, A.Y.L.; Zhan, H.; Wan, Y.; Chung, A.C.K.; Cheung, C.P.; Chen, N.; et al. Alterations in Gut Microbiota of Patients with COVID-19 during Time of Hospitalization. Gastroenterology 2020, 159, 944–955.e8. [Google Scholar] [CrossRef]
- Schmulson, M.; Ghoshal, U.C.; Barbara, G. Managing the Inevitable Surge of Post-COVID-19 Functional Gastrointestinal Disorders. Am. J. Gastroenterol. 2021, 116, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Iacobucci, G. Long covid: Damage to multiple organs presents in young, low risk patients. BMJ 2020, 371, m4470. [Google Scholar] [CrossRef]
- Zhong, P.; Xu, J.; Yang, D.; Shen, Y.; Wang, L.; Feng, Y.; Du, C.; Song, Y.; Wu, C.; Hu, X.; et al. COVID-19-associated gastrointestinal and liver injury: Clinical features and potential mechanisms. Signal. Transduct. Target Ther. 2020, 5, 256. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; the Northwell COVID-19 Research Consortium. Presenting Characteristics, Comorbidities, and Outcomes among 5700 Patients Hospitalized with COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- An, Y.W.; Song, S.; Li, W.X.; Chen, Y.X.; Hu, X.P.; Zhao, J.; Li, Z.W.; Jiang, G.Y.; Wang, C.; Wang, J.C.; et al. Liver function recovery of COVID-19 patients after discharge, a follow-up study. Int. J. Med. Sci. 2021, 18, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Marjot, T.; Moon, A.M.; Cook, J.A.; Abd-Elsalam, S.; Aloman, C.; Armstrong, M.J.; Pose, E.; Brenner, E.J.; Cargill, T.; Catana, M.A.; et al. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: An international registry study. J. Hepatol. 2021, 74, 567–577. [Google Scholar] [CrossRef]
- Wang, F.; Wang, H.; Fan, J.; Zhang, Y.; Wang, H.; Zhao, Q. Pancreatic Injury Patterns in Patients with Coronavirus Disease 19 Pneumonia. Gastroenterology 2020, 159, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhao, J.; Peng, J.; Li, X.; Deng, X.; Geng, Z.; Shen, Z.; Guo, F.; Zhang, Q.; Jin, Y.; et al. Detection of SARS-CoV-2 in saliva and characterization of oral symptoms in COVID-19 patients. Cell Prolif. 2020, 53, e12923. [Google Scholar] [CrossRef] [PubMed]
- Troyer, E.A.; Kohn, J.N.; Hong, S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav. Immun. 2020, 87, 34–39. [Google Scholar] [CrossRef]
- Coolen, T.; Lolli, V.; Sadeghi, N.; Rovai, A.; Trotta, N.; Taccone, F.S.; Creteur, J.; Henrard, S.; Goffard, J.C.; Dewitte, O.; et al. Early postmortem brain MRI findings in COVID-19 non-survivors. Neurology 2020, 95, e2016–e2027. [Google Scholar] [CrossRef] [PubMed]
- Radnis, C.; Qiu, S.; Jhaveri, M.; Da Silva, I.; Szewka, A.; Koffman, L. Radiographic and clinical neurologic manifestations of COVID-19 related hypoxemia. J. Neurol. Sci. 2020, 418, 117119. [Google Scholar] [CrossRef] [PubMed]
- Yong, S.J. Persistent Brainstem Dysfunction in Long-COVID: A Hypothesis. ACS Chem. Neurosci. 2021, 12, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Guedj, E.; Million, M.; Dudouet, P.; Tissot-Dupont, H.; Bregeon, F.; Cammilleri, S.; Raoult, D. 18F-FDG brain PET hypometabolism in post-SARS-CoV-2 infection: Substrate for persistent/delayed disorders? Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 592–595. [Google Scholar] [CrossRef] [PubMed]
- Stefano, G.B.; Ptacek, R.; Ptackova, H.; Martin, A.; Kream, R.M. Selective Neuronal Mitochondrial Targeting in SARS-CoV-2 Infection Affects Cognitive Processes to Induce ’Brain Fog’ and Results in Behavioral Changes that Favor Viral Survival. Med. Sci. Monit. 2021, 27, e930886. [Google Scholar] [CrossRef] [PubMed]
- Stefano, G.B. Historical Insight into Infections and Disorders Associated with Neurological and Psychiatric Sequelae Similar to Long COVID. Med. Sci. Monit. 2021, 27, e931447. [Google Scholar] [CrossRef] [PubMed]
- Sollini, M.; Morbelli, S.; Ciccarelli, M.; Cecconi, M.; Aghemo, A.; Morelli, P.; Chiola, S.; Gelardi, F.; Chiti, A. Long COVID hallmarks on [18F]FDG-PET/CT: A case-control study. Eur. J. Nucl. Med. Mol. Imaging 2021. [Google Scholar] [CrossRef] [PubMed]
- Mazza, M.G.; De Lorenzo, R.; Conte, C.; Poletti, S.; Vai, B.; Bollettini, I.; Melloni, E.M.T.; Furlan, R.; Ciceri, F.; Rovere-Querini, P.; et al. Anxiety and depression in COVID-19 survivors: Role of inflammatory and clinical predictors. Brain Behav. Immun. 2020, 89, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Olanipekun, T.; Abe, T.; Effoe, V.; Westney, G.; Snyder, R. Incidence and Severity of Depression among Recovered African Americans with COVID-19-Associated Respiratory Failure. J. Racial. Ethn. Health Disparities 2021. [Google Scholar] [CrossRef]
- Cai, X.; Hu, X.; Ekumi, I.O.; Wang, J.; An, Y.; Li, Z.; Yuan, B. Psychological Distress and Its Correlates among COVID-19 Survivors During Early Convalescence Across Age Groups. Am. J. Geriatr. Psychiatry 2020, 28, 1030–1039. [Google Scholar] [CrossRef]
- Hyoty, H.; Taylor, K.W. The role of viruses in human diabetes. Diabetologia 2002, 45, 1353–1361. [Google Scholar] [PubMed] [Green Version]
- Honeyman, M.C.; Stone, N.L.; Harrison, L.C. T-cell epitopes in type 1 diabetes autoantigen tyrosine phosphatase IA-2: Potential for mimicry with rotavirus and other environmental agents. Mol. Med. 1998, 4, 231–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyoty, H.; Leinikki, P.; Reunanen, A.; Ilonen, J.; Surcel, H.M.; Rilva, A.; Kaar, M.L.; Huupponen, T.; Hakulinen, A.; Makela, A.L.; et al. Mumps infections in the etiology of type 1 (insulin-dependent) diabetes. Diabetes Res. 1988, 9, 111–116. [Google Scholar]
- Pak, C.Y.; Eun, H.M.; McArthur, R.G.; Yoon, J.W. Association of cytomegalovirus infection with autoimmune type 1 diabetes. Lancet 1988, 332, 1–4. [Google Scholar] [CrossRef]
- Yang, J.K.; Lin, S.S.; Ji, X.J.; Guo, L.M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010, 47, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Papachristou, S.; Stamatiou, I.; Stoian, A.P.; Papanas, N. New-Onset Diabetes in COVID-19: Time to Frame Its Fearful Symmetry. Diabetes Ther. 2021, 12, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Boppana, A.; Traupman, J.A.; Unson, E.; Maddock, D.A.; Chao, K.; Dobesh, D.P.; Brufsky, A.; Connor, R.I. Impaired glucose metabolism in patients with diabetes, prediabetes, and obesity is associated with severe COVID-19. J. Med. Virol. 2021, 93, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Brigham, E.; O’Toole, J.; Kim, S.Y.; Friedman, M.; Daly, L.; Kaplin, A.; Swarthout, M.; Hasselfeld, B.; Lantz-Garnish, M.; Vannorsdall, T.; et al. The Johns Hopkins Post-Acute COVID-19 Team (PACT): A Multidisciplinary, Collaborative, Ambulatory Framework Supporting COVID-19 Survivors. Am. J. Med. 2021, 134, 462–467.e1. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Niloofa, R.; Jayarajah, U.; De Mel, S.; Abeysuriya, V.; Seneviratne, S.L. Hematological Abnormalities in COVID-19: A Narrative Review. Am. J. Trop. Med. Hyg. 2021, 104, 1188–1201. [Google Scholar] [CrossRef] [PubMed]
- Agbuduwe, C.; Basu, S. Haematological manifestations of COVID-19: From cytopenia to coagulopathy. Eur. J. Haematol. 2020, 105, 540–546. [Google Scholar] [CrossRef] [PubMed]
- British Thoracic Society. Guidance on Respiratory Follow up of Patients with a Clinico-Radiological Diagnosis of COVID-19 Pneumonia. 2020. Available online: https://www.brit-thoracic.org.uk (accessed on 29 July 2021).
- McGroder, C.F.; Zhang, D.; Choudhury, M.A.; Salvatore, M.M.; D’Souza, B.M.; Hoffman, E.A.; Wei, Y.; Baldwin, M.R.; Garcia, C.K. Pulmonary fibrosis 4 months after COVID-19 is associated with severity of illness and blood leucocyte telomere length. Thorax 2021. [Google Scholar] [CrossRef] [PubMed]
- Funke-Chambour, M.; Bridevaux, P.O.; Clarenbach, C.F.; Soccal, P.M.; Nicod, L.P.; von Garnier, C. Swiss Recommendations for the Follow-Up and Treatment of Pulmonary Long COVID. Respiration 2021, 100, 826–841. [Google Scholar] [CrossRef]
- Ali, R.M.M.; Ghonimy, M.B.I. Post-COVID-19 pneumonia lung fibrosis: A worrisome sequelae in surviving patients. Egypt. J. Radiol. Nucl. Med. 2021, 52, 101. [Google Scholar] [CrossRef]
- Stam, H.J.; Stucki, G.; Bickenbach, J.; European Academy of Rehabilitation Medicine. Covid-19 and Post Intensive Care Syndrome: A Call for Action. J. Rehabil. Med. 2020, 52, jrm00044. [Google Scholar] [CrossRef]
- Kiekens, C.; Boldrini, P.; Andreoli, A.; Avesani, R.; Gamna, F.; Grandi, M.; Lombardi, F.; Lusuardi, M.; Molteni, F.; Perboni, A.; et al. Rehabilitation and respiratory management in the acute and early post-acute phase. “Instant paper from the field” on rehabilitation answers to the COVID-19 emergency. Eur. J. Phys. Rehabil. Med. 2020, 56, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Chinese Association of Rehabilitation; Respiratory Rehabilitation Committee of Chinese Association of Rehabilitation; Cardiopulmonary Rehabilitation Group of Chinese Society of Physical Rehabilitation. Recommendations for respiratory rehabilitation of coronavirus disease 2019 in adult. Zhonghua Jie He He Hu Xi Za Zhi 2020, 43, 308–314. [Google Scholar]
- Sheehy, L.M. Considerations for Postacute Rehabilitation for Survivors of COVID-19. JMIR Public Health Surveill. 2020, 6, e19462. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Chau, B.; Lui, M.; Lam, G.T.; Lin, N.; Humbert, S. Physical Medicine and Rehabilitation and Pulmonary Rehabilitation for COVID-19. Am. J. Phys. Med. Rehabil. 2020, 99, 769–774. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, W.; Yang, Y.; Zhang, J.; Li, Y.; Chen, Y. Respiratory rehabilitation in elderly patients with COVID-19: A randomized controlled study. Complement. Ther. Clin. Pract. 2020, 39, 101166. [Google Scholar] [CrossRef] [PubMed]
- Parkin, A.; Davison, J.; Tarrant, R.; Ross, D.; Halpin, S.; Simms, A.; Salman, R.; Sivan, M.A. A Multidisciplinary NHS COVID-19 Service to Manage Post-COVID-19 Syndrome in the Community. J. Prim. Care Community Health 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Carvalho-Schneider, C.; Laurent, E.; Lemaignen, A.; Beaufils, E.; Bourbao-Tournois, C.; Laribi, S.; Flament, T.; Ferreira-Maldent, N.; Bruyere, F.; Stefic, K.; et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin. Microbiol. Infect. 2021, 27, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Klok, F.A.; Boon, G.; Barco, S.; Endres, M.; Geelhoed, J.J.M.; Knauss, S.; Rezek, S.A.; Spruit, M.A.; Vehreschild, J.; Siegerink, B. The Post-COVID-19 Functional Status scale: A tool to measure functional status over time after COVID-19. Eur. Respir. J. 2020, 56, 2001494. [Google Scholar] [CrossRef] [PubMed]

| Systems | Main Diagnosis | Features | Possible Mechanisms | Prognosis | References |
|---|---|---|---|---|---|
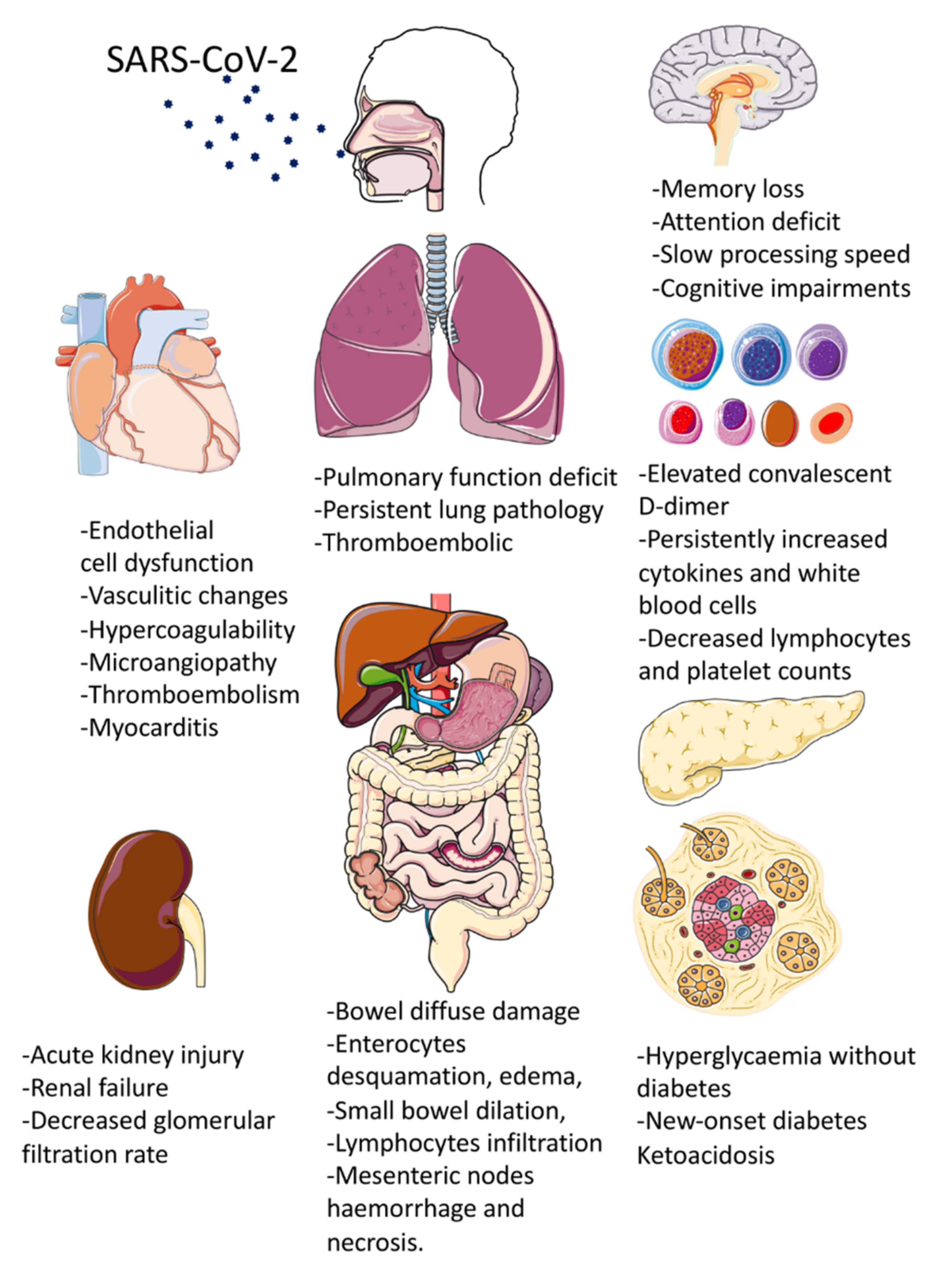
| Respiratory system | Acute respiratory distress syndrome (ARDS) | Extensive bilateral diffuse alveolar damage with cellular fibromyxoid exudates; desquamation of pneumocytes and hyaline membrane formations; diffusion impairment and pulmonary fibrosis. | SARS-CoV-2 spike S1 domain protein binding to ACE2 receptor; Post Acute Respiratory Distress Syndrome fibrosis with diffuse alveolar damage | Pulmonary function deficit 6 months after infection; extensive diffuse impairment; Long-term in-situ thrombosis | [19,20,21,22,23,24,25,26,27,28,29,30] |
| Cardiovascular system | Endothelitis; Micro-thrombosis, Capillary damage; hypercoagulability; microangiopathy; thromboembolism; myocarditis; atrial fibrillation; supraventricular tachycardia | Increased target-to-blood pool ratio; capillary disturbance; impaired oxygen diffusion. | Cytokine storm and macrophage activating syndrome-caused endothelial dysfunction. | Majority (81%) of the COVID-19 myocarditis patients survived the acute episode; ongoing subclinical myocarditis may evolve into myocardial dysfunction and sudden cardiac death. | [31,32,33,34,35] |
| Haematological system | Thromboembolism | Elevated convalescent D-dimer and C-reactive protein levels; persistently increased biomarkers of inflammation. | N/A | Prognostic biomarkers for monitoring clinical progression of Long COVID-19 patients need to be investigated | [36,37,38] |
| Urinary system | Acute kidney injury; renal failure; | Declined glomerular filtration rate (eGFR); kidney infarction | High abundance pf ACE2 expression in kidneys. | Significant risks of mortality and morbidity | [39,40,41,42] |
| Digestive system | Gastrointestinal impairment and dysfunction; hepatitic and cholestatic liver injury; pancreatic injury | Bowel diffuse damage; Enterocytes desquamation, edema, small bowel dilation, lymphocytes infiltration and mesenteric nodes hemorrhage and necrosis. | Rich in ACE2 and furin expression; fecal-oral transmission; plasma cells and lymphocytic infiltrations into lamina propria of intestinal tissues. | The liver enzymes remained persistently elevated 14 days after discharge, while the liver functions in majority survivors normalized 2 months after hospital discharge | [43,44,45,46,47,48,49] |
| Neurological system | Mood changes; cognitive difficulties; headache; fatigue; dizziness; memory loss; confusion; and attention deficit. | Hypoxic injury; microbleedings; neuronal inflammations. | Blood vessel damage, impaired oxygen supply, viral infiltration into the central nervous system and inflammatory cytokines-mediated cellular damage; indirect T-cell and microglia damage in the brain, similar to strokes and neuroinflammatory diseases. | Over 40% survivors without prior psychiatric conditions lived with depression within 90 days of recovery from severe COVID-19 associated respiratory failure, while 70% of them did not receive treatment for depression | [50,51,52,53,54,55] |
| Metabolic system | Hyperglycaemia without diabetic mellitus; new-onset diabetic mellitus; starvation ketoacidosis | High blood glucose level; impaired glucose metabolism | Intruding pancreatic β-islet cells, triggering autoimmune responses because of the exposure of the antigen from damaged islet cells. | Long-term treatment of diabetic mellitus is needed. | [56,57,58,59] |
| Systems | Main Diagnosis | Features | Useful Investigation Tools | Abnormalities to Look for | References |
|---|---|---|---|---|---|
| Respiratory system | Acute respiratory distress syndrome (ARDS) | Extensive bilateral diffuse alveolar damage with cellular fibromyxoid exudates; desquamation of pneumocytes and hyaline membrane formations; diffusion impairment. | Pulmonary function tests, high resolution CT, histology; pulmonary angiopathy | Restrictive pulmonary function test; Impaired gas transfer; reduced total lung capacity; fibrotic features on imaging; diffuse alveolar damage on histology; pulmonary vasculature | [20,25,27,28,69,71] |
| Cardiovascular system | Endothelitis; micro-thrombosis, capillary damage; Hypercoagulability; microangiopathy; thromboembolism; myocarditis; atrial fibrillation; supraventricular tachycardia | Increased target-to-blood pool ratio; capillary disturbance; impaired oxygen diffusion. |
| Microcirculation disturbance; Increased target-to-blood pool ratio; impaired oxygen diffusion; myocardial inflammation; rhythmic abnormality | [31,32,33,34,35] |
| Haematological system | Thromboembolism | Elevated convalescent D-dimer and C-reactive protein levels; Persistently increased biomarkers of inflammation. |
| Thrombocytopenia; blood cell abnormalities | [132,133] |
| Urinary system | Acute kidney injury; renal failure; | Declined glomerular filtration rate (eGFR); kidney infarction | Urine analysis; glomerular filtration rate; ultrasound scanning; MRA; renal biopsy. | Early recognition of kidney involvement; kidney injury; renal infarction | [40,41,96] |
| Digestive system | Gastrointestinal impairment and dysfunction; Hepatitic and cholestatic liver injury; pancreatic injury | Bowel diffuse damage; Enterocytes desquamation, edema, small bowel dilation, lymphocytes infiltration and mesenteric nodes hemorrhage and necrosis. | Barium beefsteak meal; colorectal transit study; computed tomography scan (CT or CAT scan); defecography; Lower gastrointestinal series; MRI; magnetic resonance cholangiopancreatography (MRCP); oropharyngeal motility (swallowing) study. | Bowel damage; high fecal calprotectin level; gastrointestinal dysfunction; liver injury; pancreatic injury; hyperamylasemia | [43,44,45,46,47,48,49] |
| Neurological system | Mood changes; cognitive difficulties; headache; fatigue; dizziness; memory loss; confusion; and attention deficit. | Hypoxic injury; microbleedings; neuronal inflammations. | CT scan; electroencephalogram; MRI; electrodiagnostic tests, such as electromyography (EMG) and nerve conduction velocity (NCV); positron emission tomography (PET); arteriogram (angiogram); lumbar puncture; evoked potentials. | Neurological symptoms; ischemic damages to cerebral white matter; blood vessel damage; hypoxic injury, microbleedings, and neuronal inflammations in different areas of the brain; brian hypometabolism | [50,51,52,53,54,55] |
| Metabolic system | Hyperglycaemia without diabetic mellitus; new-onset diabetic mellitus; starvation ketoacidosis | High blood glucose level; impaired glucose metabolism | Blood tests for blood glucose and HbA1c level; plasma amino acid analysis; plasma Carnitine level; plasma acylcarnitine profile; plasma C-peptide level, Urine organic acid analysis; Urine and plasma ketone analysis | Impaired glucose metabolism; increased ketone body | [56,57,130] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Z.; Yang, M.; Lai, C.-L. Long COVID-19 Syndrome: A Comprehensive Review of Its Effect on Various Organ Systems and Recommendation on Rehabilitation Plans. Biomedicines 2021, 9, 966. https://doi.org/10.3390/biomedicines9080966
Yan Z, Yang M, Lai C-L. Long COVID-19 Syndrome: A Comprehensive Review of Its Effect on Various Organ Systems and Recommendation on Rehabilitation Plans. Biomedicines. 2021; 9(8):966. https://doi.org/10.3390/biomedicines9080966
Chicago/Turabian StyleYan, Zhipeng, Ming Yang, and Ching-Lung Lai. 2021. "Long COVID-19 Syndrome: A Comprehensive Review of Its Effect on Various Organ Systems and Recommendation on Rehabilitation Plans" Biomedicines 9, no. 8: 966. https://doi.org/10.3390/biomedicines9080966







