Targeted Toxins for the Treatment of Prostate Cancer
Abstract
1. Prostate Cancer
2. Targeted Toxins
3. Targeted Toxins in the Clinic
4. Targeted Toxins against Prostate Cancer
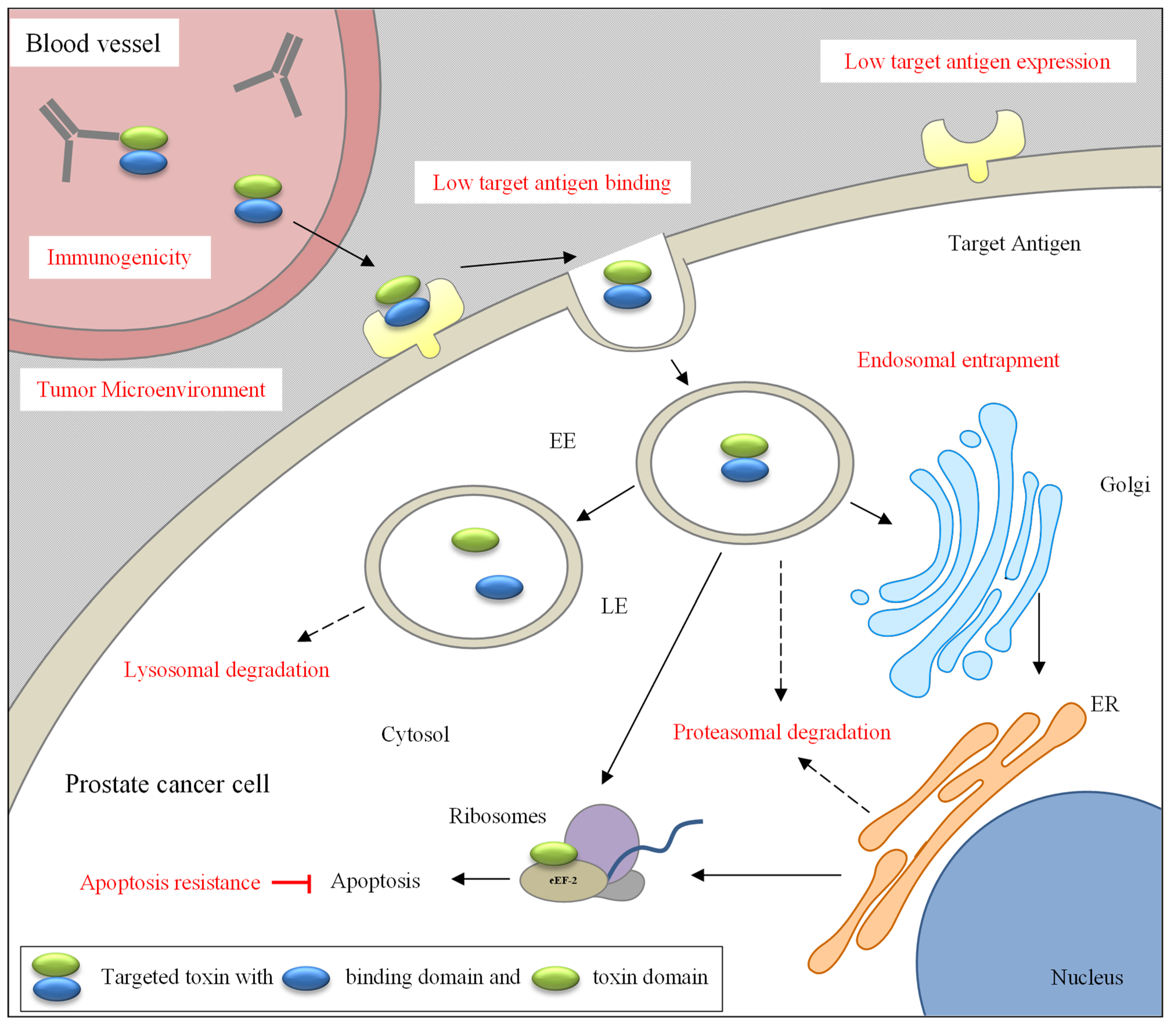
5. Effective Targeting
5.1. Challenges
5.2. Solutions for Effective Targeting
5.2.1. Surmounting the TME
5.2.2. Enhancing Tumor Penetration and Affinity
5.2.3. Enhancing Target Antigen Expression
5.2.4. Reducing On-Target/Off-Tumor Toxicities
6. Reduction of Immunogenicity
6.1. Challenges
6.2. Solutions for Reduction of Immunogenicity
6.2.1. Reducing the Immunogenicity of the Binding Domain
6.2.2. Reducing the Immunogenicity of the Toxin Domain
7. Improvement of Intracellular Trafficking
7.1. Challenges
7.2. Solutions to Improve Intracellular Trafficking
8. Overcoming Apoptosis Resistance
8.1. Challenges
8.2. Solutions to Overcome Apoptosis Resistance
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Culp, M.B.; Soerjomataram, I.; Efstathiou, J.A.; Bray, F.; Jemal, A. Recent Global Patterns in Prostate Cancer Incidence and Mortality Rates. Eur. Urol. 2019, 77, 38–52. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Litwin, M.S.; Tan, H.J. The diagnosis and treatment of prostate cancer: A review. JAMA 2017, 317, 2532–2542. [Google Scholar] [CrossRef] [PubMed]
- Crawford, E.D.; Petrylak, D.; Sartor, O. Navigating the evolving therapeutic landscape in advanced prostate cancer. Urol. Oncol. Semin. Orig. Investig. 2017, 35, S1–S13. [Google Scholar] [CrossRef]
- Shilova, O.; Shramova, E.; Proshkina, G.; Deyev, S. Natural and Designed Toxins for Precise Therapy: Modern Approaches in Experimental Oncology. Int. J. Mol. Sci. 2021, 22, 4975. [Google Scholar] [CrossRef]
- Walsh, M.J.; Dodd, J.E.; Hautbergue, G.M. Ribosome-inactivating proteins: Potent poisons and molecular tools. Virulence 2013, 4, 774–784. [Google Scholar] [CrossRef]
- Shafiee, F.; Aucoin, M.G.; Jahanian-Najafabadi, A. Targeted Diphtheria Toxin-Based Therapy: A Review Article. Front. Microbiol. 2019, 10, 2340. [Google Scholar] [CrossRef]
- Michalska, M.; Wolf, P. Pseudomonas Exotoxin A: Optimized by evolution for effective killing. Front. Microbiol. 2015, 6, 963. [Google Scholar] [CrossRef]
- Fabbrini, M.S.; Katayama, M.; Nakase, I.; Vago, R. Plant Ribosome-Inactivating Proteins: Progesses, Challenges and Biotechnological Applications (and a Few Digressions). Toxins 2017, 9, 314. [Google Scholar] [CrossRef]
- Shi, W.-W.; Mak, A.N.-S.; Wong, K.-B.; Shaw, P.-C. Structures and Ribosomal Interaction of Ribosome-Inactivating Proteins. Molecules 2016, 21, 1588. [Google Scholar] [CrossRef]
- Allahyari, H.; Heidari, S.; Ghamgosha, M.; Saffarian, P.; Amani, J. Immunotoxin: A new tool for cancer therapy. Tumor Biol. 2017, 39. [Google Scholar] [CrossRef]
- Pak, Y.; Pastan, I.; Kreitman, R.J.; Lee, B. Effect of Antigen Shedding on Targeted Delivery of Immunotoxins in Solid Tumors from a Mathematical Model. PLoS ONE 2014, 9, e110716. [Google Scholar] [CrossRef] [PubMed]
- Blum, J.; Fiani, M.; Stahl, P. Proteolytic cleavage of ricin A chain in endosomal vesicles. Evidence for the action of endosomal proteases at both neutral and acidic pH. J. Biol. Chem. 1991, 266, 22091–22095. [Google Scholar] [CrossRef]
- Nowakowska-Gołacka, J.; Sominka, H.; Sowa-Rogozińska, N.; Słomińska-Wojewódzka, M. Toxins Utilize the Endoplasmic Reticulum-Associated Protein Degradation Pathway in Their Intoxication Process. Int. J. Mol. Sci. 2019, 20, 1307. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.R. Mechanism of Diphtheria Toxin Catalytic Domain Delivery to the Eukaryotic Cell Cytosol and the Cellular Factors that Directly Participate in the Process. Toxins 2011, 3, 294–308. [Google Scholar] [CrossRef]
- Bagga, S.; Seth, D.; Batra, J.K. The Cytotoxic Activity of Ribosome-inactivating Protein Saporin-6 Is Attributed to Its rRNA N-Glycosidase and Internucleosomal DNA Fragmentation Activities. J. Biol. Chem. 2003, 278, 4813–4820. [Google Scholar] [CrossRef]
- Grela, P.; Szajwaj, M.; Horbowicz-Drożdżal, P.; Tchórzewski, M. How ricin damages the ribosome. Toxins 2019, 11, 241. [Google Scholar] [CrossRef]
- Noll, T.; Schultze-Seemann, S.; Kuckuck, I.; Michalska, M.; Wolf, P. Synergistic cytotoxicity of a prostate cancer-specific immunotoxin in combination with the BH3 mimetic ABT-737. Cancer Immunol. Immunother. 2017, 67, 413–422. [Google Scholar] [CrossRef]
- Masilamani, A.P.; Dettmer-Monaco, V.; Monaco, G.; Cathomen, T.; Kuckuck, I.; Schultze-Seemann, S.; Huber, N.; Wolf, P. An Anti-PSMA Immunotoxin Reduces Mcl-1 and Bcl2A1 and Specifically Induces in Combination with the BAD-Like BH3 Mimetic ABT-737 Apoptosis in Prostate Cancer Cells. Cancers 2020, 12, 1648. [Google Scholar] [CrossRef]
- Narayanan, S.; Surendranath, K.; Bora, N.; Surolia, A.; Karande, A.A. Ribosome inactivating proteins and apoptosis. FEBS Lett. 2005, 579, 1324–1331. [Google Scholar] [CrossRef]
- Piascik, P. Fda approves fusion protein for treatment of lymphoma. J. Am. Pharm. Assoc. 1999, 39, 571–572. [Google Scholar] [CrossRef]
- Fancher, K.M.; Lally-Montgomery, Z.C. Moxetumomab pasudotox: A first-in-class treatment for hairy cell leukemia. J. Oncol. Pharm. Pract. Official Publ. Int. Soc. Oncol. Pharm. Pract. 2019, 25, 1467–1472. [Google Scholar] [CrossRef]
- Jen, E.Y.; Gao, X.; Li, L.; Zhuang, L.; Simpson, N.E.; Aryal, B.; Wang, R.; Przepiorka, D.; Shen, Y.L.; Leong, R.; et al. FDA Approval Summary: Tagraxofusp-erzs For Treatment of Blastic Plasmacytoid Dendritic Cell Neoplasm. Clin. Cancer Res. 2019, 26, 532–536. [Google Scholar] [CrossRef]
- Kim, J.-S.; Jun, S.-Y.; Kim, Y.-S. Critical Issues in the Development of Immunotoxins for Anticancer Therapy. J. Pharm. Sci. 2019, 109, 104–115. [Google Scholar] [CrossRef] [PubMed]
- De Muga, S.; Hernández, S.; Agell, L.; Salido, M.; Juanpere, N.; Lorenzo, M.; Lorente, J.A.; Serrano, S.; Lloreta, J. Molecular alterations of EGFR and PTEN in prostate cancer: Association with high-grade and advanced-stage carcinomas. Mod. Pathol. 2010, 23, 703–712. [Google Scholar] [CrossRef]
- Di Lorenzo, G.; Tortora, G.; D’Armiento, F.P.; De Rosa, G.; Staibano, S.; Autorino, R.; D’Armiento, M.; De Laurentiis, M.; De Placido, S.; Catalano, G.; et al. Expression of epidermal growth factor receptor correlates with disease relapse and progression to androgen-independence in human prostate cancer. Clin. Cancer Res. 2002, 8, 3438–3444. [Google Scholar]
- Schlomm, T.; Kirstein, P.; Iwers, L.; Daniel, B.; Steuber, T.; Walz, J.; Chun, F.H.; Haese, A.; Kollermann, J.; Graefen, M.; et al. Clinical Significance of Epidermal Growth Factor Receptor Protein Overexpression and Gene Copy Number Gains in Prostate Cancer. Clin. Cancer Res. 2007, 13, 6579–6584. [Google Scholar] [CrossRef]
- Buhler, P.; Molnar, E.; Dopfer, E.P.; Wolf, P.; Gierschner, D.; Wetterauer, U.; Schamel, W.W.; Elsasser-Beile, U. Target-dependent t-cell activation by coligation with a psma x cd3 diabody induces lysis of prostate cancer cells. J. Immunother. 2009, 32, 565–573. [Google Scholar] [CrossRef]
- Bostad, M.; Kausberg, M.; Weyergang, A.; Olsen, C.E.; Berg, K.; Høgset, A.; Selbo, P.K. Light-Triggered, Efficient Cytosolic Release of IM7-Saporin Targeting the Putative Cancer Stem Cell Marker CD44 by Photochemical Internalization. Mol. Pharm. 2014, 11, 2764–2776. [Google Scholar] [CrossRef]
- Siva, A.C.; Wild, M.A.; Kirkland, R.E.; Nolan, M.J.; Lin, B.; Maruyama, T.; Yantiri-Wernimont, F.; Frederickson, S.; Bowdish, K.S.; Xin, H. Targeting CUB Domain-Containing Protein 1 with a Monoclonal Antibody Inhibits Metastasis in a Prostate Cancer Model. Cancer Res. 2008, 68, 3759–3766. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Wolf, I.; Fuchs, H.; Masilamani, A.P.; Wolf, P. Pseudomonas Exotoxin A Based Toxins Targeting Epidermal Growth Factor Receptor for the Treatment of Prostate Cancer. Toxins 2020, 12, 753. [Google Scholar] [CrossRef] [PubMed]
- Niesen, J.; Stein, C.; Brehm, H.; Hehmann-Titt, G.; Fendel, R.; Melmer, G.; Fischer, R.; Barth, S. Novel EGFR-specific immunotoxins based on panitumumab and cetuximab show in vitro and ex vivo activity against different tumor entities. J. Cancer Res. Clin. Oncol. 2015, 141, 2079–2095. [Google Scholar] [CrossRef] [PubMed]
- Yip, W.L.; Weyergang, A.; Berg, K.; Tønnesen, H.H.; Selbo, P.K. Targeted Delivery and Enhanced Cytotoxicity of Cetuximab—Saporin by Photochemical Internalization in EGFR-Positive Cancer Cells. Mol. Pharm. 2007, 4, 241–251. [Google Scholar] [CrossRef]
- Davol, P.; Frackelton, A.R., Jr. The mitotoxin, basic fibroblast growth factor-saporin, effectively targets human prostatic carcinoma in an animal model. J. Urol. 1996, 156, 1174–1179. [Google Scholar] [CrossRef]
- Wang, L.; Liu, B.; Schmidt, M.; Lu, Y.; Wels, W.; Fan, Z. Antitumor effect of an her2-specific antibody-toxin fusion protein on human prostate cancer cells. Prostate 2001, 47, 21–28. [Google Scholar] [CrossRef]
- Joshi, B.H.; Leland, P.; Calvo, A.; Green, J.E.; Puri, R.K. Human Adrenomedullin Up-regulates Interleukin-13 Receptor α2 Chain in Prostate Cancer In vitro and In vivo: A Novel Approach to Sensitize Prostate Cancer to Anticancer Therapy. Cancer Res. 2008, 68, 9311–9317. [Google Scholar] [CrossRef]
- Kawakami, K.; Husain, S.R.; Bright, R.K.; Puri, R.K. Gene transfer of interleukin 13 receptor α2 chain dramatically enhances the antitumor effect of IL-13 receptor–targeted cytotoxin in human prostate cancer xenografts. Cancer Gene Ther. 2001, 8, 861–868. [Google Scholar] [CrossRef]
- Maini, A.; Hillman, G.; Haas, G.P.; Wang, C.Y.; Montecillo, E.; Hamzavi, F.; Pontes, J.E.; Leland, P.; Pastan, I.; Debinski, W.; et al. Interleukin-13 receptors on human prostate carcinoma cell lines represent a novel target for a chimeric protein composed of il-13 and a mutated form of pseudomonas exotoxin. J. Urol. 1997, 158, 948–953. [Google Scholar] [CrossRef]
- Husain, S.R.; Kawakami, K.; Kawakami, M.; Puri, R.K. Interleukin-4 receptor-targeted cytotoxin therapy of androgen-dependent and -independent prostate carcinoma in xenograft models. Mol. Cancer Ther. 2003, 2, 245–254. [Google Scholar]
- Debinski, W.; Pastan, I. An immunotoxin with increased activity and homogeneity produced by reducing the number of lysine residues in recombinant Pseudomonas exotoxin. Bioconjugate Chem. 1994, 5, 40–46. [Google Scholar] [CrossRef]
- Gho, Y.S.; Chae, C.B. Luteinizing hormone releasing hormone-RNase A conjugates specifically inhibit the proliferation of LHRH-receptor-positive human prostate and breast tumor cells. Mol. Cells 1999, 9, 31–36. [Google Scholar]
- Skrepnik, N.; Zieske, A.W.; Bravo, J.C.; Gillespie, A.T.; Hunt, J.D. Recombinant oncotoxin ar209 (anti-p185erbb-2) diminishes human prostate carcinoma xenografts. J. Urol. 1999, 161, 984–989. [Google Scholar] [CrossRef]
- Xing, Y.; Xu, K.; Li, S.; Cao, L.; Nan, Y.; Li, Q.; Li, W.; Hong, Z. A Single-Domain Antibody-Based Anti-PSMA Recombinant Immunotoxin Exhibits Specificity and Efficacy for Prostate Cancer Therapy. Int. J. Mol. Sci. 2021, 22, 5501. [Google Scholar] [CrossRef]
- Michalska, M.; Schultze-Seemann, S.; Kuckuck, I.; Wolf, P. In Vitro Evaluation of Humanized/De-immunized Anti-PSMA Immunotoxins for the Treatment of Prostate Cancer. Anticancer. Res. 2018, 38, 61–69. [Google Scholar] [CrossRef]
- Meng, P.; Dong, Q.-C.; Tan, G.-G.; Wen, W.-H.; Wang, H.; Zhang, G.; Wang, Y.-Z.; Jing, Y.-M.; Wang, C.; Qin, W.-J.; et al. Anti-tumor effects of a recombinant anti-prostate specific membrane antigen immunotoxin against prostate cancer cells. BMC Urol. 2017, 17, 1–7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Michalska, M.; Schultze-Seemann, S.; Bogatyreva, L.; Hauschke, D.; Wetterauer, U.; Wolf, P. In vitro and in vivo effects of a recombinant anti-PSMA immunotoxin in combination with docetaxel against prostate cancer. Oncotarget 2016, 7, 22531–22542. [Google Scholar] [CrossRef]
- Baiz, D.; Hassan, S.; Choi, Y.A.; Flores, A.; Karpova, Y.; Yancey, D.; Pullikuth, A.; Sui, G.; Sadelain, M.; Debinski, W.; et al. Combination of the PI3K Inhibitor ZSTK474 with a PSMA-Targeted Immunotoxin Accelerates Apoptosis and Regression of Prostate Cancer. Neoplasia 2013, 15, 1172–1183, IN25–IN32. [Google Scholar] [CrossRef]
- Zhang, F.; Shan, L.; Liu, Y.; Neville, D.; Woo, J.-H.; Chen, Y.; Korotcov, A.; Lin, S.; Huang, S.; Sridhar, R.; et al. An Anti-PSMA Bivalent Immunotoxin Exhibits Specificity and Efficacy for Prostate Cancer Imaging and Therapy. Adv. Heal. Mater. 2012, 2, 736–744. [Google Scholar] [CrossRef]
- Bühler, P.; Wetterauer, D.; Gierschner, D.; Wetterauer, U.; Beile, U.E.; Wolf, P. Influence of structural variations on biological activity of anti-PSMA scFv and immunotoxins targeting prostate cancer. Anticancer. Res. 2010, 30, 3373–3379. [Google Scholar]
- Kuroda, K.; Liu, H.; Kim, S.; Guo, M.; Navarro, V.; Bander, N.H. Saporin toxin-conjugated monoclonal antibody targeting prostate-specific membrane antigen has potent anticancer activity. Prostate 2010, 70, 1286–1294. [Google Scholar] [CrossRef]
- Wolf, P.; Alt, K.; Wetterauer, D.; Bühler, P.; Gierschner, D.; Katzenwadel, A.; Wetterauer, U.; Elsässer-Beile, U. Preclinical Evaluation of a Recombinant Anti-Prostate Specific Membrane Antigen Single-Chain Immunotoxin Against Prostate Cancer. J. Immunother. 2010, 33, 262–271. [Google Scholar] [CrossRef]
- Wolf, P.; Alt, K.; Bühler, P.; Katzenwadel, A.; Wetterauer, U.; Tacke, M.; Elsässer-Beile, U. Anti-PSMA immunotoxin as novel treatment for prostate cancer? High and specific antitumor activity on human prostate xenograft tumors in SCID mice. Prostate 2007, 68, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P.; Gierschner, D.; Buhler, P.; Wetterauer, U.; Elsässer-Beile, U. A recombinant PSMA-specific single-chain immunotoxin has potent and selective toxicity against prostate cancer cells. Cancer Immunol. Immunother. 2006, 55, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Bennett, M.; Thorpe, P.E. Anti-tumor effects and lack of side effects in mice of an immunotoxin directed against human and mouse prostate-specific membrane antigen. Prostate 2004, 61, 1–11. [Google Scholar] [CrossRef]
- Fracasso, G.; Bellisola, G.; Cingarlini, S.; Castelletti, D.; Prayer-Galetti, T.; Pagano, F.; Tridente, G.; Colombatti, M. Anti-tumor effects of toxins targeted to the prostate specific membrane antigen. Prostate 2002, 53, 9–23. [Google Scholar] [CrossRef]
- Ippoliti, R.; Ginobbi, P.; Lendaro, E.; D’Agostino, I.; Ombres, D.; Benedetti, P.A.; Brunori, M.; Citro, G. The effect of monensin and chloroquine on the endocytosis and toxicity of chimeric toxins. Cell. Mol. Life Sci. 1998, 54, 866–875. [Google Scholar] [CrossRef]
- Jain, R.K. Delivery of molecular and cellular medicine to solid tumors. Adv. Drug Deliv. Rev. 2001, 46, 149–168. [Google Scholar] [CrossRef]
- Belli, C.; Trapani, D.; Viale, G.; D’Amico, P.; Duso, B.A.; Della Vigna, P.; Orsi, F.; Curigliano, G. Targeting the microenvironment in solid tumors. Cancer Treat. Rev. 2018, 65, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Rogers, O.C.; Rosen, D.M.; Antony, L.; Harper, H.M.; Das, D.; Yang, X.; Minn, I.; Mease, R.C.; Pomper, M.G.; Denmeade, S.R. Targeted delivery of cytotoxic proteins to prostate cancer via conjugation to small molecule urea-based PSMA inhibitors. Sci. Rep. 2021, 11. [Google Scholar] [CrossRef]
- Jayram, G.; Eggener, S.E. Patient selection for focal therapy of localized prostate cancer. Curr. Opin. Urol. 2009, 19, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Bos, W.V.D.; Muller, B.G.; Ahmed, H.; Bangma, C.H.; Barret, E.; Crouzet, S.; Eggener, S.E.; Gill, I.S.; Joniau, S.; Kovacs, G.; et al. Focal Therapy in Prostate Cancer: International Multidisciplinary Consensus on Trial Design. Eur. Urol. 2014, 65, 1078–1083. [Google Scholar] [CrossRef] [PubMed]
- Ahdoot, M.; Lebastchi, A.H.; Turkbey, B.; Wood, B.; Pinto, P.A. Contemporary treatments in prostate cancer focal therapy. Curr. Opin. Oncol. 2019, 31, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Tredan, O.; Galmarini, C.M.; Patel, K.; Tannock, I.F. Drug Resistance and the Solid Tumor Microenvironment. J. Natl. Cancer Inst. 2007, 99, 1441–1454. [Google Scholar] [CrossRef]
- Griffon-Etienne, G.; Boucher, Y.; Brekken, C.; Suit, H.D.; Jain, R.K. Taxane-induced apoptosis decompresses blood vessels and lowers interstitial fluid pressure in solid tumors: Clinical implications. Cancer Res. 1999, 59, 3776–3782. [Google Scholar]
- Jang, S.H.; Wientjes, M.G.; Au, J.L. Enhancement of paclitaxel delivery to solid tumors by apoptosis-inducing pretreatment: Effect of treatment schedule. J. Pharmacol. Exp. Ther. 2001, 296, 1035–1042. [Google Scholar]
- Alzubi, J.; Dettmer-Monaco, V.; Kuehle, J.; Thorausch, N.; Seidl, M.; Taromi, S.; Schamel, W.; Zeiser, R.; Abken, H.; Cathomen, T.; et al. PSMA-Directed CAR T Cells Combined with Low-Dose Docetaxel Treatment Induce Tumor Regression in a Prostate Cancer Xenograft Model. Mol. Ther. Oncolytics 2020, 18, 226–235. [Google Scholar] [CrossRef]
- Pyzik, M.; Sand, K.M.K.; Hubbard, J.J.; Andersen, J.T.; Sandlie, I.; Blumberg, R.S. The neonatal fc receptor (fcrn): A misnomer? Front. Immunol. 2019, 10, 1540. [Google Scholar] [CrossRef]
- Liu, L. Pharmacokinetics of monoclonal antibodies and Fc-fusion proteins. Protein Cell 2017, 9, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Asano, R.; Hagiwara, Y.; Koyama, N.; Masakari, Y.; Orimo, R.; Arai, K.; Ogata, H.; Furumoto, S.; Umetsu, M.; Kumagai, I. Multimerization of anti-(epidermal growth factor receptor) IgG fragments induces an antitumor effect: The case for humanized 528 scFv multimers. FEBS J. 2013, 280, 4816–4826. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Okada, R.; Kobayashi, H.; Nagaya, T.; Wei, J.; Zhou, Q.; Lee, F.; Bera, T.K.; Gao, Y.; Kuhlman, W.; et al. Site-Specific PEGylation of Anti-Mesothelin Recombinant Immunotoxins Increases Half-life and Antitumor Activity. Mol. Cancer Ther. 2019, 19, 812–821. [Google Scholar] [CrossRef]
- Elsässer-Beile, U.; Wolf, P.; Gierschner, D.; Bühler, P.; Schultze-Seemann, W.; Wetterauer, U. A new generation of monoclonal and recombinant antibodies against cell-adherent prostate specific membrane antigen for diagnostic and therapeutic targeting of prostate cancer. Prostate 2006, 66, 1359–1370. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Goenaga, A.-L.; Harms, B.D.; Zou, H.; Lou, J.; Conrad, F.; Adams, G.P.; Schoeberl, B.; Nielsen, U.B.; Marks, J.D. Impact of Intrinsic Affinity on Functional Binding and Biological Activity of EGFR Antibodies. Mol. Cancer Ther. 2012, 11, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Adams, G.P.; Schier, R.; McCall, A.M.; Simmons, H.H.; Horak, E.M.; Alpaugh, R.K.; Marks, J.D.; Weiner, L.M. High affinity restricts the localization and tumor penetration of single-chain fv antibody molecules. Cancer Res. 2001, 61, 4750–4755. [Google Scholar] [PubMed]
- Suksanpaisan, L.; Russell, S.J.; Peng, K.W. High scfv-receptor affinity does not enhance the antitumor activity of her2-retargeted measles virus. Cancer Gene Ther. 2014, 21, 256–260. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gonzalez-Moreno, O.; Calvo, A.; Joshi, B.H.; Abasolo, I.; Leland, P.; Wang, Z.; Montuenga, L.; Puri, R.K.; Green, J.E. Gene expression profiling identifies IL-13 receptor ?2 chain as a therapeutic target in prostate tumor cells overexpressing adrenomedullin. Int. J. Cancer 2004, 114, 870–878. [Google Scholar] [CrossRef]
- Kawakami, K.; Takeshita, F.; Puri, R.K. Identification of Distinct Roles for a Dileucine and a Tyrosine Internalization Motif in the Interleukin (IL)-13 Binding Component IL-13 Receptor α2 Chain. J. Biol. Chem. 2001, 276, 25114–25120. [Google Scholar] [CrossRef]
- Chen, J.; Zeng, F.; Forrester, S.J.; Eguchi, S.; Zhang, M.-Z.; Harris, R.C. Expression and Function of the Epidermal Growth Factor Receptor in Physiology and Disease. Physiol. Rev. 2016, 96, 1025–1069. [Google Scholar] [CrossRef]
- Elsasser-Beile, U.; Buhler, P.; Wolf, P. Targeted therapies for prostate cancer against the prostate specific membrane antigen. Curr. Drug Targets 2009, 10, 118–125. [Google Scholar] [CrossRef]
- Wolf, P.; Freudenberg, N.; Bühler, P.; Alt, K.; Schultze-Seemann, W.; Wetterauer, U.; Elsässer-Beile, U. Three conformational antibodies specific for different PSMA epitopes are promising diagnostic and therapeutic tools for prostate cancer. Prostate 2009, 70, 562–569. [Google Scholar] [CrossRef]
- Langbein, T.; Chaussé, G.; Baum, R.P. Salivary Gland Toxicity of PSMA Radioligand Therapy: Relevance and Preventive Strategies. J. Nucl. Med. 2018, 59, 1172–1173. [Google Scholar] [CrossRef]
- Mazor, R.; Pastan, I. Immunogenicity of Immunotoxins Containing Pseudomonas Exotoxin A: Causes, Consequences, and Mitigation. Front. Immunol. 2020, 11, 1261. [Google Scholar] [CrossRef] [PubMed]
- Sethu, S.; Govindappa, K.; Alhaidari, M.; Pirmohamed, M.; Park, K.; Sathish, J. Immunogenicity to biologics: Mechanisms, prediction and reduction. Arch. Immunol. Ther. Exp. 2012, 60, 331–344. [Google Scholar] [CrossRef]
- Bloem, K.; Hernández-Breijo, B.; Martínez-Feito, A.; Rispens, T. Immunogenicity of Therapeutic Antibodies: Monitoring Antidrug Antibodies in a Clinical Context. Ther. Drug Monit. 2017, 39, 327–332. [Google Scholar] [CrossRef]
- Klee, G.G. Human Anti-Mouse Antibodies. Arch. Pathol. Lab. Med. 2000, 124, 921–923. [Google Scholar] [CrossRef]
- Presta, L.G. Engineering of therapeutic antibodies to minimize immunogenicity and optimize function. Adv. Drug Deliv. Rev. 2006, 58, 640–656. [Google Scholar] [CrossRef]
- Frenzel, A.; Kügler, J.; Helmsing, S.; Meier, D.; Schirrmann, T.; Hust, M.; Dübel, S. Designing Human Antibodies by Phage Display. Transfus. Med. Hemotherapy 2017, 44, 312–318. [Google Scholar] [CrossRef]
- Cizeau, J.; Grenkow, D.M.; Brown, J.G.; Entwistle, J.; MacDonald, G.C. Engineering and biological characterization of vb6-845, an anti-epcam immunotoxin containing a t-cell epitope-depleted variant of the plant toxin bouganin. J. Immunother. 2009, 32, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Weldon, J.E.; Xiang, L.; Chertov, O.; Margulies, I.; Kreitman, R.J.; Fitzgerald, D.J.; Pastan, I. A protease-resistant immunotoxin against CD22 with greatly increased activity against CLL and diminished animal toxicity. Blood 2009, 113, 3792–3800. [Google Scholar] [CrossRef]
- Mazor, R.; Onda, M.; Park, D.; Addissie, S.; Xiang, L.; Zhang, J.; Hassan, R.; Pastan, I. Dual B- and T-cell de-immunization of recombinant immunotoxin targeting mesothelin with high cytotoxic activity. Oncotarget 2016, 7, 29916–29926. [Google Scholar] [CrossRef]
- Veronese, F.M.; Mero, A. The Impact of PEGylation on Biological Therapies. BioDrugs 2008, 22, 315–329. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, F.; Liu, S.; Jiang, S. Anti-PEG antibodies in the clinic: Current issues and beyond PEGylation. J. Control. Release 2016, 244, 184–193. [Google Scholar] [CrossRef]
- Lorberboum-Galski, H. Human toxin-based recombinant immunotoxins/chimeric proteins as a drug delivery system for targeted treatment of human diseases. Expert Opin. Drug Deliv. 2011, 8, 605–621. [Google Scholar] [CrossRef]
- Silke, J.; Meier, P. Inhibitor of Apoptosis (IAP) Proteins-Modulators of Cell Death and Inflammation. Cold Spring Harb. Perspect. Biol. 2013, 5, a008730. [Google Scholar] [CrossRef]
- Jordaan, S.; Akinrinmade, O.A.; Nachreiner, T.; Cremer, C.; Naran, K.; Chetty, S.; Barth, S. Updates in the Development of ImmunoRNases for the Selective Killing of Tumor Cells. Biomedicines 2018, 6, 28. [Google Scholar] [CrossRef]
- Hlongwane, P.; Mungra, N.; Madheswaran, S.; Akinrinmade, O.A.; Chetty, S.; Barth, S. Human Granzyme B Based Targeted Cytolytic Fusion Proteins. Biomed. 2018, 6, 72. [Google Scholar] [CrossRef]
- Fuchs, H.; Weng, A.; Gilabert-Oriol, R. Augmenting the Efficacy of Immunotoxins and Other Targeted Protein Toxins by Endosomal Escape Enhancers. Toxins 2016, 8, 200. [Google Scholar] [CrossRef]
- Fuchs, H.; Niesler, N.; Trautner, A.; Sama, S.; Jerz, G.; Panjideh, H.; Weng, A. Glycosylated Triterpenoids as Endosomal Escape Enhancers in Targeted Tumor Therapies. Biomedicines 2017, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Jerjes, W.; Theodossiou, T.A.; Hirschberg, H.; Høgset, A.; Weyergang, A.; Selbo, P.K.; Hamdoon, Z.; Hopper, C.; Berg, K. Photochemical Internalization for Intracellular Drug Delivery. From Basic Mechanisms to Clinical Research. J. Clin. Med. 2020, 9, 528. [Google Scholar] [CrossRef]
- Dieffenbach, M.; Pastan, I. Mechanisms of Resistance to Immunotoxins Containing Pseudomonas Exotoxin A in Cancer Therapy. Biomolecules 2020, 10, 979. [Google Scholar] [CrossRef]
- Krajewska, M.; Krajewski, S.; Epstein, J.I.; Shabaik, A.; Sauvageot, J.; Song, K.; Kitada, S.; Reed, J.C. Immunohistochemical analysis of bcl-2, bax, bcl-X, and mcl-1 expression in prostate cancers. Am. J. Pathol. 1996, 148, 1567–1576. [Google Scholar]
- Yoshino, T.; Shiina, H.; Urakami, S.; Kikuno, N.; Yoneda, T.; Shigeno, K.; Igawa, M. Bcl-2 Expression as a Predictive Marker of Hormone-Refractory Prostate Cancer Treated with Taxane-Based Chemotherapy. Clin. Cancer Res. 2006, 12, 6116–6124. [Google Scholar] [CrossRef] [PubMed]

| Antigen | Targeted Toxin | Binding Domain | Toxin Domain | Enhanced Efficacy/Safety by | Ref. |
|---|---|---|---|---|---|
| CD44 | IM7-saporin | anti-CD44 mAb (clone IM7) | Saporin | combination with PCI | [29] |
| CDPD1 | ch25A11-Sap | anti-CDCP1 mAb 25A11 | Saporin | [30] | |
| EGFR | EGF-PE40 EGF-PE24mut | EGF | PE40PE24mut | human binding domain, de-immunized toxin domain | [31] |
| scFv2112-ETA’ (from cetuximab) scFv1711-ETA’ (from panitumumab) | anti-EGFR scFv | ETA‘ | [32] | ||
| cetuximab-saporin | anti-EGFR mAb cetuximab | Saporin | combination with PCI | [33] | |
| FGF | bFGF-SAP | bFGF | saporin | [34] | |
| Her2 | scFv(FRP5)-ETA | anti-HER2 scFv | ETA | [35] | |
| IL13R | IL-13PE | human IL-13 | PE38 | human binding domain, enhancing target antigen expression, intratumoral injection | [36] |
| IL13-PE38QQR | human IL-13 | PE38QQR | human binding domain, enhancing target antigen expression | [37] | |
| IL13-PE38QQR | human IL-13 | PE38QQR | human binding domain | [38] | |
| IL4R | IL4-CTx | human IL-4 | PE | human binding domain, intratumoral injection | [39] |
| hIL4-PE4E | human IL-4 | PE mutant | human binding domain | [40] | |
| LHRH | LHRH-RNase A conjugate | LHRH | bovine RNaseA | human binding domain | [41] |
| p185 erbB-2 | AR209 | anti-p185erbB-2 scFv e23Fv | PE38KDEL | [42] | |
| PSMA | JVM-PE24X7 | anti-PSMA sd Ab | PE24X7 | de-immunized toxin domain | [43] |
| hD7-1(VL-VH)-PE40 | anti-PSMA scFv | PE40 | combination with ABT-737 | [19] | |
| hD7-1(VL-VH)-PE40 hD7-1(VL-VH)-PE24 hD7-1(VL-VH)-PE24mut | humanized anti- PSMA scFv | PE40 PE24 PE24mut | de-immunized toxin domains | [44] | |
| D7(VL-VH)-PE40 | humanized anti-PSMA scFv | PE40 | combination with ABT-737 | [18] | |
| immunocasp-3 | anti-PSMA scFv J591 | rev caspase-3 | human binding and toxin domain | [45] | |
| hD7-1(VL-VH)-PE40 | anti-PSMA scFv | PE40 | combination with docetaxel | [46] | |
| J591PE | anti-PSMA scFv (J591) | PE38QQR | combination with pan-PI3K inhibitor | [47] | |
| A-dmDT390-scfbDb(PSMA) | anti-PSMA sdAb J591 | truncated diphtheria toxin (DT) | [48] | ||
| D7-VH(Yol)VL-PE40 D7-VH(GS)VL-PE40 His D7-VH(GS)VL-PE40 D7-VL(GS)VH-PE40 His-D7-VL(GS)VH-PE40 | anti-PSMA scFv | PE40 | enhanced affinity by changing scFv domain orientation | [49] | |
| hJ591-SAZAP | hJ591 | saporin | humanized mAb as binding domain | [50] | |
| D7-PE40 | anti-PSMA scFv D7 | PE40 | [51] | ||
| A5-PE40 | anti-PSMA scFv A5 | PE40 | [52] | ||
| A5-PE40 | anti-PSMA scFv A5 | PE40 | [53] | ||
| E6-dgA | anti-PSMA mAb E6 | ricin A chain | [54] | ||
| J591-smpt-nRTA | anti-PSMA mAbs J591 PEQ226.5 PM2P079.1 | RTA, native orrecombinant | [55] | ||
| Tf | Tf-SapTf-ARCA | transferrin | Saporin or Ricin A | human binding domain, combination with monensin and chloroquine | [56] |
| Challenges | Solutions | Ref. | |
|---|---|---|---|
| Effective Targeting | |||
| Surmounting the TME | TME prevents extravasation and tumor penetration of targeted toxins | Surmounting the TME by - intratumoral injection of the targeted toxins - pre-damage of tumor masses | [36,39,46] |
| Enhancing tumor penetration and affinity | Large size of targeted toxins prevents tumor pentration | Reducing the size of the binding domain Reducing the size of the toxin domain | [31,44] |
| Targeted toxins have low binding affinity | Enhancing affinity by changing the arrangement of the functional domains of a targeted toxin | [49] | |
| Enhancing target antigen expression | Low target antigen expression on the PC cells | Enhancing target antigen expression by - gene transfer - drugs | [36,37] |
| Reducing on-target/off-tumor toxicities | Targeted toxins might harm normal cells that express the target antigen | Reducing on-target/off-tumor toxicity by - intratumoral injection of the targeted toxins - local activation of the targeted toxins | [29,33,36,39] |
| Reduction of immunogenicity | |||
| Reducing the immunogenicity of the binding domain | Targeted toxins with non-human binding domain are immunogenic in PC patients | Reducing immunogenicity by - humanization of antibody fragments - use of human ligands | [18,34,36,37,38,39,40,44,50,56] |
| Reducing the immunogenicity of the toxin domain | Targeted toxins with non-human toxin domain are immunogenic in PC patients | Reducing immunogenicity by - de-immunization - use of human toxins | [31,41,44,45] |
| Improvement of intracellular trafficking | lysosomal and proteasomal degradation of the targeted toxins | Enhancing cytosolic release by - addition of drugs - photochemical internalization | [29,33] |
| Overcoming apoptotic resistance | apoptosis resistance of PC cells | Enhancing sensitivity to apoptosis by combination with - BH3 mimetics - chemotherapy - kinase inhibitors | [18,19,46,47] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolf, P. Targeted Toxins for the Treatment of Prostate Cancer. Biomedicines 2021, 9, 986. https://doi.org/10.3390/biomedicines9080986
Wolf P. Targeted Toxins for the Treatment of Prostate Cancer. Biomedicines. 2021; 9(8):986. https://doi.org/10.3390/biomedicines9080986
Chicago/Turabian StyleWolf, Philipp. 2021. "Targeted Toxins for the Treatment of Prostate Cancer" Biomedicines 9, no. 8: 986. https://doi.org/10.3390/biomedicines9080986
APA StyleWolf, P. (2021). Targeted Toxins for the Treatment of Prostate Cancer. Biomedicines, 9(8), 986. https://doi.org/10.3390/biomedicines9080986






