Comparison of Tolerability and Impact on Metabolic Profiles of Antiretroviral Regimens Containing Darunavir/Ritonavir or Darunavir/Cobicistat in Romanian HIV Infected Patients
Abstract
1. Introduction
- -
- Is COBI a safer and better tolerated enhancer, with fewer side effects than RTV, in long-term administration?
- -
- Which one of the two enhancers, COBI or RTV, has a stronger impact on the metabolic profile?
- -
- When one enhancer or another is administered, the differences in the change of metabolic markers are sufficiently relevant and/or statistically significant to tip the balance in favor of using one of them (COBI or RTV)?
2. Materials and Methods
2.1. Design Study
2.2. Biochemical Determinations
2.3. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Biochemical Assessments of Tolerability and Safety
3.2.1. Carbohydrate Metabolism
3.2.2. Lipid Metabolism
- CT level is strongly positively correlated with age, p < 0.01;
- HDL-cholesterol strongly correlates negatively with the number of years of therapy. The same type of relationship, inversely proportional to the number of ARVs in a scheme;
- LDL-cholesterol correlates positively, p < 0.05, increase directly proportional to the number of comorbidities;
- total lipids correlate directly proportionally, strongly positive (p < 0.01) with age and positive (p < 0.05) with the number of years of therapy;
- the level of VLDL-cholesterol is directly proportional to age, number of years of therapy and number of schemes (strongly positive correlation p < 0.01) but also to the number of comorbidities (positive correlation p < 0.05);
- strong positive correlation p < 0.01 also exists between glycosylated hemoglobin and age, number of years of therapy, comorbidities, number of ARVs;
- blood glucose value was significantly correlated, strongly positive p < 0.01 only with the age of patients (Table 4).
- lipodystrophy is strongly positively correlated (p < 0.01), directly proportionally, with age. It also correlates positively (p < 0.05) with the number of comorbidities;
- dyslipidemia is strongly positively correlated with age, ART duration and number of comorbidities (p < 0.01). A positive correlation (p < 0.05) occurs between dyslipidemia and the number of ARVs in the regimen or the number of past regimens (Table 6).
3.2.3. Cardiac and Coagulation Markers
3.2.4. Liver Function
- ALT/GPT correlates directly proportionally, strongly positively (p < 0.01) with the number of comorbidities and co-infection with VHC and positively (p < 0.05) with the number of treatment regimens and pills burden;
- AST/GOT is strongly positively correlated (p < 0.01) with VHC co-infection;
- GGT with HCV co-infection correlates directly proportionally, strongly positive, (p < 0.01);
- total bilirubin is positively correlated (p < 0.05) also with co-infections with VHC;
- direct bilirubin correlates strongly positively (p < 0.01) with VHC co-infection;
- pancreatic lipase is correlated directly proportionally, strongly positive (p <0.01) with the number of therapeutic schemes and positive (p <0.05) with age and duration of therapy.
3.2.5. Renal Function
- urea correlates strongly positively, p < 0.01, with the age of patients;
- creatinine correlates positively, directly proportional, p < 0.05, with the age of patients;
- uric acid is strongly positively correlated, p < 0.01 with the age of patients and the number of comorbidities;
- creatinine clearance is directly proportional, strongly positively correlated, p < 0.01 with the duration of ART;
- the stage of renal impairment is strongly positively correlated, p < 0.01, with the duration of ART (Table 12).
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Comisia Națională de Lupta Anti-SIDA-Date Statistice (in English National Commission for the Fight against AIDS-Statistical Data). 2020. Available online: http://cnlas.ro/com_jce/date-statistice.html (accessed on 3 March 2021).
- Gokengin, D.; Oprea, C.; Begovac, J.; Horban, A.; Zeka, A.N.; Sedlacek, D.; Allabergan, B.; Almamedova, E.A.; Balayan, T.; Banhegyi, D.; et al. HIV care in central and eastern europe: How close are we to the target? Int. J. Infect. Dis. 2018, 70, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Appay, V.; Almeida, J.R.; Sauce, D.; Autran, B.; Papagno, L. Accelerated immune senescence and HIV-1 infection. Exp. Gerontol. 2007, 42, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Guaraldi, G.; Orlando, G.; Zona, S.; Menozzi, M.; Carli, F.; Garlassi, E.; Berti, A.; Rossi, E.; Roverato, A.; Palella, F. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin. Infect. Dis. 2011, 53, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Rocchetti, G.; Chadha, S.; Zengin, G.; Bungau, S.; Kumar, A.; Mehta, V.; Uddin, M.S.; Khullar, G.; Setia, D.; et al. Phytochemicals from plant foods as potential source of antiviral agents: An overview. Pharmaceuticals 2021, 14, 381. [Google Scholar] [CrossRef] [PubMed]
- Rockstroh, J.; Guaraldi, G.; Deray, G. HIV and the body: A review of multidisciplinary management. HIV Med. 2010, 11 (Suppl. 2), 1–8. [Google Scholar] [CrossRef] [PubMed]
- Streinu-Cercel, A.; Săndulescu, O.; Poiană, C.; Dorobanţu, M.; Mircescu, G.; Lăzureanu, V.E.; Dumitru, I.M.; Chirilă, O. Consensus statement on the assessment of comorbidities in people living with HIV in Romania. Germs 2019, 9, 198–210. [Google Scholar] [CrossRef]
- Nieuwkerk, P.T.; Sprangers, M.A.; Burger, D.M.; Hoetelmans, R.M.; Hugen, P.W.; Danner, S.A.; van Der Ende, M.E.; Schneider, M.M.; Schrey, G.; Meenhorst, P.L.; et al. Limited patient adherence to highly active antiretroviral therapy for HIV-1 infection in an observational cohort study. Arch. Intern. Med. 2001, 161, 1962–1968. [Google Scholar] [CrossRef]
- Paterson, D.L.; Swindells, S.; Mohr, J.; Brester, M.; Vergis, E.N.; Squier, C.; Wagener, M.M.; Singh, N. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann. Intern. Med. 2000, 133, 21–30. [Google Scholar] [CrossRef]
- Hammer, S.M.; Saag, M.S.; Schechter, M.; Montaner, J.S.; Schooley, R.T.; Jacobsen, D.M.; Thompson, M.A.; Carpenter, C.C.; Fischl, M.A.; Gazzard, B.G.; et al. Treatment for adult HIV infection: 2006 recommendations of the international AIDS society–USA panel. JAMA 2006, 14, 827–843. [Google Scholar] [CrossRef] [PubMed]
- Ministerul Sănătăţii Institutul Naţional De Boli Infecţioase. 2019. Available online: http://cnlas.ro/images/doc/30062019_rom.pdf (accessed on 3 March 2021).
- Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. 2021. Available online: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/AdultandAdolescentGL.pdf (accessed on 17 March 2021).
- Carr, A.; Cooper, D.A. Adverse effects of antiretroviral therapy. Lancet 2000, 356, 1423–1430. [Google Scholar] [CrossRef]
- Marin, R.C.; Behl, T.; Negrut, N.; Bungau, S. Management of antiretroviral therapy with boosted protease inhibitors-darunavir/ritonavir or darunavir/cobicistat. Biomedicines 2021, 9, 313. [Google Scholar] [CrossRef]
- Koh, Y.; Nakata, H.; Maeda, K.; Ogata, H.; Bilcer, G.; Devasamudram, T.; Kincaid, J.F.; Boross, P.; Wang, Y.F.; Tie, Y.; et al. Novel bis-tetrahydrofuranylurethane-containing nonpeptidic protease inhibitor (PI) UIC-94017 (TMC114) with potent activity against multi-PI-resistant human immunodeficiency virus in vitro. Antimicrob. Agents Chemother. 2003, 47, 3123–3129. [Google Scholar] [CrossRef]
- Clotet, B.; Bellos, N.; Molina, J.M.; Cooper, D.; Goffard, J.C.; Lazzarin, A.; Wöhrmann, A.; Katlama, C.; Wilkin, T.; Haubrich, R.; et al. Efficacy and safety of darunavir-ritonavir at week 48 in treatment-experienced patients with HIV-1 infection in POWER 1 and 2: A pooled subgroup analysis of data from two randomised trials. Lancet 2007, 369, 1169–1178. [Google Scholar] [CrossRef]
- Molina, J.M.; Cohen, C.; Katlama, C.; Grinsztejn, B.; Timerman, A.; Pedro Rde, J.; Vangeneugden, T.; Miralles, D.; Meyer, S.D.; Parys, W.; et al. Safety and efficacy of darunavir (TMC114) with low-dose ritonavir in treatment-experienced patients: 24-week results of POWER 3. JAIDS J. Acquir. Immune Defic. Syndr. 2007, 46, 24–31. [Google Scholar] [CrossRef]
- Acosta, E.P.; Kakuda, T.N.; Brundage, R.C.; Anderson, P.L.; Fletcher, C.V. Pharmacodynamics of human immunodeficiency virus type 1 protease inhibitors. Clin. Infect. Dis. 2000, 30 (Suppl. 2), S151–S159. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, H.; Hong, A.; Vivian, R.; Murray, B.P.; Callebaut, C.; Choi, Y.-C.; Lee, M.S.; Chau, J.; Tsai, L.K.; et al. Structure–activity relationships of diamine inhibitors of cytochrome P450 (CYP) 3A as novel pharmacoenhancers. Part II: P2/P3 region and discovery of cobicistat (GS-9350). Bioorganic Med. Chem. Lett. 2014, 24, 995–999. [Google Scholar] [CrossRef]
- Xu, L.; Liu, H.; Murray, B.P.; Callebaut, C.; Lee, M.S.; Hong, A.; Strickley, R.G.; Tsai, L.K.; Stray, K.M.; Wang, Y.; et al. Cobicistat (GS-9350): A potent and selective inhibitor of human CYP3A as a novel pharmacoenhancer. ACS Med. Chem. Lett. 2010, 1, 209–213. [Google Scholar] [CrossRef]
- Marzolini, C.; Gibbons, S.; Khoo, S.; Back, D. Cobicistat versus ritonavir boosting and differences in the drug-drug interaction profiles with co-medications. J. Antimicrob. Chemother. 2016, 71, 1755–1758. [Google Scholar] [CrossRef]
- Nathan, B.; Bayley, J.; Waters, L.; Post, F.A. Cobicistat: A novel pharmacoenhancer for co-formulation with HIV protease and integrase inhibitors. Infect. Dis. Ther. 2013, 2, 111–122. [Google Scholar] [CrossRef]
- Hsu, A.; Granneman, G.R.; Bertz, R.J. Ritonavir. Clinical pharmacokinetics and interactions with other anti-HIV agents. Clin. Pharm. 1998, 35, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Kakuda, T.N.; Opsomer, M.; Timmers, M.; Iterbeke, K.; van De Casteele, T.; Hillewaert, V.; Petrovic, R.; Hoetelmans, R.M. Pharmacokinetics of darunavir in fixed-dose combination with cobicistat compared with coadministration of darunavir and ritonavir as single agents in healthy volunteers. J. Clin. Pharmacol. 2014, 54, 949–957. [Google Scholar] [CrossRef]
- Tashima, K.; Crofoot, G.; Tomaka, F.L.; Kakuda, T.N.; Brochot, A.; Van de Casteele, T.; Opsomer, M.; Garner, W.; Margot, N.; Custodio, J.M.; et al. Cobicistat-boosted darunavir in HIV-1-infected adults: Week 48 results of a phase IIIb, open-label single-arm trial. AIDS Res. Ther. 2014, 11, 39. [Google Scholar] [CrossRef]
- World Medical Association. 2018. Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (accessed on 26 February 2021).
- European Medicines Agency. 2015. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-good-clinical-practice-e6r2-4-step-2b_en.pdf (accessed on 11 March 2021).
- Md + CALC. 2006. Available online: https://www.mdcalc.com/mdrd-gfr-equation (accessed on 18 March 2021).
- KDIGO. 2016. Available online: https://kdigo.org/guidelines/ (accessed on 21 March 2021).
- UNAIDS. 2019. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 27 March 2021).
- Streinu-Cercel, A.; Săndulescu, O.; Ceapraga, G.; Manolache, D.; Stoica, M.A.; Preoțescu, L.L. Prevalence of osteo-renal impairment in the Romanian HIV cohort. BMC Infect. Dis. 2016, 16 (Suppl. 1), 93. [Google Scholar] [CrossRef]
- Poiana, C.; Capatina, C.; Cercel, A.S.; Sandulescu, O.; Streinu Cercel, A. Hypovitaminosis D in HIV-infected patients. Acta Endocrinol. 2019, 5, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Mocroft, A.; Youle, M.; Moore, A.; Sabin, C.A.; Madge, S.; Lepri, A.C.; Tyrer, M.; Chaloner, C.; Wilson, D.; Loveday, C.; et al. Reasons for modification and discontinuation of antiretrovirals: Results from a single treatment centre. Aids 2001, 15, 185–194. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sarkar, S.; Brown, T.T. Diabetes in people living with HIV. 2019. [Updated 2019 August 27]. In Endotext Internet; Feingold, K.R., Anawalt, B., Boyce, A., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK545886/ (accessed on 5 June 2021).
- Brown, T.T.; Cole, S.R.; Li, X.; Kingsley, L.A.; Palella, F.J.; Riddler, S.A.; Visscher, B.R.; Margolick, J.B.; Dobs, A.S. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch. Intern. Med. 2005, 165, 1179–1184. [Google Scholar] [CrossRef] [PubMed]
- Hulgan, T. Factors associated with insulin resistance in adults with HIV receiving contemporary antiretroviral therapy: A brief update. Curr. HIV AIDS Rep. 2018, 15, 223–232. [Google Scholar] [CrossRef]
- Kalra, S.; Kalra, B.; Agrawal, N.; Unnikrishnan, A. Understanding diabetes in patients with HIV/AIDS. Diabetol. Metab. Syndr. 2011, 3, 2. [Google Scholar] [CrossRef]
- Echeverría, P.; Bonjoch, A.; Puig, J.; Ornella, A.; Clotet, B.; Negredo, E. Significant improvement in triglyceride levels after switching from ritonavir to cobicistat in suppressed HIV-1-infected subjects with dyslipidaemia. HIV Med. 2017, 18, 782–786. [Google Scholar] [CrossRef]
- Ridker, P.M.; Stampfer, M.J.; Rifai, N. Novel risk factors for systemic atherosclerosis: A comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a) and standard cholesterol screening as predictors of peripheral arterial disease. JAMA 2001, 285, 2481–2485. [Google Scholar] [CrossRef]
- Margolis, A.M.; Heverling, H.; Pham, P.A.; Stolbach, A. A review of the toxicity of HIV medications. J. Med. Toxicol. 2014, 10, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Xu, Y.; Han, D.; Peng, X.; Lu, X.; Brockmeyer, N.H.; Wu, N. Changes in lipid indices in HIV+ cases on HAART. Biomed. Res. Int. 2019, 2019, 2870647. [Google Scholar] [CrossRef]
- Carr, A.; Samaras, K.; Chisholm, D.J.; Cooper, D.A. Pathogenesis of HIV-1-protease inhibitor-associated peripheral lipodystrophy, hyperlipidaemia, and insulin resistance. Lancet 1998, 351, 1881–1883. [Google Scholar] [CrossRef]
- Mallon, P.W. Pathogenesis of lipodystrophy and lipid abnormalities in patients taking antiretroviral therapy. AIDS Rev. 2007, 9, 3–15. [Google Scholar] [PubMed]
- Dressman, J.; Kincer, J.; Matveev, S.V.; Guo, L.; Greenberg, R.N.; Guerin, T.; Meade, D.; Li, X.A.; Zhu, W.; Uittenbogaard, A.; et al. HIV protease inhibitors promote atherosclerotic lesion formation independent of dyslipidemia by increasing CD36-dependent cholesteryl ester accumulation in macrophages. J. Clin. Investig. 2003, 111, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Purnell, J.Q.; Zambon, A.; Knopp, R.H.; Pizzuti, D.J.; Achari, R.; Leonard, J.M.; Locke, C.; Brunzell, J.D. Effect of ritonavir on lipids and post-heparin lipase activities in normal subjects. Aids 2000, 14, 51–57. [Google Scholar] [CrossRef]
- Rosenkranz, S.L.; Yarasheski, K.E.; Para, M.F.; Reichman, R.C.; Morse, G.D. Antiretroviral drug levels and interactions affect lipid, lipoprotein and glucose metabolism in HIV-1 seronegative subjects: A pharmacokinetic-pharmacodynamic analysis. Metab. Syndr. Relat. Disord. 2007, 5, 163–173. [Google Scholar] [CrossRef]
- Friis-Møller, N.; Reiss, P.; Sabin, C.A.; Weber, R.; Monforte, A.; El-Sadr, W.; Thiébaut, R.; De Wit, S.; Kirk, O.; Fontas, E.; et al. Class of antiretroviral drugs and the risk of myocardial infarction. N. Engl. J. Med. 2007, 356, 1723–1735. [Google Scholar] [CrossRef]
- Grunfeld, C.; Tien, P. Difficulties in understanding the metabolic complications of acquired immune deficiency syndrome. Clin. Infect. Dis. 2003, 37 (Suppl. 2), S43–S46. [Google Scholar] [CrossRef][Green Version]
- Streinu-Cercel, A.; Ion, D.A.; Chivu, L.I.; Chivu, R.D. Lipodystrophy syndrome in HIV-infected patients. Clinical and diagnostic features. Rev. Med. Chir. Soc. Med. Nat. Iasi 2006, 110, 521–525. [Google Scholar] [PubMed]
- Uurlings, F.; Moutschen, M. Lipodystrophy syndrome in HIV infected patients. Rev. Med. Liege 2007, 62, 669–674. [Google Scholar] [PubMed]
- Joly, V.; Flandre, P.; Meiffredy, V.; Leturque, N.; Harel, M.; Aboulker, J.P.; Yeni, P. Increased risk of lipoatrophy under stavudine in HIV-1-infected patients: Results of a substudy from a comparative trial. Aids 2002, 16, 2447–2454. [Google Scholar] [CrossRef] [PubMed]
- Gallant, J.E.; Staszewski, S.; Pozniak, A.L.; DeJesus, E.; Suleiman, J.M.; Miller, M.D.; Coakley, D.F.; Lu, B.; Toole, J.J.; Cheng, A.K. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: A 3-year randomized trial. JAMA 2004, 292, 191–201. [Google Scholar] [CrossRef]
- Tien, P.C.; Benson, C.; Zolopa, A.R.; Sidney, S.; Osmond, D.; Grunfeld, C. The study of fat redistribution and metabolic change in HIV infection (FRAM): Methods, design and sample characteristics. Am. J. Epidemiol. 2006, 163, 860–869. [Google Scholar] [CrossRef]
- Sprinz, E.; Lazzaretti, R.K.; Kuhmmer, R.; Ribeiro, J.P. Dyslipidemia in HIV-infected individuals. Braz. J. Infect. Dis. 2010, 14, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Estrada, V.; Portilla, J. Dyslipidemia related to antiretroviral therapy. AIDS Rev. 2011, 13, 49–56. [Google Scholar]
- Sekhar, R.V. Treatment of dyslipidemia in HIV. Curr. Atheroscler. Rep. 2015, 17, 493. [Google Scholar] [CrossRef]
- Duffau, P.; Wittkop, L.; Lazaro, E.; le Marec, F.; Cognet, C.; Blanco, P.; Moreau, J.F.; Dauchy, F.A.; Cazanave, C.; Vandenhende, M.A.; et al. Association of immune-activation and senescence markers with non-AIDS-defining comorbidities in HIV-suppressed patients. Aids 2015, 29, 2099–2108. [Google Scholar] [CrossRef]
- Lagathu, C.; Béréziat, V.; Gorwood, J.; Fellahi, S.; Bastard, J.P.; Vigouroux, C.; Boccara, F.; Capeau, J. Metabolic complications affecting adipose tissue, lipid and glucose metabolism associated with HIV antiretroviral treatment. Expert Opin. Drug Saf. 2019, 18, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Arrive, E.; Viard, J.P.; Salanave, B.; Dollfus, C.; Matheron, S.; Reliquet, V.; Arezes, E.; Nailler, L.; Vigouroux, C.; Warszawski, J. Metabolic risk factors in young adults infected with HIV since childhood compared with the general population. PLoS ONE 2018, 13, e0206745. [Google Scholar] [CrossRef]
- Martin-Iguacel, R.; Llibre, J.M.; Friis-Moller, N. Risk of cardiovascular disease in an aging HIV population: Where are we now? Curr. HIV AIDS Rep. 2015, 12, 375–387. [Google Scholar] [CrossRef]
- Feinstein, M.J.; Bahiru, E.; Achenbach, C.; Longenecker, C.T.; Hsue, P.; So-Armah, K.; Freiberg, M.S.; Lloyd-Jones, D.M. Patterns of cardiovascular mortality for HIV-infected adults in the United States: 1999 to 2013. Am. J. Cardiol. 2016, 117, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Vallance, P.; Collier, J.; Bhagat, K. Infection, inflammation, and infarction: Does acute endothelial dysfunction provide a link? Lancet 1997, 349, 1391–1392. [Google Scholar] [CrossRef]
- Maggi, P.; Di Biagio, A.; Rusconi, S.; Cicalini, S.; D’Abbraccio, M.; d’Ettorre, G.; Martinelli, C.; Nunnari, G.; Sighinolfi, L.; Spagnuolo, V.; et al. Cardiovascular risk and dyslipidemia among persons living with HIV: A review. BMC Infect. Dis. 2017, 17, 551. [Google Scholar] [CrossRef]
- Friis-Møller, N.; Weber, R.; Reiss, P.; Thiébaut, R.; Kirk, O.; d’Arminio Monforte, A.; Pradier, C.; Morfeldt, L.; Mateu, S.; Law, M.; et al. Cardiovascular disease risk factors in HIV patients--association with antiretroviral therapy. Results from the DAD study. Aids 2003, 17, 1179–1193. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.S.; Lu, X.H.; Conklin, B.S.; Lin, P.H.; Lumsden, A.B.; Yao, Q.; Chen, C. HIV protease inhibitor ritonavir induces cytotoxicity of human endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1560–1566. [Google Scholar] [CrossRef]
- Muller, E.V.; Gimeno, S.G.A. Risk factors for cardiovascular disease in HIV/AIDS patients treated with highly active antiretroviral therapy (HAART) in the central-southern region of the state of Paraná-Brazil. Cienc. Saude Coletiva 2019, 24, 1903–1914. [Google Scholar] [CrossRef] [PubMed]
- Nsagha, D.S.; Assob, J.C.; Njunda, A.L.; Tanue, E.A.; Kibu, O.D.; Ayima, C.W.; Ngowe, M.N. Risk factors of cardiovascular diseases in HIV/AIDS patients on HAART. Open AIDS J. 2015, 9, 51–59. [Google Scholar] [CrossRef]
- Arildsen, H.; Sørensen, K.E.; Ingerslev, J.M.; Østergaard, L.J.; Laursen, A.L. Endothelial dysfunction, increased inflammation and activated coagulation in HIV-infected patients improve after initiation of highly active antiretroviral therapy. HIV Med. 2013, 14, 1–9. [Google Scholar] [CrossRef]
- Sinha, A.; Ma, Y.; Scherzer, R.; Hur, S.; Li, D.; Ganz, P.; Deeks, S.G.; Hsue, P.Y. Role of T-Cell dysfunction, inflammation and coagulation in microvascular disease in HIV. J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef]
- Baker, J.V. Chronic HIV disease and activation of the coagulation system. Thromb. Res. 2013, 132, 495–499. [Google Scholar] [CrossRef]
- Mooney, S.; Tracy, R.; Osler, T.; Grace, C. Elevated biomarkers of inflammation and coagulation in patients with HIV are associated with higher framingham and VACS risk index scores. PLoS ONE 2015, 10, e0144312. [Google Scholar] [CrossRef]
- Nasi, M.; Pinti, M.; De Biasi, S.; Gibellini, L.; Ferraro, D.; Mussini, C.; Cossarizza, A. Aging with HIV infection: A journey to the center of inflammAIDS, immunosenescence and neuroHIV. Immunol. Lett. 2014, 162, 329–333. [Google Scholar] [CrossRef]
- Lagathu, C.; Cossarizza, A.; Béréziat, V.; Nasi, M.; Capeau, J.; Pinti, M. Basic science and pathogenesis of ageing with HIV: Potential mechanisms and biomarkers. Aids 2017, 31 (Suppl. 2), S105–S119. [Google Scholar] [CrossRef]
- Kontorinis, N.; Dieterich, D. Hepatotoxicity of antiretroviral therapy. AIDS Rev. 2003, 5, 36–43. [Google Scholar]
- Abrescia, N.; D’Abbraccio, M.; Figoni, M.; Busto, A.; Maddaloni, A.; De Marco, M. Hepatotoxicity of antiretroviral drugs. Curr. Pharm. Des. 2005, 11, 3697–3710. [Google Scholar] [CrossRef]
- Núñez, M. Hepatotoxicity of antiretrovirals: Incidence, mechanisms and management. J. Hepatol. 2006, 44, S132–S139. [Google Scholar] [CrossRef]
- Neff, G.W.; Jayaweera, D.; Sherman, K.E. Drug-induced liver injury in HIV patients. Gastroenterol. Hepatol. 2006, 2, 430–437. [Google Scholar]
- Schwartz, M.S.; Brandt, L.J. The spectrum of pancreatic disorders in patients with the acquired immune deficiency syndrome. Am. J. Gastroenterol. 1989, 84, 459–462. [Google Scholar] [PubMed]
- Cappell, M.S. The pancreas in AIDS. Gastroenterol. Clin. N. Am. 1997, 26, 337–365. [Google Scholar] [CrossRef]
- Argiris, A.; Mathur-Wagh, U.; Wilets, I.; Mildvan, D. Abnormalities of serum amylase and lipase in HIV-positive patients. Am. J. Gastroenterol. 1999, 94, 1248–1252. [Google Scholar] [CrossRef]
- Kahn, J.O.; Lagakos, S.W.; Richman, D.D.; Cross, A.; Pettinelli, C.; Liou, S.H.; Brown, M.; Volberding, P.A.; Crumpacker, C.S.; Beall, G.; et al. A controlled trial comparing continued zidovudine with didanosine in human immunodeficiency virus infection. The NIAID AIDS clinical trials group. N. Engl. J. Med. 1992, 327, 581–587. [Google Scholar] [CrossRef]
- Powderly, W.G. Long-term exposure to lifelong therapies. J. Acquir. Immune Defic. Syndr. 2002, 29 (Suppl. 1), S28–S40. [Google Scholar] [CrossRef] [PubMed]
- Wanless, R.S.; Rugină, S.; Ruţă, S.M.; Dumitru, I.M.; Cernat, R.C.; Schwarzwald, H.L.; Calles, N.R.; Schutze, G.E.; Schweitzer, A.M.; Draper, H.R.; et al. Nine-year follow-up of HIV-infected Romanian children and adolescents receiving lopinavir/ritonavir-containing highly active antiretroviral therapy. Germs 2013, 3, 90–95. [Google Scholar] [CrossRef]
- Kalayjian, R.C. Renal issues in HIV infection. Curr. HIV AIDS Rep. 2011, 8, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.D.; Tonelli, M. Renal disease associated with antiretroviral therapy in the treatment of HIV. Nephron Clin. Pract. 2011, 118, c262–c268. [Google Scholar] [CrossRef]
- Atta, M.G.; Lucas, G.M.; Fine, D.M. HIV-associated nephropathy: Epidemiology, pathogenesis, diagnosis and management. Expert Rev. Anti Infect. Ther. 2008, 6, 365–371. [Google Scholar] [CrossRef]
- Kalayjian, R.C.; Franceschini, N.; Gupta, S.K.; Szczech, L.A.; Mupere, E.; Bosch, R.J.; Smurzynski, M.; Albert, J.M. Suppression of HIV-1 replication by antiretroviral therapy improves renal function in persons with low CD4 cell counts and chronic kidney disease. Aids 2008, 22, 481–487. [Google Scholar] [CrossRef]
- Reid, A.; Stöhr, W.; Walker, A.S.; Williams, I.G.; Kityo, C.; Hughes, P.; Kambugu, A.; Gilks, C.F.; Mugyenyi, P.; Munderi, P.; et al. Severe renal dysfunction and risk factors associated with renal impairment in HIV-infected adults in Africa initiating antiretroviral therapy. Clin. Infect. Dis. 2008, 46, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.M.; Wu, J.; Jansson, J.; Wilson, D.P. Relative risk of renal disease among people living with HIV: A systematic review and meta-analysis. BMC Public Health 2012, 12, 234. [Google Scholar] [CrossRef] [PubMed]
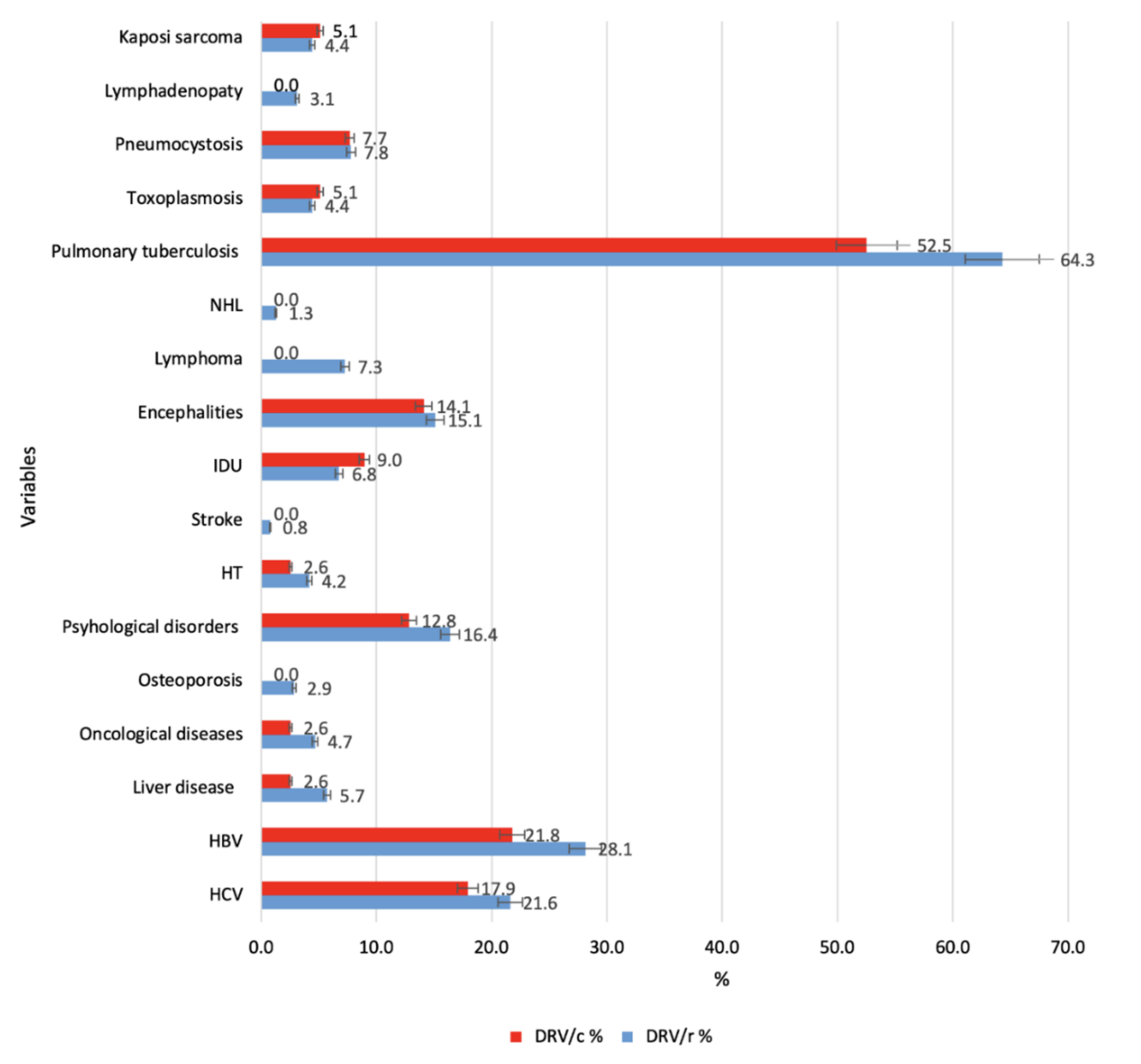


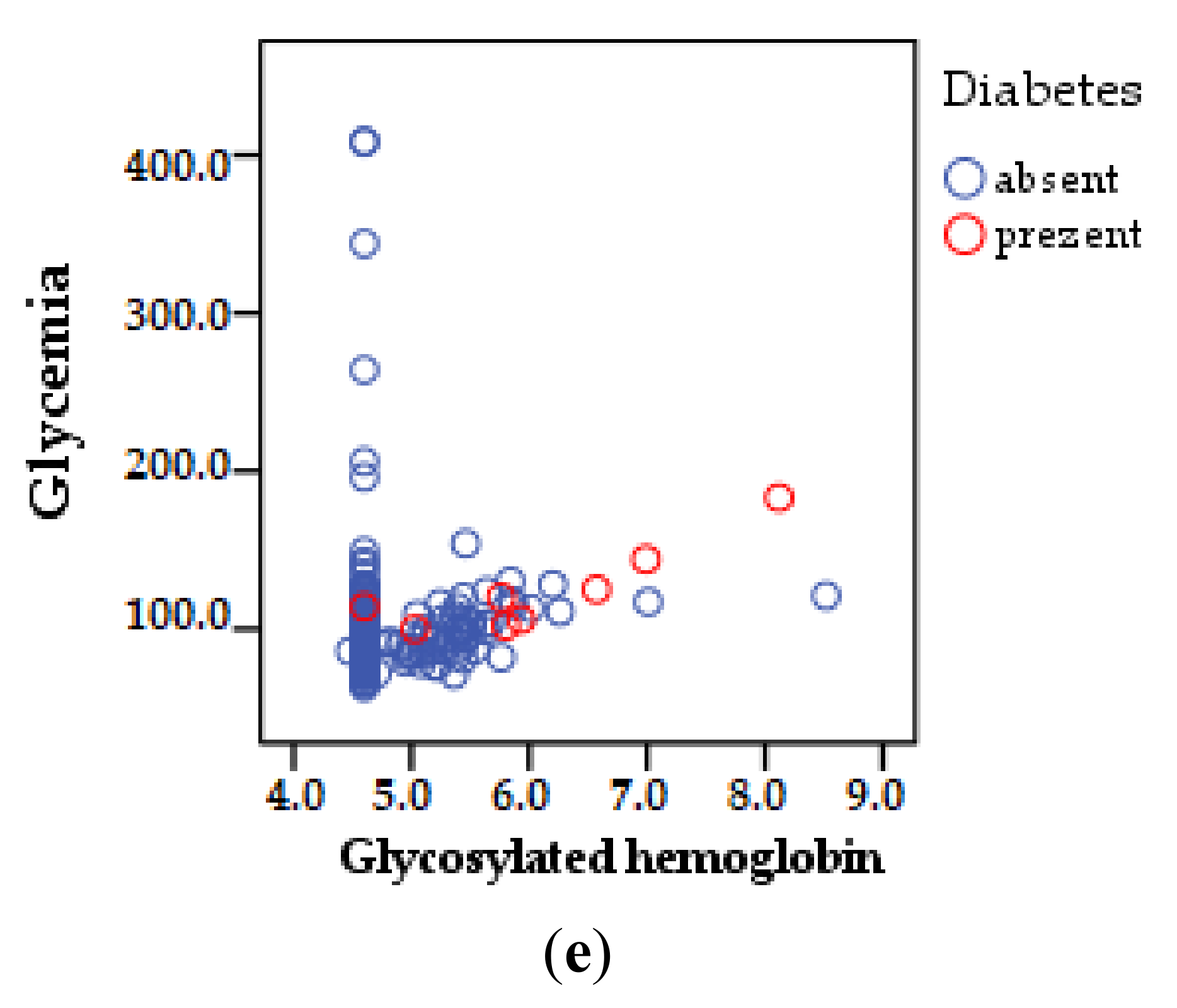
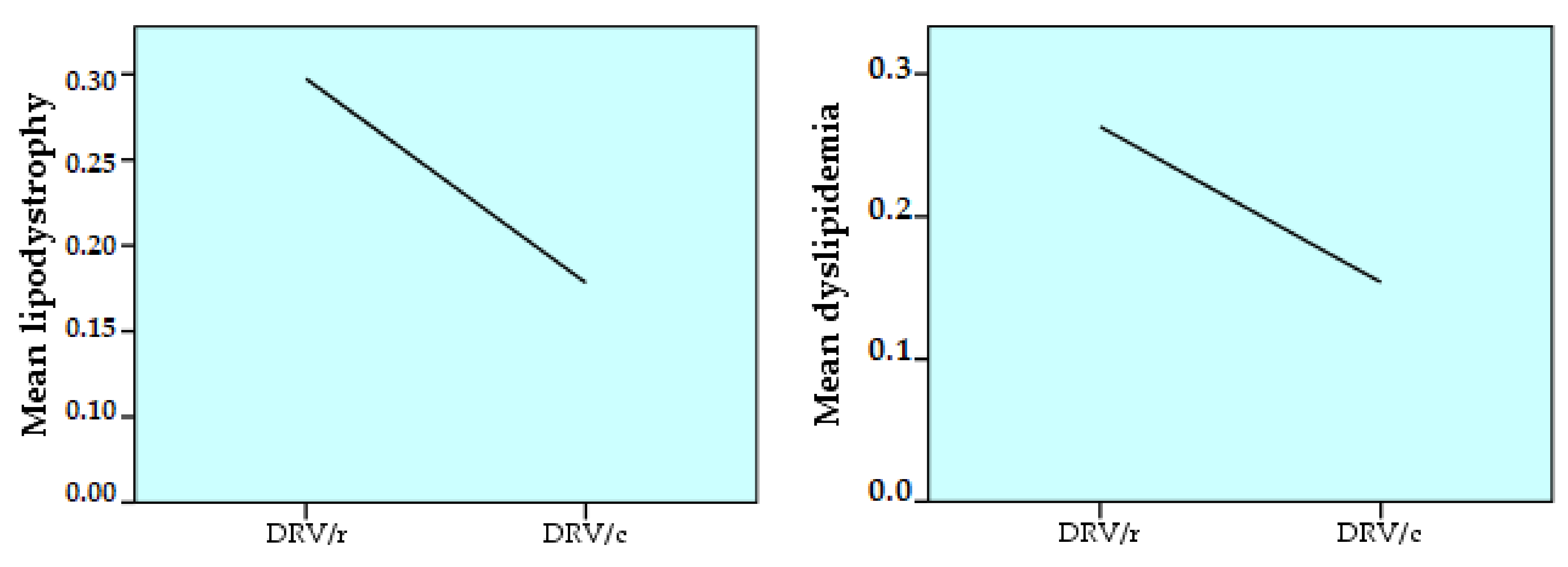

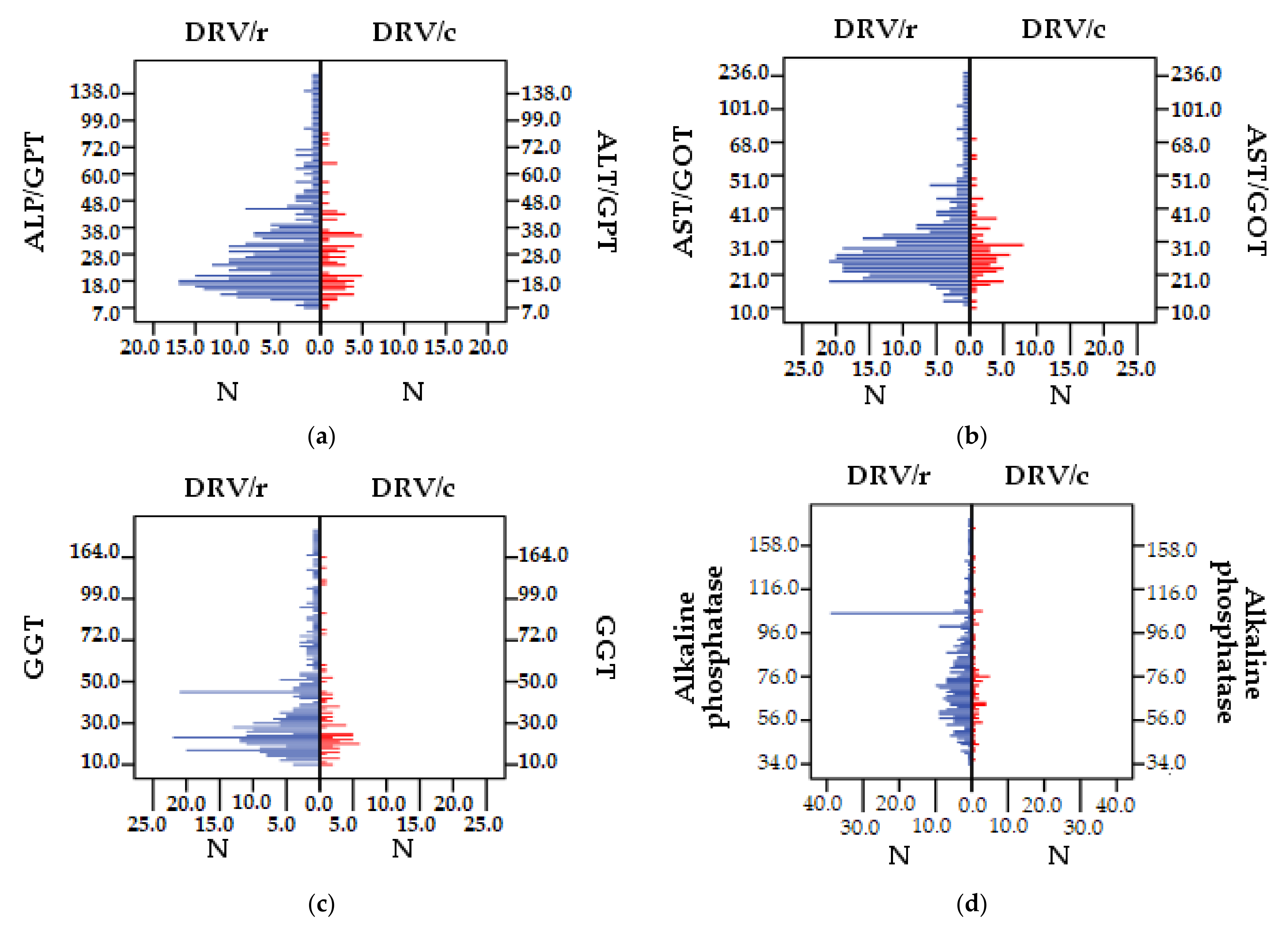


| Variable | DRV/r | DRV/c | Total | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | N | % | ||
| Demographic data | |||||||
| Number n (%) | 384 | 83.11 | 78 | 16.89 | 462 | 100 | |
| Female n % | 148 | 38.50 | 28 | 35.90 | 176 | 38.09 | |
| Male n % | 236 | 61.50 | 50 | 61.50 | 286 | 61.91 | |
| Mean age. years (±SD) | 39.41 | ±11.50 | 38.58 | ±10.45 | 39.27 | 11.32 | |
| t | 74.544 | ||||||
| p | 0.001 | ||||||
| Characteristics of the disease | |||||||
| HIV-1 RNA (copies/mL) | |||||||
| Mean (±SD) | 1.56 | ±1.04 | 1.38 | ±0.93 | 1.53 | ±1.02 | |
| t | 32.081 | ||||||
| p | 0.007 | ||||||
| Undetectable (=0) n % | 292 | 76.6 | 68 | 84.61 | 358 | 78.0 | |
| <50 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| 50–999 | 55 | 14.4 | 6 | 7.6 | 61 | 13.3 | |
| >1000 | 34 | 8.9 | 6 | 7.6 | 40 | 8.7 | |
| CD4 T-cells (cells/mm3) | |||||||
| Mean (±SD) | 3.21 | ±1.01 | 3.35 | ±0.91 | 3.23 | ±1.00 | |
| t | 69.830 | ||||||
| p | 0.001 | ||||||
| 0–199 | 37 | 9.6 | 5 | 6.4 | 42 | 9.1 | |
| 200–349 | 53 | 13.8 | 8 | 10.3 | 61 | 13.2 | |
| 350–499 | 86 | 22.4 | 20 | 25.6 | 106 | 22.9 | |
| >500 | 208 | 54.2 | 45 | 57.7 | 253 | 54.8 | |
| CDC’s HIV-1 classes | |||||||
| Mean (±SD) | 6.21 | ±3.5 | 5.17 | ±3.81 | 6.04 | ±3.5 | |
| t | 36.380 | ||||||
| p | 0.001 | ||||||
| Unknown | 63 | 16.4 | 19 | 24.3 | 82 | 17.7 | |
| A1 | 8 | 2.1 | 3 | 3.8 | 11 | 2.4 | |
| A2 | 15 | 3.9 | 5 | 6.3 | 20 | 4.3 | |
| A3 | 11 | 2.9 | 3 | 4.0 | 14 | 3.0 | |
| B1 | 2 | 0.5 | 0 | 0.0 | 2 | 0.4 | |
| B2 | 33 | 8.6 | 8 | 10.2 | 41 | 8.9 | |
| B3 | 34 | 8.9 | 5 | 6.4 | 39 | 8.4 | |
| C1 | 3 | 0.8 | 0 | 0.0 | 3 | 0.6 | |
| C2 | 18 | 4.7 | 4 | 5.1 | 22 | 4.8 | |
| C3 | 197 | 51.3 | 31 | 39.8 | 228 | 49.4 | |
| Mean duration of the infection years (±SD) | 11.05 | ±9.16 | 9.17 | ±8.19 | 10.74 | (±9.02) | |
| t | 25.576 | ||||||
| p | 0.001 | ||||||
| Duration of the infection years (%) | Unknown | 60 | 15.6 | 6 | 7.7 | 66 | 14.3 |
| 0–5 | 78 | 20.3 | 31.9 | 39.7 | 109 | 23.6 | |
| 6–10 | 59 | 15.4 | 12 | 15.3 | 71 | 15.4 | |
| 11–20 | 111 | 28.9 | 18 | 23.2 | 129 | 27.9 | |
| 21–30 | 75 | 19.5 | 11 | 14.1 | 86 | 18.6 | |
| >30 | 1 | 0.3 | 0 | 0.0 | 1 | 0.2 | |
| Mean duration of the ART years (±SD) | 11.17 | ±6.15 | 9.57 | ±6.37 | 10.90 | ±6.21 | |
| t | 37.699 | ||||||
| p | 0.001 | ||||||
| Duration of the ART years (%) | naive | 95 | 24.7 | 30 | 38.5 | 125 | 27.1 |
| 1–5 | 98 | 25.5 | 21 | 26.9 | 119 | 25.7 | |
| 6–10 | 191 | 49.8 | 27 | 34.6 | 218 | 47.2 | |
| 11–15 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| 16–20 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| Mean number of ARV. pills (±SD) | 3.48 | ±0.57 | 2.38 | ±0.54 | 3.29 | ±0.70 | |
| t | 101.166 | ||||||
| p | 0.001 | ||||||
| Mean number of therapeutic regimens (±SD) | 9.09 | ±7.42 | 7.89 | ±6.11 | 8.88 | ±7.23 | |
| t | 26.423 | ||||||
| p | 0.001 | ||||||
| Naive | 28 | 7.3 | 2 | 2.56 | 30 | 6.5 | |
| Experienced | 356 | 92.7 | 76 | 97.43 | 432 | 93.5 | |
| DRV/r (n = 384) | DRV/c (n = 78) | ||||
|---|---|---|---|---|---|
| Regimen | Patients | Regimen | Patients | ||
| n | % | n | % | ||
| ABC + 3TC + DRV + RTV | 151 | 39.33 | ABC + 3TC + DRV + COBI | 37 | 47.43 |
| FTC + TDF + DRV + RTV | 46 | 11.97 | FTC + TDF + DRV + COBI | 18 | 23.07 |
| 3TC + ZDV + DRV + RTV | 34 | 8.85 | 3TC + ZDV + DRV + COBI | 7 | 8.97 |
| ETV + RAL + DRV + RTV | 17 | 4.42 | TDF + DTG + DRV +COBI | 4 | 5.12 |
| TDF + ETV + DRV + RTV | 17 | 4.42 | FTC + TAF + DRV + COBI | 3 | 3.84 |
| TDF + DTG+ DRV + RTV | 14 | 3.64 | ETV + RAL + DRV +COBI | 2 | 2.56 |
| RAL + DRV+ RTV | 11 | 2.86 | RAL + DRV + COBI | 2 | 2.56 |
| Others | 94 | 24.47 | Others | 5 | 6.41 |
| Variables | DRV/r n = 384 | % | DRV/c n = 78 | % | Total N = 462 | % | t | |
|---|---|---|---|---|---|---|---|---|
| TC | 1 | 176 | 45.8 | 38 | 48.7 | 214 | 46.3 | 66.172 |
| 2 | 208 | 54.2 | 40 | 51.3 | 248 | 53.7 | ||
| HDL-cholesterol | 0 | 134 | 34.9 | 10 | 12.8 | 144 | 31.2 | 31.907 |
| 1 | 250 | 65.1 | 68 | 87.2 | 318 | 68.8 | ||
| LDL-cholesterol | 1 | 160 | 41.67 | 39 | 50 | 199 | 43.08 | 68.043 |
| 2 | 224 | 58.33 | 39 | 50 | 263 | 56.92 | ||
| TG | 1 | 168 | 43.8 | 44 | 56.4 | 212 | 45.9 | 66.404 |
| 2 | 216 | 56.2 | 34 | 43.6 | 250 | 54.1 | ||
| TL | 1 | 247 | 64.3 | 61 | 78.2 | 308 | 66.7 | 60.729 |
| 2 | 137 | 35.7 | 17 | 21.8 | 154 | 33.3 | ||
| VLDL-cholesterol | 1 | 301 | 78.4 | 71 | 91 | 372 | 80.5 | 2.583 |
| 2 | 83 | 21.6 | 7 | 9 | 90 | 19.5 | ||
| Variables | CT | HDL | LDL | TG | LT | VLDL | Glycosylated Hemoglobin | Glycemia |
|---|---|---|---|---|---|---|---|---|
| Age | r = 0.207 ** | r = 0.008 | r = 0.069 | r = 0.240 ** | r = 0.221 ** | r = 0.206 ** | r = 0.324 ** | r = 0.216 ** |
| (p = 0.001) | (p = 0.878) | (p = 0.139) | (p = 0.001) | (p = 0.001) | (p = 0.001) | (p = 0.001) | (p = 0.001) | |
| Duration of ART | r = 0.052 | r = −0.140 ** | r = 0.006 | r = 0.040 | r = 0.093 * | r = 0.182 ** | r = 0.189 ** | r = 0.002 |
| (p = 0.263) | (p = 0.003) | (p = 0.906) | (p = 0.393) | (p = 0.045) | (p = 0.001) | (p = 0.001) | (p = 0.974) | |
| Number of ARV regimens | r = 0.072 | r = −0.026 | r = 0.019 | r = 0.004 | r = 0.075 | r = 0.130 ** | r = 0.046 | r = 0.029 |
| (p = 0.121) | (p = 0.583) | (p = 0.682) | (p = 0.930) | (p = 0.110) | (p = 0.005) | (p = 0.325) | (p = 0.534) | |
| Number of comorbidities | r = −0.064 | r = −0.079 | r = 0.113 * | r = 0.028 | r = 0.051 | r = 0.104 * | r = 0.123 ** | r = −0.012 |
| (p = 0.170) | (p = 0.089) | (p = 0.015) | (p = 0.545) | (p = 0.278) | (p = 0.026) | (p = 0.008) | (p = 0.793) | |
| Pill’s burden | r = −0.059 | r = −0.120 ** | r = 0.020 | r = 0.068 | r = 0.066 | r = 0.107 * | r = 0.133 ** | r = 0.015 |
| (p = 0.207) | (p = 0.010) | (p = 0.673) | (p = 0.144 | (p = 0.158) | (p = 0.021) | (p = 0.004) | (p = 0.756) |
| Variables | DRV/r n = 384 | DRV/c n = 78 | Total N = 462 | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | t | |
| Lipodystrophy | 0.3542 | 0.47888 | 0.3333 | 0.47446 | 0.0173 | 0.13059 | 2.850 |
| Dyslipidemia | 0.2630 | 0.44085 | 0.1538 | 0.36314 | 0.3506 | 0.47769 | 15.778 |
| Variables | Diabetes | Lipodystrophy | Dyslipidemia |
|---|---|---|---|
| Age | r = 0.009 | r = 0.185 ** | r = 0.165 ** |
| (p = 0.851) | (p < 0.001) | (p < 0.001) | |
| Duration of ART | r = 0.008 | r = 0.127 ** | r = 0.272 ** |
| (p = 0.872) | (p = 0.006) | (p = 0.000) | |
| Number of ARV regimens | r = 0.010 | r = 0.035 | r = 0.109 * |
| (p = 0.838) | (p = 0.453) | (p = 0.019) | |
| Number of comorbidities | r = −0.048 | r = 0.105 * | r = 0.346 ** |
| (p = 0.299) | (p = 0.025) | (p < 0.001) | |
| Pill’s burden | r = −0.041 | r = 0.039 | r = 0.101 * |
| (p = 0.378) | (p = 0.397) | (p = 0.030) |
| Variables | DRV/r n = 384 | DRV/c n = 78 | Total N = 462 | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | t | |
| CK | 110.57 | 237.94 | 80.29 | 67.72 | 105.46 | 218.93 | 10.353 |
| CK-MB | 14.43 | 15.64 | 14.23 | 12.47 | 14.40 | 15.14 | 20.443 |
| PT/INR | 1.03 | 0.22 | 1.02 | 0.10 | 1.03 | 0.20 | 109.963 |
| Fibrinogen | 325.45 | 104.58 | 319.27 | 102.59 | 324.41 | 104.17 | 66.940 |
| Variables | CK | CK-MB | PT/INR | Fibrinogen |
|---|---|---|---|---|
| Age | r = −0.036 | r = −0.051 | r = 0.058 | r = 0.180 * |
| (p = 0.440) | (p = 0.276) | (p = 0.215) | (p = 0.001) | |
| Duration of ART | r = −0.030 | r = 0.024 | r = −0.052 | r = −0.029 |
| (p = 0.525) | (p = 0.614) | (p = 0.268) | (p = 0.538) | |
| Number of ARV regimens | r = −0.011 | r = 0.084 | r = −0.008 | r = 0.074 |
| (p = 0.525) | (p = 0.071) | (p = 0.860) | (p = 0.111) | |
| Number of comorbidities | r = −0.012 | r = −0.011 | r = 0.040 | r = 0.077 |
| (p = 0.799) | (p = 0.810) | (p = 0.389) | (p = 0.100) | |
| Pill’s burden | r = −0.018 | r = 0.046 | r = −0.018 | r = 0.087 |
| (p = 0.701) | (p = 0.319) | (p = 0.692) | (p = 0.063) |
| Variables | DRV/r n = 384 | DRV/c n = 78 | Total N = 462 | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | t | |
| ALT/GPT | 36.62 | 38.34 | 29.02 | 15.56 | 35.34 | 35.64 | 21.313 |
| AST/GOT | 34.94 | 31.17 | 29.07 | 10.38 | 33.95 | 28.81 | 25.331 |
| GGT | 54.55 | 140.34 | 36.48 | 29.22 | 51.50 | 128.65 | 8.605 |
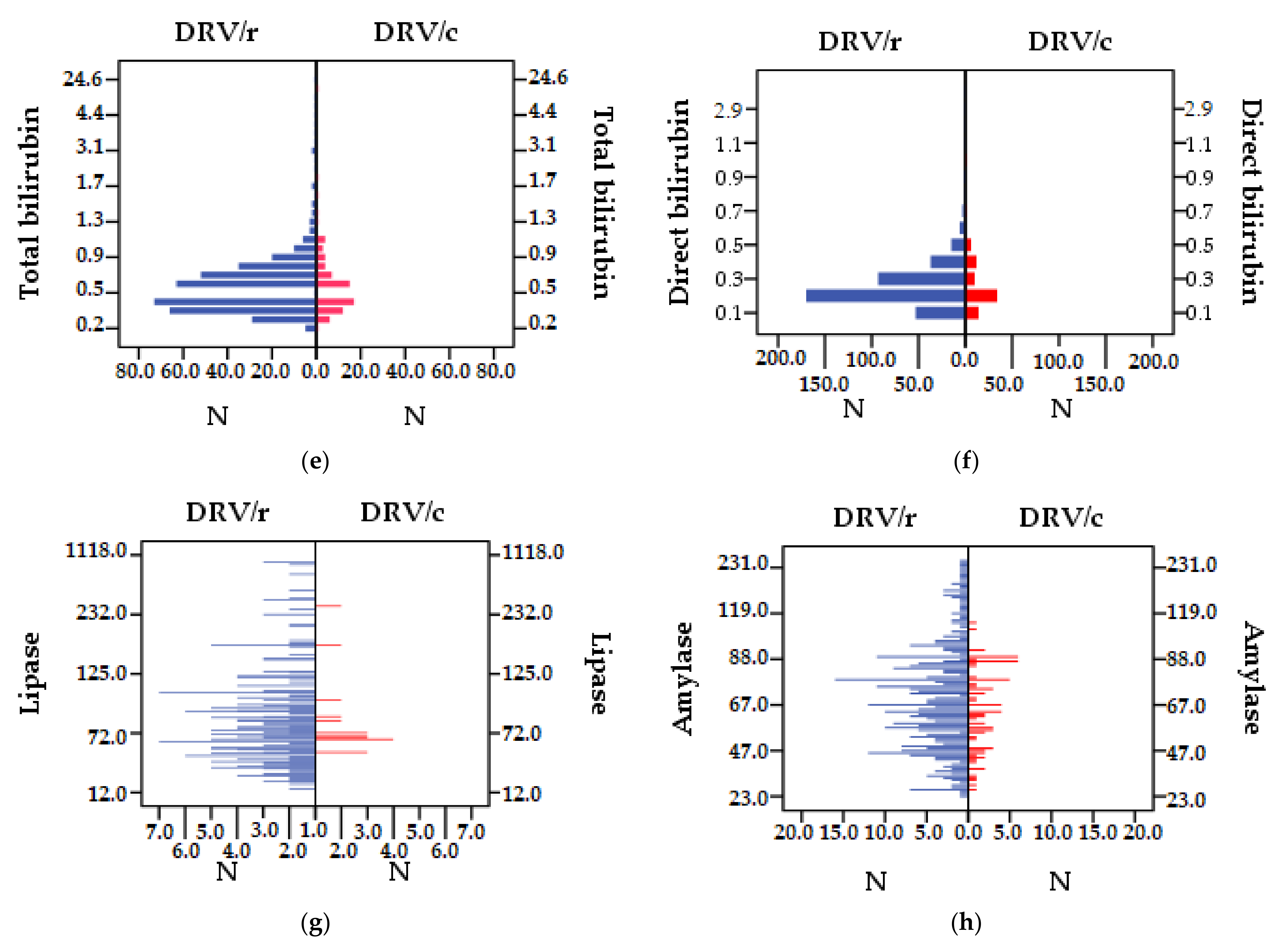
| ALP | 82.69 | 34.93 | 76.96 | 26.31 | 81.72 | 33.67 | 52.166 |
| Total bilirubin | 0.75 | 1.34 | 0.70 | 0.64 | 0.74 | 1.25 | 12.812 |
| Direct bilirubin | 0.28 | 0.41 | 0.26 | 0.15 | 0.28 | 0.38 | 16.071 |
| Lipase | 126.73 | 100.67 | 124.44 | 137.04 | 126.35 | 107.50 | 25.262 |
| Amylase | 73.91 | 35.01 | 65.85 | 18.54 | 72.55 | 32.94 | 47.338 |
| Variables | ALT/GPT | AST/GOT | GGT | Total Bilirubin | Direct Bilirubin | Lipase | Amylase |
|---|---|---|---|---|---|---|---|
| Age | r = −0.071 | r = −0.042 | r = 0.034 | r = 0.018 | r = 0.005 | r = 0.092 * | r = −0021. |
| (p = 0.129) | (p = 0.371) | (p = 0.465) | (p = 0.700) | (p = 0.922) | (p = 0.047) | (p = 0.651) | |
| Duration of ART | r = −0.052 | r = −0.059 | r = −0.032 | r = 0.018 | r = 0.010 | r = 0.120 * | r = 0.089 |
| (p = 0.263) | (p = 0.204) | (p = 0.492) | (p = 0.359) | (p = 0.833) | (p = 0.010) | (p = 0.057) | |
| Number of ARV regimens | r = −0.105 * | r = −0.021 | r = 0.006 | r = 0.043 | r = −0.026 | r = −0.114 ** | r = −0.006 |
| (p = 0.024) | (p = 0.660) | (p = 0.890) | (p = 0.696) | (p = 0.575) | (p = 0.014) | (p = 0.901) | |
| Number of comorbidities | r = 0.165 ** | r = 0.072 | r = 0.032 | r = 0.015 | r = 0.046 | r = −0.047 | r = −0.012 |
| (p = 0.001) | (p = 0.124) | (p = 0.494) | (p = 0.752) | (p = 0.322) | (p = 0.318) | (p = 0.795) | |
| Pill’s burden | r = 0.118 * | r = 0.069 | r = 0.016 | r = 0.030 | r = 0.050 | r = −0.040 | r = −0.024 |
| (p = 0.011) | (p = 0.141) | (p = 0.733) | (p = 0.522) | (p = 0.283) | (p = 0.389) | (p = 0.609) | |
| VHC co-infection | 0.200 ** | r = 0.194 ** | r = 0.194 ** | r = 0.102 * | r = 0.201 ** | r = −0.050 | r = −0.011 |
| (p = 0.001) | (p = 0.001) | (p = 0.001) | (p = 0.028) | (p = 0.001) | (p = 0.285) | (p = 0.817) | |
| VHB co-infection | r = 0.063 | r = 0.018 | r = −0.012 | r = 0.040 | r = 0.056 | r = −0.016 | r = −0.019 |
| (p = 0.178) | (p = 0.694) | (p = 0.794) | (p = 0.393) | (p = 0.227) | (p = 0.738) | (p = 0.686) |
| Variables | DRV/r n = 384 | DRV/c n = 78 | Total N = 462 | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | t | |
| Urea | 33.2544 | 18.61 | 32.5218 | 8.60 | 33.1307 | 17.32 | 41.093 * |
| Creatinine | 0.8797 | 0.67 | 0.8564 | 0.20 | 0.8758 | 0.61 | 30.404 * |
| Uric acid | 5.0146 | 1.67 | 5.3192 | 1.26 | 5.0660 | 1.61 | 67.534 * |
| Creatinine clearance | 98.90 | 30.45 | 96.46 | 23.06 | 98.49 | 29.33 | 72.176 * |
| Variables | Urea | Creatinine | Uric Acid | Creatinine Clearance | Stages of CKD |
|---|---|---|---|---|---|
| Age | r = 0.189 ** | r = 0.091 * | r = 0.148 ** | r = 0.056 | r = 0.024 |
| (p = 0.001) | (p = 0.050) | (p = 0.001) | (p = 0.228) | (p = 0.611) | |
| Duration of ART | r = −0.008 | r = −0.044 | r = −0.015 | r = −0.239 ** | r = 0.255 ** |
| (p = 0.867) | (p = 0.340) | (p = 0.748) | (p = 0.000) | (p = 0.000) | |
| Number of ARV regimens | r = 0.032 | r = −0.012 | r = −0.065 | r = 0.003 | r = 0.006 |
| (p = 0.491) | (p = 0.801) | (p = 0.163) | (p = 0.953) | (p = 0.901) | |
| Number of comorbidities | r = 0.036 | r = −0.011 | r = 0.138 ** | r = 0.040 | r = −0.042 |
| (p = 0.445) | (p = 0.818) | (p = 0.003) | (p = 0.389) | (p = 0.372) | |
| Pill’s burden | r = 0.028 | r = 0.043 | r = −0.046 | r = 0.023 | r = 0.010 |
| (p = 0.453) | (p = 0.352) | (p = 0.326) | (p = 0.618) | (p = 0.827) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marin, R.-C.; Tiț, D.M.; Săndulescu, O.; Streinu-Cercel, A.; Bungău, S.G. Comparison of Tolerability and Impact on Metabolic Profiles of Antiretroviral Regimens Containing Darunavir/Ritonavir or Darunavir/Cobicistat in Romanian HIV Infected Patients. Biomedicines 2021, 9, 987. https://doi.org/10.3390/biomedicines9080987
Marin R-C, Tiț DM, Săndulescu O, Streinu-Cercel A, Bungău SG. Comparison of Tolerability and Impact on Metabolic Profiles of Antiretroviral Regimens Containing Darunavir/Ritonavir or Darunavir/Cobicistat in Romanian HIV Infected Patients. Biomedicines. 2021; 9(8):987. https://doi.org/10.3390/biomedicines9080987
Chicago/Turabian StyleMarin, Ruxandra-Cristina, Delia Mirela Tiț, Oana Săndulescu, Adrian Streinu-Cercel, and Simona Gabriela Bungău. 2021. "Comparison of Tolerability and Impact on Metabolic Profiles of Antiretroviral Regimens Containing Darunavir/Ritonavir or Darunavir/Cobicistat in Romanian HIV Infected Patients" Biomedicines 9, no. 8: 987. https://doi.org/10.3390/biomedicines9080987
APA StyleMarin, R.-C., Tiț, D. M., Săndulescu, O., Streinu-Cercel, A., & Bungău, S. G. (2021). Comparison of Tolerability and Impact on Metabolic Profiles of Antiretroviral Regimens Containing Darunavir/Ritonavir or Darunavir/Cobicistat in Romanian HIV Infected Patients. Biomedicines, 9(8), 987. https://doi.org/10.3390/biomedicines9080987









