Collagen Bioinks for Bioprinting: A Systematic Review of Hydrogel Properties, Bioprinting Parameters, Protocols, and Bioprinted Structure Characteristics
Abstract
:1. Introduction
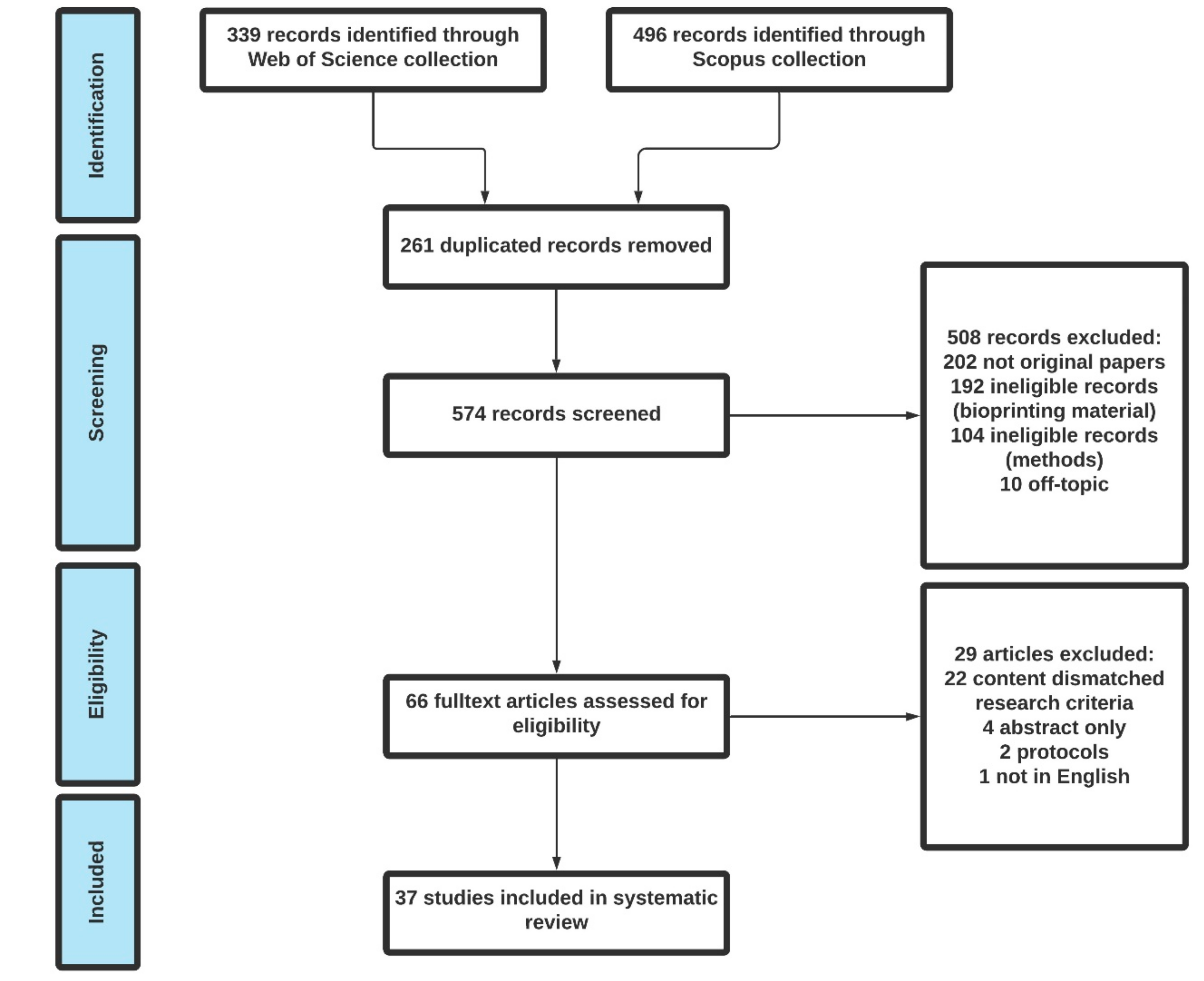
2. Methods
3. Results and Discussion
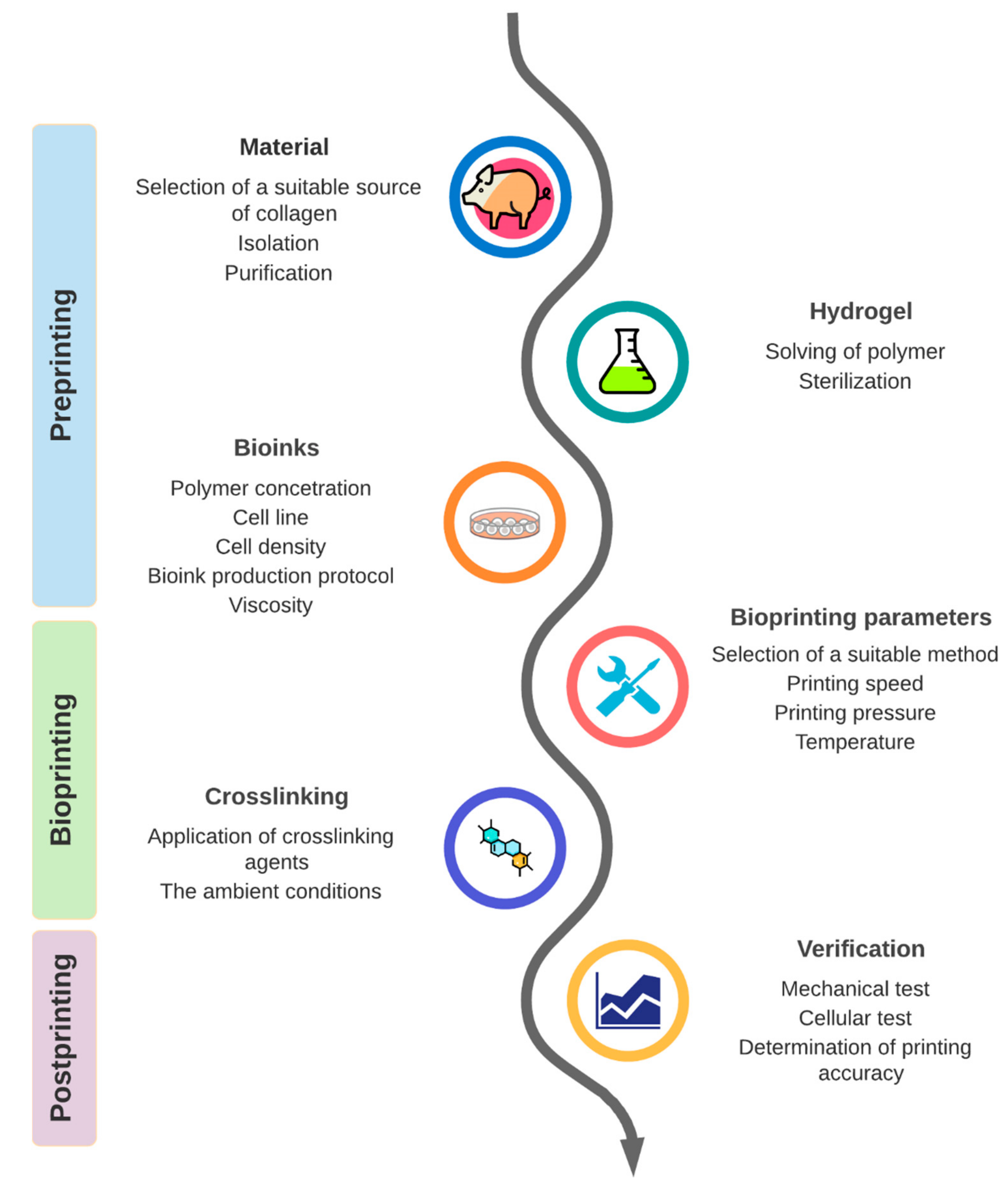
3.1. Part 1: Preprinting
3.1.1. Origin of Collagen Hydrogels
3.1.2. Collagen Extraction
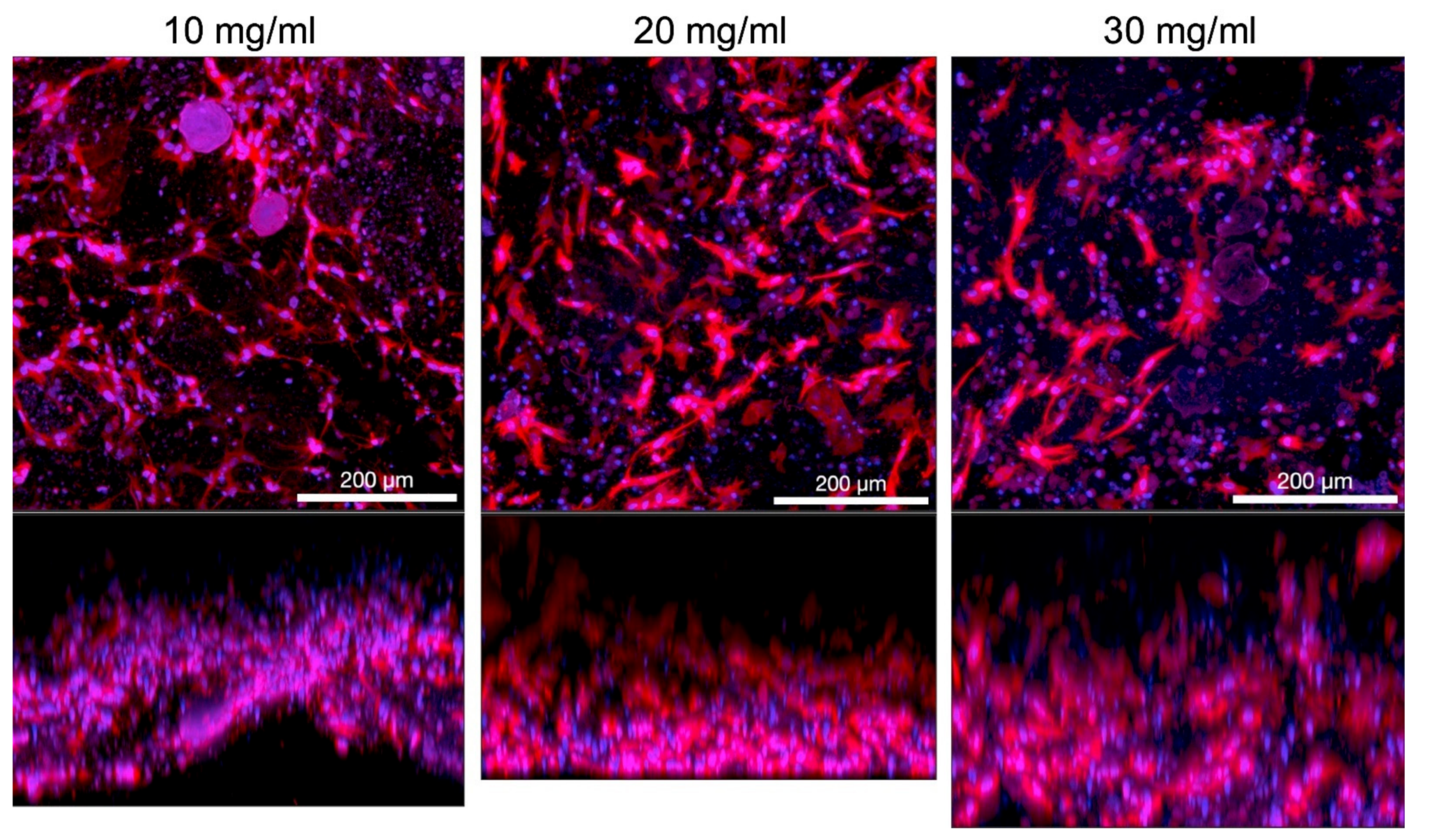
3.1.3. Collagen Concentration in Hydrogel
3.1.4. Viscosity and Cell Density
3.1.5. Protocols
3.2. Part 2: Bioprinting
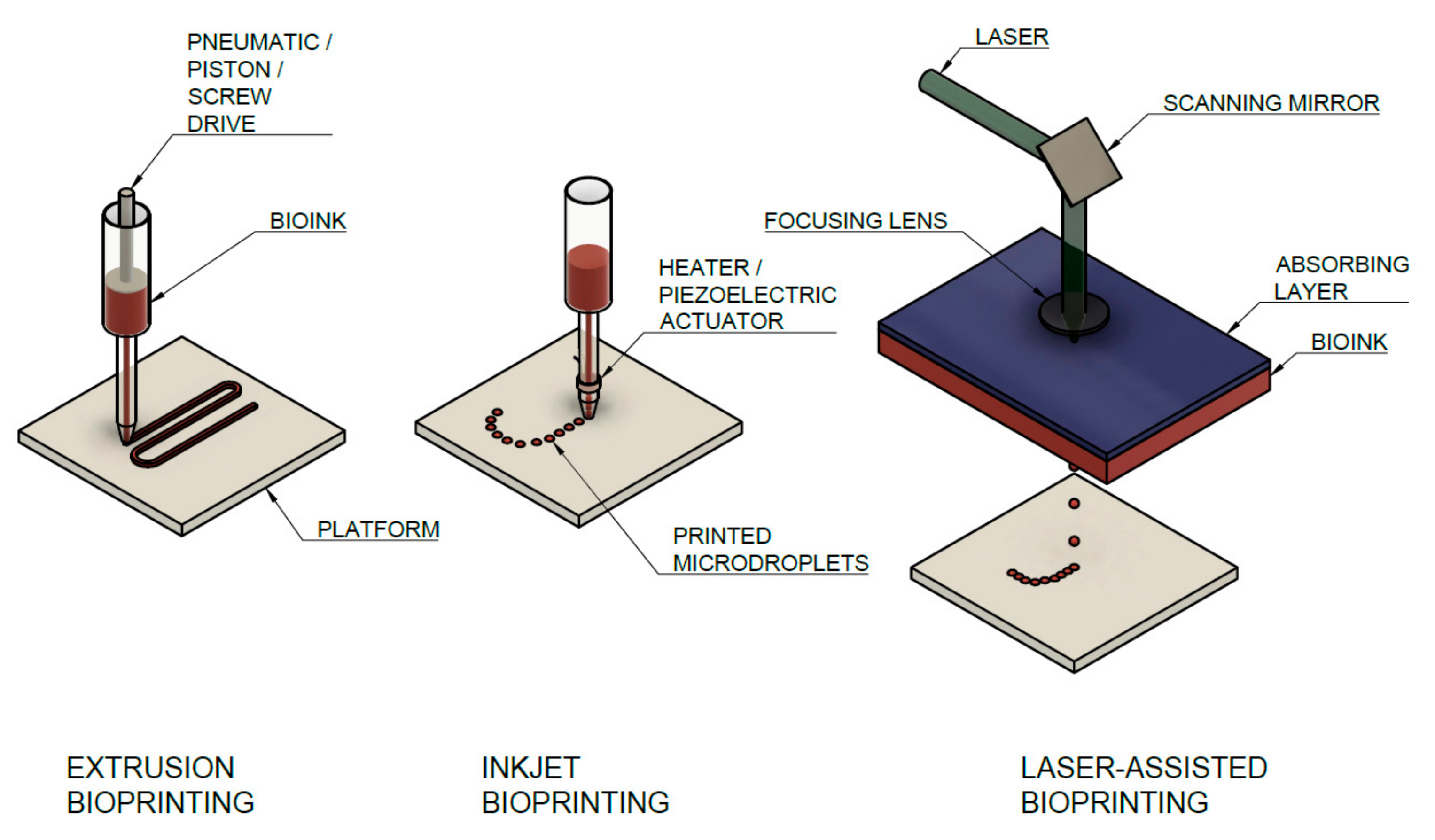
3.2.1. Types of Bioprinting Techniques Used for Collagen Bioinks and Their Parameters
- Microextrusion Printing
- Inkjet Printing
- Laser-Assisted Bioprinting (LaBP)
3.2.2. Temperature
3.2.3. Printing Pressure and Nozzle Diameter
3.2.4. Printing Speed
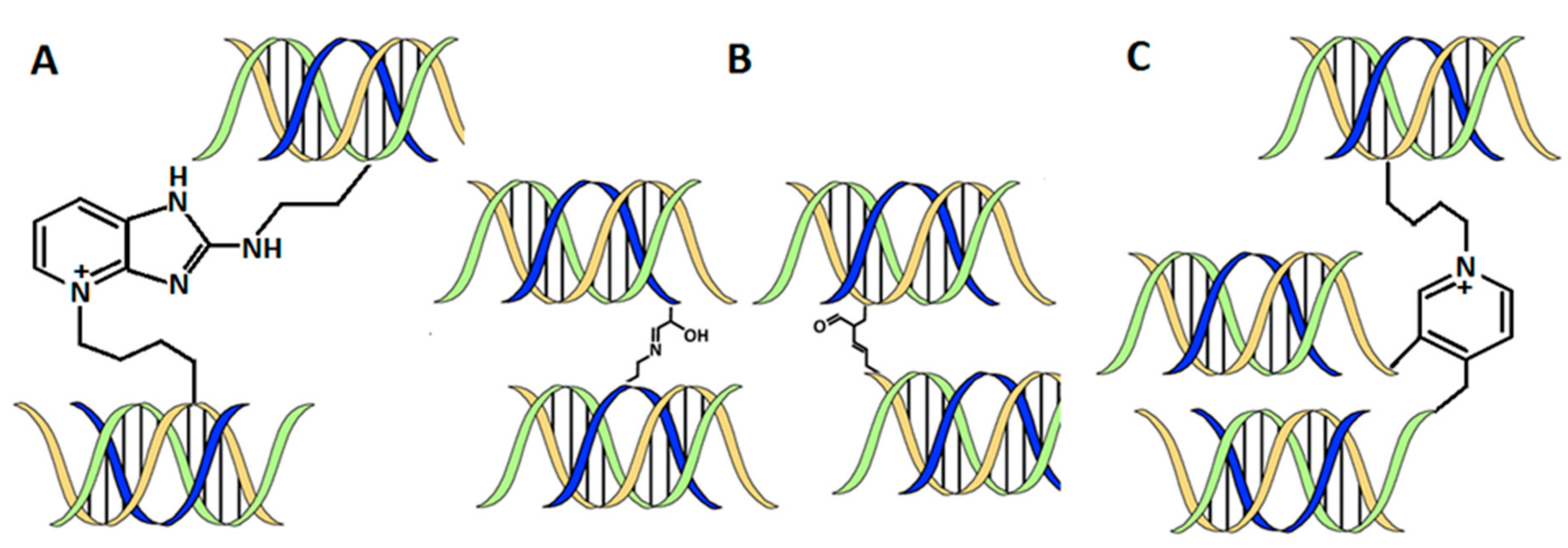
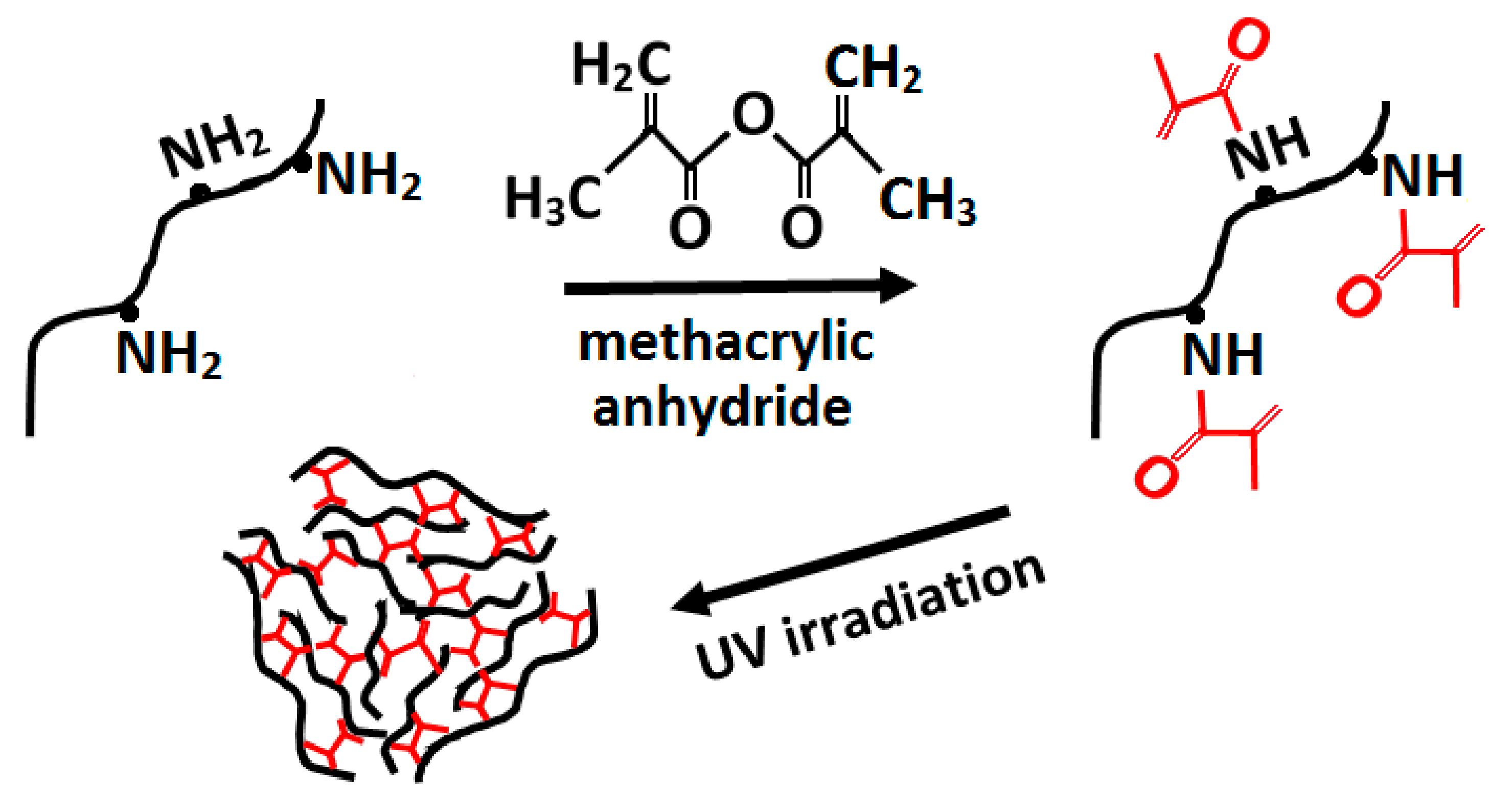
3.2.5. Crosslinking Methods
3.3. Part 3: Postprinting
3.3.1. Mechanical Tests
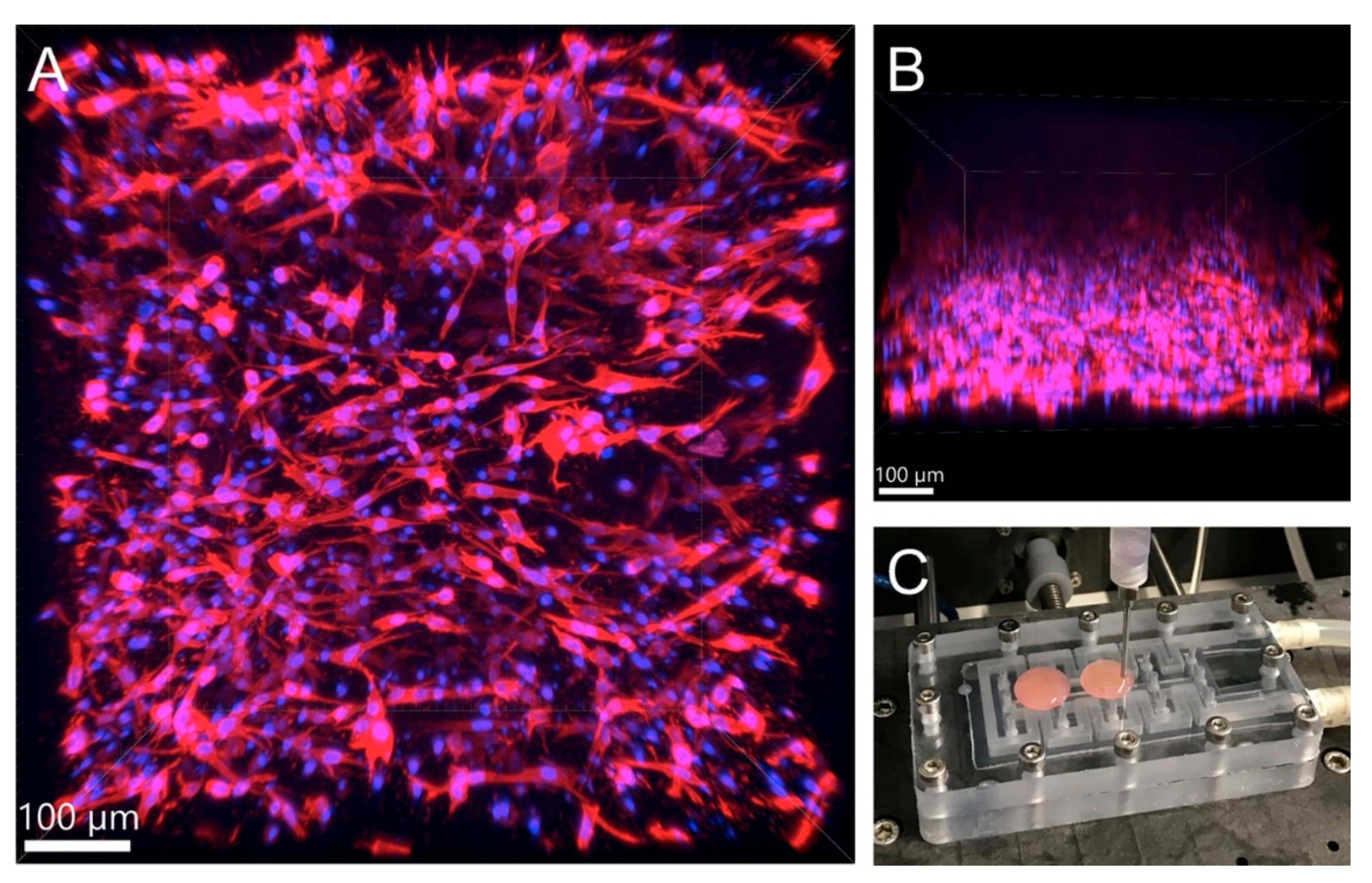
3.3.2. Viability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silva, L.P. Current Trends and Challenges in Biofabrication Using Biomaterials and Nanomaterials: Future Perspectives for 3D/4D Bioprinting. In 3D and 4D Printing in Biomedical Applications; Wiley: New York, NY, USA, 2019; pp. 373–421. [Google Scholar] [CrossRef]
- Moldovan, F. Recent Trends in Bioprinting. Procedia Manuf. 2019, 32, 95–101. [Google Scholar] [CrossRef]
- Hospodiuk, M.; Dey, M.; Sosnoski, D.; Ozbolat, I.T. The bioink: A comprehensive review on bioprintable materials. Biotechnol. Adv. 2017, 35, 217–239. [Google Scholar] [CrossRef] [Green Version]
- Arslan-Yildiz, A.; Assal, R.E.; Chen, P.; Guven, S.; Inci, F.; Demirci, U. Towards artificial tissue models: Past, present, and future of 3D bioprinting. Biofabrication 2016, 8, 014103. [Google Scholar] [CrossRef]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D bioprinting: An overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panwar, A.; Tan, L.P. Current Status of Bioinks for Micro-Extrusion-Based 3D Bioprinting. Molecules 2016, 21, 685. [Google Scholar] [CrossRef]
- Furth, M.E.; Atala, A.; Van Dyke, M.E. Smart biomaterials design for tissue engineering and regenerative medicine. Biomaterials 2007, 28, 5068–5073. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kim, Y.B.; Ahn, S.H.; Lee, J.S.; Jang, C.H.; Yoon, H.; Chun, W.; Kim, G.H. A New Approach for Fabricating Collagen/ECM-Based Bioinks Using Preosteoblasts and Human Adipose Stem Cells. Adv. Healthc. Mater. 2015, 4, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
- Ouellette, R.J.; Rawn, J.D. 28—Synthetic Polymers. In Organic Chemistry Study Guide; Ouellette, R.J., Rawn, J.D., Eds.; Elsevier: Boston, MA, USA, 2015; pp. 587–601. [Google Scholar] [CrossRef]
- Abelardo, E. 7—Synthetic material bioinks. In 3D Bioprinting for Reconstructive Surgery; Thomas, D.J., Jessop, Z.M., Whitaker, I.S., Eds.; Woodhead Publishing: Cambridge, MA, USA, 2018; pp. 137–144. [Google Scholar] [CrossRef]
- Pati, F.; Jang, J.; Ha, D.H.; Won Kim, S.; Rhie, J.W.; Shim, J.H.; Kim, D.H.; Cho, D.W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014, 5, 3935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malda, J.; Visser, J.; Melchels, F.P.; Jüngst, T.; Hennink, W.E.; Dhert, W.J.; Groll, J.; Hutmacher, D.W. 25th anniversary article: Engineering hydrogels for biofabrication. Adv. Mat. 2013, 25, 5011–5028. [Google Scholar] [CrossRef]
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical applications of collagen. Int. J. Pharm. 2001, 221, 1–22. [Google Scholar] [CrossRef]
- Roth, E.A.; Xu, T.; Das, M.; Gregory, C.; Hickman, J.J.; Boland, T. Inkjet printing for high-throughput cell patterning. Biomaterials 2004, 25, 3707–3715. [Google Scholar] [CrossRef]
- Hinton, T.J.; Jallerat, Q.; Palchesko, R.N.; Park, J.H.; Grodzicki, M.S.; Shue, H.-J.; Ramadan, M.H.; Hudson, A.R.; Feinberg, A.W. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv. 2015, 1, 1500758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, F.; Moon, S.; Emre, A.E.; Lien, C.; Turali, E.S.; Demirci, U. Cell bioprinting as a potential high-throughput method for fabricating Cell-Based Biosensors (CBBs). In Proceedings of the IEEE Sensors, Christchurch, New Zealand, 25–28 October 2009; pp. 387–391. [Google Scholar]
- Lee, W.; Debasitis, J.C.; Lee, V.K.; Lee, J.-H.; Fischer, K.; Edminster, K.; Park, J.-K.; Yoo, S.-S. Multi-layered culture of human skin fibroblasts and keratinocytes through three-dimensional freeform fabrication. Biomaterials 2009, 30, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Su, X.; Xu, Y.; Kong, B.; Sun, W.; Mi, S. Bioprinting three-dimensional cell-laden tissue constructs with controllable degradation. Sci. Rep. 2016, 6, 24474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inzana, J.A.; Olvera, D.; Fuller, S.M.; Kelly, J.P.; Graeve, O.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials 2014, 35, 4026–4034. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.; Gregory, C.A.; Molnar, P.; Cui, X.; Jalota, S.; Bhaduri, S.B.; Boland, T. Viability and electrophysiology of neural cell structures generated by the inkjet printing method. Biomaterials 2006, 27, 3580–3588. [Google Scholar] [CrossRef]
- Lee, V.; Singh, G.; Trasatti, J.P.; Bjornsson, C.; Xu, X.; Tran, T.N.; Yoo, S.-S.; Dai, G.; Karande, P. Design and Fabrication of Human Skin by Three-Dimensional Bioprinting. Tissue Eng. Part. C Methods 2013, 20, 473–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.C.; Boland, E.D.; Williams, S.K.; Hoying, J.B. Direct-write bioprinting three-dimensional biohybrid systems for future regenerative therapies. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2011, 98, 160–170. [Google Scholar] [CrossRef] [Green Version]
- Lynn, A.K.; Yannas, I.V.; Bonfield, W. Antigenicity and immunogenicity of collagen. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2004, 71, 343–354. [Google Scholar] [CrossRef]
- Nöth, U.; Rackwitz, L.; Heymer, A.; Weber, M.; Baumann, B.; Steinert, A.; Schütze, N.; Jakob, F.; Eulert, J. Chondrogenic differentiation of human mesenchymal stem cells in collagen type I hydrogels. J. Biomed. Mater. Res. A 2007, 83, 626–635. [Google Scholar] [CrossRef]
- Helary, C.; Bataille, I.; Abed, A.; Illoul, C.; Anglo, A.; Louedec, L.; Letourneur, D.; Meddahi-Pellé, A.; Giraud-Guille, M.M. Concentrated collagen hydrogels as dermal substitutes. Biomaterials 2010, 31, 481–490. [Google Scholar] [CrossRef]
- Yeleswarapu, S.; Chameettachal, S.; Bera, A.; Pati, F. Tissue-Specific Bioink from Xenogeneic Sources for 3D Bioprinting of Tissue Constructs; IntechOpen: London, UK, 2019. [Google Scholar]
- Gaudet, I.D.; Shreiber, D.I. Characterization of methacrylated type-I collagen as a dynamic, photoactive hydrogel. Biointerphases 2012, 7, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thayer, P.; Martinez, H.; Gatenholm, E. History and Trends of 3D Bioprinting. In 3D Bioprinting: Principles and Protocols; Crook, J.M., Ed.; Springer: New York, NY, USA, 2020; pp. 3–18. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [Green Version]
- Matsuo, N.; Tanaka, S.; Yoshioka, H.; Koch, M.; Gordon, M.K.; Ramirez, F. Collagen XXIV (Col24a1) gene expression is a specific marker of osteoblast differentiation and bone formation. Connect. Tissue Res. 2008, 49, 68–75. [Google Scholar] [CrossRef]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mat. 2019, 31, e1801651. [Google Scholar] [CrossRef] [Green Version]
- Gelse, K.; Pöschl, E.; Aigner, T. Collagens—Structure, function, and biosynthesis. Adv. Drug Del. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef] [Green Version]
- Bhagwat, P.K.; Dandge, P.B. Isolation, characterization and valorizable applications of fish scale collagen in food and agriculture industries. Biocatal. Agric. Biotechnol. 2016, 7, 234–240. [Google Scholar] [CrossRef]
- Alexander, B.; Daulton, T.L.; Genin, G.M.; Lipner, J.; Pasteris, J.D.; Wopenka, B.; Thomopoulos, S. The nanometre-scale physiology of bone: Steric modelling and scanning transmission electron microscopy of collagen–mineral structure. J. R. Soc. Interface 2012, 9, 1774–1786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaar, J.; Naffa, R.; Brimble, M. Enzymatic and non-enzymatic crosslinks found in collagen and elastin and their chemical synthesis. Org. Chem. Front. 2020, 7, 2789–2814. [Google Scholar] [CrossRef]
- Snedeker, J.G.; Gautieri, A. The role of collagen crosslinks in ageing and diabetes—The good, the bad, and the ugly. Muscles Ligaments Tendons J. 2014, 4, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Eyre, D.R.; Weis, M.; Rai, J. Analyses of lysine aldehyde cross-linking in collagen reveal that the mature cross-link histidinohydroxylysinonorleucine is an artifact. J. Biol. Chem. 2019, 294, 6578–6590. [Google Scholar] [CrossRef]
- Paschalis, E.P.; Verdelis, K.; Doty, S.B.; Boskey, A.L.; Mendelsohn, R.; Yamauchi, M. Spectroscopic characterization of collagen cross-links in bone. J. Bone Mineral. Res. 2001, 16, 1821–1828. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M. Processing of collagen based biomaterials and the resulting materials properties. Biomed. Eng. Online 2019, 18, 24. [Google Scholar] [CrossRef] [Green Version]
- Naffa, R.; Maidment, C.; Ahn, M.; Ingham, B.; Hinkley, S.; Norris, G. Molecular and structural insights into skin collagen reveals several factors that influence its architecture. Int. J. Biol. Macromol. 2019, 128, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, M.; Sricholpech, M. Lysine post-translational modifications of collagen. Essays Biochem. 2012, 52, 113–133. [Google Scholar] [CrossRef] [Green Version]
- Gerriets, J.E.; Curwin, S.L.; Last, J.A. Tendon hypertrophy is associated with increased hydroxylation of nonhelical lysine residues at two specific cross-linking sites in type I collagen. J. Biol. Chem. 1993, 268, 25553–25560. [Google Scholar] [CrossRef]
- Rajan, N.; Habermehl, J.; Coté, M.F.; Doillon, C.J.; Mantovani, D. Preparation of ready-to-use, storable and reconstituted type I collagen from rat tail tendon for tissue engineering applications. Nat. Protoc. 2006, 1, 2753–2758. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, A.; Bansal, M. Collagen structure: The Madras triple helix and the current scenario. IUBMB Life 2005, 57, 161–172. [Google Scholar] [CrossRef]
- Schmidt, M.M.; Dornelles, R.C.P.; Mello, R.O.; Kubota, E.H.; Mazutti, M.A.; Kempka, A.P.; Demiate, I.M. Collagen extraction process. Int. Food Res. J. 2016, 23, 913–922. [Google Scholar]
- Liu, D.; Wei, G.; Li, T.; Hu, J.; Lu, N.; Regenstein, J.M.; Zhou, P. Effects of alkaline pretreatments and acid extraction conditions on the acid-soluble collagen from grass carp (Ctenopharyngodon idella) skin. Food Chem. 2015, 172, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Regenstein, J.M.; Zhou, P. 13—Collagen and gelatin from marine by-products. In Maximising the Value of Marine By-Products; Shahidi, F., Ed.; Woodhead Publishing: Cambridge, MA, USA, 2007; pp. 279–303. [Google Scholar] [CrossRef]
- Fratzl, P. Collagen: Structure and Mechanics, an Introduction. In Collagen: Structure and Mechanics; Fratzl, P., Ed.; Springer: Boston, MA, USA, 2008; pp. 1–13. [Google Scholar] [CrossRef]
- Li, Y.; Asadi, A.; Monroe, M.R.; Douglas, E.P. pH effects on collagen fibrillogenesis in vitro: Electrostatic interactions and phosphate binding. Mater. Sci. Eng. C Mater. Biol. Appl. 2009, 29, 1643–1649. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef] [Green Version]
- Ruszczak, Z.; Friess, W. Collagen as a carrier for on-site delivery of antibacterial drugs. Adv. Drug Del. Rev. 2003, 55, 1679–1698. [Google Scholar] [CrossRef] [PubMed]
- Rýglová, Š.; Braun, M.; Suchý, T. Collagen and Its Modifications—Crucial Aspects with Concern to Its Processing and Analysis. Macromol. Mater. Eng. 2017, 302, 1600460. [Google Scholar] [CrossRef]
- Rhee, S.; Puetzer, J.L.; Mason, B.N.; Reinhart-King, C.A.; Bonassar, L.J. 3D Bioprinting of Spatially Heterogeneous Collagen Constructs for Cartilage Tissue Engineering. ACS Biomater. Sci. Eng. 2016, 2, 1800–1805. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Wu, K.; Liu, W.; Shen, L.; Li, G. Two-dimensional infrared spectroscopic study on the thermally induced structural changes of glutaraldehyde-crosslinked collagen. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 140, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Włodarczyk-Biegun, M.K.; Del Campo, A. 3D bioprinting of structural proteins. Biomaterials 2017, 134, 180–201. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, C.; Marganski, W.A.; Kim, S.; Brown, C.T.; Gunderia, V.; Dembo, M.; Wong, J.Y. Influence of Type I Collagen Surface Density on Fibroblast Spreading, Motility, and Contractility. Biophys. J. 2003, 85, 3329–3335. [Google Scholar] [CrossRef] [Green Version]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Khatiwala, C.; Law, R.; Shepherd, B.; Dorfman, S.; Csete, M. 3D cell bioprinting for regenerative medicine research and therapies. Gene Ther. Regul. 2012, 7, 1230004. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Blokzijl, M.M.; Levato, R.; Peiffer, Q.C.; Ruijter, M.D.; Hennink, W.E.; Vermonden, T.; Malda, J. Hydrogel-based reinforcement of 3D bioprinted constructs. Biofabrication 2016, 8, 035004. [Google Scholar] [CrossRef]
- Li, H.; Tan, C.; Li, L. Review of 3D printable hydrogels and constructs. Mater. Des. 2018, 159, 20–38. [Google Scholar] [CrossRef]
- Osidak, E.O.; Karalkin, P.A.; Osidak, M.S.; Parfenov, V.A.; Sivogrivov, D.E.; Pereira, F.; Gryadunova, A.A.; Koudan, E.V.; Khesuani, Y.D.; Кasyanov, V.A.; et al. Viscoll collagen solution as a novel bioink for direct 3D bioprinting. J. Mater. Sci. Mater. Med. 2019, 30, 31. [Google Scholar] [CrossRef] [PubMed]
- Diamantides, N.; Wang, L.; Pruiksma, T.; Siemiatkoski, J.; Dugopolski, C.; Shortkroff, S.; Kennedy, S.; Bonassar, L.J. Correlating rheological properties and printability of collagen bioinks: The effects of riboflavin photocrosslinking and pH. Biofabrication 2017, 9, 034102. [Google Scholar] [CrossRef]
- Lai, G.; Li, Y.; Li, G. Effect of concentration and temperature on the rheological behavior of collagen solution. Int. J. Biol. Macromol. 2008, 42, 285–291. [Google Scholar] [CrossRef]
- Duan, L.; Li, J.; Li, C.; Li, G. Effects of NaCl on the rheological behavior of collagen solution. Korea-Aust. Rheol. J. 2013, 25, 137–144. [Google Scholar] [CrossRef]
- Gr, T.S.P.; Zaman, N.T.; Alamelu, B.; Dhamankar, V.; Chu, C.; Perotta, E.; Kadiyala, I. Mechanical Characterization of Extracellular Matrix Hydrogels: Comparison of Properties Measured by Rheometer and Texture Analyzer. Asian J. Pharm. Technol. Innov. 2018, 6, 6–21. [Google Scholar]
- Choudhury, D.; Anand, S.; Naing, M.W. The Arrival of Commercial Bioprinters—Towards 3D Bioprinting Revolution! Int. J. Bioprint. 2018, 4, 139. [Google Scholar] [CrossRef]
- Derby, B.; Reis, N. Inkjet Printing of Highly Loaded Particulate Suspensions. MRS Bull. 2003, 28, 815–818. [Google Scholar] [CrossRef]
- Hölzl, K.; Lin, S.; Tytgat, L.; Van Vlierberghe, S.; Gu, L.; Ovsianikov, A. Bioink properties before, during and after 3D bioprinting. Biofabrication 2016, 8, 032002. [Google Scholar] [CrossRef]
- He, Y.; Yang, F.; Zhao, H.; Gao, Q.; Xia, B.; Fu, J. Research on the printability of hydrogels in 3D bioprinting. Sci. Rep. 2016, 6, 29977. [Google Scholar] [CrossRef]
- Chang, R.; Nam, J.; Sun, W. Effects of dispensing pressure and nozzle diameter on cell survival from solid freeform fabrication-based direct cell writing. Tissue Eng. Part. A 2008, 14, 41–48. [Google Scholar] [CrossRef]
- Galie, P.A.; Nguyen, D.H.; Choi, C.K.; Cohen, D.M.; Janmey, P.A.; Chen, C.S. Fluid shear stress threshold regulates angiogenic sprouting. Proc. Natl. Acad. Sci. USA 2014, 111, 7968–7973. [Google Scholar] [CrossRef] [Green Version]
- Chien, S. Effects of disturbed flow on endothelial cells. Ann. Biomed. Eng. 2008, 36, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Potter, C.M.; Lao, K.H.; Zeng, L.; Xu, Q. Role of biomechanical forces in stem cell vascular lineage differentiation. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2184–2190. [Google Scholar] [CrossRef] [Green Version]
- Ng, W.L.; Lee, J.M.; Yeong, W.Y.; Win Naing, M. Microvalve-based bioprinting—Process, bio-inks and applications. Biomater. Sci. 2017, 5, 632–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.; Lee, V.; Polio, S.; Keegan, P.; Lee, J.-H.; Fischer, K.; Park, J.-K.; Yoo, S.-S. On-demand three-dimensional freeform fabrication of multi-layered hydrogel scaffold with fluidic channels. Biotechnol. Bioeng. 2010, 105, 1178–1186. [Google Scholar] [CrossRef]
- Smith, C.M.; Christian, J.J.; Warren, W.L.; Williams, S.K. Characterizing environmental factors that impact the viability of tissue-engineered constructs fabricated by a direct-write bioassembly tool. Tissue Eng. 2007, 13, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.M.; Stone, A.L.; Parkhill, R.L.; Stewart, R.L.; Simpkins, M.W.; Kachurin, A.M.; Warren, W.L.; Williams, S.K. Three-dimensional bioassembly tool for generating viable tissue-engineered constructs. Tissue Eng. 2004, 10, 1566–1576. [Google Scholar] [CrossRef]
- Allevi. Follow This Guide for Cell Mixing and Bioink Loading. Available online: https://www.allevi3d.com/cell-mixing-bioink-loading/ (accessed on 21 June 2017).
- Sciences, C.L. Bioprinting Protocol: Cellink Bioink. Available online: https://www.cellink.com/wp-content/uploads/2019/03/Bioprinting-Protocol-CELLINK-Bioink_14-Jun-2021.pdf (accessed on 20 May 2021).
- Ibidi. Collagen Type I, Rat Tail, 5 mg/mL Protocol. Available online: https://ibidi.com/img/cms/products/cells_reagents/R_5020X_CollagenI/IN_5020X_CollagenI_05mg.pdf (accessed on 21 June 2017).
- Matejka, R.; Konarik, M.; Stepanovska, J.; Lipensky, J.; Chlupac, J.; Turek, D.; Prazak, I.; Broz, A.; Simunkova, Z.; Mrazova, I.; et al. Bioreactor Processed Stromal Cell Seeding and Cultivation on Decellularized Pericardium Patches for Cardiovascular Use. Appl. Sci. 2020, 10, 5473. [Google Scholar] [CrossRef]
- Liu, C.Z.; Xia, Z.D.; Han, Z.W.; Hulley, P.A.; Triffitt, J.T.; Czernuszka, J.T. Novel 3D collagen scaffolds fabricated by indirect printing technique for tissue engineering. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2008, 85, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.F.; Diogo, G.S.; Pina, S.; Oliveira, J.M.; Silva, T.H.; Reis, R.L. Collagen-based bioinks for hard tissue engineering applications: A comprehensive review. J. Mater. Sci. Mater. Med. 2019, 30, 32. [Google Scholar] [CrossRef]
- Lee, J.M.; Suen, S.K.Q.; Ng, W.L.; Ma, W.C.; Yeong, W.Y. Bioprinting of Collagen: Considerations, Potentials, and Applications. Macromol. Biosci. 2020, 21, 2000280. [Google Scholar] [CrossRef]
- Hacioglu, A.; Yilmazer, H.; Ustundag, C. 3D Printing for Tissue Engineering Applications. J. Polytech. 2018, 42, 624–638. [Google Scholar] [CrossRef] [Green Version]
- Diamantides, N.; Dugopolski, C.; Blahut, E.; Kennedy, S.; Bonassar, L.J. High density cell seeding affects the rheology and printability of collagen bioinks. Biofabrication 2019, 11, 045016. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Ahn, G.; Kim, D.; Kang, H.-W.; Yun, S.; Yun, W.-S.; Shim, J.-H.; Jin, S. Pre-set extrusion bioprinting for multiscale heterogeneous tissue structure fabrication. Biofabrication 2018, 10, 035008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moncal, K.K.; Ozbolat, V.; Datta, P.; Heo, D.N.; Ozbolat, I.T. Thermally-controlled extrusion-based bioprinting of collagen. J. Mater. Sci. Mater. Med. 2019, 30, 55. [Google Scholar] [CrossRef] [PubMed]
- Yeo, M.G.; Kim, G.H. A cell-printing approach for obtaining hASC-laden scaffolds by using a collagen/polyphenol bioink. Biofabrication 2017, 9, 025004. [Google Scholar] [CrossRef]
- Lee, J.; Yeo, M.; Kim, W.; Koo, Y.; Kim, G.H. Development of a tannic acid cross-linking process for obtaining 3D porous cell-laden collagen structure. Int. J. Biol. Macromol. 2018, 110, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Lee, J.-S.; Yim, H.; Kim, G.; Chun, W. Development of cell-laden 3D scaffolds for efficient engineered skin substitutes by collagen gelation. RSC Adv. 2016, 6, 21439–21447. [Google Scholar] [CrossRef]
- Yeo, M.; Lee, J.-S.; Chun, W.; Kim, G.H. An Innovative Collagen-Based Cell-Printing Method for Obtaining Human Adipose Stem Cell-Laden Structures Consisting of Core–Sheath Structures for Tissue Engineering. Biomacromolecules 2016, 17, 1365–1375. [Google Scholar] [CrossRef]
- Kim, Y.B.; Lee, H.; Kim, G.H. Strategy to Achieve Highly Porous/Biocompatible Macroscale Cell Blocks, Using a Collagen/Genipin-bioink and an Optimal 3D Printing Process. ACS Appl. Mater. Interfaces 2016, 8, 32230–32240. [Google Scholar] [CrossRef] [PubMed]
- Baltazar, T.; Merola, J.; Catarino, C.; Xie, C.B.; Kirkiles-Smith, N.C.; Lee, V.; Hotta, S.; Dai, G.; Xu, X.; Ferreira, F.C.; et al. Three Dimensional Bioprinting of a Vascularized and Perfusable Skin Graft Using Human Keratinocytes, Fibroblasts, Pericytes, and Endothelial Cells. Tissue Eng. Part. A 2020, 26, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Duarte Campos, D.F.; Blaeser, A.; Korsten, A.; Neuss, S.; Jäkel, J.; Vogt, M.; Fischer, H. The Stiffness and Structure of Three-Dimensional Printed Hydrogels Direct the Differentiation of Mesenchymal Stromal Cells toward Adipogenic and Osteogenic Lineages. Tissue Eng. Part. A 2014, 21, 740–756. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Wang, F.; Chen, C.; Gong, X.; Yin, L.; Yang, L. Engineering zonal cartilage through bioprinting collagen type II hydrogel constructs with biomimetic chondrocyte density gradient. BMC Musculoskelet. Disord. 2016, 17, 301. [Google Scholar] [CrossRef] [Green Version]
- Wilson, W.C., Jr.; Boland, T. Cell and organ printing 1: Protein and cell printers. Anat. Rec. 2003, 272, 491–496. [Google Scholar] [CrossRef]
- Park, T.-M.; Kang, D.; Jang, I.; Yun, W.-S.; Shim, J.-H.; Jeong, Y.H.; Kwak, J.-Y.; Yoon, S.; Jin, S. Fabrication of In Vitro Cancer Microtissue Array on Fibroblast-Layered Nanofibrous Membrane by Inkjet Printing. Int. J. Mol. Sci. 2017, 18, 2348. [Google Scholar] [CrossRef] [Green Version]
- Park, J.Y.; Choi, J.-C.; Shim, J.-H.; Lee, J.-S.; Park, H.; Kim, S.W.; Doh, J.; Cho, D.-W. A comparative study on collagen type I and hyaluronic acid dependent cell behavior for osteochondral tissue bioprinting. Biofabrication 2014, 6, 035004. [Google Scholar] [CrossRef]
- Murphy, S.V.; Skardal, A.; Atala, A. Evaluation of hydrogels for bio-printing applications. J. Biomed. Mater. Res. A 2013, 101, 272–284. [Google Scholar] [CrossRef]
- Duarte Campos, D.F.; Rohde, M.; Ross, M.; Anvari, P.; Blaeser, A.; Vogt, M.; Panfil, C.; Yam, G.H.-F.; Mehta, J.S.; Fischer, H.; et al. Corneal bioprinting utilizing collagen-based bioinks and primary human keratocytes. J. Biomed. Mater. Res. A 2019, 107, 1945–1953. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Pinckney, J.; Lee, V.; Lee, J.-H.; Fischer, K.; Polio, S.; Park, J.-K.; Yoo, S.-S. Three-dimensional bioprinting of rat embryonic neural cells. Neuroreport 2009, 20, 798–803. [Google Scholar] [CrossRef]
- Moon, S.; Hasan, S.K.; Song, Y.S.; Xu, F.; Keles, H.O.; Manzur, F.; Mikkilineni, S.; Hong, J.W.; Nagatomi, J.; Haeggstrom, E.; et al. Layer by layer three-dimensional tissue epitaxy by cell-laden hydrogel droplets. Tissue Eng. Part. C Methods 2010, 16, 157–166. [Google Scholar] [CrossRef]
- Benning, L.; Gutzweiler, L.; Tröndle, K.; Riba, J.; Zengerle, R.; Koltay, P.; Zimmermann, S.; Stark, G.B.; Finkenzeller, G. Cytocompatibility testing of hydrogels toward bioprinting of mesenchymal stem cells. J. Biomed. Mater. Res. A 2017, 105, 3231–3241. [Google Scholar] [CrossRef] [PubMed]
- Benning, L.; Gutzweiler, L.; Tröndle, K.; Riba, J.; Zengerle, R.; Koltay, P.; Zimmermann, S.; Stark, G.B.; Finkenzeller, G. Assessment of hydrogels for bioprinting of endothelial cells. J. Biomed. Mater. Res. A 2018, 106, 935–947. [Google Scholar] [CrossRef]
- Reid, J.A.; Palmer, X.-L.; Mollica, P.A.; Northam, N.; Sachs, P.C.; Bruno, R.D. A 3D bioprinter platform for mechanistic analysis of tumoroids and chimeric mammary organoids. Sci. Rep. 2019, 9, 7466. [Google Scholar] [CrossRef]
- Xu, F.; Emre, A.E.; Turali, E.S.; Hasan, S.K.; Moon, S.; Nagatomi, J.; Khademhosseini, A.; Demirci, U. Cell proliferation in bioprinted cell-laden collagen droplets. In Proceedings of the 2009 IEEE 35th Annual Northeast Bioengineering Conference, Cambridge, MA, USA, 3–5 April 2009; pp. 1–2. [Google Scholar]
- Michael, S.; Sorg, H.; Peck, C.-T.; Koch, L.; Deiwick, A.; Chichkov, B.; Vogt, P.M.; Reimers, K. Tissue engineered skin substitutes created by laser-assisted bioprinting form skin-like structures in the dorsal skin fold chamber in mice. PLoS ONE 2013, 8, e57741. [Google Scholar] [CrossRef]
- Koch, L.; Deiwick, A.; Schlie, S.; Michael, S.; Gruene, M.; Coger, V.; Zychlinski, D.; Schambach, A.; Reimers, K.; Vogt, P.M.; et al. Skin tissue generation by laser cell printing. Biotechnol. Bioeng. 2012, 109, 1855–1863. [Google Scholar] [CrossRef] [PubMed]
- Keriquel, V.; Oliveira, H.; Rémy, M.; Ziane, S.; Delmond, S.; Rousseau, B.; Rey, S.; Catros, S.; Amédée, J.; Guillemot, F.; et al. In situ printing of mesenchymal stromal cells, by laser-assisted bioprinting, for in vivo bone regeneration applications. Sci. Rep. 2017, 7, 1778. [Google Scholar] [CrossRef] [PubMed]
- Sorkio, A.; Koch, L.; Koivusalo, L.; Deiwick, A.; Miettinen, S.; Chichkov, B.; Skottman, H. Human stem cell based corneal tissue mimicking structures using laser-assisted 3D bioprinting and functional bioinks. Biomaterials 2018, 171, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Bourget, J.-M.; Kérourédan, O.; Medina, M.; Rémy, M.; Thébaud, N.B.; Bareille, R.; Chassande, O.; Amédée, J.; Catros, S.; Devillard, R. Patterning of Endothelial Cells and Mesenchymal Stem Cells by Laser-Assisted Bioprinting to Study Cell Migration. BioMed Res. Int. 2016, 2016, 3569843. [Google Scholar] [CrossRef] [Green Version]
- Kérourédan, O.; Bourget, J.-M.; Rémy, M.; Crauste-Manciet, S.; Kalisky, J.; Catros, S.; Thébaud, N.B.; Devillard, R. Micropatterning of endothelial cells to create a capillary-like network with defined architecture by laser-assisted bioprinting. J. Mater. Sci. Mater. Med. 2019, 30, 28. [Google Scholar] [CrossRef] [PubMed]
- Kérourédan, O.; Hakobyan, D.; Rémy, M.; Ziane, S.; Dusserre, N.; Fricain, J.-C.; Delmond, S.; Thébaud, N.B.; Devillard, R. In situ prevascularization designed by laser-assisted bioprinting: Effect on bone regeneration. Biofabrication 2019, 11, 045002. [Google Scholar] [CrossRef]
- Yoon, S.; Park, J.A.; Lee, H.-R.; Yoon, W.H.; Hwang, D.S.; Jung, S. Inkjet–Spray Hybrid Printing for 3D Freeform Fabrication of Multilayered Hydrogel Structures. Adv. Healthc. Mater. 2018, 7, 1800050. [Google Scholar] [CrossRef] [PubMed]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef] [Green Version]
- Foyt, D.A.; Norman, M.D.A.; Yu, T.T.L.; Gentleman, E. Exploiting Advanced Hydrogel Technologies to Address Key Challenges in Regenerative Medicine. Adv. Healthc. Mater. 2018, 7, e1700939. [Google Scholar] [CrossRef] [PubMed]
- Khalil, S.; Nam, J.; Sun, W. Multi-nozzle deposition for construction of 3D biopolymer tissue scaffolds. Rapid Prototyp. J. 2005, 11, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Dababneh, A.B.; Ozbolat, I.T. Bioprinting Technology: A Current State-of-the-Art Review. J. Manuf. Sci. Eng. 2014, 136, 061016. [Google Scholar] [CrossRef]
- Vozzi, G.; Previti, A.; De Rossi, D.; Ahluwalia, A. Microsyringe-based deposition of two-dimensional and three-dimensional polymer scaffolds with a well-defined geometry for application to tissue engineering. Tissue Eng. 2002, 8, 1089–1098. [Google Scholar] [CrossRef] [Green Version]
- Suntornnond, R.; Tan, E.Y.S.; An, J.; Chua, C.K. A Mathematical Model on the Resolution of Extrusion Bioprinting for the Development of New Bioinks. Materials 2016, 9, 756. [Google Scholar] [CrossRef] [Green Version]
- Ozbolat, I.T.; Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016, 76, 321–343. [Google Scholar] [CrossRef] [Green Version]
- Boland, T.; Mironov, V.; Gutowska, A.; Roth, E.A.; Markwald, R.R. Cell and organ printing 2: Fusion of cell aggregates in three-dimensional gels. Anat. Rec. 2003, 272, 497–502. [Google Scholar] [CrossRef]
- Chung, J.H.Y.; Naficy, S.; Yue, Z.; Kapsa, R.; Quigley, A.; Moulton, S.E.; Wallace, G.G. Bio-ink properties and printability for extrusion printing living cells. Biomater. Sci. 2013, 1, 763–773. [Google Scholar] [CrossRef] [Green Version]
- Levato, R.; Visser, J.; Planell, J.A.; Engel, E.; Malda, J.; Mateos-Timoneda, M.A. Biofabrication of tissue constructs by 3D bioprinting of cell-laden microcarriers. Biofabrication 2014, 6, 035020. [Google Scholar] [CrossRef]
- Angelopoulos, I.; Allenby, M.C.; Lim, M.; Zamorano, M. Engineering inkjet bioprinting processes toward translational therapies. Biotechnol. Bioeng. 2020, 117, 272–284. [Google Scholar] [CrossRef]
- Calvert, P. Inkjet printing for materials and devices. Chem. Mater. 2001, 13, 3299–3305. [Google Scholar] [CrossRef]
- Gao, G.; Yonezawa, T.; Hubbell, K.; Dai, G.; Cui, X. Inkjet-bioprinted acrylated peptides and PEG hydrogel with human mesenchymal stem cells promote robust bone and cartilage formation with minimal printhead clogging. Biotechnol. J. 2015, 10, 1568–1577. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Jin, J.; Gregory, C.; Hickman, J.J.; Boland, T. Inkjet printing of viable mammalian cells. Biomaterials 2005, 26, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Boland, T.; D’Lima, D.D.; Lotz, M.K. Thermal inkjet printing in tissue engineering and regenerative medicine. Recent Pat. Drug Deliv. Formul. 2012, 6, 149–155. [Google Scholar] [CrossRef]
- Chahal, D.; Ahmadi, A.; Cheung, K.C. Improving piezoelectric cell printing accuracy and reliability through neutral buoyancy of suspensions. Biotechnol. Bioeng. 2012, 109, 2932–2940. [Google Scholar] [CrossRef] [PubMed]
- Puhlev, I.; Guo, N.; Brown, D.R.; Levine, F. Desiccation tolerance in human cells. Cryobiology 2001, 42, 207–217. [Google Scholar] [CrossRef] [Green Version]
- Guillotin, B.; Souquet, A.; Catros, S.; Duocastella, M.; Pippenger, B.; Bellance, S.; Bareille, R.; Rémy, M.; Bordenave, L.; Amédée, J.; et al. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials 2010, 31, 7250–7256. [Google Scholar] [CrossRef]
- Schiele, N.R.; Corr, D.T.; Huang, Y.; Raof, N.A.; Xie, Y.; Chrisey, D.B. Laser-based direct-write techniques for cell printing. Biofabrication 2010, 2, 032001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillemot, F.; Guillotin, B.; Fontaine, A.; Ali, M.; Catros, S.; Kériquel, V.; Fricain, J.C.; Rémy, M.; Bareille, R.; Amédée-Vilamitjana, J. Laser-assisted bioprinting to deal with tissue complexity in regenerative medicine. MRS Bull. 2011, 36, 1015–1019. [Google Scholar] [CrossRef]
- Singh, M.; Haverinen, H.M.; Dhagat, P.; Jabbour, G.E. Inkjet printing-process and its applications. Adv. Mat. 2010, 22, 673–685. [Google Scholar] [CrossRef]
- Antoine, E.E.; Vlachos, P.P.; Rylander, M.N. Review of collagen I hydrogels for bioengineered tissue microenvironments: Characterization of mechanics, structure, and transport. Tissue Eng. Part. B Rev. 2014, 20, 683–696. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.L.; Motte, S.; Kaufman, L.J. Pore size variable type I collagen gels and their interaction with glioma cells. Biomaterials 2010, 31, 5678–5688. [Google Scholar] [CrossRef] [PubMed]
- Chrobak, K.M.; Potter, D.R.; Tien, J. Formation of perfused, functional microvascular tubes in vitro. Microvasc. Res. 2006, 71, 185–196. [Google Scholar] [CrossRef]
- Kim, G.; Ahn, S.; Yoon, H.; Kim, Y.; Chun, W. A cryogenic direct-plotting system for fabrication of 3D collagen scaffolds for tissue engineering. J. Mater. Chem. 2009, 19, 8817–8823. [Google Scholar] [CrossRef]
- Blaeser, A.; Duarte Campos, D.F.; Puster, U.; Richtering, W.; Stevens, M.M.; Fischer, H. Controlling Shear Stress in 3D Bioprinting is a Key Factor to Balance Printing Resolution and Stem Cell Integrity. Adv. Healthc. Mater. 2016, 5, 326–333. [Google Scholar] [CrossRef]
- Sun, J.; Ng, J.H.; Fuh, Y.H.; Wong, Y.S.; Loh, H.T.; Xu, Q. Comparison of micro-dispensing performance between micro-valve and piezoelectric printhead. Microsyst. Technol. 2009, 15, 1437–1448. [Google Scholar] [CrossRef]
- Xu, J.; Zheng, S.; Hu, X.; Li, L.; Li, W.; Parungao, R.; Wang, Y.; Nie, Y.; Liu, T.; Song, K. Advances in the Research of Bioinks Based on Natural Collagen, Polysaccharide and Their Derivatives for Skin 3D Bioprinting. Polymers 2020, 12, 1237. [Google Scholar] [CrossRef]
- Saunders, R.E.; Derby, B. Inkjet printing biomaterials for tissue engineering: Bioprinting. Int. Mater. Rev. 2014, 59, 430–448. [Google Scholar] [CrossRef]
- Koch, L.; Kuhn, S.; Sorg, H.; Gruene, M.; Schlie, S.; Gaebel, R.; Polchow, B.; Reimers, K.; Stoelting, S.; Ma, N.; et al. Laser printing of skin cells and human stem cells. Tissue Eng. Part. C Methods 2010, 16, 847–854. [Google Scholar] [CrossRef]
- Delgado, L.M.; Bayon, Y.; Pandit, A.; Zeugolis, D.I. To cross-link or not to cross-link? Cross-linking associated foreign body response of collagen-based devices. Tissue Eng. Part. B Rev. 2015, 21, 298–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamiak, K.; Sionkowska, A. Current methods of collagen cross-linking: Review. Int. J. Biol. Macromol. 2020, 161, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Nimni, M.E.; Cheung, D.; Strates, B.; Kodama, M.; Sheikh, K. Chemically modified collagen: A natural biomaterial for tissue replacement. J. Biomed. Mater. Res. 1987, 21, 741–771. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Song, W.; Cao, X.; Wang, Y. 3D Bioplotting of Gelatin/Alginate Scaffolds for Tissue Engineering: Influence of Crosslinking Degree and Pore Architecture on Physicochemical Properties. J. Mater. Sci. Technol. 2016, 32, 889–900. [Google Scholar] [CrossRef]
- Gough, J.E.; Scotchford, C.A.; Downes, S. Cytotoxicity of glutaraldehyde crosslinked collagen/poly(vinyl alcohol) films is by the mechanism of apoptosis. J. Biomed. Mater. Res. 2002, 61, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Hermanson, G. Bioconjugate Techniques, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1–1146. [Google Scholar] [CrossRef]
- Mu, C.; Zhang, K.; Lin, W.; Li, D. Ring-opening polymerization of genipin and its long-range crosslinking effect on collagen hydrogel. J. Biomed. Mater. Res. A 2013, 101, 385–393. [Google Scholar] [CrossRef]
- Touyama, R.; Takeda, Y.; Inoue, K.; Kawamura, I.; Yatsuzuka, M.; Ikumoto, T.; Shingu, T.; Yokoi, T.; Inouye, H. Studies on the Blue Pigments Produced from Genipin and Methylamine. I. Structures of the Brownish-Red Pigments, Intermediates Leading to the Blue Pigments. Chem. Pharm. Bull. 1994, 42, 668–673. [Google Scholar] [CrossRef] [Green Version]
- Koob, T.J.; Hernandez, D.J. Material properties of polymerized NDGA–collagen composite fibers: Development of biologically based tendon constructs. Biomaterials 2002, 23, 203–212. [Google Scholar] [CrossRef]
- Jus, S.; Stachel, I.; Schloegl, W.; Pretzler, M.; Friess, W.; Meyer, M.; Birner-Gruenberger, R.; Guebitz, G.M. Cross-linking of collagen with laccases and tyrosinases. Mater. Sci. Eng. C Mater. Biol. Appl. 2011, 31, 1068–1077. [Google Scholar] [CrossRef]
- Folk, J.E. Transglutaminases. Annu. Rev. Biochem. 1980, 49, 517–531. [Google Scholar] [CrossRef]
- Siegel, R.C.; Pinnell, S.R.; Martin, G.R. Cross-linking of collagen and elastin. Properties of lysyl oxidase. Biochemistry 1970, 9, 4486–4492. [Google Scholar] [CrossRef] [PubMed]
- Khor, E. Methods for the treatment of collagenous tissues for bioprostheses. Biomaterials 1997, 18, 95–105. [Google Scholar] [CrossRef]
- Haugh, M.G.; Jaasma, M.J.; O’Brien, F.J. The effect of dehydrothermal treatment on the mechanical and structural properties of collagen-GAG scaffolds. J. Biomed. Mater. Res. A 2009, 89, 363–369. [Google Scholar] [CrossRef]
- Miles, C.A.; Bailey, A.J. Thermally labile domains in the collagen molecule. Micron 2001, 32, 325–332. [Google Scholar] [CrossRef]
- Koide, M.; Osaki, K.; Konishi, J.; Oyamada, K.; Katakura, T.; Takahashi, A.; Yoshizato, K. A new type of biomaterial for artificial skin: Dehydrothermally cross-linked composites of fibrillar and denatured collagens. J. Biomed. Mater. Res. 1993, 27, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Goldich, Y.; Marcovich, A.L.; Barkana, Y.; Avni, I.; Zadok, D. Safety of corneal collagen cross-linking with UV-A and riboflavin in progressive keratoconus. Cornea 2010, 29, 409–411. [Google Scholar] [CrossRef] [Green Version]
- Bax, D.V.; Davidenko, N.; Hamaia, S.W.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Impact of UV- and carbodiimide-based crosslinking on the integrin-binding properties of collagen-based materials. Acta Biomater. 2019, 100, 280–291. [Google Scholar] [CrossRef]
- Schacht, E.H. Polymer chemistry and hydrogel systems. J. Phys. Conf. Ser. 2004, 3, 22–28. [Google Scholar] [CrossRef]
- Sionkowska, A. UV Light as a Tool for Surface Modification of Polymeric Biomaterials. Key Eng. Mater. 2014, 583, 80–86. [Google Scholar] [CrossRef]
- Pamfil, D.; Schick, C.; Vasile, C. New Hydrogels Based on Substituted Anhydride Modified Collagen and 2-Hydroxyethyl Methacrylate. Synthesis and Characterization. Ind. Eng. Chem. Res. 2014, 53, 11239–11248. [Google Scholar] [CrossRef]
- Weadock, K.S.; Miller, E.J.; Bellincampi, L.D.; Zawadsky, J.P.; Dunn, M.G. Physical crosslinking of collagen fibers: Comparison of ultraviolet irradiation and dehydrothermal treatment. J. Biomed. Mater. Res. 1995, 29, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.K.K. Engineering of nanometer-sized cross-linked hydrogels for biomedical applications. Can. J. Chem. 2010, 88, 173–184. [Google Scholar] [CrossRef]
- Maitra, J.; Shukla, V. Cross-linking in hydrogels—A review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar]
- Chuang, C.-H.; Lin, R.-Z.; Melero-Martin, J.M.; Chen, Y.-C. Comparison of covalently and physically cross-linked collagen hydrogels on mediating vascular network formation for engineering adipose tissue. Artif. Cells Nanomed. Biotechnol. 2018, 46, 434–447. [Google Scholar] [CrossRef] [PubMed]
- d’Angelo, M.; Benedetti, E.; Tupone, M.G.; Catanesi, M.; Castelli, V.; Antonosante, A.; Cimini, A. The Role of Stiffness in Cell Reprogramming: A Potential Role for Biomaterials in Inducing Tissue Regeneration. Cells 2019, 8, 1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valero, C.; Amaveda, H.; Mora, M.; García-Aznar, J.M. Combined experimental and computational characterization of crosslinked collagen-based hydrogels. PLoS ONE 2018, 13, e0195820. [Google Scholar] [CrossRef] [Green Version]
- Handorf, A.M.; Zhou, Y.; Halanski, M.A.; Li, W.J. Tissue stiffness dictates development, homeostasis, and disease progression. Organogenesis 2015, 11, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Provenzano, P.P.; Inman, D.R.; Eliceiri, K.W.; Knittel, J.G.; Yan, L.; Rueden, C.T.; White, J.G.; Keely, P.J. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008, 6, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandran, P.L.; Barocas, V.H. Microstructural mechanics of collagen gels in confined compression: Poroelasticity, viscoelasticity, and collapse. J. Biomech. Eng. 2004, 126, 152–166. [Google Scholar] [CrossRef]
- Gribova, V.; Crouzier, T.; Picart, C. A material’s point of view on recent developments of polymeric biomaterials: Control of mechanical and biochemical properties. J. Mater. Chem. 2011, 21, 14354–14366. [Google Scholar] [CrossRef]
- Wells, R.G. The role of matrix stiffness in regulating cell behavior. Hepatology 2008, 47, 1394–1400. [Google Scholar] [CrossRef]
- Yang, Y.-l.; Leone, L.M.; Kaufman, L.J. Elastic Moduli of Collagen Gels Can Be Predicted from Two-Dimensional Confocal Microscopy. Biophys. J. 2009, 97, 2051–2060. [Google Scholar] [CrossRef] [Green Version]
- Reddy, N.; Reddy, R.; Jiang, Q. Crosslinking biopolymers for biomedical applications. Trends Biotechnol. 2015, 33, 362–369. [Google Scholar] [CrossRef]
- Wen, Q.; Basu, A.; Janmey, P.A.; Yodh, A.G. Non-affine deformations in polymer hydrogels. Soft Matter 2012, 8, 8039–8049. [Google Scholar] [CrossRef] [Green Version]
- Kurniawan, N.A.; Wong, L.H.; Rajagopalan, R. Early Stiffening and Softening of Collagen: Interplay of Deformation Mechanisms in Biopolymer Networks. Biomacromolecules 2012, 13, 691–698. [Google Scholar] [CrossRef]
- Nyambat, B.; Manga, Y.B.; Chen, C.-H.; Gankhuyag, U.; Pratomo WP, A.; Kumar Satapathy, M.; Chuang, E.-Y. New Insight into Natural Extracellular Matrix: Genipin Cross-Linked Adipose-Derived Stem Cell Extracellular Matrix Gel for Tissue Engineering. Int. J. Mol. Sci. 2020, 21, 4864. [Google Scholar] [CrossRef]
- Sánchez-Cid, P.; Jiménez-Rosado, M.; Perez-Puyana, V.; Guerrero, A.; Romero, A. Rheological and Microstructural Evaluation of Collagen-Based Scaffolds Crosslinked with Fructose. Polymers 2021, 13, 632. [Google Scholar] [CrossRef]
- Kreger, S.T.; Bell, B.J.; Bailey, J.; Stites, E.; Kuske, J.; Waisner, B.; Voytik-Harbin, S.L. Polymerization and matrix physical properties as important design considerations for soluble collagen formulations. Biopolymers 2010, 93, 690–707. [Google Scholar] [CrossRef] [PubMed]
- Miron-Mendoza, M.; Seemann, J.; Grinnell, F. The differential regulation of cell motile activity through matrix stiffness and porosity in three dimensional collagen matrices. Biomaterials 2010, 31, 6425–6435. [Google Scholar] [CrossRef] [Green Version]
- Roeder, B.A.; Kokini, K.; Voytik-Harbin, S.L. Fibril microstructure affects strain transmission within collagen extracellular matrices. J. Biomech. Eng. 2009, 131, 031004. [Google Scholar] [CrossRef]
- Saddiq, Z.A.; Barbenel, J.C.; Grant, M.H. The mechanical strength of collagen gels containing glycosaminoglycans and populated with fibroblasts. J. Biomed. Mater. Res. 2009, 89, 697–706. [Google Scholar] [CrossRef]
- Lam, D.; Enright, H.A.; Peters, S.K.G.; Moya, M.L.; Soscia, D.A.; Cadena, J.; Alvarado, J.A.; Kulp, K.S.; Wheeler, E.K.; Fischer, N.O. Optimizing cell encapsulation condition in ECM-Collagen I hydrogels to support 3D neuronal cultures. J. Neurosci. Methods 2020, 329, 108460. [Google Scholar] [CrossRef] [PubMed]
- Heid, S.; Boccaccini, A.R. Advancing bioinks for 3D bioprinting using reactive fillers: A review. Acta Biomater. 2020, 113, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, Y.; Martin, J.A.; Ozbolat, I.T. Evaluation of cell viability and functionality in vessel-like bioprintable cell-laden tubular channels. J. Biomech. Eng. 2013, 135, 91011. [Google Scholar] [CrossRef] [Green Version]
- Ringeisen, B.R.; Kim, H.; Barron, J.A.; Krizman, D.B.; Chrisey, D.B.; Jackman, S.; Auyeung, R.Y.C.; Spargo, B.J. Laser Printing of Pluripotent Embryonal Carcinoma Cells. Tissue Eng. 2004, 10, 483–491. [Google Scholar] [CrossRef]
- Lin, Y.; Huang, Y.; Wang, G.; Tzeng, T.-R.J.; Chrisey, D. Effect of laser fluence on yeast cell viability in laser-assisted cell transfer. Int. J. Appl. Phys. 2009, 106, 043106. [Google Scholar] [CrossRef]
- Sun, H.; Zhou, J.; Huang, Z.; Qu, L.; Lin, N.; Liang, C.; Dai, R.; Tang, L.; Tian, F. Carbon nanotube-incorporated collagen hydrogels improve cell alignment and the performance of cardiac constructs. Int. J. Nanomed. 2017, 12, 3109–3120. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Method | Bioink Components | Collagen Concentration (mg/mL) | Cell Density (mil./mL) | Crosslinking Agent | Temperature (°C) | Pressure (kPa) | Nozzle Diameter (µm) | Actuator | Power (μJ) | Droplet Volume (nL) | Application | Reference | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydrogel | Nozzle | Platform | ||||||||||||
| Extrusion | 1× PBS, 10× PBS, NaOH | 4, 8, 12 | 10 | riboflavin | On ice | n.s. | 37 | n.s. | 250 | - | - | - | Measuring print accuracy and rheological properties with and without riboflavin crosslinking | [63] |
| Extrusion | 1× PBS, 10× PBS, NaOH | 8 | 0.5, 10, 25, 100 | n.s. | On ice | n.s. | 37 | n.s. | 250 | - | - | - | The effects of incorporating cells on the rheology and printability of collagen bioinks | [87] |
| Extrusion | 10× DMEM, buffer (NaHCO3, HEPES, NaOH, H2O) | 3 | 20/30 | T, pH | On ice | n.s. | n.s. | n.s. | 200, 250, 400, 610, 840 | - | - | - | Heterogenous tissue structure fabrication | [88] |
| Extrusion | n.s. | 3, 6 | 10 | Pluronic F127 | On ice | 37 | 37 | 50–85 | 1000 | - | - | - | Optimization of bioprinting time and extrusion profile to fabricate 3D collagen constructs | [89] |
| Extrusion | n.s. | 4 | 2 | Tannic acid | On ice | 30 | 30 | 140–600 | 310 | - | - | - | Study of metabolic activity, cell viability and proliferation of cell-laden collagen structures | [90] |
| Extrusion | 10× DMEM | 5 | 5 | Tannic acid | n.s. | 5-10 | 35–37 | 220 | 150 | - | - | - | The effect of crosslinking agent on physical properties and cellular activity | [91] |
| Extrusion | 4× DMEM, NaOH | 3 | 10-20 | T, pH | 10 | 10 | n.s. | 12/10 | 90/250 | - | - | - | The use of 3D direct-write cell deposition system to construct spatially organized viable structures | [77] |
| Extrusion | n.s. | 3 | 0.1, 1, 10 | T, pH | 7 | 7 | no heat or 29–31 | 14, 28, 36, 60, 120 | 90, 250 | - | - | - | Evaluating the use of a direct-write, 3D bioassembly tool capable of extruding cells and matrix into spatially organized 3D constructs | [78] |
| Extrusion | PBS, 10× PBS, NaOH | 12, 15, 17.5 | 10 | n.s. | n.s. | n.s. | 37 | n.s. | n.s. | - | - | - | Developing and evaluating a method of printing soft tissue implants with high-density collagen hydrogels using a commercially available 3D printer | [54] |
| Extrusion | 10× DMEM | n.s. | 0.5, 3.2 | T, pH | n.s. | n.s. | 25–60 | 150 | 180 | - | - | - | Fabrication of 3D cell-laden scaffolds for better skin tissue regeneration | [92] |
| Extrusion | n.s. | 2 | 5, 6.7 | T, pH | n.s. | n.s. | 32 | 12 | n.s. | - | - | - | Developing multilayered cell-laden mesh structure and a collagen-based cell-laden bioink | [93] |
| Extrusion | 10× DMEM | 3, 5, 7 | 1 | genipin solution | 10 | 10 | 35 | 110–300 | 310 | - | - | - | A printing strategy with optimal condition including a safe cross-linking procedure for obtaining a 3D porous cell-block composed of a biocompatible collagen bioink | [94] |
| Extrusion | 10× HAM-F12, NaOH, NaHCO3, HEPES | 3.5 | 1.75 | T, pH | On ice | n.s. | n.s. | 41 | n.s. | - | - | - | The fabrication of an implantable multilayered vascularized bioengineered skin graft using 3D bioprinting | [95] |
| Extrusion | 10× DMEM, NaOH | 3 | 1.6 | T, pH | n.s. | n.s. | n.s. | n.s. | n.s. | - | - | - | The structure and stiffness of printable hydrogel matrices under different conditions | [96] |
| Extrusion | NaOH | 10 | 5, 10, 30 | T, pH | On ice | 20 | n.s. | n.s. | 250 | - | - | - | Collagen type II hydrogel/chondrocyte constructs fabricated using a bioprinter with three different total cell seeding densities in collagen type II pre-gel | [97] |
| Inkjet | 10× DMEM, NaOH | 3.3 | n.s. | n.s. | On ice | n.s. | n.s. | n.s. | n.s. | piezocrystal | - | 15 | Using of thermosensitive gels for generating 3D constructs | [98] |
| Inkjet | PBS | 1 | 0.15 | T, pH | On ice | n.s. | n.s. | n.s. | 20-30 | piezocrystal | - | 0.01–0.02 | Applying high-throughput inkjet printing to control cellular attachment and proliferation by precise, automated deposition of collagen | [14] |
| Inkjet | n.s. | 0.5 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | piezocrystal | - | n.s. | Preparation a cancer microtissue array in a multi-well format by continuous deposition of collagen-suspended Hela cells on a fibroblast-layered nanofibrous membrane via inkjet printing | [99] |
| Inkjet | n.s. | 2 | 2 | T, pH | n.s. | n.s. | n.s. | n.s. | n.s. | piezocrystal | - | n.s. | The behaviour of chondrocytes and osteoblasts to hyaluronic acid and type I collagen hydrogels | [100] |
| Inkjet | 10× DMEM, H2O, NaOH | 1.25 | 0.25 | T, pH | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | - | n.s. | Evaluation and comparison of 12 hydrogels to determine suitability for bioprinting | [101] |
| Inkjet | n.s. | 0.3 | 1 | T, pH | n.s. | 30 | n.s. | 50 | 300 | microvalve | - | n.s. | A freeform and cell-friendly drop-on-demand bioprinting strategy for creating corneal stromal 3D models as suitable implants | [102] |
| Inkjet | 1× PBS | 2.05 | 1 | T, pH (NaHCO3) | On ice | 5–40 | 5–40 | 13.8 | 150 | microvalve | - | 7.6 | Freeform fabrication technique, based on direct cell dispensing, implemented using a robotic platform that prints collagen hydrogel precursor, fibroblasts and keratinocytes. | [17] |
| Inkjet | 1× PBS | 1.12 | 1, 3 | T, pH (NaHCO3) | On ice | 5–40 | 5–40 | 13.8 | 150 | microvalve | - | 7.6 | A direct cell printing technique to pattern neural cells in a three-dimensional (3D) multilayered collagen gel | [103] |
| Inkjet | 1× PBS | 2.23 | 1 | T, pH (NaHCO3) | On ice | 5–40 | 5–40 | 11–13.8 | 150 | microvalve | - | n.s. | 3D direct printing technique to construct hydrogel scaffolds containing fluidic channels | [76] |
| Inkjet | 1× PBS | 3 | 0.5-5 | T, pH (NaHCO3) | On ice | n.s. | n.s. | 18 | n.s. | microvalve | - | 52 | The potential of 3D bioprinting for tissue engineering using human skin as a prototypical example | [21] |
| Inkjet | 10× PBS, H2O, NaOH, FBS, DMEM | n.s. | 1, 5, 10 | T, pH | On ice | n.s. | n.s. | 34.4 | n.s. | piezocrystal | - | 6 | A bioprinter that can be used to print 3D patches of smooth muscle cells encapsulated within collagen | [104] |
| Inkjet | NaOH | 3 | 2 | T, pH | n.s. | n.s. | n.s. | n.s. | n.s. | piezocrystal | - | n.s. | Direct comparisons between commercially available hydrogels in the context of their cytocompatibility toward MSCs and their physicochemical parameters | [105] |
| Inkjet | NaOH | 3 | n.s. | T, pH | n.s. | n.s. | n.s. | n.s. | n.s. | piezocrystal | - | n.s. | Comparison of popular commercially available bioprinting hydrogels in the context of their physicochemical and other parameters | [106] |
| Inkjet | 1× PBS, NaOH | 1.3 | 0.06 | T, pH | On ice | n.s. | 37 | n.s. | n.s. | n.s. | - | n.s. | The use of this system for the study of tumorigenesis and microenvironmental redirection of breast cancer cells | [107] |
| Inkjet | n.s. | n.s. | 0.5 | T, pH | On ice | n.s. | n.s. | n.s. | n.s. | n.s. | - | 100 | The proliferation of primary rat bladder smooth muscle cells in printed cell-laden collagen droplets | [108] |
| LaBP | 10× PBS, NaOH | n.s. | 35 | n.s. | - | - | - | - | - | - | n.s. | n.s. | Fully cellularized skin substitute made of fibroblasts and keratinocytes on top of a stabilizing matrix (Matriderm®) | [109] |
| LaBP | 10× DMEM/Ham’s F12, NaHCO3 | 3 | 35 | T, pH | - | - | - | - | - | - | n.s. | 0.1–1 | The 3D arrangement of vital cells by LaBP as multicellular grafts analogous to native archetype and the formation of tissue by these cells | [110] |
| LaBP | n.s. | 2 | 120 | T, pH | - | - | - | - | - | - | 50 | n.s. | In situ printing of mesenchymal stromal cells, associated with collagen and nano-hydroxyapatite, in order to favour bone regeneration, in a calvaria defect model in mice | [111] |
| LaBP | 10× PBS, NaOH, EDTA, thrombin, TBS | 3 | 30 | n.s. | - | - | - | - | - | - | 18 | n.s. | 3D cornea-mimicking tissues using human stem cells and laser-assisted bioprinting | [112] |
| LaBP | n.s. | 2 | 100 | T, pH | - | - | - | - | - | - | n.s. | n.s. | The effect of distance between printed cell islets and the influence of coprinted mesenchymal cells on migration | [113] |
| LaBP | 1× DMEM | 2 | 70 | T, pH | - | - | - | - | - | - | 26 | n.s. | A microvascular network following a defined pattern and its preservation while preparing the surface to print another layer of endothelial cells | [114] |
| LaBP | n.s. | 2 | n.s. | T, pH | - | - | - | - | - | - | 28 | n.s. | Organizing endothelial cells in situ, in a mouse calvaria bone defect, to generate a prevascularization with a defined architecture, and promote in vivo bone regeneration | [115] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stepanovska, J.; Supova, M.; Hanzalek, K.; Broz, A.; Matejka, R. Collagen Bioinks for Bioprinting: A Systematic Review of Hydrogel Properties, Bioprinting Parameters, Protocols, and Bioprinted Structure Characteristics. Biomedicines 2021, 9, 1137. https://doi.org/10.3390/biomedicines9091137
Stepanovska J, Supova M, Hanzalek K, Broz A, Matejka R. Collagen Bioinks for Bioprinting: A Systematic Review of Hydrogel Properties, Bioprinting Parameters, Protocols, and Bioprinted Structure Characteristics. Biomedicines. 2021; 9(9):1137. https://doi.org/10.3390/biomedicines9091137
Chicago/Turabian StyleStepanovska, Jana, Monika Supova, Karel Hanzalek, Antonin Broz, and Roman Matejka. 2021. "Collagen Bioinks for Bioprinting: A Systematic Review of Hydrogel Properties, Bioprinting Parameters, Protocols, and Bioprinted Structure Characteristics" Biomedicines 9, no. 9: 1137. https://doi.org/10.3390/biomedicines9091137
APA StyleStepanovska, J., Supova, M., Hanzalek, K., Broz, A., & Matejka, R. (2021). Collagen Bioinks for Bioprinting: A Systematic Review of Hydrogel Properties, Bioprinting Parameters, Protocols, and Bioprinted Structure Characteristics. Biomedicines, 9(9), 1137. https://doi.org/10.3390/biomedicines9091137








