Secondary Cerebellar Cortex Injury in Albino Male Rats after MCAO: A Histological and Biochemical Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biochemicals
2.2. Animals
2.3. Induction of Middle Cerebral Artery Occlusion (MCAO)
2.4. Experimental Groups
2.5. Blood Sample Collection and Preparation
2.6. Biochemical Measurements
2.7. Histological and Immune-Histochemical Studies for Light Microscopic Examination
2.8. Statistical Analysis
3. Results
3.1. Histological Results
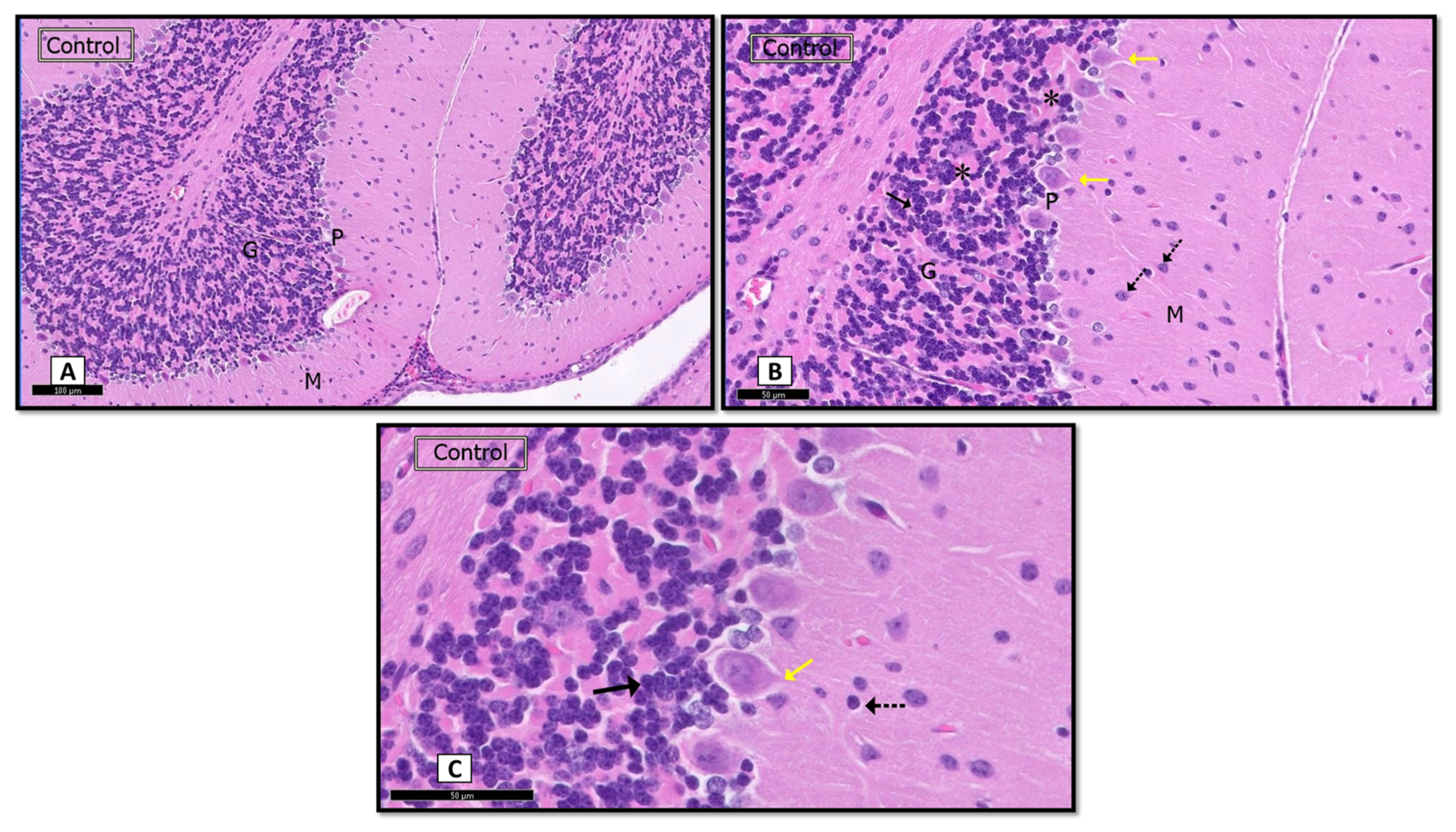
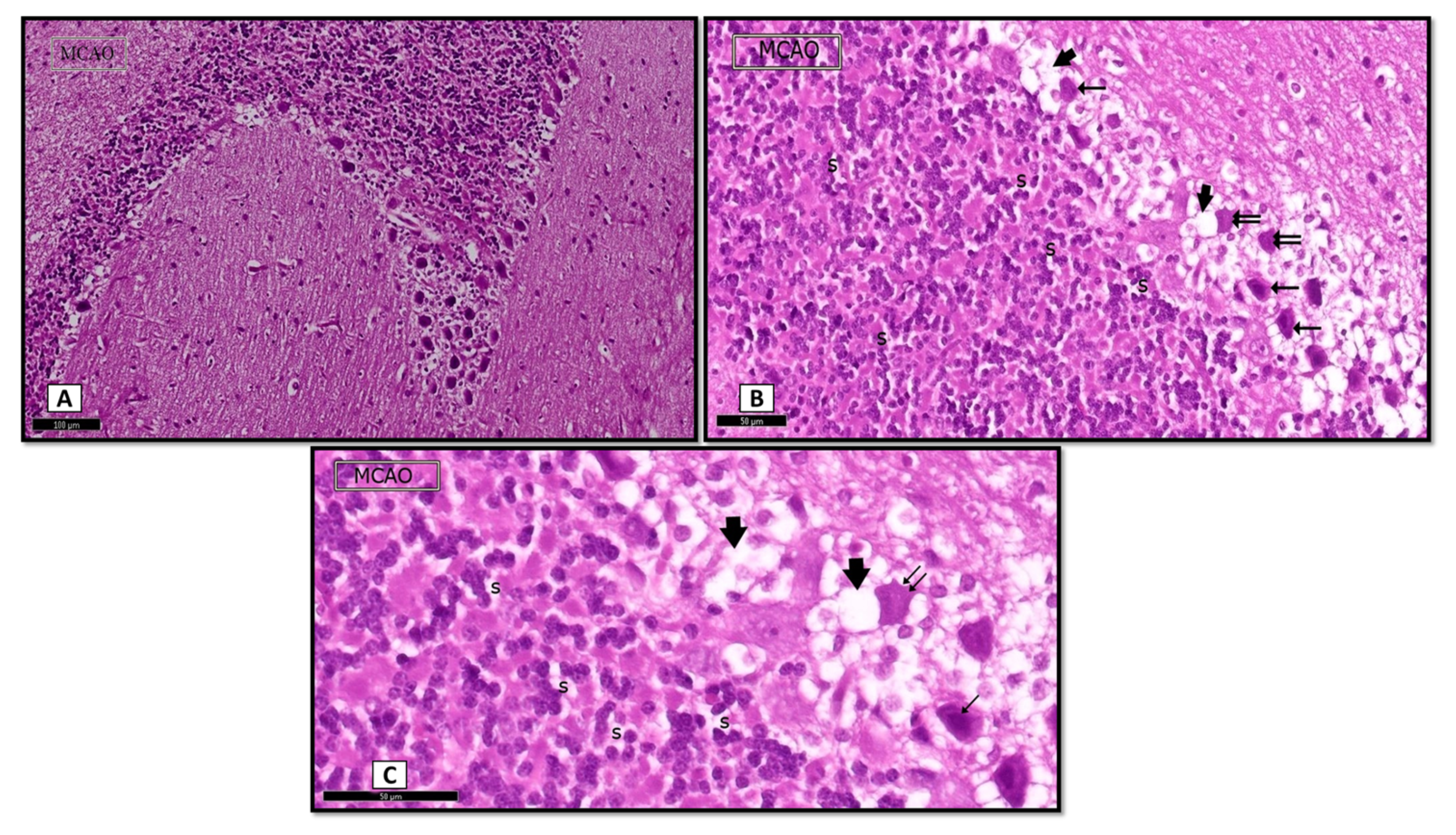
3.1.1. Hematoxylin and Eosin (H&E)
Sham Control Group
MCAO Group
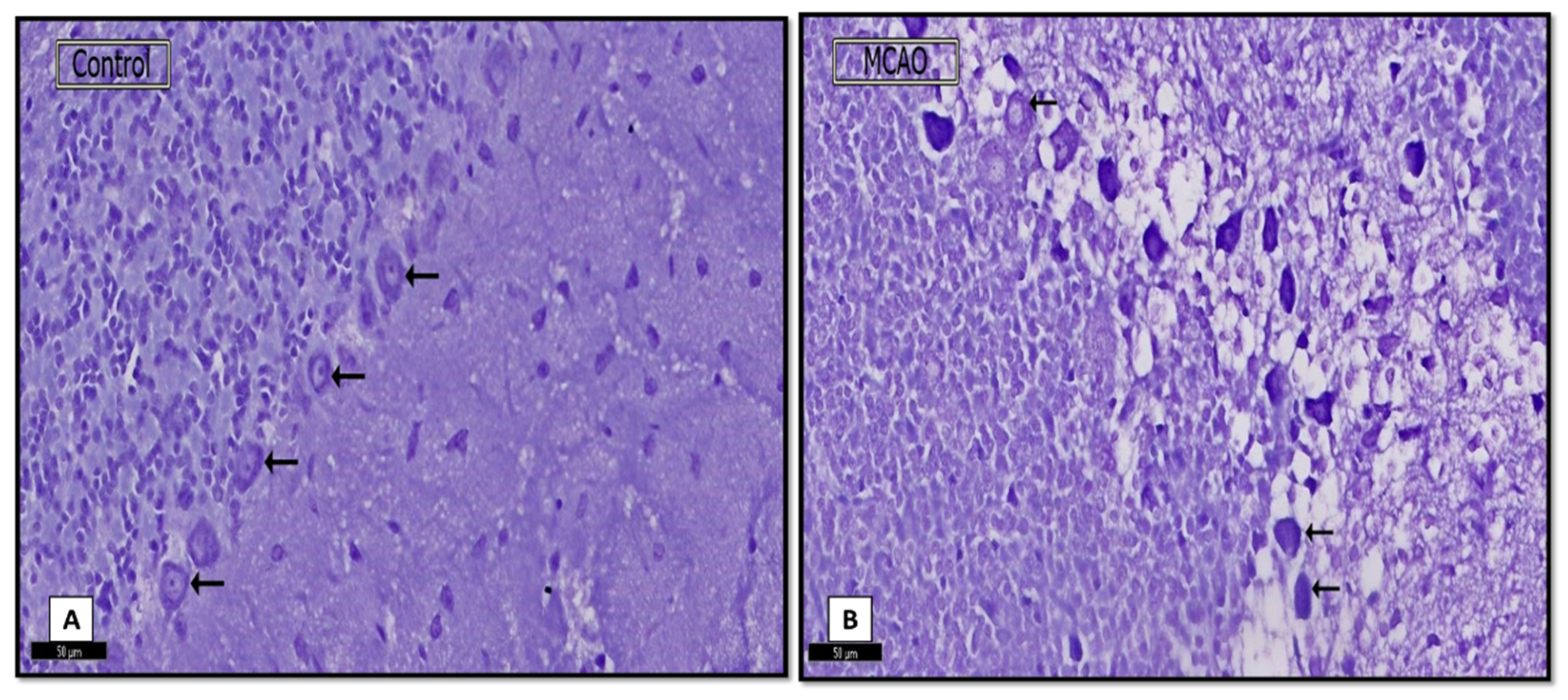
3.1.2. Nissl Stain
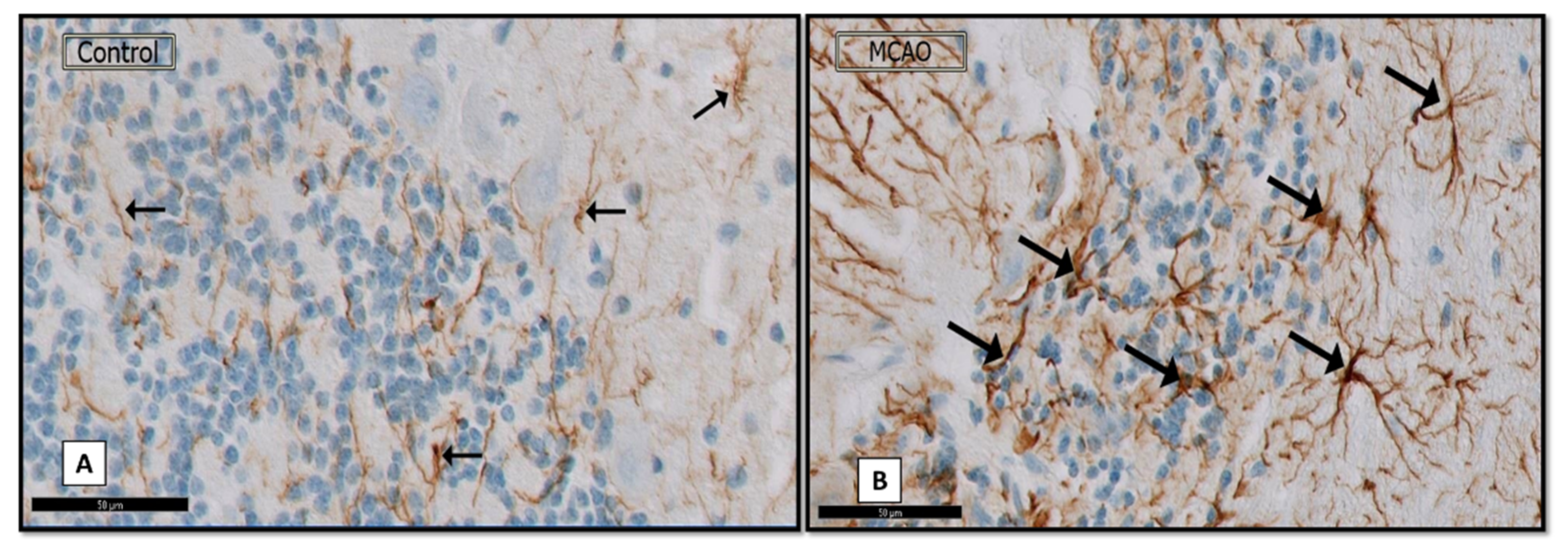
3.1.3. Immunostained GFAP Sections
3.2. Biochemical Results
3.2.1. Angiopoietin-2 (pg/mL)
3.2.2. Granulocyte Colony-Stimulating Factor (G-CSF)
3.2.3. Endoglin
3.2.4. Endothelin-1 (ET-1)
3.2.5. Vascular Endothelial Growth Factor-A (VEGF-A)
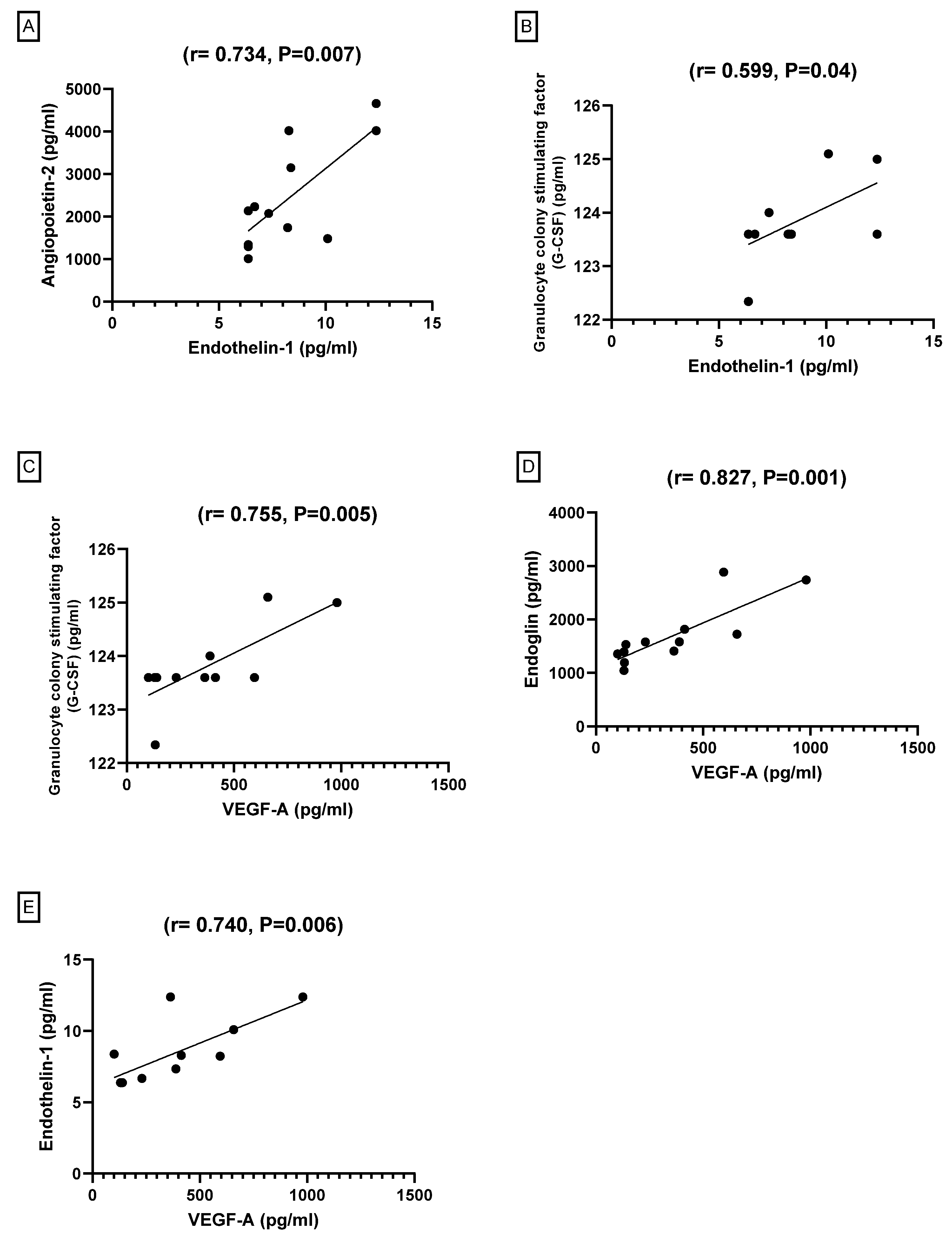
3.2.6. Pearson’s Correlation between Angiogenesis Markers among the Groups
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Genovese, T.; Impellizzeri, D.; Ahmad, A.; Cornelius, C.; Campolo, M.; Cuzzocrea, S.; Esposito, E. Post-ischaemic thyroid hormone treatment in a rat model of acute stroke. Brain Res. 2013, 1513, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Asirvatham, A.R.; Marwan, M.Z. Stroke in Saudi Arabia: A review of the recent literature. Pan Afr. Med. J. 2014, 17, 14. [Google Scholar] [CrossRef]
- Tran, J.; Mirzaei, M.; Anderson, L.; Leeder, S.R. The epidemiology of stroke in the Middle East and North Africa. J. Neurol. Sci. 2010, 295, 38–40. [Google Scholar] [CrossRef] [PubMed]
- Al-Jadid, M.S.; Robert, A.A. Determinants of length of stay in an inpatient stroke rehabilitation unit in Saudi Arabia. Saudi Med. J. 2010, 31, 189–192. [Google Scholar] [PubMed]
- Finger, S.; Koehler, P.J.; Jagella, C. The Monakow concept of diaschisis: Origins and perspectives. Arch. Neurol. 2004, 61, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.C.; Bousser, M.G.; Comar, D.; Castaigne, P. ‘Crossed cerebellar diaschisis’ in human supratentorial brain infarction. Trans. Am. Neurol. Assoc. 1981, 105, 459–461. [Google Scholar]
- Kidani, N.; Hishikawa, T.; Hiramatsu, M.; Nishihiro, S.; Kin, K.; Takahashi, Y.; Murai, S.; Sugiu, K.; Yasuhara, T.; Miyazaki, I.; et al. Cerebellar Blood Flow and Gene Expression in Crossed Cerebellar Diaschisis after Transient Middle Cerebral Artery Occlusion in Rats. Int. J. Mol. Sci. 2020, 21, 4137. [Google Scholar] [CrossRef]
- Gold, L.; Lauritzen, M. Neuronal deactivation explains decreased cerebellar blood flow in response to focal cerebral ischemia or suppressed neocortical function. Proc. Natl. Acad. Sci. USA 2002, 99, 7699–7704. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Karonen, J.; Nuutinen, J.; Vanninen, E.; Kuikka, J.T.; Vanninen, R.L. Crossed cerebellar diaschisis in acute ischemic stroke: A study with serial SPECT and MRI. J. Cereb. Blood Flow Metab. 2007, 27, 1724–1732. [Google Scholar] [CrossRef]
- Vendrame, M.; Gemma, C.; De Mesquita, D.; Collier, L.; Bickford, P.; Sanberg, C.D.; Sanberg, P.R.; Pennypacker, K.R.; Willing, A.E. Anti-inflammatory Effects of Human Cord Blood Cells in a Rat Model of Stroke. Stem Cells Dev. 2005, 14, 595–604. [Google Scholar] [CrossRef]
- Hu, Z.; Xia, H.; Yang, Y.; Zheng, H.; Zhao, L.; Chen, Y.; Zhuge, Q.; Xia, N.; Gao, H.; Chen, W. Metabolic alterations in the rat cerebellum following acute middle cerebral artery occlusion, as determined by 1H NMR spectroscopy. Mol. Med. Rep. 2018, 17, 531–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhang, R.; Xing, X.; Guo, J.; Xie, F.; Zhang, G.; Qin, X. Repulsive guidance molecule a suppresses angiogenesis after ischemia/reperfusion injury of middle cerebral artery occlusion in rats. Neurosci. Lett. 2018, 662, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Lennmyr, F.; Terént, A.; Syvänen, A.C.; Barbany, G. Vascular endothelial growth factor gene expression in middle cerebral artery occlusion in the rat. Acta Anaesthesiol. Scand. 2005, 49, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ma, Y.; Dou, S.; Wang, Y.; La, D.; Liu, J.; Ma, Z. Hemodynamic changes in a rat parietal cortex after endothelin-1-induced middle cerebral artery occlusion monitored by optical coherence tomography. J. Biomed. Opt. 2016, 21, 75014. [Google Scholar] [CrossRef]
- Lo, A.C.Y.; Chen, A.Y.S.; Hung, V.K.L.; Yaw, L.P.; Fung, M.K.L.; Ho, M.C.Y.; Tsang, M.C.S.; Chung, S.S.M.; Chung, S.K. Endothelin-1 overexpression leads to further water accumulation and brain edema after middle cerebral artery occlusion via aquaporin 4 expression in astrocytic end-feet. J. Cereb. Blood Flow Metab. 2005, 25, 998–1011. [Google Scholar] [CrossRef] [Green Version]
- Shyu, W.-C.; Lin, S.-Z.; Lee, C.-C.; Liu, D.D.; Li, H. Granulocyte colony-stimulating factor for acute ischemic stroke: A randomized controlled trial. CMAJ 2006, 174, 927–933. [Google Scholar] [CrossRef] [Green Version]
- Schaäbitz, W.-R.; Schneider, A. Developing granulocyte-colony stimulating factor for the treatment of stroke: Current status of clinical trials. Stroke 2006, 37, 1654. [Google Scholar] [CrossRef] [Green Version]
- Matchett, G.A.; Calinisan, J.B.; Matchett, G.C.; Martin, R.D.; Zhang, J.H. The effect of granulocyte-colony stimulating factor in global cerebral ischemia in rats. Brain Res. 2007, 1136, 200–207. [Google Scholar] [CrossRef] [Green Version]
- Longa, E.Z.; Weinstein, P.R.; Carlson, S.; Cummins, R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989, 20, 84–91. [Google Scholar] [CrossRef] [Green Version]
- Boyko, M.; Zlotnik, A.; Gruenbaum, B.F.; Gruenbaum, S.E.; Ohayon, S.; Goldsmith, T.; Kotz, R.; Leibowitz, A.; Sheiner, E.; Shapira, Y.; et al. An experimental model of focal ischemia using an internal carotid artery approach. J. Neurosci. Methods 2010, 193, 246–253. [Google Scholar] [CrossRef]
- Shah, F.A.; Li, T.; Al Kury, L.T.; Zeb, A.; Khatoon, S.; Liu, G.; Yang, X.; Liu, F.; Yao, H.; Khan, A.-U.; et al. Pathological Comparisons of the Hippocampal Changes in the Transient and Permanent Middle Cerebral Artery Occlusion Rat Models. Front. Neurol. 2019, 10, 1178. [Google Scholar] [CrossRef]
- Boyko, M.; Kutz, R.; Gruenbaum, B.F.; Cohen, H.; Kozlovsky, N.; Gruenbaum, S.E.; Shapira, Y.; Zlotnik, A. The influence of aging on poststroke depression using a rat model via middle cerebral artery occlusion. Cogn. Affect. Behav. Neurosci. 2013, 13, 847–859. [Google Scholar] [CrossRef]
- Jie, L.; Yuqin, C.; Yao, D.; Shuai, H.; Yuanyuan, W. Occlusion of middle cerebral artery induces apoptosis of cerebellar cortex neural cells via caspase-3 in rats. Turk. Neurosurg. 2011, 21, 567–574. [Google Scholar] [CrossRef]
- Zhao, F.; Kuroiwa, T.; Miyasaka, N.; Nagaoka, T.; Nakane, M.; Tamura, A.; Mizusawa, H. Characteristic changes in T2-value, apparent diffusion coefficient, and ultrastructure of substantia nigra evolving exofocal postischemic neuronal death in rats. Brain Res. 2001, 895, 238–244. [Google Scholar] [CrossRef]
- Dihné, M.; Grommes, C.; Lutzenburg, M.; Witte, O.W.; Block, F. Different mechanisms of secondary neuronal damage in thalamic nuclei after focal cerebral ischemia in rats. Stroke 2002, 33, 3006–3011. [Google Scholar] [CrossRef] [Green Version]
- Loos, M.; Dihne, M.; Block, F. Tumor necrosis factor-α expression in areas of remote degeneration following middle cerebral artery occlusion of the rat. Neuroscience 2003, 122, 373–380. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Xing, S.; Liang, Z.; Zeng, J. Secondary neurodegeneration in remote regions after focal cerebral infarction: A new target for stroke management? Stroke 2012, 43, 1700–1705. [Google Scholar] [CrossRef] [PubMed]
- Afifi, O.K. Effect of sodium fluoride on the cerebellar cortex of adult albino rats and the possible protective role of vitamin B6: A light and electron microscopic study. Egypt. J. Histol. 2009, 32, 358–367. [Google Scholar]
- El-Dien, S.H.M.; el Gamal, D.A.; Mubarak, H.A.; Saleh, S.M. Effect of fluoride on rat cerebellar cortex: Light and electron microscopic studies. Egypt. J. Histol. 2010, 33, 245–246. [Google Scholar]
- Hanz, S.; Fainzilber, M. Retrograde signaling in injured nerve–the axon reaction revisited. J. Neurochem. 2006, 99, 13–19. [Google Scholar] [CrossRef]
- Kassab, A.A. Wheat germ oil attenuates deltamethrin-induced injury in rat cerebellar cortex: Histological and immunohistochemical study. Egypt. J. Histol. 2018, 41, 182–191. [Google Scholar] [CrossRef]
- Hashem, H.E.; Safwat, M.D.E.-D.; Algaidi, S. The effect of monosodium glutamate on the cerebellar cortex of male albino rats and the protective role of vitamin C (histological and immunohistochemical study). J. Mol. Histol. 2012, 43, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, Y.; Bai, S.; Peng, L.; Zhang, B.; Lu, H. Granulocyte colony stimulating factor therapy for stroke: A pairwise meta-analysis of randomized controlled trial. PLoS ONE 2017, 12, e0175774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, S.J.; Love, C.; Spector, M.; Cool, S.M.; Nurcombe, V.; Lo, E.H. Endogenous regeneration: Engineering growth factors for stroke. Neurochem. Int. 2017, 107, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Sobrino, T.; Rodríguez-Yáñez, M.; Campos, F.; Iglesias-Rey, R.; Millán, M.; De La Ossa, N.P.; Dávalos, A.; Delgado-Mederos, R.; Martínez-Domeño, A.; Martí-Fábregas, J.; et al. Association of High Serum Levels of Growth Factors with Good Outcome in Ischemic Stroke: A Multicenter Study. Transl. Stroke Res. 2019, 11, 653–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, A.; Krüger, C.; Steigleder, T.; Weber, D.; Pitzer, C.; Laage, R.; Aronowski, J.; Maurer, M.; Gassler, N.; Mier, W.; et al. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J. Clin. Investig. 2005, 115, 2083–2098. [Google Scholar] [CrossRef] [Green Version]
- Shyu, W.-C.; Lin, S.-Z.; Yang, H.-I.; Tzeng, Y.-S.; Pang, C.-Y.; Yen, P.-S.; Li, H. Functional recovery of stroke rats induced by granulocyte colony-stimulating factor–stimulated stem cells. Circulation 2004, 110, 1847–1854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morita, Y.; Takizawa, S.; Kamiguchi, H.; Uesugi, T.; Kawada, H.; Takagi, S. Administration of hematopoietic cytokines increases the expression of anti-inflammatory cytokine (IL-10) mRNA in the subacute phase after stroke. Neurosci. Res. 2007, 58, 356–360. [Google Scholar] [CrossRef]
- Ohab, J.J.; Fleming, S.; Blesch, A.; Carmichael, S.T. A neurovascular niche for neurogenesis after stroke. J. Neurosci. 2006, 26, 13007–13016. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.; Chen, J.; Zacharek, A.; Roberts, C.; Yang, Y.; Chopp, M. Nitric oxide donor up-regulation of SDF1/CXCR4 and Ang1/Tie2 promotes neuroblast cell migration after stroke. J. Neurosci. Res. 2009, 87, 86–95. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.G.; Zhang, L.; Croll, S.D.; Chopp, M. Angiopoietin-1 reduces cerebral blood vessel leakage and ischemic lesion volume after focal cerebral embolic ischemia in mice. Neuroscience 2002, 113, 683–687. [Google Scholar] [CrossRef]
- Hui, Z.; Sha, D.-J.; Wang, S.-L.; Li, C.-S.; Qian, J.; Wang, J.-Q.; Zhao, Y.; Zhang, J.-H.; Cheng, H.-Y.; Yang, H.; et al. Panaxatriol saponins promotes angiogenesis and enhances cerebral perfusion after ischemic stroke in rats. BMC Complement. Altern. Med. 2017, 17, 70. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Chen, L.; Shu, B.; Tang, J.; Zhang, L.; Xie, J.; Qi, S.; Xu, Y. Granulocyte/macrophage colony-stimulating factor influences angiogenesis by regulating the coordinated expression of VEGF and the Ang/Tie system. PLoS ONE 2014, 9, e92691. [Google Scholar] [CrossRef]
- Góra-Kupilas, K.; Jośko, J. The neuroprotective function of vascular endothelial growth factor (VEGF). Folia Neuropathol. 2005, 43, 31–39. [Google Scholar]
- Cao, L.; Jiao, X.; Zuzga, D.S.; Liu, Y.; Fong, D.M.; Young, D.; During, M.J. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat. Genet. 2004, 36, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Dzietko, M.; Derugin, N.; Wendland, M.F.; Vexler, Z.S.; Ferriero, D.M. Delayed VEGF treatment enhances angiogenesis and recovery after neonatal focal rodent stroke. Transl. Stroke Res. 2013, 4, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Nishijima, K.; Ng, Y.-S.; Zhong, L.; Bradley, J.; Schubert, W.; Jo, N.; Akita, J.; Samuelsson, S.J.; Robinson, G.S.; Adamis, A.P.; et al. Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am. J. Pathol. 2007, 171, 53–67. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Zechariah, A.; Qu, Y.; Hermann, D.M. Effects of vascular endothelial growth factor in ischemic stroke. J. Neurosci. Res. 2012, 90, 1873–1882. [Google Scholar] [CrossRef]
- Sahota, P.; Savitz, S.I. Investigational therapies for ischemic stroke: Neuroprotection and neurorecovery. Neurotherapeutics 2011, 8, 434–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marti, H.J.; Bernaudin, M.; Bellail, A.; Schoch, H.; Euler, M.; Petit, E.; Risau, W. Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. Am. J. Pathol. 2000, 156, 965–976. [Google Scholar] [CrossRef] [Green Version]
- Schoch, H.J.; Fischer, S.; Marti, H.H. Hypoxia-induced vascular endothelial growth factor expression causes vascular leakage in the brain. Brain 2002, 125, 2549–2557. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhang, F.; Li, Q.-Y.; Gong, A.; Lan, Q. Protective effect of Ad-VEGF-Bone mesenchymal stem cells on cerebral infarction. Turk. Neurosurg. 2016, 26, 8–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.-T.; Zhang, P.; Gao, Y.; Li, C.-L.; Wang, H.-J.; Chen, L.-C.; Feng, Y.; Li, R.-Y.; Li, Y.-L.; Jiang, C.-L. Early VEGF inhibition attenuates blood-brain barrier disruption in ischemic rat brains by regulating the expression of MMPs. Mol. Med. Rep. 2017, 15, 57–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, S.-W.; Duan, C.-L.; Chen, X.-H.; Wang, Y.-Q.; Sun, X.; Zhang, Q.-W.; Cui, H.-R.; Sun, F.-Y. Neurogenic effect of VEGF is related to increase of astrocytes transdifferentiation into new mature neurons in rat brains after stroke. Neuropharmacology 2016, 108, 451–461. [Google Scholar] [CrossRef]
- Kanazawa, M.; Igarashi, H.; Kawamura, K.; Takahashi, T.; Kakita, A.; Takahashi, H.; Nakada, T.; Nishizawa, M.; Shimohata, T. Inhibition of VEGF signaling pathway attenuates hemorrhage after tPA treatment. J. Cereb. Blood Flow Metab. 2011, 31, 1461–1474. [Google Scholar] [CrossRef]
- Cosky, E.E.P.; Ding, Y. The role of vascular endothelial growth factor in angiogenesis and brain circulation after stroke. Brain Circ. 2018, 4, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Zacharek, A.; Chen, J.; Li, A.; Cui, X.; Li, Y.; Roberts, C.; Feng, Y.; Gao, Q.; Chopp, M. Angiopoietin1/Tie2 and VEGF/Flk1 induced by MSC treatment amplifies angiogenesis and vascular stabilization after stroke. J. Cereb. Blood Flow Metab. 2007, 27, 1684–1691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Lee, K.; Kim, Y.; Kim, S.H.; Koh, S.; Lee, Y.-J. Serum VEGF levels in acute ischaemic strokes are correlated with long-term prognosis. Eur. J. Neurol. 2010, 17, 45–51. [Google Scholar] [CrossRef]
- Matsuo, R.; Ago, T.; Kamouchi, M.; Kuroda, J.; Kuwashiro, T.; Hata, J.; Sugimori, H.; Fukuda, K.; Gotoh, S.; Makihara, N.; et al. Clinical significance of plasma VEGF value in ischemic stroke-research for biomarkers in ischemic stroke (REBIOS) study. BMC Neurol. 2013, 13, 32. [Google Scholar] [CrossRef] [Green Version]
- Bus, P.; Gerrits, T.; Heemskerk, S.A.; Zandbergen, M.; Wolterbeek, R.; Bruijn, J.A.; Baelde, H.J.; Scharpfenecker, M. Endoglin Mediates Vascular Endothelial Growth Factor-A-Induced Endothelial Cell Activation by Regulating Akt Signaling. Am. J. Pathol. 2018, 188, 2924–2935. [Google Scholar] [CrossRef] [Green Version]
- Torsney, E.; Charlton, R.; Parums, D.; Collis, M.; Arthur, H.M. Inducible expression of human endoglin during inflammation and wound healing in vivo. Inflamm. Res. 2002, 51, 464–470. [Google Scholar] [CrossRef]
- Dijke, P.T.; Goumans, M.-J.; Pardali, E. Endoglin in angiogenesis and vascular diseases. Angiogenesis 2008, 11, 79–89. [Google Scholar] [CrossRef]
- Moldes, O.; Sobrino, T.; Millán, M.; Castellanos, M.; de la Ossa, N.P.; Leira, R.; Serena, J.; Vivancos, J.; Dávalos, A.; Castillo, J. High serum levels of endothelin-1 predict severe cerebral edema in patients with acute ischemic stroke treated with t-PA. Stroke 2008, 39, 2006–2010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Dirksen, W.P.; Zweier, J.L.; Periasamy, M. Endothelin-1-induced responses in isolated mouse vessels: The expression and function of receptor types. Am. J. Physiol. Circ. Physiol. 2004, 287, H573–H578. [Google Scholar] [CrossRef] [PubMed]
- Andresen, J.; Shafi, N.I.; Bryan, R.M., Jr. Endothelial influences on cerebrovascular tone. J. Appl. Physiol. 2006, 100, 318–327. [Google Scholar] [CrossRef]
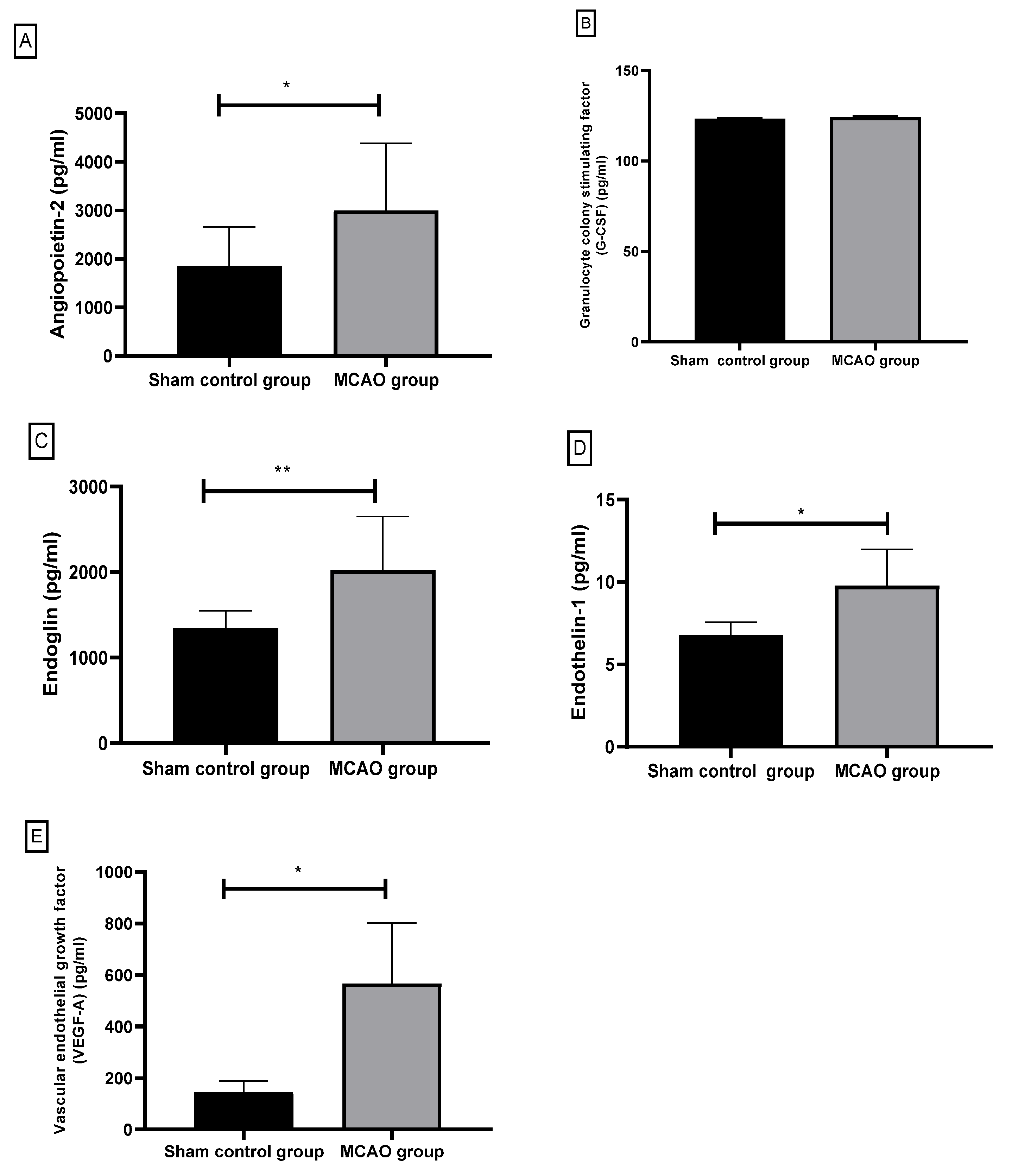
| Group | N | Mean ± Std. Deviation | Significance | |
|---|---|---|---|---|
| Angiopoietin-2 (pg/mL) | sham control group | 6 | 1859.66 ± 798.30 | 0.013 * |
| MACO group | 6 | 2997.50 ± 1383.26 | ||
| Granulocyte colony stimulating factor (G-CSF) (pg/mL) | sham control group | 6 | 123.39 ± 0.51 | 0.197 |
| MACO group | 6 | 124.15 ± 0.71 | ||
| Endoglin (pg/mL) | sham control group | 6 | 1349.50 ± 202.77 | 0.008 ** |
| MACO group | 6 | 2025.70 ± 626.25 | ||
| Endothelin-1 (ET-1) (pg/mL) | sham control group | 6 | 6.76 ± 0.80 | 0.01 * |
| MACO group | 6 | 9.78 ± 2.19 | ||
| VEGF-A (pg/mL) | sham control group | 6 | 144.12 ± 44.50 | 0.02 * |
| MACO group | 6 | 566.68 ± 235.12 |
| Angiopoietin-2 (pg/mL) | G-CSF (pg/mL) | Endoglin (pg/mL) | Endothelin-1 (ET-1) (pg/mL) | ||
|---|---|---|---|---|---|
| G-CSF (pg/mL) | Pearson Correlation | 0.303 | |||
| Sig. (2-tailed) | 0.339 | ||||
| Endoglin (pg/mL) | Pearson Correlation | 0.344 | 0.474 | ||
| Sig. (2-tailed) | 0.274 | 0.120 | |||
| Endothelin-1 (ET-1) (pg/mL) | Pearson Correlation | 0.734 ** | 0.599 * | 0.462 | |
| Sig. (2-tailed) | 0.007 | 0.040 | 0.131 | ||
| VEGF-A (pg/mL) | Pearson Correlation | 0.472 | 0.755 ** | 0.827 ** | 0.740 ** |
| Sig. (2-tailed) | 0.121 | 0.005 | 0.001 | 0.006 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alrafiah, A.R. Secondary Cerebellar Cortex Injury in Albino Male Rats after MCAO: A Histological and Biochemical Study. Biomedicines 2021, 9, 1267. https://doi.org/10.3390/biomedicines9091267
Alrafiah AR. Secondary Cerebellar Cortex Injury in Albino Male Rats after MCAO: A Histological and Biochemical Study. Biomedicines. 2021; 9(9):1267. https://doi.org/10.3390/biomedicines9091267
Chicago/Turabian StyleAlrafiah, Aziza R. 2021. "Secondary Cerebellar Cortex Injury in Albino Male Rats after MCAO: A Histological and Biochemical Study" Biomedicines 9, no. 9: 1267. https://doi.org/10.3390/biomedicines9091267
APA StyleAlrafiah, A. R. (2021). Secondary Cerebellar Cortex Injury in Albino Male Rats after MCAO: A Histological and Biochemical Study. Biomedicines, 9(9), 1267. https://doi.org/10.3390/biomedicines9091267






