Nutraceuticals and Herbs in Reducing the Risk and Improving the Treatment of COVID-19 by Targeting SARS-CoV-2
Abstract
:1. Introduction
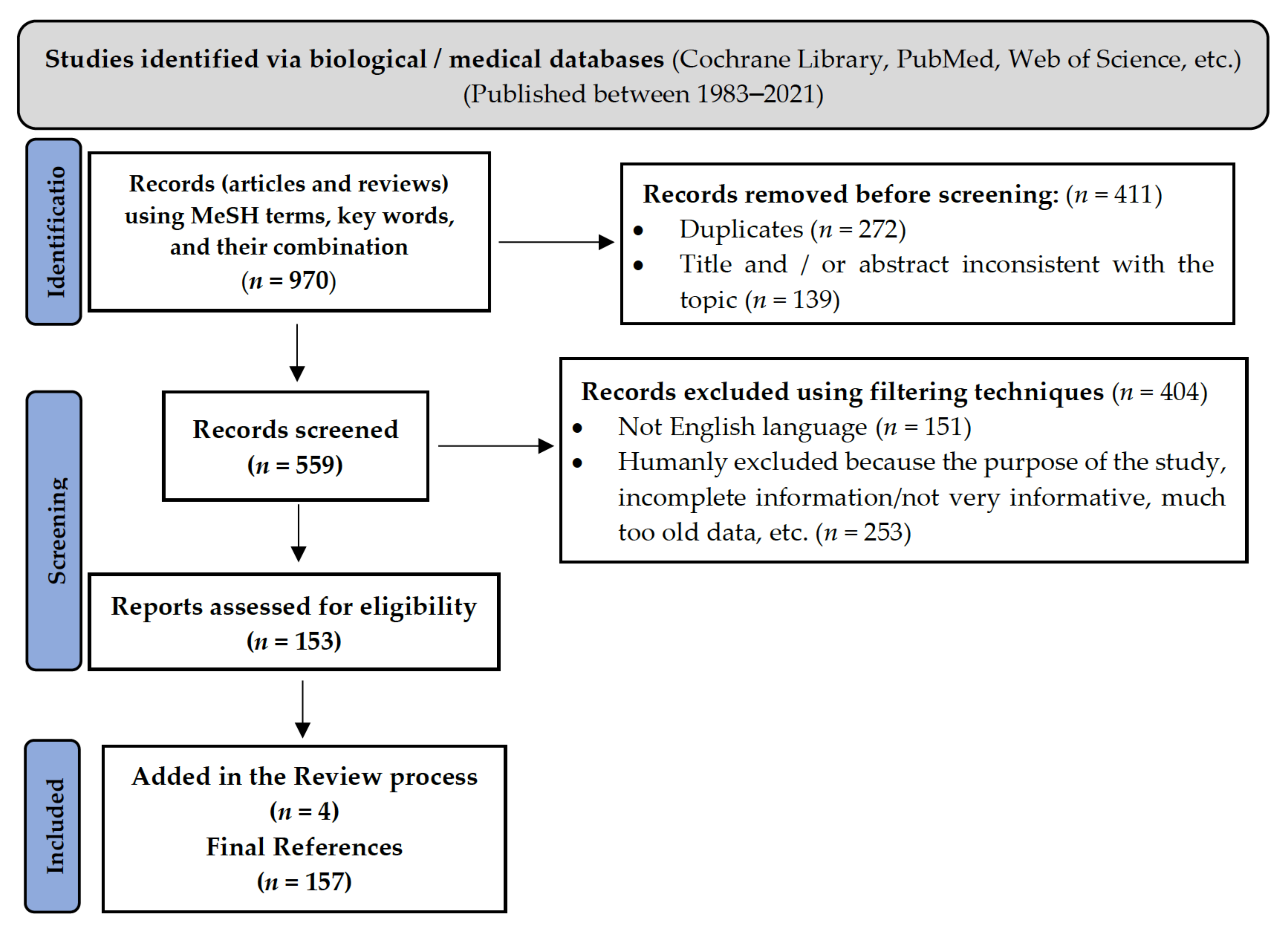
2. Methodology
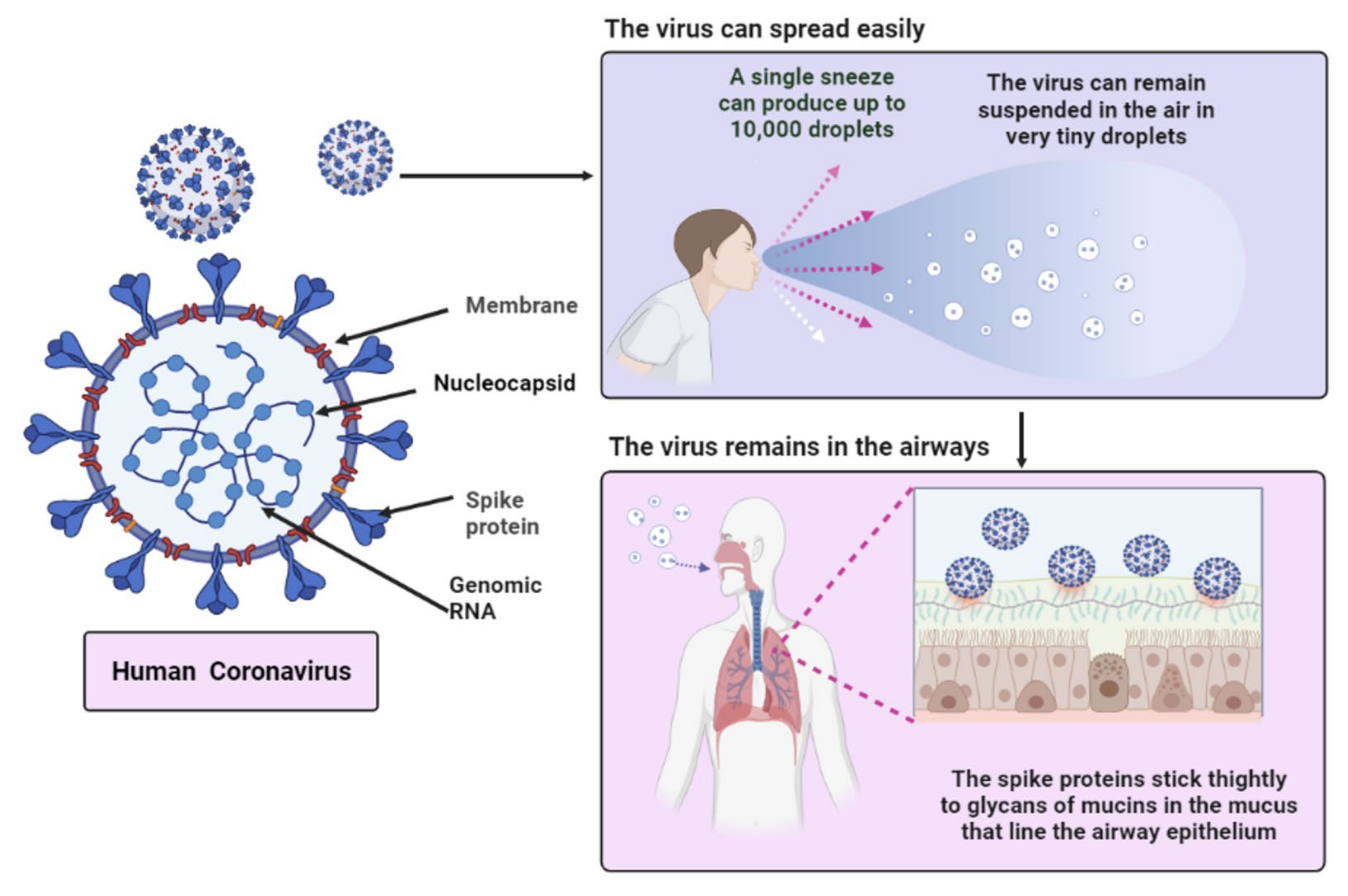
3. Characteristics of SARS-CoV-2
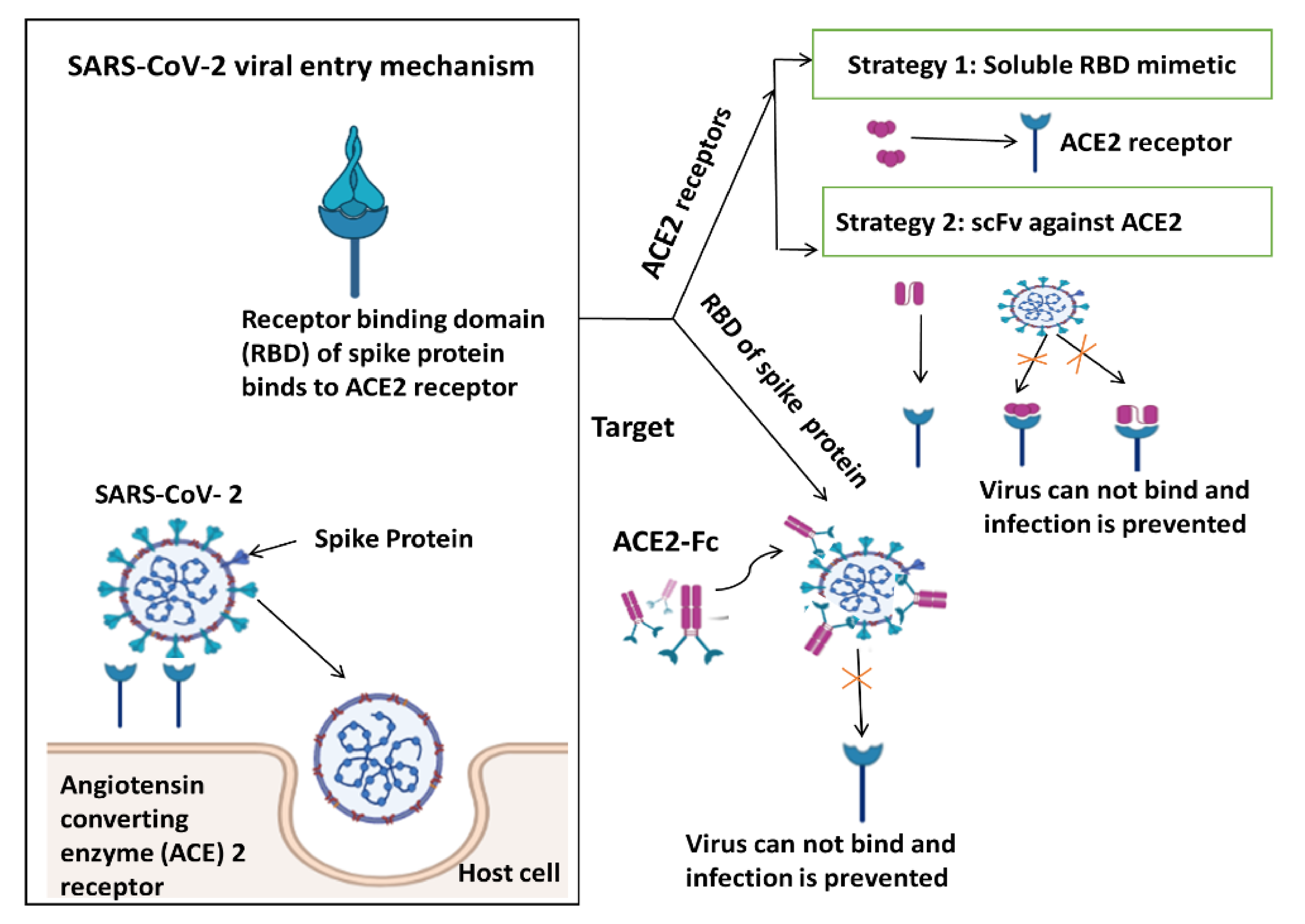
4. Immune Response from Various Perspectives and Targeting SARS-CoV Viral Entry Mechanism
5. Nutraceuticals or Supplements Targeting COVID-19
5.1. Vitamins
5.1.1. Vitamin C
5.1.2. Vitamin D
5.2. Minerals—Zinc
5.3. Probiotics
5.4. Natural Polyphenols
5.4.1. Resveratrol
5.4.2. Quercetin
6. Herbs Considered Usable for the Treatment of COVID-19
6.1. Curcuma longa
6.2. Cinchona officinalis
6.3. Allium sativum
6.4. Sambucus nigra
6.5. Piper nigrum L.
6.6. Withania somnifera
6.7. Ocimum sanctum
6.8. Astragalus membranaceus
6.9. Nigella sativa
6.10. Tinospora cordifolia
6.11. Glycyrrhiza glabra
6.12. Zingiber officinalis
7. Conclusions and Future Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Rahimi, F.; Talebi Bezmin Abadi, A. Ethical and sensible dissemination of information during the COVID-19 pandemic. Am. J. Bioeth. 2020, 20, W4–W6. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Batra, S.; Gupta, S.; Sharma, V.K.; Rahman, M.H.; Kamal, M.A. Persons with co-existing neurological disorders: Risk analysis, considerations and management in COVID-19 pandemic. CNS Neurol. Disord. Drug Targets 2021, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.R.; Martin, M.R.; Martin, J.D.; Kuhn, P.; Hicks, J.B. Modeling the onset of symptoms of COVID-19. Front. Public Health 2020, 8, 473. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Tuberculosis. Available online: https://www.who.int/en/news-room/fact-sheets/detail/tuberculosis (accessed on 14 June 2021).
- Otrisal, P.; Bungau, C.; Obsel, V.; Melicharik, Z.; Tont, G. Selected Respiratory Protective Devices: Respirators and Significance of Some Markings. Sustainability 2021, 13, 4988. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Li, X.-L.; Yan, Z.-R.; Sun, X.-P.; Han, J.; Zhang, B.-W. Potential neurological symptoms of COVID-19. Ther. Adv. Neurol. Disord. 2020, 13, 1756286420917830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouaziz, J.; Duong, T.; Jachiet, M.; Velter, C.; Lestang, P.; Cassius, C.; Arsouze, A.; Domergue Than Trong, E.; Bagot, M.; Begon, E. Vascular skin symptoms in COVID-19: A french observational study. J. Eur. Acad. Derm. Venereol. 2020, 34, e451–e452. [Google Scholar] [CrossRef]
- Rahman, M.H.; Akter, R.; Behl, T.; Chowdhury, M.A.; Mohammed, M.; Bulbul, I.J.; Elshenawy, S.E.; Kamal, M.A. COVID-19 outbreak and emerging management through pharmaceutical therapeutic strategy. Curr. Pharm. Des. 2020, 26, 5224–5240. [Google Scholar] [CrossRef] [PubMed]
- Infusino, F.; Marazzato, M.; Mancone, M.; Fedele, F.; Mastroianni, C.M.; Severino, P.; Ceccarelli, G.; Santinelli, L.; Cavarretta, E.; Marullo, A.G. Diet supplementation, probiotics, and nutraceuticals in SARS-CoV-2 infection: A scoping review. Nutrients 2020, 12, 1718. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.J. Pathogenesis of COVID-19 from a cell biology perspective. Eur. Respir. Soc. 2020, 55, 2000607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabir, M.T.; Uddin, M.S.; Hossain, M.F.; Abdulhakim, J.A.; Alam, M.A.; Ashraf, G.M.; Bungau, S.G.; Bin-Jumah, M.N.; Abdel-Daim, M.M.; Aleya, L. nCOVID-19 pandemic: From molecular pathogenesis to potential investigational therapeutics. Front. Cell Dev. Biol. 2020, 8, 616. [Google Scholar] [CrossRef] [PubMed]
- Rockx, B.; Kuiken, T.; Herfst, S.; Bestebroer, T.; Lamers, M.M.; Oude Munnink, B.B.; de Meulder, D.; van Amerongen, G.; van den Brand, J.; Okba, N.M.A.; et al. Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science 2020, 368, 1012–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahase, E. Hydroxychloroquine for COVID-19: The end of the line? BMJ 2020, 369, m2378. [Google Scholar] [CrossRef] [PubMed]
- Boehmer, T.K.; DeVies, J.; Caruso, E.; van Santen, K.L.; Tang, S.; Black, C.L.; Hartnett, K.P.; Kite-Powell, A.; Dietz, S.; Lozier, M.; et al. Changing Age Distribution of the COVID-19 Pandemic-United States, May-August 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1404–1409. [Google Scholar] [CrossRef] [PubMed]
- Mrityunjaya, M.; Pavithra, V.; Neelam, R.; Janhavi, P.; Halami, P.M.; Ravindra, P.V. Immune-Boosting, Antioxidant and Anti-inflammatory Food Supplements Targeting Pathogenesis of COVID-19. Front. Immunol. 2020, 11, 570122. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.S.; Radford, S.; Kow, C.S.; Zaidi, S.T.R. Venous thromboembolism in critically ill COVID-19 patients receiving prophylactic or therapeutic anticoagulation: A systematic review and meta-analysis. J. Thromb. Thrombolysis 2020, 50, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Waikar, S.; Oli, A. COVID-19: Ophthalmic prophylactic and therapeutic measures. Indian J. Ophthalmol. 2020, 68, 1223–1224. [Google Scholar] [CrossRef] [PubMed]
- Bar-On, Y.M.; Flamholz, A.; Phillips, R.; Milo, R. SARS-CoV-2 (COVID-19) by the numbers. eLife 2020, 9. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Alhenc-Gelas, F.; Bouby, N.; Girolami, J.-P. Kallikrein/K1, Kinins, and ACE/Kininase II in Homeostasis and in Disease Insight From Human and Experimental Genetic Studies, Therapeutic Implication. Front. Med. 2019, 6, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaduganathan, M.; Vardeny, O.; Michel, T.; McMurray, J.J.V.; Pfeffer, M.A.; Solomon, S.D. Renin-Angiotensin-Aldosterone System Inhibitors in Patients with COVID-19. N. Engl. J. Med. 2020, 382, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Abobaker, A.; Alzwi, A.; Alraied, A.H.A. Overview of the possible role of vitamin C in management of COVID-19. Pharm. Rep. 2020, 72, 1517–1528. [Google Scholar] [CrossRef] [PubMed]
- Tahir, A.H.; Javed, M.M.; Hussain, Z. Nutraceuticals and herbal extracts: A ray of hope for COVID-19 and related infections (Review). Int. J. Funct. Nutr. 2020, 1, 6. [Google Scholar] [CrossRef]
- De Almeida Brasiel, P.G. The key role of zinc in elderly immunity: A possible approach in the COVID-19 crisis. Clin. Nutr. ESPEN 2020, 38, 65–66. [Google Scholar] [CrossRef] [PubMed]
- Panfili, F.M.; Roversi, M.; D’Argenio, P.; Rossi, P.; Cappa, M.; Fintini, D. Possible role of vitamin D in COVID-19 infection in pediatric population. J. Endocrinol. Investig. 2021, 44, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Kodchakorn, K.; Poovorawan, Y.; Suwannakarn, K.; Kongtawelert, P. Molecular modelling investigation for drugs and nutraceuticals against protease of SARS-CoV-2. J. Mol. Graph. Model. 2020, 101, 107717. [Google Scholar] [CrossRef] [PubMed]
- Savant, S.; Srinivasan, S.; Kruthiventi, A.K. Potential Nutraceuticals for COVID-19. Nutr. Diet. Suppl. 2021, 13, 25. [Google Scholar] [CrossRef]
- Heller, R.A.; Sun, Q.; Hackler, J.; Seelig, J.; Seibert, L.; Cherkezov, A.; Minich, W.B.; Seemann, P.; Diegmann, J.; Pilz, M.; et al. Prediction of survival odds in COVID-19 by zinc, age and selenoprotein P as composite biomarker. Redox Biol. 2021, 38, 101764. [Google Scholar] [CrossRef] [PubMed]
- Romani, L.; Tomino, C.; Puccetti, P.; Garaci, E. Off-label therapy targeting pathogenic inflammation in COVID-19. Cell Death Discov. 2020, 6, 49. [Google Scholar] [CrossRef]
- Xu, Y.; Baylink, D.J.; Chen, C.S.; Reeves, M.E.; Xiao, J.; Lacy, C.; Lau, E.; Cao, H. The importance of vitamin d metabolism as a potential prophylactic, immunoregulatory and neuroprotective treatment for COVID-19. J. Transl. Med. 2020, 18, 322. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M.; Aldawoud, T.M.S.; Rizou, M.; Rowan, N.J.; Ibrahim, S.A. Food Ingredients and Active Compounds against the Coronavirus Disease (COVID-19) Pandemic: A Comprehensive Review. Foods 2020, 9, 1701. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Barrea, L.; Savastano, S.; Colao, A. Nutritional recommendations for COVID-19 quarantine. Eur. J. Clin. Nutr. 2020, 74, 850–851. [Google Scholar] [CrossRef]
- Long, C.; Xu, H.; Shen, Q.; Zhang, X.; Fan, B.; Wang, C.; Zeng, B.; Li, Z.; Li, X.; Li, H. Diagnosis of the Coronavirus disease (COVID-19): rRT-PCR or CT? Eur. J. Radiol. 2020, 126, 108961. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, Z.; Chang, R.; Wang, H.; Xu, C.; Yu, X.; Tsamlag, L.; Dong, Y.; Wang, H.; Cai, Y. COVID-19 containment: China provides important lessons for global response. Front. Med. 2020, 14, 215–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Moher, D. Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. J. Clin. Epidemiol. 2021, 134, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Cao, X. COVID-19: Immunopathology and its implications for therapy. Nat. Rev. Immunol. 2020, 20, 269–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thevarajan, I.; Nguyen, T.H.O.; Koutsakos, M.; Druce, J.; Caly, L.; van de Sandt, C.E.; Jia, X.; Nicholson, S.; Catton, M.; Cowie, B.; et al. Breadth of concomitant immune responses prior to patient recovery: A case report of non-severe COVID-19. Nat. Med. 2020, 26, 453–455. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Zhou, X.; Zhu, C.; Song, Y.; Feng, F.; Qiu, Y.; Feng, J.; Jia, Q.; Song, Q.; Zhu, B.; et al. Immune Phenotyping Based on the Neutrophil-to-Lymphocyte Ratio and IgG Level Predicts Disease Severity and Outcome for Patients With COVID-19. Front. Mol. Biosci. 2020, 7, 157. [Google Scholar] [CrossRef] [PubMed]
- Boni, M.F.; Lemey, P.; Jiang, X.; Lam, T.T.-Y.; Perry, B.W.; Castoe, T.A.; Rambaut, A.; Robertson, D.L. Evolutionary origins of the SARS-CoV-2 sarbecovirus lineage responsible for the COVID-19 pandemic. Nat. Microbiol. 2020, 5, 1408–1417. [Google Scholar] [CrossRef]
- Zheng, J. SARS-CoV-2: An Emerging Coronavirus that Causes a Global Threat. Int. J. Biol. Sci. 2020, 16, 1678–1685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. Discovery of a novel coronavirus associated with the recent pneumonia outbreak in humans and its potential bat origin. bioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Kimball, A.; Hatfield, K.M.; Arons, M.; James, A.; Taylor, J.; Spicer, K.; Bardossy, A.C.; Oakley, L.P.; Tanwar, S.; Chisty, Z. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility—King County, Washington, March 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrario, C.M.; Ahmad, S.; Groban, L. Mechanisms by which angiotensin-receptor blockers increase ACE2 levels. Nat. Rev. Cardiol. 2020, 17, 378. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kutateladze, T.G. Molecular structure analyses suggest strategies to therapeutically target SARS-CoV-2. Nat. Commun. 2020, 11, 2920. [Google Scholar] [CrossRef] [PubMed]
- Allam, M.; Cai, S.; Ganesh, S.; Venkatesan, M.; Doodhwala, S.; Song, Z.; Hu, T.; Kumar, A.; Heit, J.; Study Group, C.; et al. COVID-19 Diagnostics, Tools, and Prevention. Diagnostics 2020, 10, 409. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, R.; Sooriyaarachchi, P.; Chourdakis, M.; Jeewandara, C.; Ranasinghe, P. Enhancing immunity in viral infections, with special emphasis on COVID-19: A review. Diabetes Metab. Syndr. 2020, 14, 367–382. [Google Scholar] [CrossRef]
- Kumar, V.; Kancharla, S.; Jena, M.K. In silico virtual screening-based study of nutraceuticals predicts the therapeutic potentials of folic acid and its derivatives against COVID-19. Virusdisease 2021, 32, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ratha, S.K.; Renuka, N.; Rawat, I.; Bux, F. Prospective options of algae-derived nutraceuticals as supplements to combat COVID-19 and human coronavirus diseases. Nutrition 2021, 83, 111089. [Google Scholar] [CrossRef] [PubMed]
- Clohisey, S.; Baillie, J.K. Host susceptibility to severe influenza A virus infection. Crit. Care 2019, 23, 303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, N.B. Does COVID-19-related cachexia mimic cancer-related cachexia? Examining mechanisms, clinical biomarkers, and potential targets for clinical management. J. Cachexia Sarcopenia Muscle 2021, 12, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Sikander, M.; Malik, S.; Rodriguez, A.; Yallapu, M.M.; Narula, A.S.; Satapathy, S.K.; Dhevan, V.; Chauhan, S.C.; Jaggi, M. Role of Nutraceuticals in COVID-19 Mediated Liver Dysfunction. Molecules 2020, 25, 5905. [Google Scholar] [CrossRef] [PubMed]
- Nasri, H.; Baradaran, A.; Shirzad, H.; Rafieian-Kopaei, M. New concepts in nutraceuticals as alternative for pharmaceuticals. Int. J. Prev. Med. 2014, 5, 1487–1499. [Google Scholar]
- Dhalaria, R.; Verma, R.; Kumar, D.; Puri, S.; Tapwal, A.; Kumar, V.; Nepovimova, E.; Kuca, K. Bioactive Compounds of Edible Fruits with Their Anti-Aging Properties: A Comprehensive Review to Prolong Human Life. Antioxidants 2020, 9, 1123. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.; Heaven Taylor, B.; Winchester, C. COVID-19–Where should we go now? Integr. Med. Res. 2020, 9, 100468. [Google Scholar] [CrossRef]
- Ayseli, Y.I.; Aytekin, N.; Buyukkayhan, D.; Aslan, I.; Ayseli, M.T. Food policy, nutrition and nutraceuticals in the prevention and management of COVID-19: Advice for healthcare professionals. Trends Food Sci. Technol. 2020, 105, 186–199. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; McCarty, M. Thrombotic complications of COVID-19 may reflect an upregulation of endothelial tissue factor expression that is contingent on activation of endosomal NADPH oxidase. Open Heart 2020, 7, e001337. [Google Scholar] [CrossRef] [PubMed]
- Verweij, P.E.; Gangneux, J.P.; Bassetti, M.; Brüggemann, R.J.M.; Cornely, O.A.; Koehler, P.; Lass-Flörl, C.; van de Veerdonk, F.L.; Chakrabarti, A.; Hoenigl, M. Diagnosing COVID-19-associated pulmonary aspergillosis. Lancet Microbe 2020, 1, e53–e55. [Google Scholar] [CrossRef]
- Ntyonga-Pono, M.P. COVID-19 infection and oxidative stress: An under-explored approach for prevention and treatment? Pan. Afr. Med. J. 2020, 35, 12. [Google Scholar] [CrossRef]
- McCarty, M.F.; Iloki Assanga, S.B.; Lewis Luján, L.; O’keefe, J.H.; DiNicolantonio, J.J. Nutraceutical strategies for suppressing NLRP3 inflammasome activation: Pertinence to the management of COVID-19 and beyond. Nutrients 2021, 13, 47. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Shen, H.; Cheng, Q. Chinese herbal medicine for SARS and SARS-CoV-2 treatment and prevention, encouraging using herbal medicine for COVID-19 outbreak. Acta Agric. Scand. Sect. B—Soil Plant. Sci. 2020, 70, 437–443. [Google Scholar] [CrossRef]
- Carr, A.C.; Rowe, S. The Emerging Role of Vitamin C in the Prevention and Treatment of COVID-19. Nutrients 2020, 12, 3286. [Google Scholar] [CrossRef] [PubMed]
- Kaur, I.; Behl, T.; Aleya, L.; Rahman, H.; Kumar, A.; Arora, S.; Bulbul, I.J. Artificial intelligence as a fundamental tool in management of infectious diseases and its current implementation in COVID-19 pandemic. Env. Sci. Pollut. Res. Int. 2021, 28, 40515–40532. [Google Scholar] [CrossRef] [PubMed]
- Weir, E.K.; Thenappan, T.; Bhargava, M.; Chen, Y. Does vitamin D deficiency increase the severity of COVID-19? Clin. Med. 2020, 20, e107–e108. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilz, S.; März, W.; Cashman, K.D.; Kiely, M.E.; Whiting, S.J.; Holick, M.F.; Grant, W.B.; Pludowski, P.; Hiligsmann, M.; Trummer, C.; et al. Rationale and Plan for Vitamin D Food Fortification: A Review and Guidance Paper. Front. Endocrinol. 2018, 9, 373. [Google Scholar] [CrossRef]
- Brenner, H. Vitamin D Supplementation to Prevent COVID-19 Infections and Deaths-Accumulating Evidence from Epidemiological and Intervention Studies Calls for Immediate Action. Nutrients 2021, 13, 411. [Google Scholar] [CrossRef] [PubMed]
- Faniyi, A.A.; Lugg, S.T.; Faustini, S.E.; Webster, C.; Duffy, J.E.; Hewison, M.; Shields, A.; Nightingale, P.; Richter, A.G.; Thickett, D.R. Vitamin D status and seroconversion for COVID-19 in UK healthcare workers. Eur. Respir. J. 2021, 57. [Google Scholar] [CrossRef] [PubMed]
- Breslin, N.; Baptiste, C.; Gyamfi-Bannerman, C.; Miller, R.; Martinez, R.; Bernstein, K.; Ring, L.; Landau, R.; Purisch, S.; Friedman, A.M.; et al. Coronavirus disease 2019 infection among asymptomatic and symptomatic pregnant women: Two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am. J. Obs. Gynecol MFM 2020, 2, 100118. [Google Scholar] [CrossRef]
- Tagde, P.; Kulkarni, G.T.; Mishra, D.K.; Kesharwani, P. Recent advances in folic acid engineered nanocarriers for treatment of breast cancer. J. Drug Deliv. Sci. Technol. 2020, 56, 101613. [Google Scholar] [CrossRef]
- Singh, S. Covariation of Zinc Deficiency with COVID-19 Infections and Mortality in European Countries: Is Zinc Deficiency a Risk Factor for COVID-19? medRxiv 2020. [Google Scholar] [CrossRef]
- Xue, J.; Moyer, A.; Peng, B.; Wu, J.; Hannafon, B.N.; Ding, W.-Q. Chloroquine is a zinc ionophore. PLoS ONE 2014, 9, e109180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calder, P.C.; Carr, A.C.; Gombart, A.F.; Eggersdorfer, M. Optimal Nutritional Status for a Well-Functioning Immune System Is an Important Factor to Protect against Viral Infections. Nutrients 2020, 12, 1181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, K.K.; Baker, W.L.; Sobieraj, D.M. Myth Busters: Dietary Supplements and COVID-19. Ann. Pharm. 2020, 54, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Rao, A. Probiotics: A potential immunomodulator in COVID-19 infection management. Nutr. Res. 2021, 87, 1–12. [Google Scholar] [CrossRef]
- Kanauchi, O.; Andoh, A.; AbuBakar, S.; Yamamoto, N. Probiotics and Paraprobiotics in Viral Infection: Clinical Application and Effects on the Innate and Acquired Immune Systems. Curr. Pharm. Des. 2018, 24, 710–717. [Google Scholar] [CrossRef]
- Sundararaman, A.; Ray, M.; Ravindra, P.V.; Halami, P.M. Role of probiotics to combat viral infections with emphasis on COVID-19. Appl. Microbiol. Biotechnol. 2020, 104, 8089–8104. [Google Scholar] [CrossRef] [PubMed]
- Gohil, K.; Samson, R.; Dastager, S.; Dharne, M. Probiotics in the prophylaxis of COVID-19: Something is better than nothing. 3 Biotech 2021, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, E.; Sankari, L.S.; Malathi, L.; Krupaa, J.R. Naturally occurring products in cancer therapy. J. Pharm. Bioallied. Sci. 2015, 7, S181–S183. [Google Scholar] [CrossRef]
- Wang, D.; Sun-Waterhouse, D.; Li, F.; Xin, L.; Li, D. MicroRNAs as molecular targets of quercetin and its derivatives underlying their biological effects: A preclinical strategy. Crit. Rev. Food Sci. Nutr. 2019, 59, 2189–2201. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.-C.; Ho, C.-T.; Chuo, W.-H.; Li, S.; Wang, T.T.; Lin, C.-C. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect. Dis. 2017, 17, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramdani, L.H.; Bachari, K. Potential therapeutic effects of Resveratrol against SARS-CoV-2. Acta Virol. 2020, 64, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Xu, J.; Song, X.; Jia, R.; Yin, Z.; Cheng, A.; Jia, R.; Zou, Y.; Li, L.; Yin, L.; et al. Antiviral effect of resveratrol in ducklings infected with virulent duck enteritis virus. Antivir. Res. 2016, 130, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Wahedi, H.M.; Ahmad, S.; Abbasi, S.W. Stilbene-based natural compounds as promising drug candidates against COVID-19. J. Biomol. Struct. Dyn. 2021, 39, 3225–3234. [Google Scholar] [CrossRef]
- Shawon, J.; Akter, Z.; Hossen, M.M.; Akter, Y.; Sayeed, A.; Junaid, M.; Afrose, S.S.; Khan, M.A. Current Landscape of Natural Products against Coronaviruses: Perspectives in COVID-19 Treatment and Anti-viral Mechanism. Curr. Pharm. Des. 2020, 26, 5241–5260. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Gautam, A.; Chhetri, S.; Bhattarai, U. Immunity Against COVID-19: Potential Role of Ayush Kwath. J. Ayurveda Integr. Med. 2020. [Google Scholar] [CrossRef]
- Shree, P.; Mishra, P.; Selvaraj, C.; Singh, S.K.; Chaube, R.; Garg, N.; Tripathi, Y.B. Targeting COVID-19 (SARS-CoV-2) main protease through active phytochemicals of ayurvedic medicinal plants-Withania somnifera (Ashwagandha), Tinospora cordifolia (Giloy) and Ocimum sanctum (Tulsi)—A molecular docking study. J. Biomol. Struct. Dyn. 2020, 1–4. [Google Scholar] [CrossRef]
- Tripathi, M.K.; Singh, P.; Sharma, S.; Singh, T.P.; Ethayathulla, A.S.; Kaur, P. Identification of bioactive molecule from Withania somnifera (Ashwagandha) as SARS-CoV-2 main protease inhibitor. J. Biomol. Struct. Dyn. 2021, 39, 5668–5681. [Google Scholar] [CrossRef]
- Saggam, A.; Limgaokar, K.; Borse, S.; Chavan-Gautam, P.; Dixit, S.; Tillu, G.; Patwardhan, B. Withania somnifera (L.) Dunal: Opportunity for Clinical Repurposing in COVID-19 Management. Front. Pharmacol. 2021, 12. [Google Scholar] [CrossRef]
- Derosa, G.; Maffioli, P.; D’Angelo, A.; Di Pierro, F. A role for quercetin in coronavirus disease 2019 (COVID-19). Phytother. Res. 2021, 35, 1230–1236. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.H.; Bajgai, J.; Fadriquela, A.; Sharma, S.; Trinh Thi, T.; Akter, R.; Goh, S.H.; Kim, C.-S.; Lee, K.-J. Redox Effects of Molecular Hydrogen and Its Therapeutic Efficacy in the Treatment of Neurodegenerative Diseases. Processes 2021, 9, 308. [Google Scholar] [CrossRef]
- Huang, J.; Tao, G.; Liu, J.; Cai, J.; Huang, Z.; Chen, J.X. Current Prevention of COVID-19: Natural Products and Herbal Medicine. Front. Pharm. 2020, 11, 588508. [Google Scholar] [CrossRef] [PubMed]
- Fadus, M.C.; Lau, C.; Bikhchandani, J.; Lynch, H.T. Curcumin: An age-old anti-inflammatory and anti-neoplastic agent. J. Tradit. Complement. Med. 2017, 7, 339–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heydarian, M.; Abdorrahimian, F.; Emami, S.M.A.; Beheshti, S.I. The provenance and distribution of Early Bronze ceramics in the Kolyaei Plain, central Zagros, Iran. Archaeometry 2020, 62, 694–711. [Google Scholar] [CrossRef]
- Suravajhala, R.; Gupta, S.; Kumar, N.; Suravajhala, P. Deciphering LncRNA-protein interactions using docking complexes. J. Biomol. Struct. Dyn. 2020, 1–8. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.J.; Su, C.; Zhang, D.M.; Xu, L.P.; He, R.R.; Wang, L.; Zhang, J.; Zhang, X.Q.; Ye, W.C. Cytotoxic quassinoids from Ailanthus altissima. Bioorg. Med. Chem. Lett. 2013, 23, 654–657. [Google Scholar] [CrossRef]
- Soleimani, V.; Sahebkar, A.; Hosseinzadeh, H. Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances: Review. Phytother. Res. 2018, 32, 985–995. [Google Scholar] [CrossRef]
- Manoharan, Y.; Haridas, V.; Vasanthakumar, K.C.; Muthu, S.; Thavoorullah, F.F.; Shetty, P. Curcumin: A Wonder Drug as a Preventive Measure for COVID19 Management. Indian J. Clin. Biochem. 2020, 35, 373–375. [Google Scholar] [CrossRef]
- Li, H.Y.; Yang, M.; Li, Z.; Meng, Z. Curcumin inhibits angiotensin II-induced inflammation and proliferation of rat vascular smooth muscle cells by elevating PPAR-γ activity and reducing oxidative stress. Int. J. Mol. Med. 2017, 39, 1307–1316. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.R.; Kim, W.K.; Ha, A.W. Effects of Phytochemicals on Blood Pressure and Neuroprotection Mediated Via Brain Renin-Angiotensin System. Nutrients 2019, 11, 2761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otobone, F.J.; Sanches, A.C.; Nagae, R.; Martins, J.V.; Sela, V.R.; de Mello, J.C.; Audi, E.A. Effect of lyophilized extracts from guaraná seeds [Paullinia cupana var. sorbilis (Mart.) Ducke] on behavioral profiles in rats. Phytother. Res. 2007, 21, 531–535. [Google Scholar] [CrossRef]
- Odeh, N.D.; Babkair, H.; Abu-Hammad, S.; Borzangy, S.; Abu-Hammad, A.; Abu-Hammad, O. COVID-19: Present and Future Challenges for Dental Practice. Int. J. Env. Res. Public Health 2020, 17, 3151. [Google Scholar] [CrossRef] [PubMed]
- McBride, D.A.; Kerr, M.D.; Dorn, N.C.; Ogbonna, D.A.; Santos, E.C.; Shah, N.J. Triggers, Timescales, and Treatments for Cytokine-Mediated Tissue Damage. Eur. Med. J. Innov. 2021, 5, 52–62. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, B.; Lv, J.T.; Sa, R.N.; Zhang, X.M.; Lin, Z.J. The clinical benefits of Chinese patent medicines against COVID-19 based on current evidence. Pharm. Res. 2020, 157, 104882. [Google Scholar] [CrossRef] [PubMed]
- Adaki, S.; Adaki, R.; Shah, K.; Karagir, A. Garlic: Review of literature. Indian J. Cancer 2014, 51, 577–581. [Google Scholar] [CrossRef]
- Arreola, R.; Quintero-Fabián, S.; López-Roa, R.I.; Flores-Gutiérrez, E.O.; Reyes-Grajeda, J.P.; Carrera-Quintanar, L.; Ortuño-Sahagún, D. Immunomodulation and anti-inflammatory effects of garlic compounds. J. Immunol. Res. 2015, 2015, 401630. [Google Scholar] [CrossRef]
- Petrovic, V.; Nepal, A.; Olaisen, C.; Bachke, S.; Hira, J.; Søgaard, C.K.; Røst, L.M.; Misund, K.; Andreassen, T.; Melø, T.M.; et al. Anti-Cancer Potential of Homemade Fresh Garlic Extract Is Related to Increased Endoplasmic Reticulum Stress. Nutrients 2018, 10, 450. [Google Scholar] [CrossRef] [Green Version]
- Donma, M.M.; Donma, O. The effects of allium sativum on immunity within the scope of COVID-19 infection. Med. Hypotheses 2020, 144, 109934. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Sánchez, M.A.; Zepeda-Morales, A.S.M.; Carrera-Quintanar, L.; Viveros-Paredes, J.M.; Franco-Arroyo, N.N.; Godínez-Rubí, M.; Ortuño-Sahagun, D.; López-Roa, R.I. Alliin, an Allium sativum Nutraceutical, ReducesMetaflammation Markers in DIO Mice. Nutrients 2020, 12, 624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Saber Batiha, G.; Magdy Beshbishy, A.; Wasef, L.G.; Elewa, Y.H.A.; Al-Sagan, A.A.; El-Hack, M.E.; Taha, A.E.; Abd-Elhakim, Y.M.; Prasad Devkota, H. Chemical Constituents and Pharmacological Activities of Garlic (Allium sativum L.): A Review. Nutrients 2020, 12, 872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlachojannis, J.E.; Cameron, M.; Chrubasik, S. A systematic review on the sambuci fructus effect and efficacy profiles. Phytother. Res. 2010, 24, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Torabian, G.; Valtchev, P.; Adil, Q.; Dehghani, F. Anti-influenza activity of elderberry (Sambucus nigra). J. Funct. Foods 2019, 54, 353–360. [Google Scholar] [CrossRef]
- Kaack, K.; Austed, T. Interaction of vitamin C and flavonoids in elderberry (Sambucus nigra L.) during juice processing. Plant. Foods Hum. Nutr 1998, 52, 187–198. [Google Scholar] [CrossRef]
- Veberic, R.; Jakopic, J.; Stampar, F.; Schmitzer, V. European elderberry (Sambucus nigra L.) rich in sugars, organic acids, anthocyanins and selected polyphenols. Food Chem. 2009, 114, 511–515. [Google Scholar] [CrossRef]
- Christensen, L.P.; Kaack, K.; Fretté, X.C. Selection of elderberry (Sambucus nigra L.) genotypes best suited for the preparation of elderflower extracts rich in flavonoids and phenolic acids. Eur. Food Res. Technol. 2008, 227, 293–305. [Google Scholar] [CrossRef]
- Takooree, H.; Aumeeruddy, M.Z.; Rengasamy, K.R.R.; Venugopala, K.N.; Jeewon, R.; Zengin, G.; Mahomoodally, M.F. A systematic review on black pepper (Piper nigrum L.): From folk uses to pharmacological applications. Crit. Rev. Food Sci. Nutr. 2019, 59, S210–S243. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, P.; Chakdar, H.; Singh, D.; Selvaraj, C.; Singh, S.K.; Kumar, S.; Saxena, A. Computational studies reveal piperine, the predominant oleoresin of black pepper (Piper nigrum) as a potential inhibitor of SARS-CoV-2 (COVID-19). Curr. Sci. 2020, 119, 1333–1342. [Google Scholar] [CrossRef]
- McCubrey, J.A.; Lertpiriyapong, K.; Steelman, L.S.; Abrams, S.L.; Yang, L.V.; Murata, R.M.; Rosalen, P.L.; Scalisi, A.; Neri, L.M.; Cocco, L.; et al. Effects of resveratrol, curcumin, berberine and other nutraceuticals on aging, cancer development, cancer stem cells and microRNAs. Aging 2017, 9, 1477–1536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alharris, E.; Alghetaa, H.; Seth, R.; Chatterjee, S.; Singh, N.P.; Nagarkatti, M.; Nagarkatti, P. Resveratrol Attenuates Allergic Asthma and Associated Inflammation in the Lungs Through Regulation of miRNA-34a That Targets FoxP3 in Mice. Front. Immunol. 2018, 9, 2992. [Google Scholar] [CrossRef] [Green Version]
- Burns, J.; Yokota, T.; Ashihara, H.; Lean, M.E.; Crozier, A. Plant foods and herbal sources of resveratrol. J. Agric. Food Chem. 2002, 50, 3337–3340. [Google Scholar] [CrossRef]
- Balkrishna, A.; Pokhrel, S.; Singh, H.; Joshi, M.; Mulay, V.P.; Haldar, S.; Varshney, A. Withanone from Withania somnifera Attenuates SARS-CoV-2 RBD and Host ACE2 Interactions to Rescue Spike Protein Induced Pathologies in Humanized Zebrafish Model. Drug Des. Dev. 2021, 15, 1111–1133. [Google Scholar] [CrossRef]
- Kumar, V.; Dhanjal, J.K.; Bhargava, P.; Kaul, A.; Wang, J.; Zhang, H.; Kaul, S.C.; Wadhwa, R.; Sundar, D. Withanone and Withaferin-A are predicted to interact with transmembrane protease serine 2 (TMPRSS2) and block entry of SARS-CoV-2 into cells. J. Biomol. Struct. Dyn. 2020, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, B.; Mrigesh, M.; Chauhan, P.; Rastogi, S.K. Dietary supplementation with Withania somnifera root powder ameliorates experimentally induced Infectious Bursal Disease in chicken. Trop. Anim. Health Prod. 2020, 52, 1195–1206. [Google Scholar] [CrossRef]
- Jamshidi, N.; Cohen, M.M. The Clinical Efficacy and Safety of Tulsi in Humans: A Systematic Review of the Literature. Evid.-Based Complementary Altern. Med. Ecam. 2017, 2017, 9217567. [Google Scholar] [CrossRef]
- Harsha, M.; Mohan Kumar, K.P.; Kagathur, S.; Amberkar, V.S. Effect of Ocimum sanctum extract on leukemic cell lines: A preliminary in-vitro study. J. Oral Maxillofac. Pathol. 2020, 24, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.A.; Mohammed, A.A.; Ahmad, A.; Arshad Husain, R. Ocimum sanctum: Role in Diseases Management through Modulating Various Biological Activity. Pharmacogn. J. 2020, 12, 5. [Google Scholar]
- Cohen, M.M. Tulsi-Ocimum sanctum: A herb for all reasons. J. Ayurveda Integr. Med. 2014, 5, 251–259. [Google Scholar] [CrossRef] [Green Version]
- Das, S.K.; Chandra, A.; Agarwal, S.S.; Singh, N. Ocimum sanctum (Tulsi) in the treatment of viral encephalitis. Antiseptic 1983, 80(7), 323–327. [Google Scholar]
- Ak, S.; Jp, C.; Khan, R.; Dhand, C.; Verma, S. Role of Medicinal Plants of Traditional Use in Recuperating Devastating COVID-19 Situation. Med. Aromat. Plants 2020, 9, 1–16. [Google Scholar]
- Islam, M.T.; Khan, M.R.; Mishra, S.K. An updated literature-based review: Phytochemistry, pharmacology and therapeutic promises of Nigella sativa L. Orient. Pharm. Exp. Med. 2019, 19, 115–129. [Google Scholar] [CrossRef]
- Hunt, L.M.; de Voogd, K.B. Are good intentions good enough? Informed consent without trained interpreters. J. Gen. Intern. Med. 2007, 22, 598–605. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, C.; Mondal, M.; Torequl Islam, M.; Martorell, M.; Docea, A.O.; Maroyi, A.; Sharifi-Rad, J.; Calina, D. Potential Therapeutic Options for COVID-19: Current Status, Challenges, and Future Perspectives. Front. Pharmacol. 2020, 11, 572870. [Google Scholar] [CrossRef]
- Sharma, B.R.; Park, C.M.; Kim, H.A.; Kim, H.J.; Rhyu, D.Y. Tinospora cordifolia preserves pancreatic beta cells and enhances glucose uptake in adipocytes to regulate glucose metabolism in diabetic rats. Phytother. Res. 2019, 33, 2765–2774. [Google Scholar] [CrossRef]
- Nayak, P.; Tiwari, P.; Prusty, S.; Sahu, P. Phytochemistry and Pharmacology of Tinospora cordifolia: A Review. Syst. Rev. Pharm. 2018, 9, 70–78. [Google Scholar] [CrossRef]
- Ghatpande, N.S.; Misar, A.V.; Waghole, R.J.; Jadhav, S.H.; Kulkarni, P.P. Tinospora cordifolia protects against inflammation associated anemia by modulating inflammatory cytokines and hepcidin expression in male Wistar rats. Sci. Rep. 2019, 9, 10969. [Google Scholar] [CrossRef] [Green Version]
- George, M.; Josepha, L.; Mathew, M. A research on screening of learning and memory enhancing activity of whole plant extract of Tinospora cordifolia (Willd). Pharm. Innov. J. 2016, 5, 104–107. [Google Scholar]
- Sagar, V.; Kumar, A.H.S. Efficacy of natural compounds from Tinospora cordifolia against SARS-CoV-2 protease, surface glycoprotein and RNA polymerase. Virology 2020, 1–10. Available online: https://assets.researchsquare.com/files/rs-27375/v1/ec9bf967-ea5b-4362-b609-1859a5e370a1.pdf?c=1631834057 (accessed on 23 April 2021). [CrossRef]
- Agarwal, S.; Ramamurthy, P.H.; Fernandes, B.; Rath, A.; Sidhu, P. Assessment of antimicrobial activity of different concentrations of Tinospora cordifolia against Streptococcus mutans: An in vitro study. Dent. Res. J. 2019, 16, 24–28. [Google Scholar]
- Thakur, A.; Raj, P. Pharmacological perspective of Glycyrrhiza glabra Linn: A mini-review. J. Anal. Pharm. Res. 2017, 5, 00156. [Google Scholar] [CrossRef] [Green Version]
- Bailly, C.; Vergoten, G. Glycyrrhizin: An alternative drug for the treatment of COVID-19 infection and the associated respiratory syndrome? Pharmacol. Ther. 2020, 214, 107618. [Google Scholar] [CrossRef] [PubMed]
- Cinatl, J.; Morgenstern, B.; Bauer, G.; Chandra, P.; Rabenau, H.; Doerr, H.W. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet 2003, 361, 2045–2046. [Google Scholar] [CrossRef] [Green Version]
- Mao, Q.-Q.; Xu, X.-Y.; Cao, S.-Y.; Gan, R.-Y.; Corke, H.; Beta, T.; Li, H.-B. Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murck, H. Symptomatic Protective Action of Glycyrrhizin (Licorice) in COVID-19 Infection? Front. Immunol. 2020, 11, 1239. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Varoni, E.M.; Salehi, B.; Sharifi-Rad, J.; Matthews, K.R.; Ayatollahi, S.A.; Kobarfard, F.; Ibrahim, S.A.; Mnayer, D.; Zakaria, Z.A.; et al. Plants of the Genus Zingiber as a Source of Bioactive Phytochemicals: From Tradition to Pharmacy. Molecules 2017, 22, 2145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathinavel, T.; Palanisamy, M.; Srinivasan, P.; Subramanian, A.; Thangaswamy, S. Phytochemical 6-Gingerol -A promising Drug of choice for COVID-19. Int. J. Adv. Sci. Eng. 2020, 6. [Google Scholar] [CrossRef]
- Lestari, S.; Rifa, M. Regulatory T cells and anti-inflammatory cytokine profile of mice fed a high-fat diet after single-bulb garlic (Allium sativum L.) oil treatment. Trop. J. Pharm. Res. 2018, 17, 2157–2162. [Google Scholar] [CrossRef] [Green Version]
- Akter, R.; Chowdhury, M.A.R.; Rahman, M.H. Flavonoids and Polyphenolic Compounds as Potential Talented Agents for the Treatment of Alzheimer’s Disease and their Antioxidant Activities. Curr. Pharm. Des. 2021, 27, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Cui, Q.; Fu, Q.; Song, X.; Jia, R.; Yang, Y.; Zou, Y.; Li, L.; He, C.; Liang, X.; et al. Antiviral properties of resveratrol against pseudorabies virus are associated with the inhibition of IκB kinase activation. Sci. Rep. 2017, 7, 8782. [Google Scholar] [CrossRef] [PubMed]
- Baldassarre, M.E.; Di Mauro, A.; Labellarte, G.; Pignatelli, M.; Fanelli, M.; Schiavi, E.; Mastromarino, P.; Capozza, M.; Panza, R.; Laforgia, N. Resveratrol plus carboxymethyl-β-glucan in infants with common cold: A randomized double-blind trial. Heliyon 2020, 6, e03814. [Google Scholar] [CrossRef]
- Thakkar, S.S.; Shelat, F.; Thakor, P. Magical bullets from an indigenous Indian medicinal plant Tinospora cordifolia: An in silico approach for the antidote of SARS-CoV-2. Egypt. J. Pet. 2021, 30, 53–66. [Google Scholar] [CrossRef]
- Saeed, M.; Naveed, M.; Leskovec, J.; Ali kamboh, A.; Kakar, I.; Ullah, K.; Ahmad, F.; Sharif, M.; Javaid, A.; Rauf, M.; et al. Using Guduchi (Tinospora cordifolia) as an eco-friendly feed supplement in human and poultry nutrition. Poult. Sci. 2020, 99, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Ivanović, M.; Makoter, K.; Islamčević Razboršek, M. Comparative Study of Chemical Composition and Antioxidant Activity of Essential Oils and Crude Extracts of Four Characteristic Zingiberaceae Herbs. Plants 2021, 10, 501. [Google Scholar] [CrossRef] [PubMed]
- Di Sotto, A.; Vitalone, A.; Di Giacomo, S. Plant-Derived Nutraceuticals and Immune System Modulation: An Evidence-Based Overview. Vaccines 2020, 8, 468. [Google Scholar] [CrossRef]
- Zhou, J.; Huang, J. Current Findings Regarding Natural Components with Potential Anti-2019-nCoV Activity. Front. Cell Dev. Biol. 2020, 8, 589. [Google Scholar] [CrossRef]
- Yu, M.S.; Lee, J.; Lee, J.M.; Kim, Y.; Chin, Y.W.; Jee, J.G.; Keum, Y.S.; Jeong, Y.J. Identification of myricetin and scutellarein as novel chemical inhibitors of the SARS coronavirus helicase, nsP13. Bioorg. Med. Chem. Lett. 2012, 22, 4049–4054. [Google Scholar] [CrossRef] [PubMed]
- Van Doremalen, N.; Lambe, T.; Spencer, A.; Belij-Rammerstorfer, S.; Purushotham, J.N.; Port, J.R.; Avanzato, V.A.; Bushmaker, T.; Flaxman, A.; Ulaszewska, M.; et al. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature 2020, 586, 578–582. [Google Scholar] [CrossRef] [PubMed]
| Supplements | Daily Requirement | Intervention | Clinical Trial Phase | Reference |
|---|---|---|---|---|
| Vitamin C | 250 and 1000 mg | Reduces the duration and severity of upper respiratory infections (viral infections). Scavenges ROS, prevents lipid peroxidation, and protein alkylation and thus protect cells from oxidative Decreases pro-inflammatory cytokines, TNF-α and IFN-γ and increases anti-inflammatory IL-10 production. | Phase II | [22,65,66,67,68,69] |
| Vitamin D | 1000 to 4000 IU of supplemental vitamin D per day | Blocks NF-κB p65 activation via up-regulation of I-kappa-B-alpha (IKB-α). Decreases the expression of the pro-inflammatory type 1 cytokines: IL-12, IL-16, IL-8, TNF-α and IFN-γ Increases type 2 cytokines IL-4, IL-5, IL-1 | Phase II | [70,71] |
| Zinc | 11 mg for men and 8 mg for non-pregnant women, daily | Protects against oxidative stress and inhibit TNF-α, IFN-γ, FasR and JAK-STAT signaling pathways. Modulates the viral entry, fusion, replication, viral protein translation and virus budding of respiratory viruses | Phase I + II | [72,73,74,75] |
| Probiotics | Two capsules a day (one closed capsule to swallow and one open capsule mixed with maple syrup) from day 1 to 10 and one closed capsule to swallow from day 11 to 25, for maximum 25 days. | Lactobacillus plantarum DR7 suppress proinflammatory cytokines TNF-α, IFN-γ, enhancing anti-inflammatory cytokines IL-10, IL-4. | Not applicable | [71,75] |
| Quercetin | Patients will receive a daily dose of 400 mg of oral quercetin phytosomes | Dietary supplement: quercetin phytosomes | Phase 3 | [74,75] |
| Plant/Family | Potential Bioactive Compound | Interventions | Ref. |
|---|---|---|---|
Curcuma Longa/Zingiberaceae | Curcumin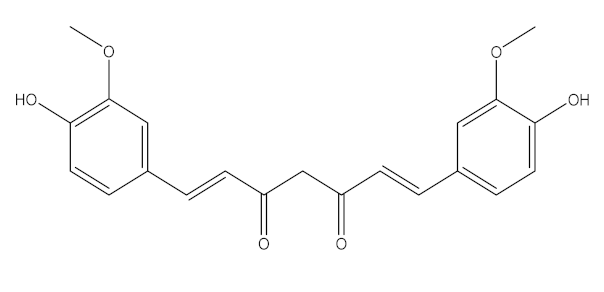 | Increase the development of pro-inflammatory cytokines Rise in ACE2, accelerating the disease’s development | [21,97,100] |
Cinchona officinalis/Rubiaceae | Quinine | Chloroquine and Hydroxychloroquine are synthetic analogs of quinine, and both have promising role in reducing the SARSCoV-2 viral load in COVID-19 patients. LCCD widely used in treating COVID-19 patients. | [104,107,108] |
Allium sativum/Alliaceae | Allicin | Reversal any of the signs and effects shown in these patients Restoration or recovery of the sensorial sensation that has been lost or diminished, Boosts the amount of Treg cells in the body Boosts the number of cytotoxic and helper T cells Lowers leptin, leptin receptor, and PPAR-I levels CD4+, Interleukin-2 receptor alpha chain, FoxP3+ regulatory T cells (Treg) cells from being inhibited Reduces the amount of IL-6 in the body Activate NK cells, Reduces TNF- and C-reactive protein levels | [109,110,147] |
Sambucus nigra/Adoxaceae | Quercetin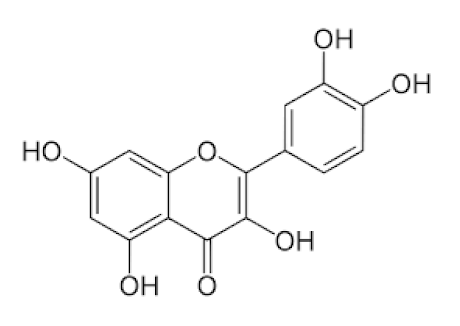 | Reduces upper respiratory symptoms caused by viral infections and has potential immunological mechanisms of action | [116,117,118,148] |
Piper Nigrum L./Piperaceae | Piperine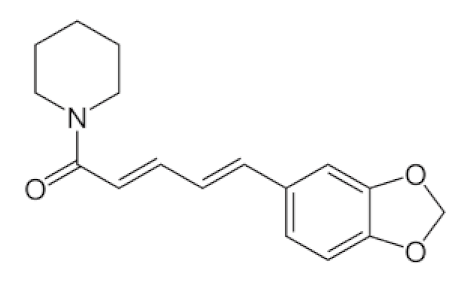 | Reduces respiratory system infection have antiviral & antibacterial properties | [119,120,121] |
Vaccinium berries/Ericaceae | Resveratrol | Blocks the key pathways involved in SARS-CoV-2 pathogenesis Controls RAS and expression of ACE, body’s immune activation Down-regulation of pro-inflammatory cytokines release. | [82,86,89,149,150] |
Withania somnifera/Solanaceae | Withaferin A | Lowering of pulmonary hypertension through decreasing inflammation, oxidative stress, epithelial dysfunction, respiratory cell apoptosis, and pulmonary respiratory system tolerance. Triggers the innate immune system and regulate the immune response of Th1 cells | [122,123,124,126,127] |
Ocimum sanctum/Lamiaceae | Eugenol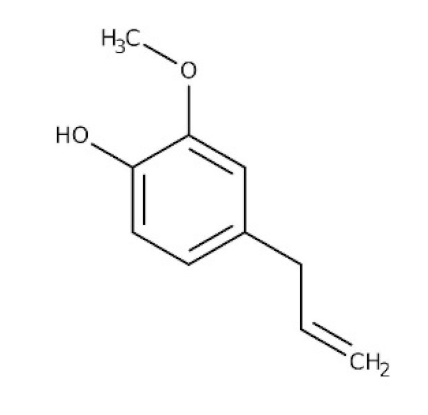 | Cures lung infections and relieves pain, diarrhea, and coughing. | [87,130] |
Astragalus membranaceus/Leguminosae | Astragalus polysaccharides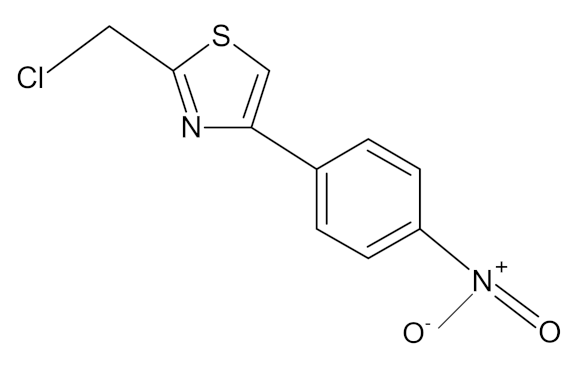 | Immunomodulator and antiviral activity. Interferes with the entry process of virus by blocking the positive charge of the pathogen surface receptors, to prevent them from binding to HSPGs on the surface of host | [133] |
| Nigella sativa/ Ranunculaceae | Thymoquinone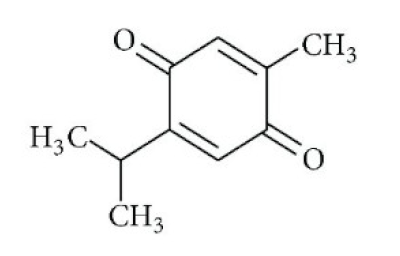 | Antioxidant, anti-inflammatory, immunomodulatory, epigenetic modulation, antiviral activity, docking studies on anti-COVID-19 activity, antibacterial and anticoagulant effects for the treatment of COVID-19. | [134,135,136,151] |
| Tinospora cordifolia/ Menispermaceae | Tinocordiside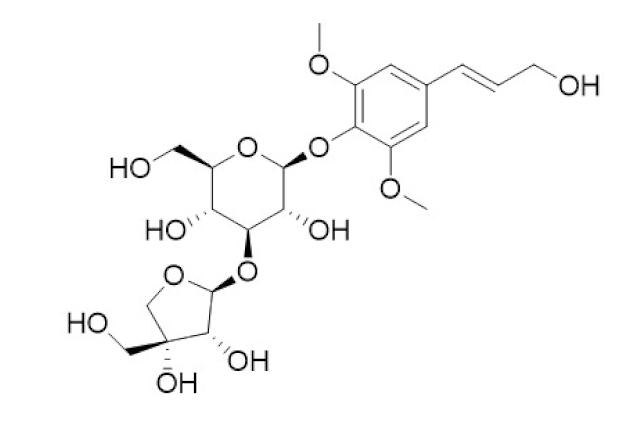 | Highest binding affinity as compared to built-in ligand N3 for SARS-CoV-2 Mpro as per YASARA scoring. | [87,137,138,142,152] |
| Glycyrrhiza glabra L./ Fabaceae | Glycyrrhizin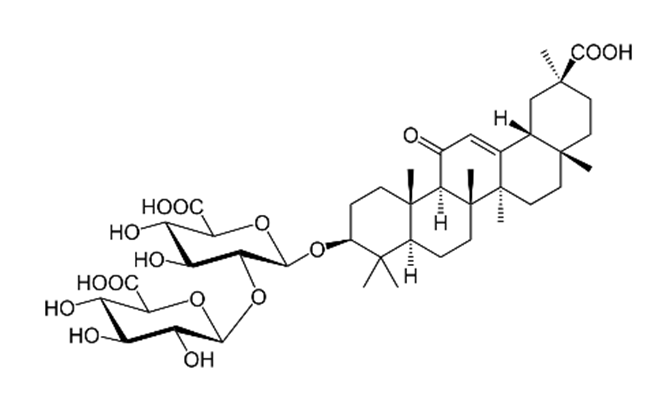 | Interferes specifically with ACE2 Stopped FFM-1 and FFM-2 viruses from replicating and disrupted their biosorption and osmotic replication cycles. | [142,144,145] |
| Zingiber officinalis/ Zingiberaceae | 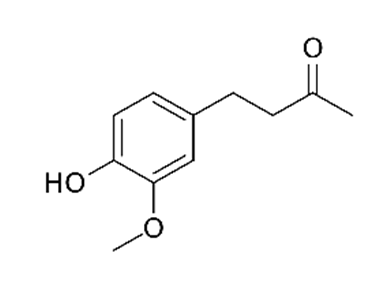 Zingerone 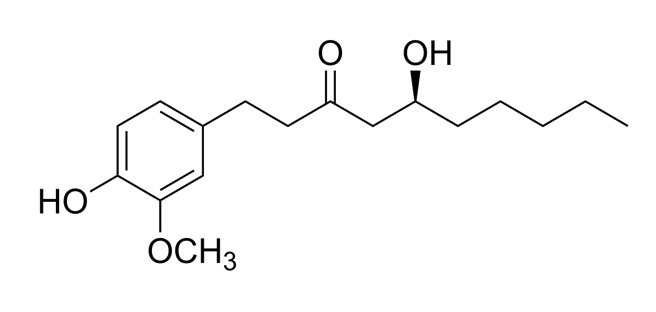 6-gingerol 6-gingerol | Antimicrobial, antioxidant, and anti-inflammatory activities. Inhibits human respiratory syncytial virus | [146,153] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tagde, P.; Tagde, S.; Tagde, P.; Bhattacharya, T.; Monzur, S.M.; Rahman, M.H.; Otrisal, P.; Behl, T.; ul Hassan, S.S.; Abdel-Daim, M.M.; et al. Nutraceuticals and Herbs in Reducing the Risk and Improving the Treatment of COVID-19 by Targeting SARS-CoV-2. Biomedicines 2021, 9, 1266. https://doi.org/10.3390/biomedicines9091266
Tagde P, Tagde S, Tagde P, Bhattacharya T, Monzur SM, Rahman MH, Otrisal P, Behl T, ul Hassan SS, Abdel-Daim MM, et al. Nutraceuticals and Herbs in Reducing the Risk and Improving the Treatment of COVID-19 by Targeting SARS-CoV-2. Biomedicines. 2021; 9(9):1266. https://doi.org/10.3390/biomedicines9091266
Chicago/Turabian StyleTagde, Priti, Sandeep Tagde, Pooja Tagde, Tanima Bhattacharya, Shams Minhaz Monzur, Md. Habibur Rahman, Pavel Otrisal, Tapan Behl, Syed Shams ul Hassan, Mohamed M. Abdel-Daim, and et al. 2021. "Nutraceuticals and Herbs in Reducing the Risk and Improving the Treatment of COVID-19 by Targeting SARS-CoV-2" Biomedicines 9, no. 9: 1266. https://doi.org/10.3390/biomedicines9091266
APA StyleTagde, P., Tagde, S., Tagde, P., Bhattacharya, T., Monzur, S. M., Rahman, M. H., Otrisal, P., Behl, T., ul Hassan, S. S., Abdel-Daim, M. M., Aleya, L., & Bungau, S. (2021). Nutraceuticals and Herbs in Reducing the Risk and Improving the Treatment of COVID-19 by Targeting SARS-CoV-2. Biomedicines, 9(9), 1266. https://doi.org/10.3390/biomedicines9091266













