Current Role of Monoclonal Antibody Therapy in Pediatric IBD: A Special Focus on Therapeutic Drug Monitoring and Treat-to-Target Strategies
Abstract
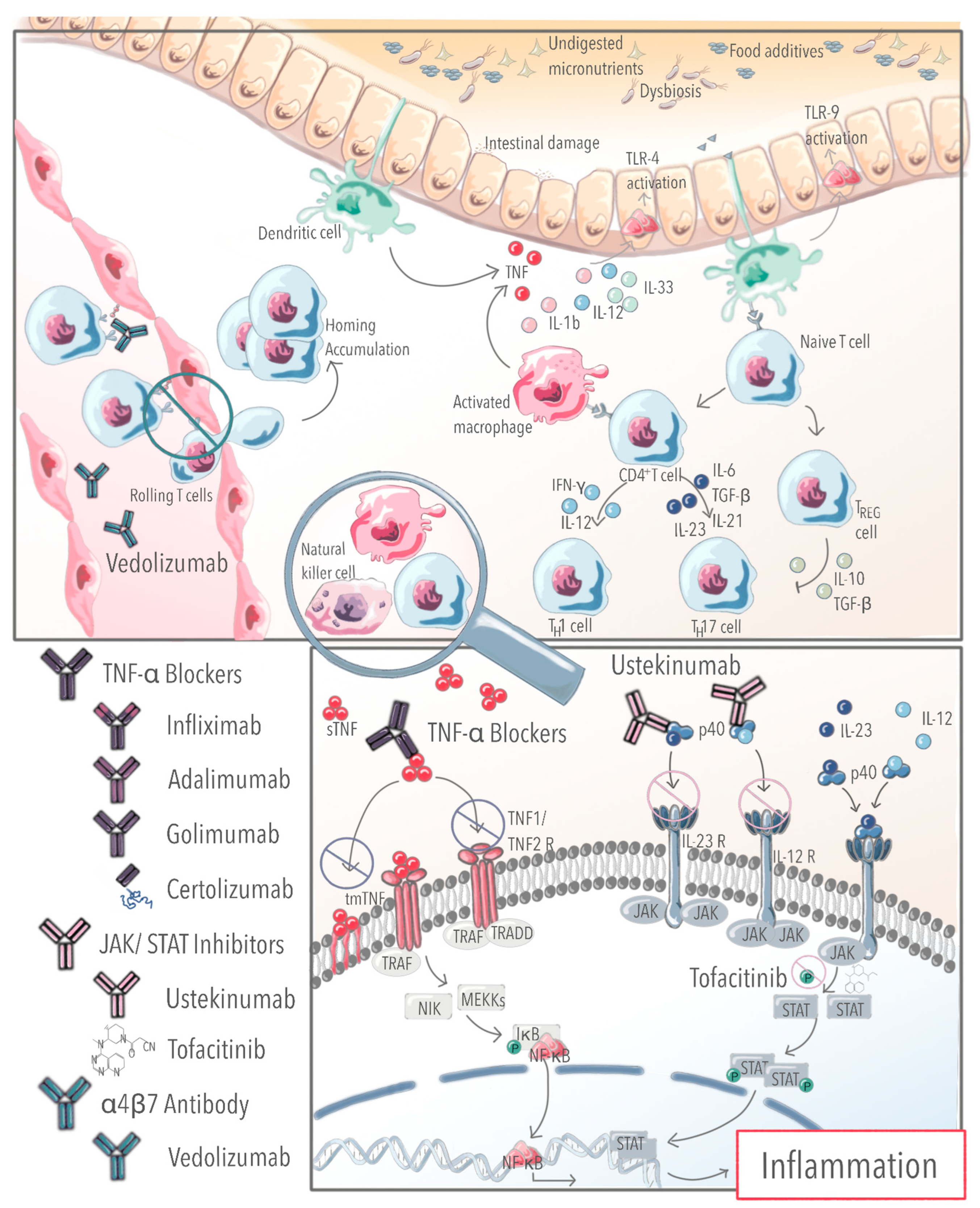
1. Introduction
2. Clinical Effect of Biologics
3. Methods and Selection Criteria
4. Early and Effective Use of TNF-α Blockers Prevents Disease Progression and Disease Complications
5. Occurrence and Frequency of Adverse Treatment Events
6. Therapeutic Drug Monitoring to Optimize the Treatment Strategy and Maintain the Efficacy of Biological Agents
| Induction | Maintenance | |
|---|---|---|
| Infliximab | >18 µg/mL before 3rd infusion to achieve clinical remission in CD [63] >12.7 µg/mL before 4th infusion for fistula healing and >9.1 to prevent treatment failure in CD [64,65] >13 ug/mL before 4th infusion for fistula healing (*) [66] | >7µg/mL to prevent treatment failure in CD [42]—>8.3 µg/mL for clinical remission in CD [67] >10.1 µg/mL for fistula healing in CD [92] |
| Adalimumab | >13.85 µg/mL at the end of induction for long term clinical remission in UC and CD [73] | ≥10.1 μg/mL–12 µg/mL (*) to prevent treatment failure [42] In case of loss of response—>new induction dose or weekly application [71,100] |
| Golimumab | >0.97 μg/mL at week 14 for clinical response in UC [101] | |
| Ustekinumab | ≈6.6 µg/mL at week 8 (associated with steroid-free remission week 52) in all IBD subtypes [10] | |
| Vedolizumab | >37 µg/mL before 3rd infusion and >20 µg/mL before 4th infusion to achieve steroid free-clinical remission in UC and CD [77] >30 µg/mL in week 2 (*) for endoscopic remission, clinical remission in CD and UC [102] | <30 kg: >7 μg/mL for steroid free and EEN-free remission in all IBD subtypes [75] >30 kg: ≥11.5 µg/mL for clinical and biochemical remission in CD and UC (*) [76] |
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADA | Anti-drug Antibodies |
| BMI | Body mass index |
| CD CEDATA CMV | Crohn’s disease Registry of Pediatric Patients with IBD in German-speaking countries Cytomegaly virus |
| CRP | C-reactive Protein |
| EIM | Extraintestinal manifestations |
| ESPGHAN | European Society for Pediatric Gastroenterology Hepatology and Nutrition |
| GPGE | German Association of Pediatric Gastroenterology |
| IBD | Inflammatory bowel disease |
| IFX | Infliximab |
| PCDAI | Pediatric Crohn’s disease activity index |
| PUCAI UC | Pediatric ulcerative colitis activity index Ulcerative colitis |
| TNF | Tumor necrosis factor |
| TDM | Therapeutic drug monitoring |
References
- Claßen, M.; de Laffolie, J.; Claßen, M.; Schnell, A.; Sohrabi, K.; Hoerning, A. Significant Advantages for First Line Treatment with TNF-Alpha Inhibitors in Pediatric Patients with Inflammatory Bowel Disease—Data from the Multicenter CEDATA-GPGE Registry Study. Front. Pediatr. 2022, 10, 903677. [Google Scholar] [CrossRef] [PubMed]
- van Rheenen, P.F.; Aloi, M.; Assa, A.; Bronsky, J.; Escher, J.C.; Fagerberg, U.L.; Gasparetto, M.; Gerasimidis, K.; Griffiths, A.; Henderson, P.; et al. The Medical Management of Paediatric Crohn’s Disease: An ECCO-ESPGHAN Guideline Update. J Crohns Colitis 2021, 15, 171–194. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Ruemmele, F.M.; Orlanski-Meyer, E.; Griffiths, A.M.; de Carpi, J.M.; Bronsky, J.; Veres, G.; Aloi, M.; Strisciuglio, C.; Braegger, C.P.; et al. Management of Paediatric Ulcerative Colitis, Part 1: Ambulatory Care—An Evidence-Based Guideline from European Crohn’s and Colitis Organization and European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 257–291. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.L.; Liu, C.; King, E.C.; Bass, J.A.; Patel, A.S.; Tung, J.; Chen, S.; Lissoos, T.; Candela, N.; Saeed, S.; et al. Use, Durability, and Risks for Discontinuation of Initial and Subsequent Biologics in a Large Pediatric-Onset IBD Cohort. J. Pediatr. Gastroenterol. Nutr. 2023, e003734. [Google Scholar] [CrossRef] [PubMed]
- Cozijnsen, M.; Duif, V.; Kokke, F.; Kindermann, A.; van Rheenen, P.; de Meij, T.; Schaart, M.; Damen, G.; Norbruis, O.; Pelleboer, R.; et al. Adalimumab Therapy in Children with Crohn Disease Previously Treated With Infliximab. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 205–210. [Google Scholar] [CrossRef]
- Danese, S.; Vuitton, L.; Peyrin-Biroulet, L. Biologic Agents for IBD: Practical Insights. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 537–545. [Google Scholar] [CrossRef]
- Garcia-Romero, R.; Martinez de Zabarte Fernandez, J.M.; Pujol-Muncunill, G.; Donat-Aliaga, E.; Segarra-Cantón, O.; Irastorza-Terradillos, I.; Medina-Benitez, E.; Ruiz-Hernández, C.J.; Carrillo-Palau, M.; Ros-Arnal, I.; et al. Safety and Effectiveness of Vedolizumab in Paediatric Patients with Inflammatory Bowel Disease: An Observational Multicentre Spanish Study. Eur. J. Pediatr. 2021, 180, 3029–3038. [Google Scholar] [CrossRef]
- Yerushalmy-Feler, A.; Pujol-Muncunill, G.; Martin-de-Carpi, J.; Kolho, K.-L.; Levine, A.; Olbjørn, C.; Granot, M.; Bramuzzo, M.; Rolandsdotter, H.; Mouratidou, N.; et al. Safety and Potential Efficacy of Escalating Dose of Ustekinumab in Pediatric Crohn Disease (the Speed-up Study): A Multicenter Study from the Pediatric IBD Porto Group of ESPGHAN. J. Pediatr. Gastroenterol. Nutr. 2022, 75, 717–723. [Google Scholar] [CrossRef]
- Dhaliwal, J.; McKay, H.E.; Deslandres, C.; Debruyn, J.; Wine, E.; Wu, A.; Huynh, H.; Carman, N.; Crowley, E.; Church, P.C.; et al. One-Year Outcomes with Ustekinumab Therapy in Infliximab-Refractory Paediatric Ulcerative Colitis: A Multicentre Prospective Study. Aliment. Pharmacol. Ther. 2021, 53, 1300–1308. [Google Scholar] [CrossRef]
- Dayan, J.R.; Dolinger, M.; Benkov, K.; Dunkin, D.; Jossen, J.; Lai, J.; Phan, B.L.; Pittman, N.; Dubinsky, M.C. Real World Experience with Ustekinumab in Children and Young Adults at a Tertiary Care Pediatric Inflammatory Bowel Disease Center. J. Pediatr. Gastroenterol. Nutr. 2019, 69, 61–67. [Google Scholar] [CrossRef]
- deBruyn, J.C.; Jacobson, K.; El-Matary, W.; Carroll, M.; Wine, E.; Wrobel, I.; Van Woudenberg, M.; Huynh, H.Q. Long-Term Outcomes of Infliximab Use for Pediatric Crohn Disease: A Canadian Multicenter Clinical Practice Experience. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Aardoom, M.A.; Veereman, G.; de Ridder, L. A Review on the Use of Anti-TNF in Children and Adolescents with Inflammatory Bowel Disease. Int. J. Mol. Sci. 2019, 20, 2529. [Google Scholar] [CrossRef] [PubMed]
- Bronsky, J.; Copova, I.; Kazeka, D.; Lerchova, T.; Mitrova, K.; Pospisilova, K.; Sulovcova, M.; Zarubova, K.; Hradsky, O. Adalimumab vs Infliximab in Pediatric Patients with Crohn’s Disease: A Propensity Score Analysis and Predictors of Treatment Escalation. Clin. Transl. Gastroenterol. 2022, 13, e00490. [Google Scholar] [CrossRef] [PubMed]
- Björkesten, C.-G.a.; Nieminen, U.; Turunen, U.; Arkkila, P.E.; Sipponen, T.; Färkkilä, M.A. Endoscopic Monitoring of Infliximab Therapy in Crohn’s Disease. Inflamm. Bowel Dis. 2011, 17, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Ribaldone, D.G.; Caviglia, G.P.; Abdulle, A.; Pellicano, R.; Ditto, M.C.; Morino, M.; Fusaro, E.; Saracco, G.M.; Bugianesi, E.; Astegiano, M. Adalimumab Therapy Improves Intestinal Dysbiosis in Crohn’s Disease. J. Clin. Med. 2019, 8, 1646. [Google Scholar] [CrossRef] [PubMed]
- Scarallo, L.; Alvisi, P.; Bolasco, G.; Di Toma, M.; Lanari, M.; Cangiari, A.; Paci, M.; Naldini, S.; Renzo, S.; Barp, J.; et al. Mucosal and Histologic Healing in Children with Inflammatory Bowel Disease Treated With Antitumor Necrosis Factor-Alpha. J. Pediatr. Gastroenterol. Nutr. 2021, 72, 728–735. [Google Scholar] [CrossRef]
- Kierkus, J.; Dadalski, M.; Szymanska, E.; Oracz, G.; Wegner, A.; Gorczewska, M.; Szymanska, S.; Woynarowski, M.; Ryzko, J. The Impact of Infliximab Induction Therapy on Mucosal Healing and Clinical Remission in Polish Pediatric Patients with Moderate-to-Severe Crohn’s Disease. Eur. J. Gastroenterol. Hepatol. 2012, 24, 495–500. [Google Scholar] [CrossRef]
- Lee, D.; Baldassano, R.N.; Otley, A.R.; Albenberg, L.; Griffiths, A.M.; Compher, C.; Chen, E.Z.; Li, H.; Gilroy, E.; Nessel, L.; et al. Comparative Effectiveness of Nutritional and Biological Therapy in North American Children with Active Crohn’s Disease. Inflamm. Bowel Dis. 2015, 21, 1786–1793. [Google Scholar] [CrossRef]
- Luo, Y.; Yu, J.; Lou, J.; Fang, Y.; Chen, J. Exclusive Enteral Nutrition versus Infliximab in Inducing Therapy of Pediatric Crohn’s Disease. Gastroenterol. Res. Pract. 2017, 2017, e6595048. [Google Scholar] [CrossRef]
- van Rheenen, H.; van Rheenen, P.F. Long-Term Efficacy of Anti-Tumor Necrosis Factor Agents in Pediatric Luminal Crohn’s Disease: A Systematic Review of Real-World Evidence Studies. Pediatr. Gastroenterol. Hepatol. Nutr. 2020, 23, 121–131. [Google Scholar] [CrossRef]
- Carnovale, C.; Maffioli, A.; Zaffaroni, G.; Mazhar, F.; Battini, V.; Mosini, G.; Pozzi, M.; Radice, S.; Clementi, E.; Danelli, P. Efficacy of Tumour Necrosis Factor-Alpha Therapy in Paediatric Crohn’s Disease Patients with Perianal Lesions: A Systematic Review. Expert Opin. Biol. Ther. 2020, 20, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Hyams, J.; Damaraju, L.; Blank, M.; Johanns, J.; Guzzo, C.; Winter, H.S.; Kugathasan, S.; Cohen, S.; Markowitz, J.; Escher, J.C.; et al. Induction and Maintenance Therapy with Infliximab for Children with Moderate to Severe Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2012, 10, 391–399.e1. [Google Scholar] [CrossRef] [PubMed]
- Bolia, R.; Rajanayagam, J.; Hardikar, W.; Alex, G. Impact of Changing Treatment Strategies on Outcomes in Pediatric Ulcerative Colitis. Inflamm. Bowel Dis. 2019, 25, 1838–1844. [Google Scholar] [CrossRef] [PubMed]
- Croft, N.M.; Faubion, W.A.; Kugathasan, S.; Kierkus, J.; Ruemmele, F.M.; Shimizu, T.; Mostafa, N.M.; Venetucci, M.; Finney-Hayward, T.; Gonzalez, Y.S.; et al. Efficacy and Safety of Adalimumab in Paediatric Patients with Moderate-to-Severe Ulcerative Colitis (ENVISION I): A Randomised, Controlled, Phase 3 Study. Lancet Gastroenterol. Hepatol. 2021, 6, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Ley, D.; Leroyer, A.; Dupont, C.; Sarter, H.; Bertrand, V.; Spyckerelle, C.; Guillon, N.; Wils, P.; Savoye, G.; Turck, D.; et al. New Therapeutic Strategies Have Changed the Natural History of Pediatric Crohn’s Disease: A Two-Decade Population-Based Study. Clin. Gastroenterol. Hepatol. 2022, 20, 2588–2597.e1. [Google Scholar] [CrossRef] [PubMed]
- Boros, K.K.; Veres, G.; Cseprekál, O.; Pintér, H.K.; Richter, É.; Cseh, Á.; Dezsőfi-Gottl, A.; Arató, A.; Reusz, G.; Dohos, D.; et al. Body Composition, Physical Activity, and Quality of Life in Pediatric Patients with Inflammatory Bowel Disease on Anti-TNF Therapy—An Observational Follow-up Study. Eur. J. Clin. Nutr. 2023, 77, 380–385. [Google Scholar] [CrossRef]
- D’Arcangelo, G.; Abi Nader, E.; Charbit-Henrion, F.; Talbotec, C.; Goulet, O.; Ruemmele, F.M.; Pigneur, B. Increased Use of Anti-Tumor Necrosis Factor Following the Implementation of the ECCO-ESPGHAN Guidelines and Its Impact on the Outcome of Pediatric Crohn’s Disease: A Retrospective Single-Center Study. J. Pediatr. Gastroenterol. Nutr. 2022, 74, 79–84. [Google Scholar] [CrossRef]
- Kim, H.J.; Oh, S.H.; Lee, S.H.; Kim, Y.-B.; Kim, D.Y.; Park, S.H.; Ye, B.D.; Yang, S.-K.; Kim, K.M. Risk Factors for Disease Behavior Evolution and Efficacy of Biologics in Reducing Progression in Pediatric Patients with Nonstricturing, Nonpenetrating Crohn’s Disease at Diagnosis: A Single-Center Experience in Korea. Gut Liver 2021, 15, 851–857. [Google Scholar] [CrossRef]
- Walters, T.D.; Kim, M.-O.; Denson, L.A.; Griffiths, A.M.; Dubinsky, M.; Markowitz, J.; Baldassano, R.; Crandall, W.; Rosh, J.; Pfefferkorn, M.; et al. Increased Effectiveness of Early Therapy with Anti-Tumor Necrosis Factor-α vs an Immunomodulator in Children with Crohn’s Disease. Gastroenterology 2014, 146, 383–391. [Google Scholar] [CrossRef]
- Choe, Y.J.; Han, K.; Shim, J.O. Treatment Patterns of Anti-Tumour Necrosis Factor-Alpha and Prognosis of Paediatric and Adult-Onset Inflammatory Bowel Disease in Korea: A Nationwide Population-Based Study. Aliment. Pharmacol. Ther. 2022, 56, 980–988. [Google Scholar] [CrossRef]
- Kugathasan, S.; Denson, L.A.; Walters, T.D.; Kim, M.-O.; Marigorta, U.M.; Schirmer, M.; Mondal, K.; Liu, C.; Griffiths, A.; Noe, J.D.; et al. Prediction of Complicated Disease Course for Children Newly Diagnosed with Crohn’s Disease: A Multicentre Inception Cohort Study. Lancet 2017, 389, 1710–1718. [Google Scholar] [CrossRef] [PubMed]
- Kandavel, P.; Eder, S.J.; Adler, J.; The ImproveCareNow Network Pediatric IBD Learning Health. Reduced Systemic Corticosteroid Use among Pediatric Patients with Inflammatory Bowel Disease in a Large Learning Health System. J. Pediatr. Gastroenterol. Nutr. 2021, 73, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Sherlock, M.E.; Zachos, M.; Issenman, R.M.; Mulder, D.J. Clinical and Laboratory Characteristics Are Associated with Biologic Therapy Use in Pediatric Inflammatory Bowel Disease: A Retrospective Cohort Study. J. Can. Assoc. Gastroenterol. 2021, 4, e92–e100. [Google Scholar] [CrossRef] [PubMed]
- Nuti, F.; Viola, F.; Civitelli, F.; Alessandri, C.; Aloi, M.; Dilillo, A.; Del Giudice, E.; Cucchiara, S. Biological Therapy in a Pediatric Crohn Disease Population at a Referral Center. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 582–587. [Google Scholar] [CrossRef]
- Beukelman, T.; Xie, F.; Chen, L.; Horton, D.B.; Lewis, J.D.; Mamtani, R.; Mannion, M.M.; Saag, K.G.; Curtis, J.R. Risk of Malignancy Associated with Paediatric Use of Tumour Necrosis Factor Inhibitors. Anna. Rheum. Dis. 2018, 77, 1012–1016. [Google Scholar] [CrossRef]
- Hradsky, O.; Kazeka, D.; Copova, I.; Lerchova, T.; Mitrova, K.; Pospisilova, K.; Sulovcova, M.; Zarubova, K.; Bronsky, J. Risk Factors for Dermatological Complications of Anti-TNF Therapy in a Cohort of Children with Crohn’s Disease. Eur. J. Pediatr. 2021, 180, 3001–3008. [Google Scholar] [CrossRef]
- Dolinger, M.T.; Rolfes, P.; Spencer, E.; Stoffels, G.; Dunkin, D.; Dubinsky, M.C. Outcomes of Children with Inflammatory Bowel Disease Who Develop Anti-Tumour Necrosis Factor-Induced Skin Reactions. J. Crohns Colitis 2022, 16, 1420–1427. [Google Scholar] [CrossRef]
- Baggett, K.; Brandon, T.G.; Xiao, R.; Valenzuela, Z.; Buckley, L.H.; Weiss, P.F. Incidence Rates of Psoriasis in Children with Inflammatory Bowel Disease and Juvenile Arthritis Treated with Tumor Necrosis Factor Inhibitors and Disease-Modifying Antirheumatic Drugs. J. Rheumatol. 2022, 49, 935–941. [Google Scholar] [CrossRef]
- Zvuloni, M.; Matar, M.; Levi, R.; Shouval, D.S.; Shamir, R.; Assa, A. High Anti-TNFα Concentrations Are Not Associated with More Adverse Events in Pediatric Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2021, 73, 717–721. [Google Scholar] [CrossRef]
- Eindor-Abarbanel, A.; Meleady, L.; Lawrence, S.; Hamilton, Z.; Krikler, G.; Lakhani, A.; Zhang, Q.; Jacobson, K. Progression to Anti-TNF Treatment in Very Early Onset Inflammatory Bowel Disease Patients. J. Pediatr. Gastroenterol. Nutr. 2022, 75, 473–479. [Google Scholar] [CrossRef]
- Kerur, B.; Fiedler, K.; Stahl, M.; Hyams, J.; Stephens, M.; Lu, Y.; Pfefferkorn, M.; Alkhouri, R.; Strople, J.; Kelsen, J.; et al. Utilization of Antitumor Necrosis Factor Biologics in Very Early Onset Inflammatory Bowel Disease: A Multicenter Retrospective Cohort Study from North America. J. Pediatr. Gastroenterol. Nutr. 2022, 75, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, N.A.; Heap, G.A.; Green, H.D.; Hamilton, B.; Bewshea, C.; Walker, G.J.; Thomas, A.; Nice, R.; Perry, M.H.; Bouri, S.; et al. Predictors of Anti-TNF Treatment Failure in Anti-TNF-Naive Patients with Active Luminal Crohn’s Disease: A Prospective, Multicentre, Cohort Study. Lancet Gastroenterol. Hepatol. 2019, 4, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Azor, B.; Martín-Masot, R.; Dayaldasani Khialani, A.; Fernández-Martín, J.M.; Gallego Fernández, C.; Navas-López, V.M. Proactive Monitoring of Anti-TNF Agents Improves Follow-up of Paediatric Patients with Crohn Disease. Anales de Pediatría (Engl. Ed.) 2023, 98, 165–174. [Google Scholar] [CrossRef]
- Salvador-Martín, S.; Zapata-Cobo, P.; Velasco, M.; Palomino, L.M.; Clemente, S.; Segarra, O.; Sánchez, C.; Tolín, M.; Moreno-Álvarez, A.; Fernández-Lorenzo, A.; et al. Association between HLA DNA Variants and Long-Term Response to Anti-TNF Drugs in a Spanish Pediatric Inflammatory Bowel Disease Cohort. Int. J. Mol. Sci. 2023, 24, 1797. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.Z.; Schoen, B.T.; Kugathasan, S.; Sauer, C.G. Management of Anti-Drug Antibodies to Biologic Medications in Children With Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2019, 69, 551. [Google Scholar] [CrossRef]
- Colman, R.J.; Portocarrero-Castillo, A.; Chona, D.; Hellmann, J.; Minar, P.; Rosen, M.J. Favorable Outcomes and Anti-TNF Durability After Addition of an Immunomodulator for Anti-Drug Antibodies in Pediatric IBD Patients. Inflamm. Bowel Dis. 2021, 27, 507–515. [Google Scholar] [CrossRef]
- Sassine, S.; Djani, L.; Cambron-Asselin, C.; Savoie, M.; Lin, Y.F.; Qaddouri, M.; Zekhnine, S.; Grzywacz, K.; Groleau, V.; Dirks, M.; et al. Risk Factors of Clinical Relapses in Pediatric Luminal Crohn’s Disease: A Retrospective Cohort Study. Am. J. Gastroenterol. 2022, 117, 637–646. [Google Scholar] [CrossRef]
- Scarallo, L.; Bolasco, G.; Barp, J.; Bianconi, M.; di Paola, M.; Di Toma, M.; Naldini, S.; Paci, M.; Renzo, S.; Labriola, F.; et al. Anti-Tumor Necrosis Factor-Alpha Withdrawal in Children with Inflammatory Bowel Disease in Endoscopic and Histologic Remission. Inflamm. Bowel Dis. 2022, 28, 183–191. [Google Scholar] [CrossRef]
- Weigl, E.; Schwerd, T.; Lurz, E.; Häberle, B.; Koletzko, S.; Hubertus, J. Children with Localized Crohn’s Disease Benefit from Early Ileocecal Resection and Perioperative Anti-Tumor Necrosis Factor Therapy. Eur. J. Pediatr. Surg. 2023. [Google Scholar] [CrossRef]
- Jongsma, M.M.E.; Aardoom, M.A.; Cozijnsen, M.A.; van Pieterson, M.; de Meij, T.; Groeneweg, M.; Norbruis, O.F.; Wolters, V.M.; van Wering, H.M.; Hojsak, I.; et al. First-Line Treatment with Infliximab versus Conventional Treatment in Children with Newly Diagnosed Moderate-to-Severe Crohn’s Disease: An Open-Label Multicentre Randomised Controlled Trial. Gut 2020, 71, 34–42. [Google Scholar] [CrossRef]
- Jongsma, M.M.E.; Winter, D.A.; Huynh, H.Q.; Norsa, L.; Hussey, S.; Kolho, K.-L.; Bronsky, J.; Assa, A.; Cohen, S.; Lev-Tzion, R.; et al. Infliximab in Young Paediatric IBD Patients: It Is All about the Dosing. Eur. J. Pediatr. 2020, 179, 1935–1944. [Google Scholar] [CrossRef] [PubMed]
- Church, P.C.; Ho, S.; Sharma, A.; Tomalty, D.; Frost, K.; Muise, A.; Walters, T.D.; Griffiths, A.M. Intensified Infliximab Induction Is Associated with Improved Response and Decreased Colectomy in Steroid-Refractory Paediatric Ulcerative Colitis. J. Crohn’s Colitis 2019, 13, 982–989. [Google Scholar] [CrossRef] [PubMed]
- van Hoeve, K.; Dreesen, E.; Hoffman, I.; Van Assche, G.; Ferrante, M.; Gils, A.; Vermeire, S. Adequate Infliximab Exposure During Induction Predicts Remission in Paediatric Patients with Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Schnell, A.; Schwarz, B.; Wahlbuhl, M.; Allabauer, I.; Hess, M.; Weber, S.; Werner, F.; Schmidt, H.; Rechenauer, T.; Siebenlist, G.; et al. Distribution and Cytokine Profile of Peripheral B Cell Subsets Is Perturbed in Pediatric IBD and Partially Restored During a Successful IFX Therapy. Inflamm. Bowel Dis. 2021, 27, 224–235. [Google Scholar] [CrossRef]
- Cheifetz, A.S.; Vande Casteele, N.; Wang, Z.; Dubinsky, M.C.; Papamichael, K. Higher Postinduction Infliximab Concentrations Are Associated with Favorable Clinical Outcomes in Pediatric Crohn’s Disease: A Post Hoc Analysis of the REACH Trial. AJG Am. J. Gastroenterol. 2023, 118, 485–490. [Google Scholar] [CrossRef]
- Lawrence, S.; Faytrouni, F.; Harris, R.E.; Irvine, M.; Carrion, E.; Scott, G.; Clarke, B.; Garrick, V.; Curtis, L.; Gervais, L.; et al. Optimized Infliximab Induction Predicts Better Long-Term Clinical and Biomarker Outcomes Compared to Standard Induction Dosing. J. Pediatr. Gastroenterol. Nutr. 2022, 75, 601–607. [Google Scholar] [CrossRef]
- Chung, A.; Carroll, M.; Almeida, P.; Petrova, A.; Isaac, D.; Mould, D.; Wine, E.; Huynh, H. Early Infliximab Clearance Predicts Remission in Children with Crohn’s Disease. Dig. Dis. Sci. 2022. [Google Scholar] [CrossRef]
- Kwon, Y.; Kim, E.-S.; Kim, Y.-Z.; Choe, Y.-H.; Kim, M.-J. Cytokine Profile at Diagnosis Affecting Trough Concentration of Infliximab in Pediatric Crohn’s Disease. Biomedicines 2022, 10, 2372. [Google Scholar] [CrossRef]
- Constant, B.D.; Khushal, S.; Jiang, J.; Bost, J.E.; Chaisson, E.; Conklin, L.S. Early Inflammatory Markers Are Associated with Inadequate Post-Induction Infliximab Trough in Pediatric Crohn’s Disease. J. Pediatr. Gastroenterol. Nutr. 2021, 72, 410–416. [Google Scholar] [CrossRef]
- Merras-Salmio, L.; Kolho, K.-L. Clinical Use of Infliximab Trough Levels and Antibodies to Infliximab in Pediatric Patients with Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 272. [Google Scholar] [CrossRef]
- Dave, M.B.; Dherai, A.J.; Desai, D.C.; Mould, D.R.; Ashavaid, T.F. Optimization of Infliximab Therapy in Inflammatory Bowel Disease Using a Dashboard Approach-an Indian Experience. Eur. J. Clin. Pharmacol. 2021, 77, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Curci, D.; Lucafò, M.; Cifù, A.; Fabris, M.; Bramuzzo, M.; Martelossi, S.; Franca, R.; Decorti, G.; Stocco, G. Pharmacogenetic Variants of Infliximab Response in Young Patients with Inflammatory Bowel Disease. Clin. Transl. Sci. 2021, 14, 2184–2192. [Google Scholar] [CrossRef] [PubMed]
- Clarkston, K.; Tsai, Y.-T.; Jackson, K.; Rosen, M.J.; Denson, L.A.; Minar, P. Development of Infliximab Target Concentrations During Induction in Pediatric Crohn Disease Patients. J. Pediatr. Gastroenterol. Nutr. 2019, 69, 68–74. [Google Scholar] [CrossRef] [PubMed]
- El-Matary, W.; Walters, T.D.; Huynh, H.Q.; deBruyn, J.; Mack, D.R.; Jacobson, K.; Sherlock, M.E.; Church, P.; Wine, E.; Carroll, M.W.; et al. Higher Postinduction Infliximab Serum Trough Levels Are Associated with Healing of Fistulizing Perianal Crohn’s Disease in Children. Inflamm. Bowel Dis. 2019, 25, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Stein, R.; Lee, D.; Leonard, M.B.; Thayu, M.; Denson, L.A.; Chuang, E.; Herskovitz, R.; Kerbowski, T.; Baldassano, R.N. Serum Infliximab, Antidrug Antibodies, and Tumor Necrosis Factor Predict Sustained Response in Pediatric Crohn’s Disease. Inflamm. Bowel Dis. 2016, 22, 1370–1377. [Google Scholar] [CrossRef]
- Drobne, D.; Kurent, T.; Golob, S.; Svegl, P.; Rajar, P.; Terzic, S.; Kozelj, M.; Novak, G.; Smrekar, N.; Plut, S.; et al. Success and Safety of High Infliximab Trough Levels in Inflammatory Bowel Disease. Scand. J. Gastroenterol. 2018, 53, 940–946. [Google Scholar] [CrossRef]
- Courbette, O.; Aupiais, C.; Viala, J.; Hugot, J.-P.; Roblin, X.; Candon, S.; Louveau, B.; Chatenoud, L.; Martinez-Vinson, C. Trough Levels of Infliximab at Week 6 Are Predictive of Remission at Week 14 in Pediatric Crohn’s Disease. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 310–317. [Google Scholar] [CrossRef]
- Crombé, V.; Salleron, J.; Savoye, G.; Dupas, J.-L.; Vernier-Massouille, G.; Lerebours, E.; Cortot, A.; Merle, V.; Vasseur, F.; Turck, D.; et al. Long-Term Outcome of Treatment with Infliximab in Pediatric-Onset Crohn’s Disease: A Population-Based Study. Inflamm. Bowel Dis. 2011, 17, 2144–2152. [Google Scholar] [CrossRef]
- Assa, A.; Matar, M.; Turner, D.; Broide, E.; Weiss, B.; Ledder, O.; Guz-Mark, A.; Rinawi, F.; Cohen, S.; Topf-Olivestone, C.; et al. Proactive Monitoring of Adalimumab Trough Concentration Associated with Increased Clinical Remission in Children with Crohn’s Disease Compared With Reactive Monitoring. Gastroenterology 2019, 157, 985–996.e2. [Google Scholar] [CrossRef]
- Matar, M.; Shamir, R.; Turner, D.; Broide, E.; Weiss, B.; Ledder, O.; Guz-Mark, A.; Rinawi, F.; Cohen, S.; Topf-Olivestone, C.; et al. Combination Therapy of Adalimumab with an Immunomodulator Is Not More Effective Than Adalimumab Monotherapy in Children with Crohn’s Disease: A Post Hoc Analysis of the PAILOT Randomized Controlled Trial. Inflamm. Bowel Dis. 2020, 26, 1627–1635. [Google Scholar] [CrossRef]
- Dubinsky, M.C.; Rosh, J.; Faubion, W.A.; Kierkus, J.; Ruemmele, F.; Hyams, J.S.; Eichner, S.; Li, Y.; Huang, B.; Mostafa, N.M.; et al. Efficacy and Safety of Escalation of Adalimumab Therapy to Weekly Dosing in Pediatric Patients with Crohn’s Disease. Inflamm. Bowel Dis. 2016, 22, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Payen, E.; Neuraz, A.; Zenzeri, L.; Talbotec, C.; Abi Nader, E.; Chatenoud, L.; Chhun, S.; Goulet, O.; Ruemmele, F.M.; Pigneur, B. Adalimumab Therapy in Pediatric Crohn Disease: A 2-Year Follow-Up Comparing “Top-Down” and “Step-Up” Strategies. J. Pediatr. Gastroenterol. Nutr. 2023, 76, 166. [Google Scholar] [CrossRef] [PubMed]
- Lucafò, M.; Curci, D.; Bramuzzo, M.; Alvisi, P.; Martelossi, S.; Silvestri, T.; Guastalla, V.; Labriola, F.; Stocco, G.; Decorti, G. Serum Adalimumab Levels After Induction Are Associated with Long-Term Remission in Children with Inflammatory Bowel Disease. Front. Pediatr. 2021, 9, 244. [Google Scholar] [CrossRef] [PubMed]
- Hyams, J.S.; Dubinsky, M.C.; Baldassano, R.N.; Colletti, R.B.; Cucchiara, S.; Escher, J.; Faubion, W.; Fell, J.; Gold, B.D.; Griffiths, A.; et al. Infliximab Is Not Associated with Increased Risk of Malignancy or Hemophagocytic Lymphohistiocytosis in Pediatric Patients with Inflammatory Bowel Disease. Gastroenterology 2017, 152, 1901–1914.e3. [Google Scholar] [CrossRef] [PubMed]
- Atia, O.; Shavit-Brunschwig, Z.; Mould, D.R.; Stein, R.; Matar, M.; Aloi, M.; Ledder, O.; Focht, G.; Urlep, D.; Hyams, J.; et al. Outcomes, Dosing, and Predictors of Vedolizumab Treatment in Children with Inflammatory Bowel Disease (VEDOKIDS): A Prospective, Multicentre Cohort Study. Lancet Gastroenterol. Hepatol. 2023, 8, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, R.C.; Yarur, A.; Jossen, J.; Phan, B.L.; Chefitz, E.; Sehgal, P.; Kamal, K.; Bruss, A.; Beniwal-Patel, P.; Fox, C.; et al. Higher Trough Vedolizumab Concentrations During Maintenance Therapy Are Associated with Corticosteroid-Free Remission in Inflammatory Bowel Disease. J. Crohn’s Colitis 2019, 13, 963–969. [Google Scholar] [CrossRef]
- Colman, R.J.; Mizuno, T.; Fukushima, K.; Haslam, D.B.; Hyams, J.S.; Boyle, B.; Noe, J.D.; D’Haens, G.R.; Van Limbergen, J.; Chun, K.; et al. Real World Population Pharmacokinetic Study in Children and Young Adults with Inflammatory Bowel Disease Discovers Novel Blood and Stool Microbial Predictors of Vedolizumab Clearance. Aliment. Pharmacol. Ther. 2023, 57, 524–539. [Google Scholar] [CrossRef]
- Jansson, S.; Malham, M.; Paerregaard, A.; Jakobsen, C.; Wewer, V. Extraintestinal Manifestations Are Associated with Disease Severity in Pediatric Onset Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2020, 71, 40–45. [Google Scholar] [CrossRef]
- D’Arcangelo, G.; Distante, M.; Raso, T.; Rossetti, D.; Catassi, G.; Aloi, M. Safety of Biological Therapy in Children with Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2021, 72, 736–741. [Google Scholar] [CrossRef]
- Russi, L.; Scharl, M.; Rogler, G.; Biedermann, L. The Efficacy and Safety of Golimumab as Third- or Fourth-Line Anti-TNF Therapy in Patients with Refractory Crohn’s Disease: A Case Series. IID 2017, 2, 131–138. [Google Scholar] [CrossRef]
- Conrad, M.A.; Kelsen, J.R. The Treatment of Pediatric Inflammatory Bowel Disease with Biologic Therapies. Curr. Gastroenterol. Rep. 2020, 22, 36. [Google Scholar] [CrossRef] [PubMed]
- Roda, G.; Jharap, B.; Neeraj, N.; Colombel, J.-F. Loss of Response to Anti-TNFs: Definition, Epidemiology, and Management. Clin. Transl. Gastroenterol. 2016, 7, e135. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-Y.; Park, H.-M.; Lee, M.-Y.; Jeon, J.-Y.; Yoo, H.-J.; Ye, B.D. Real-World Incidence of Suboptimal Response to Anti-Tumor Necrosis Factor Therapy for Ulcerative Colitis: A Nationwide Population-Based Study. Gut Liver 2021, 15, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Ricciuto, A.; Lewis, A.; D’Amico, F.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W.; et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target Strategies in IBD. Gastroenterology 2021, 160, 1570–1583. [Google Scholar] [CrossRef] [PubMed]
- Feuerstein, J.D.; Nguyen, G.C.; Kupfer, S.S.; Falck-Ytter, Y.; Singh, S.; American Gastroenterological Association Institute Clinical Guidelines Committee. American Gastroenterological Association Institute Guideline on Therapeutic Drug Monitoring in Inflammatory Bowel Disease. Gastroenterology 2017, 153, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Gomollón, F.; Dignass, A.; Annese, V.; Tilg, H.; Van Assche, G.; Lindsay, J.O.; Peyrin-Biroulet, L.; Cullen, G.J.; Daperno, M.; Kucharzik, T.; et al. 3rd European Evidence-Based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 1: Diagnosis and Medical Management. J. Crohns Colitis 2017, 11, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, G.R.; Loftus, E.V.; Isaacs, K.L.; Regueiro, M.D.; Gerson, L.B.; Sands, B.E. ACG Clinical Guideline: Management of Crohn’s Disease in Adults. Am. J. Gastroenterol. 2018, 113, 481–517. [Google Scholar] [CrossRef]
- Mitrev, N.; Vande Casteele, N.; Seow, C.H.; Andrews, J.M.; Connor, S.J.; Moore, G.T.; Barclay, M.; Begun, J.; Bryant, R.; Chan, W.; et al. Review Article: Consensus Statements on Therapeutic Drug Monitoring of Anti-Tumour Necrosis Factor Therapy in Inflammatory Bowel Diseases. Aliment. Pharmacol. Ther. 2017, 46, 1037–1053. [Google Scholar] [CrossRef]
- Steinhart, A.H.; Panaccione, R.; Targownik, L.; Bressler, B.; Khanna, R.; Marshall, J.K.; Afif, W.; Bernstein, C.N.; Bitton, A.; Borgaonkar, M.; et al. Clinical Practice Guideline for the Medical Management of Perianal Fistulizing Crohn’s Disease: The Toronto Consensus. Inflamm. Bowel Dis. 2019, 25, 1–13. [Google Scholar] [CrossRef]
- Cheifetz, A.S.; Abreu, M.T.; Afif, W.; Cross, R.K.; Dubinsky, M.C.; Loftus, E.V.; Osterman, M.T.; Saroufim, A.; Siegel, C.A.; Yarur, A.J.; et al. A Comprehensive Literature Review and Expert Consensus Statement on Therapeutic Drug Monitoring of Biologics in Inflammatory Bowel Disease. Am. J. Gastroenterol. 2021, 116, 2014–2025. [Google Scholar] [CrossRef]
- Syversen, S.W.; Jørgensen, K.K.; Goll, G.L.; Brun, M.K.; Sandanger, Ø.; Bjørlykke, K.H.; Sexton, J.; Olsen, I.C.; Gehin, J.E.; Warren, D.J.; et al. Effect of Therapeutic Drug Monitoring vs Standard Therapy During Maintenance Infliximab Therapy on Disease Control in Patients with Immune-Mediated Inflammatory Diseases: A Randomized Clinical Trial. JAMA 2021, 326, 2375–2384. [Google Scholar] [CrossRef]
- Yarur, A.J.; Kanagala, V.; Stein, D.J.; Czul, F.; Quintero, M.A.; Agrawal, D.; Patel, A.; Best, K.; Fox, C.; Idstein, K.; et al. Higher Infliximab Trough Levels Are Associated with Perianal Fistula Healing in Patients with Crohn’s Disease. Aliment. Pharmacol. Ther. 2017, 45, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Ordás, I.; Mould, D.R.; Feagan, B.G.; Sandborn, W.J. Anti-TNF Monoclonal Antibodies in Inflammatory Bowel Disease: Pharmacokinetics-Based Dosing Paradigms. Clin. Pharmacol. Ther. 2012, 91, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Yarur, A.J.; Jain, A.; Sussman, D.A.; Barkin, J.S.; Quintero, M.A.; Princen, F.; Kirkland, R.; Deshpande, A.R.; Singh, S.; Abreu, M.T. The Association of Tissue Anti-TNF Drug Levels with Serological and Endoscopic Disease Activity in Inflammatory Bowel Disease: The ATLAS Study. Gut 2016, 65, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Brandse, J.F.; van den Brink, G.R.; Wildenberg, M.E.; van der Kleij, D.; Rispens, T.; Jansen, J.M.; Mathôt, R.A.; Ponsioen, C.Y.; Löwenberg, M.; D’Haens, G.R.A.M. Loss of Infliximab into Feces Is Associated With Lack of Response to Therapy in Patients With Severe Ulcerative Colitis. Gastroenterology 2015, 149, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Winter, D.A.; Joosse, M.E.; de Wildt, S.N.; Taminiau, J.; de Ridder, L.; Escher, J.C. Pharmacokinetics, Pharmacodynamics, and Immunogenicity of Infliximab in Pediatric Inflammatory Bowel Disease: A Systematic Review and Revised Dosing Considerations. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 763–776. [Google Scholar] [CrossRef]
- Turner, D.; Ruemmele, F.M.; Orlanski-Meyer, E.; Griffiths, A.M.; De Carpi, J.M.; Bronsky, J.; Veres, G.; Aloi, M.; Strisciuglio, C.; Braegger, C.P.; et al. Management of Paediatric Ulcerative Colitis, Part 2: Acute Severe Colitis—An Evidence-Based Consensus Guideline from the European Crohn’s and Colitis Organization and the European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 292–310. [Google Scholar] [CrossRef]
- Desai, D.C.; Dherai, A.J.; Strik, A.; Mould, D.R. Personalized Dosing of Infliximab in Patients with Inflammatory Bowel Disease Using a Bayesian Approach: A Next Step in Therapeutic Drug Monitoring. J. Clin. Pharmacol. 2023, 63, 480–489. [Google Scholar] [CrossRef]
- Irving, P.M.; Gecse, K.B. Optimizing Therapies Using Therapeutic Drug Monitoring: Current Strategies and Future Perspectives. Gastroenterology 2022, 162, 1512–1524. [Google Scholar] [CrossRef]
- Bodini, G.; Giannini, E.G.; Savarino, V.; Del Nero, L.; Pellegatta, G.; De Maria, C.; Baldissarro, I.; Savarino, E. Adalimumab Trough Serum Levels and Anti-Adalimumab Antibodies in the Long-Term Clinical Outcome of Patients with Crohn’s Disease. Scand. J. Gastroenterol. 2016, 51, 1081–1086. [Google Scholar] [CrossRef]
- Hyams, J.S.; Chan, D.; Adedokun, O.J.; Padgett, L.; Turner, D.; Griffiths, A.; Veereman, G.; Heyman, M.B.; Rosh, J.R.; Wahbeh, G.; et al. Subcutaneous Golimumab in Pediatric Ulcerative Colitis: Pharmacokinetics and Clinical Benefit. Inflamm. Bowel Dis. 2017, 23, 2227–2237. [Google Scholar] [CrossRef] [PubMed]
- Dreesen, E.; Verstockt, B.; Bian, S.; de Bruyn, M.; Compernolle, G.; Tops, S.; Noman, M.; Van Assche, G.; Ferrante, M.; Gils, A.; et al. Evidence to Support Monitoring of Vedolizumab Trough Concentrations in Patients with Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2018, 16, 1937–1946.e8. [Google Scholar] [CrossRef] [PubMed]
- Mechie, N.-C.; Burmester, M.; Mavropoulou, E.; Pilavakis, Y.; Kunsch, S.; Ellenrieder, V.; Amanzada, A. Evaluation of Ustekinumab Trough Levels during Induction and Maintenance Therapy with Regard to Disease Activity Status in Difficult to Treat Crohn Disease Patients. Medicine 2021, 100, e25111. [Google Scholar] [CrossRef]
- Baldassano, R.N.; Han, P.D.; Jeshion, W.C.; Berlin, J.A.; Piccoli, D.A.; Lautenbach, E.; Mick, R.; Lichtenstein, G.R. Pediatric Crohn’s Disease: Risk Factors for Postoperative Recurrence. Am. J. Gastroenterol. 2001, 96, 2169–2176. [Google Scholar] [CrossRef]
- Amil-Dias, J.; Kolacek, S.; Turner, D.; Pærregaard, A.; Rintala, R.; Afzal, N.A.; Karolewska-Bochenek, K.; Bronsky, J.; Chong, S.; Fell, J.; et al. Surgical Management of Crohn Disease in Children: Guidelines from the Paediatric IBD Porto Group of ESPGHAN. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 818–835. [Google Scholar] [CrossRef] [PubMed]
- Regueiro, M.; Feagan, B.G.; Zou, B.; Johanns, J.; Blank, M.A.; Chevrier, M.; Plevy, S.; Popp, J.; Cornillie, F.J.; Lukas, M.; et al. Infliximab Reduces Endoscopic, but Not Clinical, Recurrence of Crohn’s Disease After Ileocolonic Resection. Gastroenterology 2016, 150, 1568–1578. [Google Scholar] [CrossRef] [PubMed]
- Regueiro, M.; Schraut, W.; Baidoo, L.; Kip, K.E.; Sepulveda, A.R.; Pesci, M.; Harrison, J.; Plevy, S.E. Infliximab Prevents Crohn’s Disease Recurrence after Ileal Resection. Gastroenterology 2009, 136, 441–450.e1. [Google Scholar] [CrossRef]
- De Cruz, P.; Kamm, M.A.; Hamilton, A.L.; Ritchie, K.J.; Krejany, E.O.; Gorelik, A.; Liew, D.; Prideaux, L.; Lawrance, I.C.; Andrews, J.M.; et al. Efficacy of Thiopurines and Adalimumab in Preventing Crohn’s Disease Recurrence in High-Risk Patients—A POCER Study Analysis. Aliment. Pharmacol. Ther. 2015, 42, 867–879. [Google Scholar] [CrossRef]
- Bouhuys, M.; Lexmond, W.S.; Dijkstra, G.; Lobatón, T.; Louis, E.; van Biervliet, S.; Groen, H.; Guardiola, J.; Rheenen, P. van Efficacy of Anti-TNF Dosing Interval Lengthening in Adolescents and Young Adults with Inflammatory Bowel Disease in Sustained Remission (FREE-Study): Protocol for a Partially Randomised Patient Preference Trial. BMJ Open 2021, 11, e054154. [Google Scholar] [CrossRef]
| Study | N TNF | Age (Yrs) (CD, UC) | m % (CD, UC) | Study Type | Observation Period | Country | IBD Type | Biologic | Time from Diagnosis to Biologic | Comparison-Group | Post-Assessments | Outcomes | Results |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Claßen et al., 2022 [1] | 487 | 11.9 | 59.1 | Retrospective registry | 2004–2020 | Germany, Austria, Switzerland | CD, CU, IBDu | all | 19 months | First-line vs. Second line | Laboratory markers, clinical scores, side effects, treatment failure | Patients with CD significantly benefitted from early treatment, with lower clinical scores, fewer EIMs and lower risk for treatment failure | |
| D’Arcangelo et al., 2021 [27] | 185 | 13 | 58 | Retrospective, observational cohort Single-center | 2012–2020 | Italy | CD, UC, IBDu | IFX, ADL, UST, VEDO | 2 yrs | Immediate and delayed AEs | 32.8% biologic-related Aes 10% immediate reactions, 45% delayed 14% treatment discontinuation because of AEs | ||
| Kaplan et al., 2023 [4] | 17,649 | Retrospective, observational cohort | 2006–2016 | USA | CD, UC | all | Use, discontinuation | 43% of pediatric IBD patients treated with biologic, more likely for CD, discontinuation significantly higher in UC | |||||
| TNF-α inhibitors | |||||||||||||
| Bronsky et al., 2022 [13] | 62 | 11.64–16.27 | 55–68 | Prospective observational cohort | 2013–2017 | Czech Republic | CD | IFX, ADL | 0.6–1.04 yrs | IFX vs. ADL | Up to 24 months | Treatment escalation Non-response Serious AEs | No difference between IFX and ADL in efficacy and safety |
| Lee et al., 2015 [18] | 52 | 13.9 | 46 | Observational cohort | Canada, USA | CD | IFX (1xADL) | 0.7 yr | EEN (n = 22), PEN (n = 16) | 8 weeks | PCDAI, QoL, mucosal healing via FCP | Clinical response: 64% PEN, 88% EEN, 84% TNFi Mucosal healing: PEN 14%, EEN 45%, TNFi 62% QoL not statistically significant | |
| Scarallo et al., 2021 [16] | 134 | 10.9, 10.3 | 65.4, 50 | retrospective, observational (two centers) | 2008–2018 | Italy | 78 (CD) 56 (UC) | IFX, ADL | Endoscopically assessed mucosal remission | Mucosal remission in 41% of CD patients and 53.6% of UC patients, histological remission in 33.3% of CD patients and 39.3% of UC patients | |||
| Boros et al., 2023 [26] | 32 | 15.2, 16.4 | 49 | Prospective, observational follow up Single-center | 2016–2018 | Hungary | CD, UC | TNFi | 1.4 yrs, 3 yrs | Healthy controls | 2 & 6 months | Body composition, health-related quality of life, physical activity | Body composition and physical activity significantly improved after 6 months and caught up to healthy controls, no change in health-related quality of life 58% of CD 37.5% of UC patients in remission |
| Kim et al., 2021 [28] | 84 | 15 | 74.1 | Retrospective single-center | 2000–2013 | Korea | CD | TNFi | Thiopurine treatment (N = 287) | Up to 13 yrs | Disease behavior evolution | Early treatment (within 3 months after diagnosis) was associated with lower risk of disease behavior progression | |
| Walters et al., 2014 [29] | 68 | 11.8 | 61 | Retrospective multicenter | 2008–2012 | North America | CD | IFX, ADL | Within 3 months | Immunomodulator (IM) (N = 68), no IM (N 68) | Steroid-free and surgery-free remission, growth | 85.3% in remission with TNFi, significantly more than other groups, growth improved in biologic group only | |
| Ley et al., 2022 [25] | 1007 | Retrospective multicenter | 1988–2011 | France | CD | TNFi | M = 8.8 yrs | Intestinal resection, disease progression, hospitalizations | Reduction in intestinal resection and disease progression, no change in hospitalization over time | ||||
| Choe et al., 2022 [30] | Pediatric + adult | Population-based | 2006–2015 | Korea | CD, UC | TNFi | TNFi prescription, fistulectomy, surgery | Lower odds of surgery in CD patients under TNFi therapy | |||||
| Kugathasan et al., 2017 [31] | 913 | Prospective inception cohort | 2008–2012 | USA, Canada (28 sites) | CD | TNFi | Disease complications | Early TNFi admission reduced risk for penetrating complications but not stricturing complications | |||||
| Kandavel et al., 2021 [32] | 27,321 | Retrospective cohort, multicenter | 2007–2018 | US, UK, Qatar | CD, UC, IBDu | TNFi | Use of corticosteroids | Appliance of TNFi within the first 120 days after diagnosis reduces risk for need of steroids later in CD not in UC | |||||
| Sherlock et al., 2021 [33] | 198 | 10.5 | 59.1 | Retrospective cohort, single-center | 2001–2015 | Canada | CD, UC, IBDu | 21.5 months | M = 47.8 | Biologic therapy associated with older age, higher PCDAI/ PUCAI hypoalbuminemia in UC and CD | |||
| Nuti et al., 2014 [34] | 78 | 15 | 63 | Single-center cohort | 2001–2011 | Italy | CD | IFX, ADL | 40.6 months | 1, 2, 3 yrs | Clinical activity (PCDAI), discontinuation, AEs | 81% continuation yr 1, 54% yr 2, 33% yr 3, no serious AEs | |
| Beukelmann et al., 2018 [35] | 6808 | 43 | Retrospective, cohort | US | IBD, JIA, PsA | TNFi | No TNFi use (N = 20,049) | Malignancies | TNFi use in combination with thiopurines increased the risk for malignancies | ||||
| Hradsky et al., 2021 [36] | 100 | 15 | 57–65 | Retrospective | CD | TNFi | Skin complications | After 2 yrs of treatment 35% of patients developed at least one skin complication | |||||
| Dolinger et al., 2022 [37] | 638 | Retrospective | IFX, ADL | 6 months | Skin reactions | 21% infliximab patients, 11% adalimumab patients | |||||||
| Baggett et al., 2022 [38] | 3794 | Retrospective | 2008–2020 | IFX, ADL, etanercept | Non-TNFi exposure | Incidence of psoriasis | Higher risk of psoriasis in patients treated with TNFi (highest in adalimumab) | ||||||
| Zvuloni et al., 2021 [39] | 135 | 12.9 | 56.3 | Retrospective, cohort single-center | 2015–2020 | Israel | CD, UC | IFX, ADL | MD = 1.7 yrs | Incidence of AEs | 37% of patients had AEs, psoriatiform rashes (45%), elevated transaminases (32%) and infusion reactions (13%) | ||
| Eindor-Abarbanel et al., 2022 [40] | 89 | 3.8 | 62.8 | Retrospective | 2005–2019 | United states | VEO IBD | TNFi | TNFi-naive | 1 yr | Disease course, dose, and dose interval of IFX | 39.5% of VEO IBD patients received TNFi, higher disease activity was associated with TNFi-treatment, clinical remission on first biologic agent in 61,8% | |
| Kerur et al., 2022 [41] | 294 | Retrospective, cohort Multicenter | 2008–2013 | North America | VEO IBD | IFX, ADL | 1, 3, 5 yrs | Utilization and durability of TNFi | 55% of patients treated with TNFi between 0–6 yrs old, durability 90% after one yr, 55% after 5 yrs, lower durability in UC und IBDu | ||||
| Kennedy et al., 2019 [42] | 219 pediatric | Adult + pediatric | 49 | Prospective, observational cohort, multicenter | 2013–2016 | UK, Korea, USA | CD | IFX, ADL | 2.3–3.3 yrs | 12 months | Disease activity, AEs, discontinuation, treatment failure, anti-drug antibodies | Low drug concentration the only predictor for primary non-response in week 14, and remission by week 54 62.8% ADAs in IFX, 28.5% in ADL predicted by suboptimal drug concentration in week 14 | |
| Rodriguez Azor et al., 2023 [43] | 30 | 11.3 | 70 | Prospective observational | 2015–2020 | CD | IFX, ADL | 9.9 months | M = 27.1 months | Clinical remission, mucosal healing, laboratory markers | 87.1% in clinical remission (wPCDAI), 83% achieved mucosal healing (MINI) | ||
| Salvador-Martín et al., 2023 [44] | 340 | 11.2 | 60.3 | Observational, multicenter | Spain | CD, UC, IBDu | IFX, ADL | 6.1 months | Responders vs. non-responders | 9 yrs | Treatmtent failure | Only in adults association of HLA polymorphisms and treatment failure | |
| Cohen et al., 2019 [45] | 234 | 13 | 54.2 | Retrospective, single-center | USA | CD, UC | IFX, ADL | With and without ADAs | ADAs | 24.8% developed ADAs, 48% of those underwent dose optimization and of those 54% had undetectable ADAs on follow-up, Patients switching to another agent were not more likely to develop ADAs | |||
| Colman et al., 2021 [46] | 89 | 12.2–17.7 | 58.7 | Retrospective cohort, single-center | 2014–2018 | USA | CD, UC, IBDu | IFX, ADL | With and without immunomodulator (IM) | 6, 12 months | Clinical and biochemical remission, discontinuation, ADAs | Significantly more patients in combination therapy with TNFi and IM were in remission after one yr than without IM (53.9% vs. 26.8%) Without IM ADAs were unlikely to reverse if titer > 329 ng/ml | |
| Sassine et al., 2022 [47] | 639 | 14 | 56 | Retrospective cohort study | 2009–2019 | lCD | TNFi | Clinical relapse | Use of TNFi reduced risk for relapse compared to immunomodulators | ||||
| Scarallo et al., 2021b [48] | 170 | 12 | 65.6, 46.7 | Retrospective Two centers | 2008–2018 | Italy | CD, UC | IFX, ADL | 1–1.5 | Endoscopic (mucosal and histological) remission | MH was achieved by 32 patients with CD (41%) and 30 patients with UC (53.6%); 26 patients with CD (33.3%) and 22 patients with UC (39.3%) achieved HH Withdrawal of TNFi associated with relapse | ||
| Weigl et al., 2023 [49] | 13 | 52 | Retrospective | Germany | CD | TNFi | No perioperative TNFi (N = 16) | Weight, height, disease activity, infections | Improvement of weight, height after ileocecal resection, significantly more improvement in disease activity in TNFi group, no increase in infections | ||||
| Infliximab | |||||||||||||
| deBruyn et al., 2018 [11] | 180 | 14.3 | 54.4 | Retrospective, multicenter | 2008–2012 | Canada | CD | IFX | 1.5 yrs | Discontinuation, dose optimization | Dose escalation occurred in 57.3% primarily due to loss of response Annual discontinuation 3.2% per yr | ||
| Kierkus et al., 2012 [17] | 66 | 14.1 | 43.9 | Prospective cohort | Poland | CD | IFX | 5.6 yrs | 2, 6, 10 weeks | Disease activity (clinical, laboratory & endoscopic) | 33% reached clinical remission, 28% non-responders, endoscopic improvement in week 10 | ||
| Luo et al., 2017 [19] | 13 | 11.7 | 46.2 | Prospective | China | CD | IFX | 12 months | EEN (n = 13) | 8 weeks | PCDAI, growth, AEs | Significantly higher percentage of clinical response, growth, and AEs in IFX group | |
| Hyams et al., 2012 [22] | 60 | 14.5 | 53.3 | Randomized | 2006–2010 | USA, Canada | UC | IFX | 1.4 yrs | Dosing interval 8 vs. 12 weeks | 8, 54 weeks | Clinical remission, AEs | Response at week 8 73.3%, overall remission rate at week 54 was 28.6%, no serious AEs |
| Bolia et al., 2019 [23] | 204 | 12 | 50 | Retrospective | 2005–2016 | Australia | UC | IFX | Colectomy rates | Reduction in colectomy rates after introduction of IFX | |||
| Jongsma et al., 2020 [50] | 50 | Multicenter open label randomized controlled trial | CD | IFX | Conventional treatment (N = 50) Steroids/EEN | 10, 52 weeks | Clinical and endoscopic remission | Higher percentage of patients in TNFi group achieved clinical (59%) and endoscopic remission (59%) at week 10, no significant difference in week 52, less treatment escalation needed in TNFi group at week 52 | |||||
| Jongsma et al., 2020b [51] | 2015 | 9.22 | 57 | Retrospective, case–control, multicenter | 2015–2019 | Europe, Canada | CD, UC, IBDu | IFX | Start IFX < 10 yrs of age vs. >10 yrs | 1 yr | Dosing, treatment intervals, trough levels, discontinuation, clinical remission | Equal amount of patients maintained therapy with IFX, younger patients on significantly higher dosage per kg, no effect of proactive drug monitoring | |
| Church et al., 2019 [52] | 125 | 14 | 54–70 | Retrospective Single-center | 2000–2015 | Canada | SR UC | IFX | 0.7 yrs | Standard vs. intensified induction | M = 1.4 yrs | Colectomy, remission, mucosal healing, AEs | Lower chance of colectomy in intensified regimen, other long-term outcomes are similar, 66% mucosal healing, AEs were rare |
| van Hoeve et al., 2019 [53] | 35 | retrospective | 2012–2018 | CD, UC | IFX | Remission at week 52 vs. non remission | 52 weeks | Clinical, biological remission, trough levels | Trough levels just before maintenance were the only predictors for clinical and biological remission | ||||
| Schnell et al., 2021 [54] | 42 | 13.3, 14.27 | 64.3 | Prospective, controlled, single-center | Germany | CD, UC | IFX | Healthy matched controls | 2, 6, 12 months | Biological remission, trough levels, cytokines | Higher trough levels in patients responding to treatment after 6 months, no effect of comedication with azathioprine Before treatment different cytokine profiles in IBD patients and healthy controls | ||
| Cheifetz et al., 2022 [55] | 103 | Post hoc REACH trial | CD | IFX | 10, 30, 54 weeks | Clinical remission | Higher infliximab concentration at week 10 was associated with clinical remission at week 10, and 30 | ||||||
| Lawrence et al., 2022 [56] | 140 | 14,1 | 54% | trial | 2016–2018 | Canada, Scotland | IFX | Standard induction vs. Optimization-based induction | 52 weeks | Clinical remission | Higher rates of clinical remission in optimized induction | ||
| Chung et al., 2022 [57] | 85 | Single-center retrospective | CD | IFX | Pharmacokinetic model of infliximab clearance, clinical remission | CRP and Albumin predict trough levels, induction trough levels predict remission | |||||||
| Kwon et al., 2022 [58] | 30 | 13.7 | 80 | Prospective | 2020–2021 | Korea | CD | IFX | Cytokines, trough levels, clinical and biochemical remission | Higher cytokine profiles in patients not achieving remission than in patients in remission, Cut-off for higher IFX doses TNFi concentration > 27.6 pg/ml | |||
| Constant et al., 2021 [59] | 55 | 13.1 | 69 | Retrospective single-center | 2013–2019 | USA | CD | IFX | 2, 8 weeks | Laboratory markers, IFX trough levels | Baseline laboratory markers (CRP, hypoalbuminemia, ESR) significantly associated with inadequate post-induction IFX trough concentration | ||
| Merras-Salmio et al., 2017 [60] | 146 | 14.8 | 57 | Retrospective, Single-center | 2003–2014 | Norway | CD, UC, IBDu | IFX | 1.8 | IFX trough levels, IFX ADAs | 63% of patients had loss of response, trough level significantly higher in patients in remission or ongoing therapy | ||
| Dave et al., 2021 [61] | 30 | 14.3–33.5 | 60 | Part prospective, part retrospective | 2017–2019 | India | CD, UC | IFX | 5 | IFX trough level, ADAs, evaluation of iDose software | iDose predicted 70% of patients’ trough concentrations correctly Of 11 patients managed with iDose, 8 achieved clinical remission, 2 showed partial response, one developed antibody | ||
| Curci et al., 2021 [62] | 76 | 14.7 | 47.4 | Prospective, two centers | Italy | CD, UC | IFX | 8, 22, 52 weeks | Clinical response | single-nucleotide polymorphisms (SNPs) rs396991 in FCGR3A variant had significantly lower trough levels, higher chance of ADAs and reduced clinical response | |||
| Clarkston et al., 2019 [63] | 72 | 13.6 | 65 | Prospective cohort, Single-center | 2014–2018 | US | CD | IFX | 51 days | 1 yr | Clinical response (wPCDAI), biological response, maintenance concentrations | Clinical response 64%, fecal calprotectin improvement in 54% | |
| El-Matary et al., 2019 [64] | 52 | 13.5 | 60.8 | Cohort, multicenter | 2014–2017 | Canada | fCD | IFX | 24 weeks | Fistula healing, trough levels | Correlation between pre-fourth infusion trough levels and fistula healing | ||
| Stein et al., 2016 [65] | 77 | 14.8 | 63 | Prospective single-center | 2006–2011 | US | CD | IFX | 1.66 yrs | 1 yr | Ongoing treatment with IFX CRP, ADAs, trough levels | 78% remained on IFX associated higher week 10 trough levels | |
| Drobne et al., 2018 [66] | 183 | 15.4–40 | 57 | Cohort, single-center | 2010–2015 | Slovenia | CD, UC, IBDu | IFX | 7.3, 5.7 | Trough level, CRP, fecal calprotectin | Higher trough levels were associated with lower levels of CRP and fecal calprotectin, no higher number of infections in higher trough levels | ||
| Courbette et al., 2020 [67] | 111 | 11.6 | 59 | Retrospective single-center | 2002–2014 | France | CD | IFX | 14 weeks | Clinical response, predictors for response, through levels | 38.7% in clinical remission plus 36% partial response Normal growth and normal albumin levels at first application associated with clinical response | ||
| Crombé et al., 2011 [68] | 120 | 14.5 | 45 | Retrospective registry | 1988–2004 | France | CD | IFX | 41 months | Short- and long-term efficacy, rate of resection surgery, AEs | 58% response rate, reduced risk for surgery in responder group, 13% of AEs that led to discontinuation | ||
| Adalimumab | |||||||||||||
| Cozijnsen et al., 2015 [5] | 53 | 11 | 49.1 | Observational cohort | Netherlands | CD | ADL | 3 yrs | MD = 12 months | Categorized cPCDAI, discontinuation/treatment failure | 64% remission after three months, maintained by 50% for two yrs, more IFX primary non-responders failed ADL than Patients with loss of response | ||
| Croft et al., 2021 [24] | 93 | Double blind nulticenter | 2014–2018 | 10 countries | UC | ADL | High dose induction vs. standard dose | 8, 52 weeks | Clinical remission, mucosal healing, AEs | Remission rates in ADL group better than in placebo groups, high dose induction had higher rate of remission in week 8 and week 52 | |||
| Assa et al., 2019 [69] | 78 | 14.3 | 71 | Randomized controlled trial | 2015–2018 | Israel | CD | ADL | Proactive vs. reactive drug monitoring | 8–72 weeks | Steroid-free remission, biologic remission, discontinuation | Significantly higher proportion of patients achieved steroid-free remission in the proactive group than in the reactive group (82% vs. 48%), as well as drug intensification (87% vs. 60%) | |
| Matar et al., 2020 [70] | 78 | 14.3 | 71 | Randomized controlled trial multicenter | 2015–2018 | Israel | CD | ADL | With and without immunomodulator (IM) | Sustained steroid-free remission, laboratory markers, trough levels, ADAs, AEs | No difference in steroid-free remission between groups with and without IM (73% vs. 63%), or laboratory markers, trough levels, ADAs, occurrence of AEs | ||
| Dubinsky et al., 2016 [71] | 188 | 51, 57 | Randomized controlled trial multicenter | 8 countries | CD | ADL | 3 yrs | High dose, low dose weekly | 4, 26, 52 weeks | Remission, response rate, AEs | Significantly higher proportion of patients in high dose group responded (31.4% vs. 18.8%) and achieved remission (57.1% vs. 47.9%), same rate of AEs | ||
| Payen et al., 2023 [72] | 120 | 2008–2019 | CD | ADL | Top-down vs. step-up | 12, 24 months | Steroid -, EEN-free remission, clinical remission | Top-down strategy more effective, higher serum levels of ADL, no serious AEs | |||||
| Lucafò et al., 2021 [73] | 32 | 14.9 | 62.5 | Retrospective cross sectional multicenter | 2013–2019 | Italy | CD, UC | ADL | 41.73 | 4, 52, 82 weeks | Disease activity (PUCAI, PCDAI), trough levels | Around 50% remission rate, higher trough levels in patients with sustained clinical remission | |
| Golimumab | |||||||||||||
| Hyams et al., 2017 [74] | 35 | 15 | Open-label multicenter | 2014–2015 | North America, Europe, Israel | UC | GOL | 15 yrs | 2, 4, 6 weeks | Serum concentration, clinical outcomes, AEs | 60% clinical response, 34% clinical remission, and 54% mucosal healing, no safety concerns | ||
| Vedolizumab | |||||||||||||
| Garcia Romero et al., 2021 [7] | 42 | 12.6 | 52.4 | Retrospective, multicenter | 2017–2019 | Spain | CD UC | VEDO | 2.6 yrs (CD), 4.1 yrs (UC) | 14, 30, 52 weeks | Laboratory markers, activity indices, AEs | 52.4% overall remission rate at week 14, more in UC, 84.5% remained remission in week 52 | |
| Atia et al., 2023 [75] | 142 | 13.6 | 46% | Multicenter, prospective cohort, multicenter | 2016–2022 | 6 countries | CD, UC, IBDu | VEDO | 14 weeks | Steroid-free -/EEN-free remission | 42% UC in remission under vedolizumab, 32% CD, optimal drug concentration at week 14—> 7µg/ml | ||
| Ungaro et al., 2019 [76] | 22 pediatric | adult + pediatric | Cross-sectional, two centers | USA | CD, UC | VEDO | Clinical -, steroid-free -, biochemical remission, drug concentration | Vedolizumab concentration > 11.5 µg/mL was associated with steroid free and biochemical remission | |||||
| Colman et al., 2023 [77] | 74 | 16 | 51 | Prospective observational | 2014–2019 | USA | CD, UC, IBDu | VEDO | 33 months | Pharmacokinetic model, clinical remission, through levels | Final model includes weight, erythrocyte sedimentation rate, and hypoalbuminemia | ||
| Ustekinumab | |||||||||||||
| Yerushalmy-Feler et al., 2022 [8] | 69 | 15.8 | Retrospective, Multicenter | Europe | CD | UST | 4.3 yrs | 3 months | Clinical remission, CRP, fecal Calprotectin, endoscopic, histological healing | Reduction in inflammatory markers, 16% endoscopic, 13% histological mucosal healing | |||
| Dhaliwal et al., 2021 [9] | 25 | 14.8 | 28 | Prospective, multicenter | 2018–2019 | Canada | UC | UST | 2.3 yrs | 26, 52 weeks | Steroid-free remission, PUCAI, endoscopic remission, AEs | 69% steroid free remission, significantly more of whom only failed TNFi treatment before (instead of TNFi and VEDO also) | |
| Dayan et al., 2019 [10] | 52 | 16.8 | 50, 20 | Observational cohort | 2014–2018 | USA | CD, UC, IBDu | UST | 4.9 yrs (CD) and 1.8 yrs (UC/IBDu) | 52 weeks | Steroid-free remission, clinical and biomarker remission | 75% maintained on UST after one yr, 50% of bio-exposed and 90% of bio-naïve in steroid free remission | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Claßen, M.; Hoerning, A. Current Role of Monoclonal Antibody Therapy in Pediatric IBD: A Special Focus on Therapeutic Drug Monitoring and Treat-to-Target Strategies. Children 2023, 10, 634. https://doi.org/10.3390/children10040634
Claßen M, Hoerning A. Current Role of Monoclonal Antibody Therapy in Pediatric IBD: A Special Focus on Therapeutic Drug Monitoring and Treat-to-Target Strategies. Children. 2023; 10(4):634. https://doi.org/10.3390/children10040634
Chicago/Turabian StyleClaßen, Merle, and André Hoerning. 2023. "Current Role of Monoclonal Antibody Therapy in Pediatric IBD: A Special Focus on Therapeutic Drug Monitoring and Treat-to-Target Strategies" Children 10, no. 4: 634. https://doi.org/10.3390/children10040634
APA StyleClaßen, M., & Hoerning, A. (2023). Current Role of Monoclonal Antibody Therapy in Pediatric IBD: A Special Focus on Therapeutic Drug Monitoring and Treat-to-Target Strategies. Children, 10(4), 634. https://doi.org/10.3390/children10040634





