Abstract
The diagnosis of Kawasaki disease (KD) is challenging and often delayed mainly in case of young infants and in presence of an incomplete disease and atypical features. Facial nerve palsy is one of the rare neurologic symptoms of KD, associated with a higher incidence of coronary arteries lesions and may be an indicator of a more severe disease. Here, we describe a case of lower motor neuron facial nerve palsy complicating KD and perform an extensive literature review to better characterize clinical features and treatment of patients with KD-associated facial nerve palsy. The patient was diagnosed at the sixth day of disease and presented extensive coronary artery lesions. A prompt treatment with intravenous immunoglobulins, aspirin and steroids obtained a good clinical and laboratory response, with resolution of facial nerve palsy and improvement of coronary lesions. The incidence of facial nerve palsy is 0.9–1.3%; it is often unilateral, transient, more frequent on the left and seemingly associated with coronary impairment. Our literature review showed coronary artery involvement in the majority of reported cases (27/35, 77%) of KD with facial nerve palsy. Unexplained facial nerve palsy in young children with a prolonged febrile illness should prompt consideration of echocardiography to exclude KD and start the appropriate treatment.
1. Introduction
Kawasaki disease (KD) is an acute, self-limited heterogeneous disease of unknown etiology that predominantly affects infants and children less than 5 years of age [1]. No diagnostic test is currently available for this condition, and typical or classic KD is diagnosed on a clinical basis in presence of specific criteria and after other similar clinical entities have been excluded. Classic KD is characterized by the presence of ≥5 days of fever and ≥4 of the following main clinical features: bilateral non-exudative conjunctivitis, erythema of lips and oral mucosa, changes in the extremities, skin rash, and cervical lymphadenopathy [1]. Patients who do not fulfill complete diagnostic criteria for KD are often referred to as atypical or incomplete KD [1,2].
The most feared complication of KD is the development of coronary artery lesions (CALs) that make KD the most common cause of acquired cardiac disorder in children [3]. In other cases, patients present clinical manifestations not included in the diagnostic criteria such as myocarditis, pericarditis, peri-bronchial and interstitial infiltrates on chest radiography, abdominal pain, behavioral changes and irritability, aseptic meningitis, and peripheral facial nerve palsy (FNP) [2]. This last manifestation is rare and nearly all available literature is limited to isolated case reports. We describe a young infant with KD presenting with FNP, lung nodules and severe CALs. We also performed an extensive literature review to better characterize clinical features and treatment of patients with KD-associated FNP.
2. Case Presentation
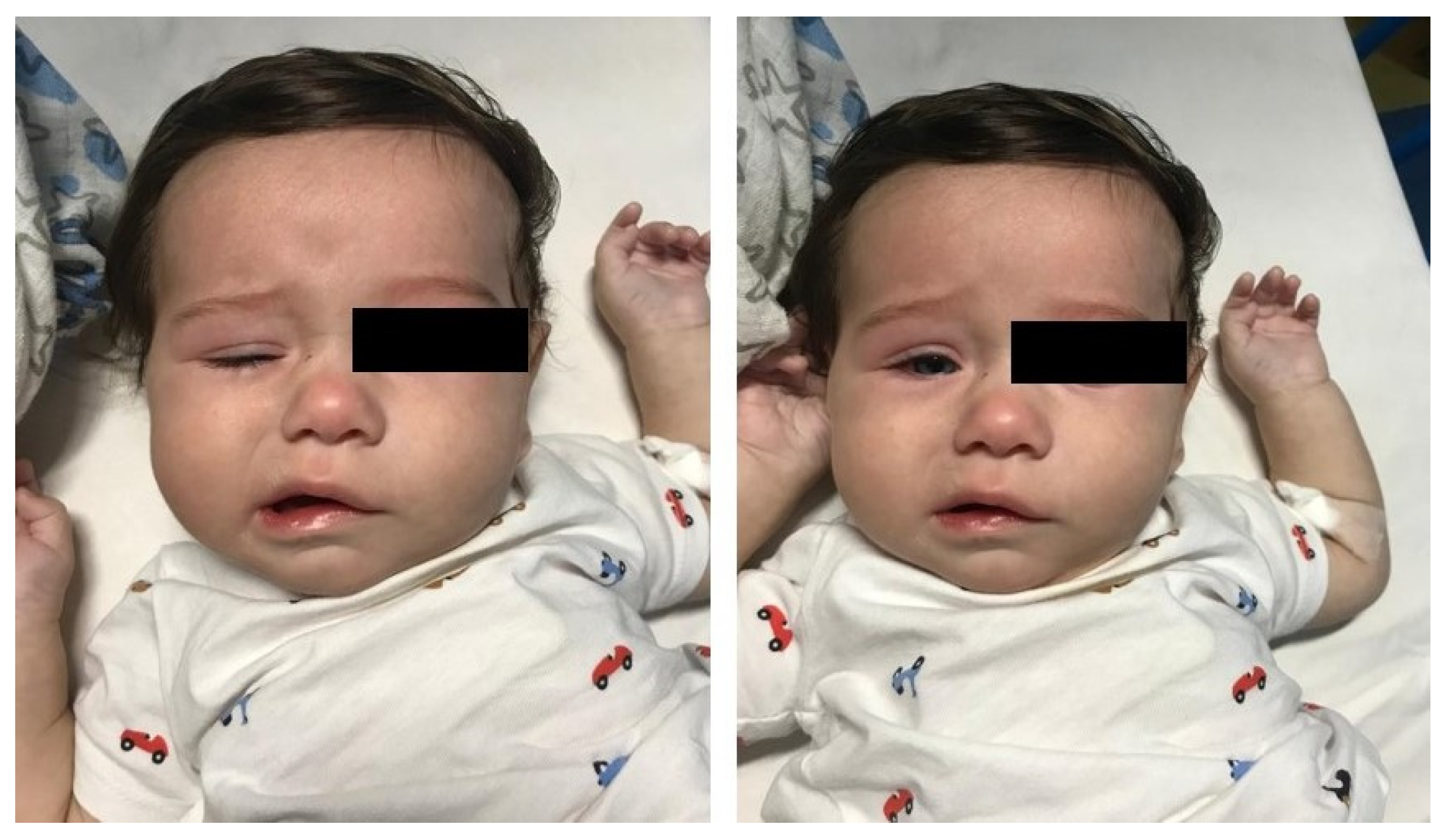
A 4-month-old boy presented with a 6-day history of fever treated with antibiotics. On day 4 of fever, bilateral conjunctival injection and a confluent, erythematous and papular rash of distal extremities appeared. As fever persisted, and given the abnormal facial movements noted by the parents, he presented to the Pediatric Emergency Department. At hospital admission, physical examination revealed impaired facial expression when he cried and food refusal. Neurologic assessment confirmed a lower motor-neuron-type palsy of the right facial nerve with no other neurological deficit (Figure 1).
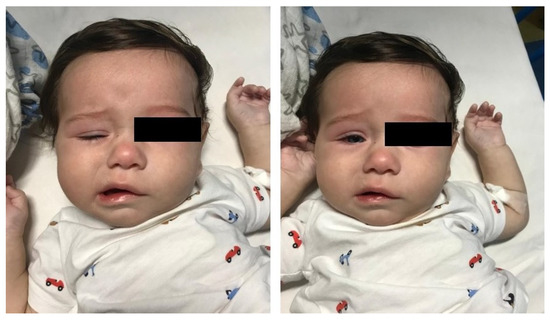
Figure 1.
Right facial nerve palsy in the 4-month-old infant. These clinical features were observed at hospital admission (closed eyes on the left; open eyes on the right).
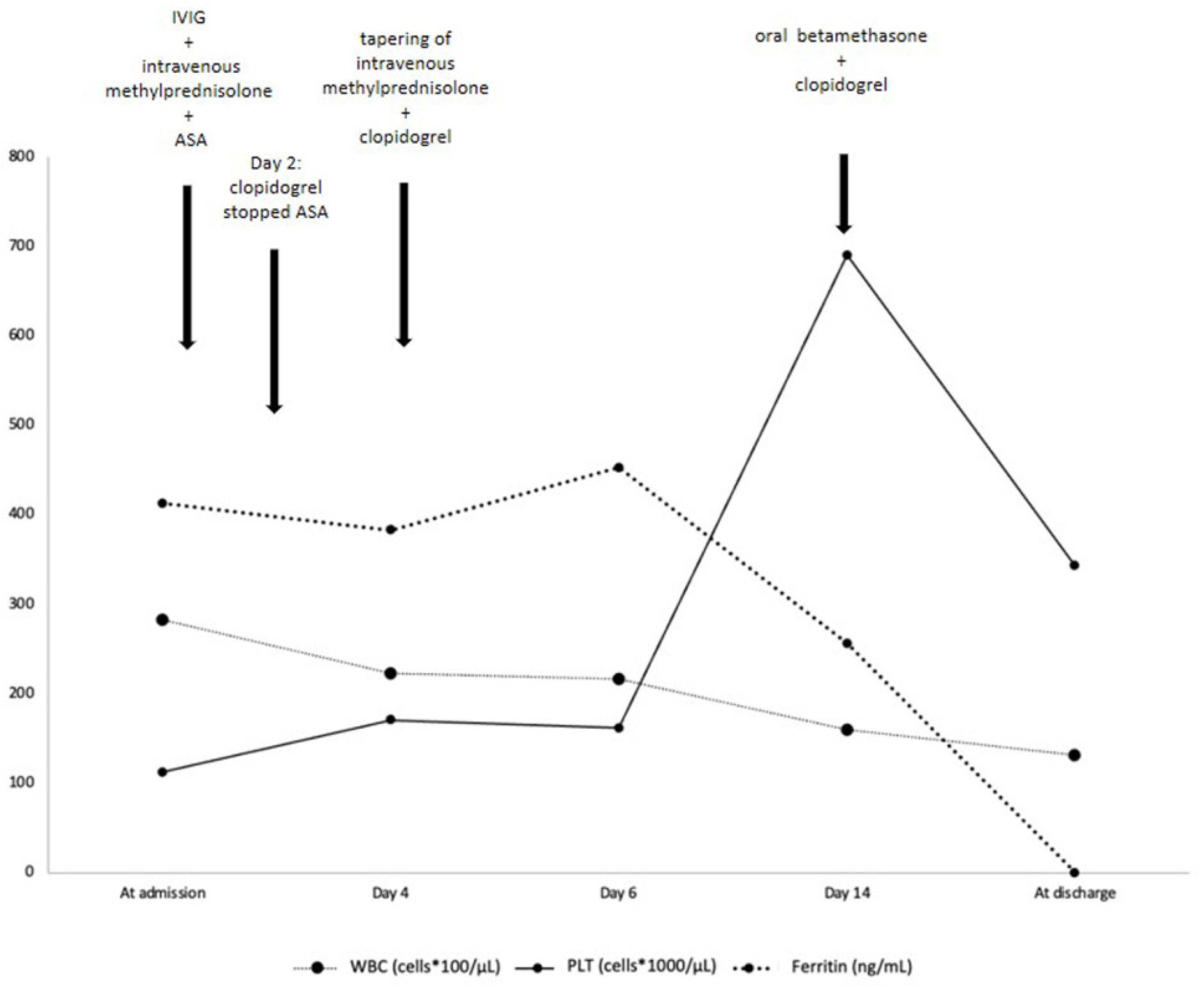
A head computed tomography (CT) showed no focal abnormalities. Brain magnetic resonance imaging and lumbar puncture were not performed. Blood tests at admission (Table 1 and Figure 2) showed leukocytosis, normocytic normochromic anemia, increased platelets count and C-reactive protein (CRP), and a slightly increased procalcitonin (PCT, 0.82 ng/mL, reference value < 0.5). He also presented elevated serum ferritin and reduced serum albumin levels (21 g/L). The most important markers of cardiac injury (cardiac myoglobin, creatine kinase-MB and brain natriuretic peptide, BNP) were within the normal range.

Table 1.
Laboratory findings during hospital stay.
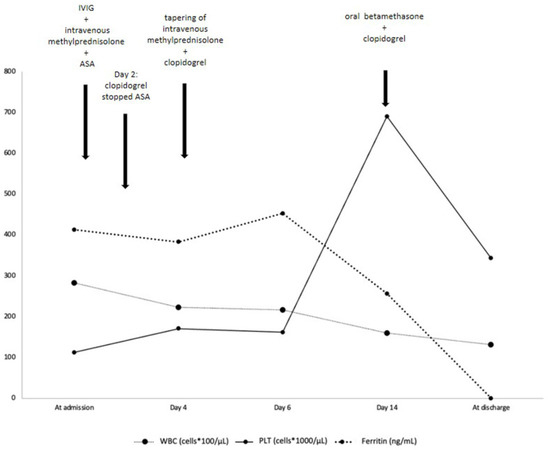
Figure 2.
Timeline evolution of most relevant laboratory findings and treatment. IVIG = intravenous immunoglobulin; ASA = acetylsalicylic acid; WBC= white blood cells; PLT = platelets. Ferritin (ng/mL) reference value < 320.
Serum cytokines analysis showed an increased tumor necrosis factor alpha (TNF-α), and a slight increase in interleukin (IL)-10, IL-1-β and IL-17A (Table 2).

Table 2.
Serum cytokine analysis.
Blood, throat, stool, and urine cultures were negative. Polymerase chain reaction for bacterial and viral pathogens on throat swab showed positivity for human rhinovirus/enterovirus and Parainfluenza virus 1. Chest X-ray was unremarkable. Serologic tests were negative for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), herpes simplex virus (HSV) types 1 and 2, enterovirus, adenovirus, Mycoplasma pneumoniae, Epstein–Barr virus and cytomegalovirus. An empiric antibiotic therapy with ceftazidime was started, while the laboratory results were pending.
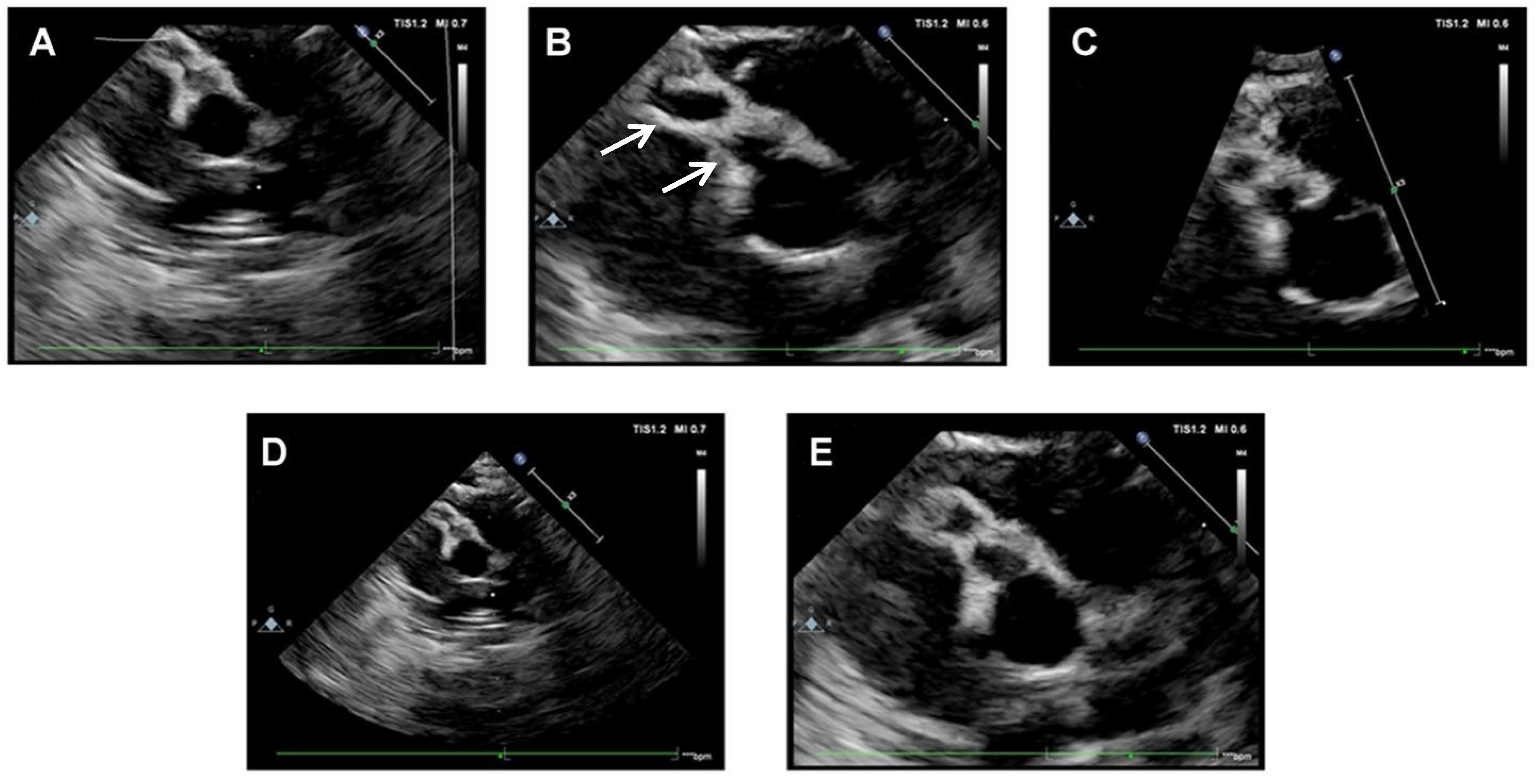
Based on patient’s medical history and clinical and laboratory findings, an incomplete KD was suspected. A cardiac assessment with electrocardiogram (ECG) and echocardiography was performed. At admission, echocardiography showed medium-size fusiform aneurysms of the right CA (4 mm, z-score 9), medium-size dilatation of the proximal right CA (3.3 mm, z-score 6) and small-size dilatation of the left CA (2.8 mm, z-score 3.9) (Figure 3).
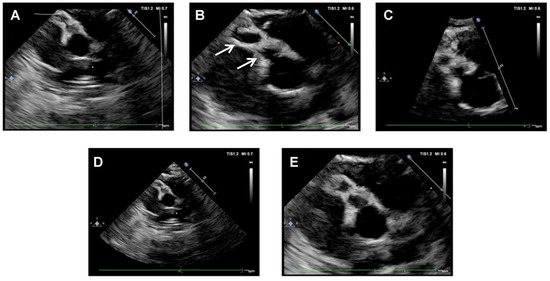
Figure 3.
Echocardiographic scans at hospital admission. (A–D): medium-size fusiform aneurysms of right CA (beaded appearance). In Figure (B), arrows indicated aneurysms of right CA. (E): medium-size dilatation of proximal right CA.
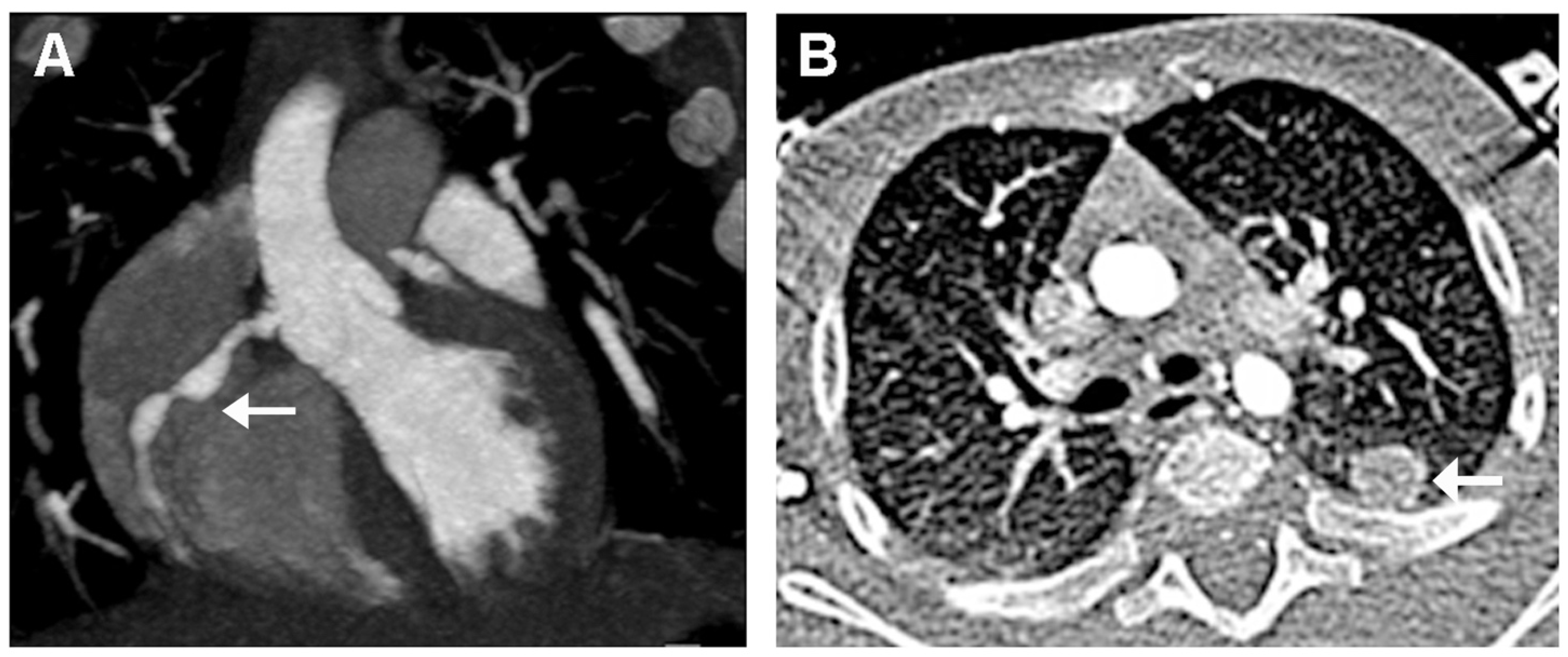
The diagnosis of KD was then confirmed. Therapy with intravenous immunoglobulin (IVIG 2 g/kg) associated to corticosteroids (intravenous methylprednisolone 30 mg/kg/day for three days) and oral acetylsalicylic acid (ASA, 50 mg/kg/day) was started. Resolution of fever and irritability was observed within 24 h. Laboratory findings showed a progressive decrease of CRP, ferritin, leukocytes, and platelets (Table 1 and Figure 2). On day 4 of hospitalization, he underwent a cardiac CT that showed a typical “beaded appearance” of right (dominant) CA due to aneurysmatic dilatations in the absence of thrombi. The proximal tract of right CA showed a 7 mm-long aneurysmatic dilatation with maximum diameters of 4 × 3 mm, while the medium tract showed a 7 mm-long aneurysmatic dilatation with maximum diameters of 5 × 4 mm. The proximal tract of the conal branch also showed a focal saccular dilatation (diameters of 3 × 2 mm). At its origin, the posterolateral branch showed a focal aneurysmatic dilatation (maximum diameter, 3 mm); the left anterior descending artery branch showed a focal aneurysmatic dilatation (diameters 3 × 3.5 mm). Two lung nodules (10 × 9 mm and 4 mm in diameter, respectively) located in the left upper lobe were also detected (Figure 4).
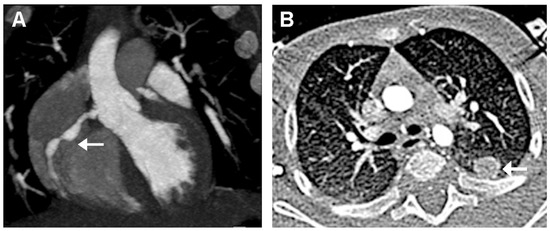
Figure 4.
Cardiac CT performed 4 days after hospital admission. (A): “Beaded appearance” (indivated by arrow) of the aneurysmatic dilatations of the right CA. (B): Lung nodule (indicated by arrow) in the left upper lobe.
On the basis of the severity of the coronary lesions and the of the z-score value, 4 days after admission, clopidogrel (1.3 mg/day) was started and ASA was stopped. Steroids were tapered and then switched to oral route.
The patient was discharged after 23 days with a strict cardiological follow-up. His left facial weakness was unappreciable at discharge. Three months after discharge, echocardiographic evaluation showed an improvement of CA lesions with a mild dilatation (2.8 mm, z-score 5) of the right CA in its median tract, with complete resolution of the lesions of the proximal tract of right CA and of left CA.
3. Discussion
Clinical diagnosis of KD in infants below 1 year of age, and even more below 6 months, can be very challenging since patients often do not present the typical signs and symptoms, and the risk of diagnostic delay is therefore greater. Mastrangelo and coworkers described clinical and laboratory findings in infants below 12 months of age with KD and reported a common presentation with incomplete disease [4]. In addition, KD in early infancy is associated with an increased risk of cardiac involvement that is detected in about 60% of patients in both complete and incomplete KD [4]. Coronary artery aneurysms (CAA) are detected in 50% of patients, most of whom have an incomplete form of KD [4]. Similarly, Salgado reported a higher incidence of dilated or aneurysmal CA in infants <6 months of age compared with those older than 6 months (43.4% vs. 19.5%), even when treatment was started within the first 10 days after fever onset [5].
Our patient was only 4 months old, and we made a diagnosis of incomplete KD because of fever persisting for more than 5 days associated with skin polymorphous rash, conjunctivitis, and changes in the extremities. The diagnosis was strengthened by several supporting laboratory findings, namely thrombocytosis, anemia, hypoalbuminemia and raised inflammatory markers. In addition, the child developed a left FNP and cardiac damage. FNP is a rare neurological manifestation of KD [6]. The first case of FNP in KD was reported in 1974 by Murayama in a 4-month-old male [7]. To date, about 50 cases of infants with KD-associated FNP have been described. Table 3 summarizes the clinical features, treatment, and prognosis of 35 patients with KD and FNP [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29].

Table 3.
Published articles including children with KD and concomitant facial nerve palsy.
The incidence of FNP in KD is 0.9–1.3% [29], and it is often unilateral, transient with a spontaneous resolution, more frequent on the left side and seemingly associated with coronary impairment [20]. Although FNP may develop at any time of KD, its onset has been estimated to occur, on average, at the 16th day of illness [22]. In our patient, FNP appeared earlier in comparison with most literature reports (Table 3). This early onset may be due to the severe inflammation observed in our child, as demonstrated by a significant increase in inflammatory markers at admission (CRP, ferritin, platelet, and cytokine levels).
A study conducted by Poon et al. reported 28 patients with FNP as a complication of KD in patients with 3 to 25 months of age, mainly occurring in females, and lasting from 2 days to 3 months, more frequent on the left side and with associated CAA at least in half of the patients [13]. A prospective study by Alves and coworkers summed KD complications and its rare clinical features [20]. He reported an incidence of central nervous system (CNS) involvement in KD ranging from 1.1% to 3.7%: reported manifestations included ataxia, FNP and sensorineural auditory loss [20]. In this review, the only patient showing FNP experienced it during the subacute phase and presented concomitant ataxia, hearing loss and a small left CAA [20]. In a recently published observational study including nine KD patients presenting FNP, its duration ranged from 10 to 130 days. Only one patient showed bilateral FNP [29].
A strict association between presentation with FNP and CAA has been reported, as coronary involvement is present in about two thirds of cases [22]. In the current analysis, CA involvement was described in most cases (27/35, 77%) of KD with FNP. A possible explanation of this strict association may be that the presence of incomplete KD and FNP lead to a delayed diagnosis and thus to a delayed treatment of the condition. Wright et al. described a child with a missed diagnosis of KD during the acute phase because of attribution of a neurologic sign to a possible concurrent otitis media [16]. Nevertheless, in another study comparing patients with FNP to matched KD patients without FNP, the incidence of CALs in KD patients with FNP was much higher than that of the matched KD patients without FNP [29]. These data indicated that KD patients with FNP appeared to have a real higher incidence of CALs.
In our patient, cardiac assessment was based on basal and serial echocardiography and cardiac CT performed on the 4th day after hospitalization. Cardiac CT (CCT) is superior to echocardiography in CA visualization and characterization, especially for distal CA segments [30]. In our case, CT scans allowed to exclude the presence of CA thrombosis or stenosis and to better characterize CA involvement. Furthermore, it identified two lung nodules that were not revealed by chest X-ray. It is important to note that our patient had no associated respiratory symptoms. Lung lesions in KD remain a diagnostic challenge. Pulmonary nodules have an inflammatory nature and rapidly regressed with the standard KD treatment [31,32]. Given the absence of respiratory manifestations and the reported spontaneous resolution of nodules in the literature, we did not perform a follow-up chest CT scan. Indeed, clinical improvement with proper treatment of KD allows to avoid serial CT scans in order to reduce both radiation exposure and frequency of general anesthesia, which is needed for image acquisition in young children.
As for the pathogenesis of FNP in KD, several etiologies have been hypothesized. It is likely that ischemic vasculitis of the arteries supplying the facial nerve contributes to FNP [11] together with an inflammatory process of the facial nerve itself [22,26]. This hypothesis may explain the positive effect of IVIG on FNP, likely due to a modulating effect on the synthesis and release of proinflammatory cytokines [33]. In a recent study, FNP occurred in 44.4% of patients despite the use of IVIG [29]. It seems that a prompt therapy with IVIG may resolve FNP more quickly, but it is not able to decrease the excessive inflammatory activity in these patients [22].
The current treatment for KD is a high dose (2 g/kg) of IVIG, administered within the first 10 days after disease onset [34,35]. Additionally, high-dose aspirin is advised by the American Heart Association. Most patients show a quick clinical response after IVIG therapy, yet approximately 10–20% of all patients do not respond well or have recurrent fever within 36–48 h after treatment [36]. Age of <6 months has been reported to be an important variable in predicting IVIG resistance [37,38]. In presence of high-risk patients with KD (children less than 12 months or children having CRP higher than 200 mg/l, severe anemia at disease onset, albumin level below 2.5 g/dl, liver disease, overt coronary artery aneurysms, macrophage activation syndrome or septic shock), Italian guidelines recommended high dose of intravenous methylprednisolone and ASA at anti-platelet dose [34]. Treatment of KD with FNP is less standardized. In a recent review including nine patients, all children received IVIG (2 g/kg), ASA (30–50 mg/kg/day) and short-term dexamethasone [29]. Our patient was considered a high-risk infant and was treated with IVIG, high dose of steroids and ASA. Treatment obtained a prompt clinical and laboratory response, while CA damage improved at a medium-term follow up. As expected, CAAs require more time to recover than CA dilation. In presence of CAA, a frequent and long-term follow-up echocardiography is mandatory.
4. Conclusions
This case highlights the importance to bear in mind KD diagnosis in any child with prolonged unexplained high fever and FNP. FNP is an unusual manifestation of KD but its presence may be associated to a high risk of severe CAA. For this reason, it is important to not delay the diagnosis of KD in order to start a prompt treatment. Despite its incomplete presentation, the diagnosis of KD was not delayed in our child, and treatment was started a few hours after hospital admission. Unlike CAA, the paralysis normally evolves towards a complete resolution without sequelae, as in the described child. A long-term echocardiography follow-up is required in patients with KD and CAAs.
Author Contributions
M.M.: Conceptualized and designed the case report; acqured data; analyzed and interpreted data; and wrote the manuscript. M.G. (Michela Grieco), R.C., G.D.N. and G.M.D.M.: Performed cardiac assessment; designed figures; and writing of the manuscript. A.B., M.C.L. and R.M.I.: Collected data and review articles and participated in the creation of tables. M.G. (Monica Gelzo) and G.C.: Performed the immunological assay and analysis and interpretation of results. C.T.: performed the neurological evaluation at hospital admission and during the entire period of observation, and revised clinical data. V.T.: Revised clinical data and analyzed the in-formation critically for important correlation content. A.G.: Conceptualized and designed the case report; acquired data; analyzed and interpreted data; performed literature review and verified the writing and content of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. During our cohort, no experimental drugs nor experimental investigation were used.
Informed Consent Statement
Informed consent was obtained from caregivers of patient involved in the study.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Santobono-Pausilipon Foundation for the support to our patients and their families.
Conflicts of Interest
The authors have no conflict of interest relevant to this article to disclose.
Abbreviations
| KD | Kawasaki disease |
| CALs | coronary artery lesions |
| FNP | facial nerve palsy |
| CT | computed tomography |
| WBC | white blood cells |
| CRP | c-reactive protein |
| PCT | procalcitonin |
| BNP | brain natriuretic peptide |
| TNF | tumor necrosis factor |
| IL | interleukin |
| SARS-CoV-2 | acute respiratory syndrome coronavirus 2 |
| HSV | herpes simplex virus |
| ECG | electrocardiogram |
| ASA | acetyl salicylic acid |
| intravenous immunoglobulin | IVIG |
| CAA | coronary artery aneurysm |
| CA | coronary artery |
| CNS | central nervous system |
References
- Marchesi, A.; Tarissi de Jacobis, I.; Rigante, D.; Rimini, A.; Malorni, W.; Corsello, G.; Bossi, G.; Buonuomo, S.; Cardinale, F.; Cortis, E.; et al. Kawasaki disease: Guidelines of the Italian Society of Pediatrics, part I—Definition, epidemiology, etiopathogenesis, clinical expression and management of the acute phase. Ital. J. Pediatr. 2018, 44, 102. [Google Scholar] [CrossRef] [PubMed]
- Rife, E.; Gedalia, A. Kawasaki Disease: An Update. Curr. Rheumatol. Rep. 2020, 22, 75. [Google Scholar] [CrossRef]
- Seki, M.; Minami, T. Kawasaki Disease: Pathology, Risks, and Management. Vasc. Health Risk Manag. 2022, 18, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, G.; Cimaz, R.; Calabri, G.B.; Simonini, G.; Lasagni, D.; Resti, M.; Trapani, S. Kawasaki disease in infants less than one year of age: An Italian cohort from a single center. BMC Pediatr. 2019, 19, 321. [Google Scholar] [CrossRef] [PubMed]
- Salgado, A.P. High risk of coronary artery aneurysms in infants younger than 6 months with Kawasaki disease. J. Pediatr. 2017, 185, 112–116.e1. [Google Scholar] [CrossRef]
- Tizard, E.J. Complications of Kawasaki disease. Curr. Paediatr. 2005, 15, 62–68. [Google Scholar] [CrossRef]
- Murayama, J. An autopsy case of acute febrile mucocutaneous lymph node syndrome with coronary embolism. Acta Paediatr. Jpn. 1974, 78, 115. [Google Scholar]
- Terasawa, K.; Ichinose, E.; Matsuishi, T.; Kato, H. Neurological complications in Kawasaki disease. Brain Dev. 1983, 5, 371–374. [Google Scholar] [CrossRef]
- Hattori, T.; Tokugawa, K.; Fukushige, J.; Tasaki, H.; Nanri, T.; Ueda, K. Facial palsy in Kawasaki disease. Report of two cases and a review. Eur. J. Pediatr. 1987, 146, 601–602. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; Lee, H.Y.; Kim, H.M.; Yang, J.S.; Lim, B.K.; Kim, J.S. Facial nerve paralysis associated with Kawasaki disease. Yonsei Med. J. 1991, 32, 279–282. [Google Scholar] [CrossRef]
- Bushara, K.; Wilson, A.; Rust, R.S. Facial Palsy in Kawasaki syndrome. Pediatr. Neurol. 1997, 17, 362–364. [Google Scholar] [CrossRef]
- McDonald, D.; Buttery, J.; Pike, M. Neurological complications of Kawasaki disease. Arch. Dis. Child. 1998, 79, 200. [Google Scholar] [CrossRef] [PubMed]
- Poon, L.K.H.; Lun, K.S.; Ng, Y.M. Facial nerve palsy and Kawasaki disease. HKMJ 2000, 6, 224–227. [Google Scholar] [PubMed]
- Biezeveld, M.H.; Voorbrood, B.S.; Clur, S.A.; Kuijpers, T.W. Facial nerve palsy in a thirteen-year-old male youth with Kawasaki disease. Pediatr. Infect. Dis. J. 2002, 21, 442–443. [Google Scholar] [CrossRef] [PubMed]
- Larralde, M.; Santos-Muñoz, A.; Rutiman, R. Kawasaki disease with facial nerve paralysis. Pediatr. Dermatol. 2003, 20, 511–513. [Google Scholar] [CrossRef]
- Wright, H.; Waddington, C.; Geddes, J.; Newburger, J.W.; Burgner, D. Facial nerve palsy complicating Kawasaki disease. Pediatrics 2008, 122, e783–e785. [Google Scholar] [CrossRef]
- Li, S.T.; Chiu, N.C.; Ho, C.S.; Chen, M.R. Facial palsy in Kawasaki disease: Report of two cases. Acta Paediatr. Taiwan 2008, 49, 24–27. [Google Scholar] [PubMed]
- Lim, T.C.; Yeo, W.S.; Loke, K.Y.; Quek, S.C. Bilateral facial nerve palsy in Kawasaki disease. Ann. Acad. Med. Singap. 2009, 38, 737–738. [Google Scholar] [CrossRef]
- Kaur, S.; Kulkarni, K.P.; Dubey, P.N. Facial palsy in a 2-month-old infant with Kawasaki disease. Rheumatol. Int. 2010, 30, 1407–1408. [Google Scholar] [CrossRef] [PubMed]
- Alves, N.R.; Magalhães, C.M.; Almeida, R.d.F.; Santos, R.C.; Gandolfi, L.; Pratesi, R. Prospective study of Kawasaki disease complications: Review of 115 cases. Rev. Assoc. Med. Bras. 2011, 57, 295–300. [Google Scholar] [CrossRef]
- Khubchandani, R.P.; Dhanrajani, A. Facial nerve palsy complicating a case of Kawasaki disease. Indian J. Pediatr. 2014, 81, 406–407. [Google Scholar] [CrossRef]
- Stowe, R.C. Facial nerve palsy, Kawasaki disease and coronary artery aneurysm. Eur. J. Paediatr. Neurol. 2015, 19, 607–609. [Google Scholar] [CrossRef]
- Rodriguez-Gonzalez, M.; Castellano-Martinez, A.; Perez-Reviriego, A.A. Atypical Presentation of Incomplete Kawasaki Disease: A Peripheral Facial Nerve Palsy. J. Emerg. Med. 2018, 55, 118–120. [Google Scholar] [CrossRef] [PubMed]
- Orgun, A.; Karagöl, C.; Pamuk, U.; Gürsu, H.A.; Çetin, İ. A rare cause of facial nerve palsy in a young infant: Kawasaki disease. Turk. J. Pediatr. 2018, 60, 433–435. [Google Scholar] [CrossRef]
- Yuan, Y.; Lu, N. Facial nerve palsy presenting as rare neurological complication of Kawasaki disease: A case report. Medicine 2019, 98, e16888. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Hao, Y.; Zhang, Y.; Yang, N.; Li, H.; Liang, J. Kawasaki disease manifesting as bilateral facial nerve palsy and meningitis: A case report and literature review. J. Int. Med. Res. 2019, 47, 4014–4018. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Liu, X.; Wang, Y.; Lu, N.; Wang, M.; Sun, L. Kawasaki disease complicating bilateral facial nerve palsy and giant coronary artery aneurysms: A case report. Medicine 2019, 98, 14395–14399. [Google Scholar] [CrossRef]
- Peña-Juárez, A.; Medina-Andrade, M.A.; Olivares, I.E.R.; Colín-Ortíz, J.L.; Yamazaki-Nakashimada, M.A.; Garrido-Garcia, L.M. Multiresistant Kawasaki Disease Complicated with Facial Nerve Palsy, Bilateral Giant Coronary Artery Aneurysms, and Stenosis of the Right Coronary Artery in an Infant. J. Clin. Rheumatol. 2021, 27, S351–S354. [Google Scholar] [CrossRef]
- Chen, J.; Liu, P.; Hu, W.; Xu, Y.; Deng, J. Facial nerve palsy may indicate coronary artery lesions in Kawasaki disease. Clin. Rheumatol. 2021, 40, 4191. [Google Scholar] [CrossRef]
- Gellis, L.; Castellanos, D.A.; Oduor, R.; Gauvreau, K.; Dionne, A.; Newburger, J.; Friedman, K.G. Comparison of coronary artery measurements between echocardiograms and cardiac CT in Kawasaki disease patients with aneurysms. J. Cardiovasc. Comput. Tomogr. 2022, 16, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Liu, X. Kawasaki disease with pulmonary nodules: Two case reports and literature review. Transl. Pediatr. 2021, 10, 1952–1959. [Google Scholar] [CrossRef] [PubMed]
- Freeman, A.F.; Crawford, S.E.; Finn, L.S.; López-Andreu, J.A.; Ferrando-Monleón, S.; Pérez-Tamarit, D.; Cornwall, M.L.; Shulman, S.T.; Rowley, A.H. Inflammatory pulmonary nodules in Kawasaki disease. Pediatr. Pulmonol. 2003, 36, 102–106. [Google Scholar] [CrossRef]
- Burns, J.C.; Franco, A. The immunomodulatory effects of intravenous immunoglobulin therapy in Kawasaki disease. Expert Rev. Clin. Immunol. 2015, 11, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, A.; Rigante, D.; Cimaz, R.; Ravelli, A.; Tarissi de Jacobis, I.; Rimini, A.; Cardinale, F.; Cattalini, M.; De Zorzi, A.; Dellepiane, R.M.; et al. Revised recommendations of the Italian Society of Pediatrics about the general management of Kawasaki disease. Ital. J. Pediatr. 2021, 47, 16. [Google Scholar] [CrossRef]
- Gorelik, M.; Chung, S.A.; Ardalan, K.; Binstadt, B.A.; Friedman, K.; Hayward, K.; Imundo, L.F.; Lapidus, S.K.; Kim, S.; Son, M.B.; et al. 2021 American College of Rheumatology/Vasculitis Foundation Guideline for the Management of Kawasaki Disease. Arthritis Care Res. (Hoboken) 2022, 74, 538–548. [Google Scholar] [CrossRef] [PubMed]
- McCrindle, B.W.; Rowley, A.H.; Newburger, J.W.; Burns, J.C.; Bolger, A.F.; Gewitz, M.; Baker, A.L.; Jackson, M.A.; Takahashi, M.; Shah, P.B.; et al. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals from the American Heart Association. Circulation 2017, 135, e927–e999. [Google Scholar] [CrossRef]
- Egami, K.; Muta, H.; Ishii, M.; Suda, K.; Sugahara, Y.; Iemura, M.; Matsuishi, T. Prediction of resistance to intravenous immunoglobulin treatment in patients with Kawasaki disease. J. Pediatr. 2006, 149, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Inoue, Y.; Takeuchi, K.; Okada, Y.; Tamura, K.; Tomomasa, T.; Kobayashi, T.; Morikawa, A. Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation 2006, 113, 2606–2612. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).