Targeting Sarcopenia as an Objective Clinical Outcome in the Care of Children with Spinal Cord-Related Paralysis: A Clinician’s View
Abstract
:1. Introduction
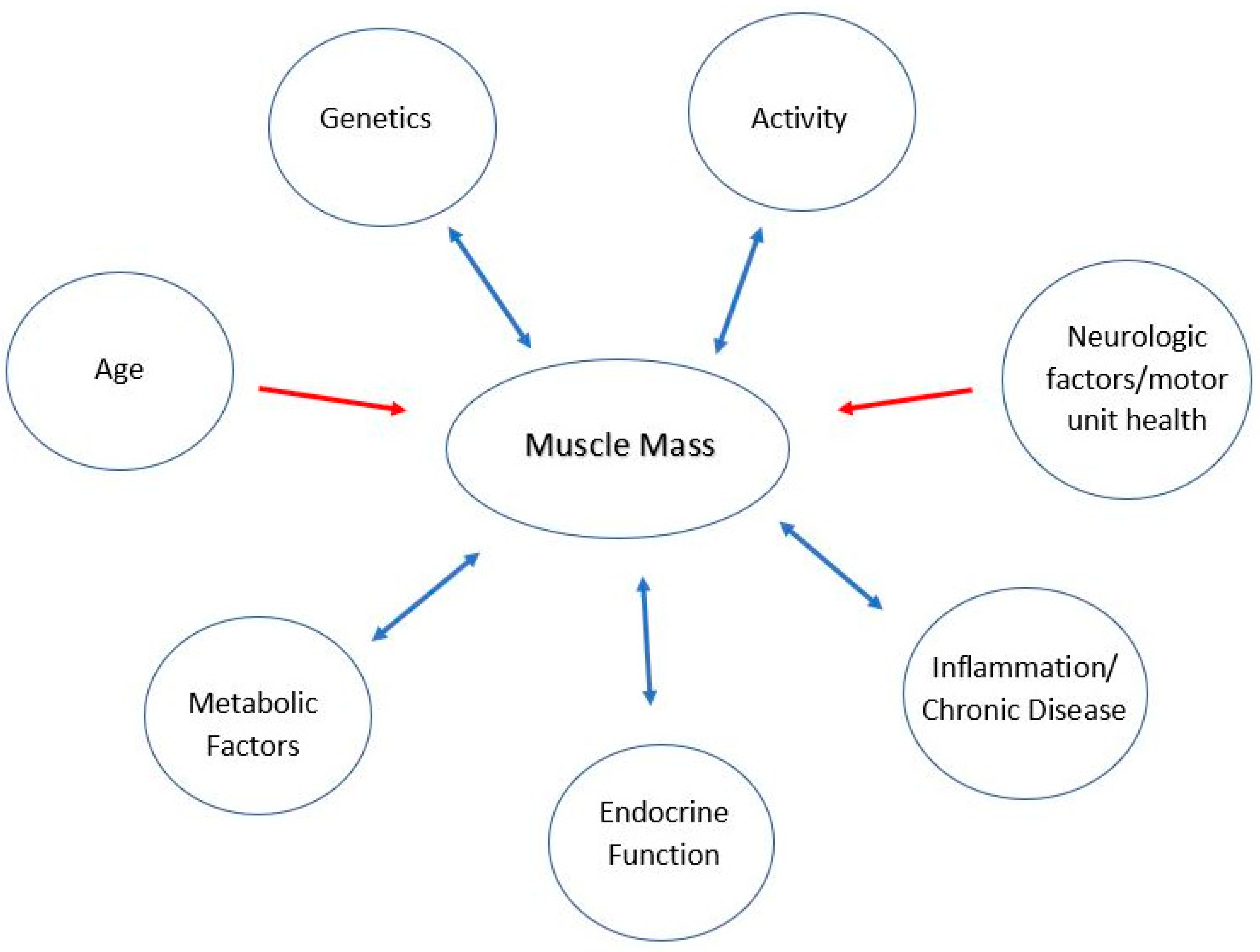
- Simple measurements and indices: anthropometrics [14], skin fold (SF), body mass index (BMI), and waist circumference (WC)
- Age
- Activity
- Endocrine function
- Genetics
- Inflammation and chronic conditions
- Metabolic factors
- Neurologic factors/motor unit health
1.1. Age and Muscle Mass
1.2. Activity and Muscle Mass
1.3. Endocrine Function and Muscle Mass
1.4. Genetics Factors and Muscle Mass
1.5. Inflammation and Chronic Conditions and Muscle Mass
1.6. Metabolic Factors and Muscle Mass
1.7. Neurologic Factors/Motor Unit Health and Muscle Mass
2. Muscle Mass-Function Connection
3. Studies of Muscle Mass in Paralysis Related to Spinal Cord Disease
4. Sarcopenia Reversal and Other Strategies to Increase Muscle Mass
4.1. Exercise
4.2. Diet
5. Summary
Funding
Informed Consent Statement
Conflicts of Interest
References
- Boo, S.-H.; Joo, M.C.; Lee, J.M.; Kim, S.C.; Yu, Y.M.; Kim, M.-S. Association between skeletal muscle mass and cardiorespiratory fitness in community-dwelling elderly men. Aging Clin. Exp. Res. 2019, 31, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Fomon, S.J.; Haschke, F.; Ziegler, E.E.; Nelson, S.E. Body composition of reference children from birth to age 10 years. Am. J. Clin. Nutr. 1982, 35, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Maltin, C.A.; I Delday, M.; Sinclair, K.D.; Steven, J.; Sneddon, A. Impact of manipulations of myogenesis in utero on the performance of adult skeletal muscle. Reproduction 2001, 122, 359–374. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Chen, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Chou, M.-Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef]
- Steffl, M.; Chrudimsky, J.; Tufano, J.J. Using relative handgrip strength to identify children at risk of sarcopenic obesity. PLoS ONE 2017, 12, e0177006. [Google Scholar] [CrossRef]
- Castro, M.J.; Apple, D.F., Jr.; Hillegass, E.A.; Dudley, G.A. Influence of complete spinal cord injury on skeletal muscle cross-sectional area within the first 6 months of injury. Eur. J. Appl. Physiol. Occup. Physiol. 1999, 80, 373–378. [Google Scholar] [CrossRef]
- de Groot, S.; Post, M.W.; Hoekstra, T.; Valent, L.J.; Faber, W.X.; van der Woude, L. Trajectories in the Course of Body Mass Index After Spinal Cord Injury. Arch. Phys. Med. Rehabil. 2014, 95, 1083–1092. [Google Scholar] [CrossRef]
- Cauley, J.A. An Overview of Sarcopenic Obesity. J. Clin. Densitom. 2015, 18, 499–505. [Google Scholar] [CrossRef]
- Pelletier, C.A.; Miyatani, M.; Giangregorio, L.; Craven, B. Sarcopenic Obesity in Adults with Spinal Cord Injury: A Cross-Sectional Study. Arch. Phys. Med. Rehabil. 2016, 97, 1931–1937. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-W.; Yang, K.-C.; Chang, H.-H.; Lee, L.-T.; Chen, C.-Y.; Huang, K.-C. Sarcopenic obesity is closely associated with metabolic syndrome. Obes. Res. Clin. Pract. 2013, 7, e301–e307. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.C.K.; Fewtrell, M.S. Measuring body composition. Arch. Dis. Child. 2006, 91, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Trowbridge, F.L.; Hiner, C.D.; Robertson, A.D. Arm muscle indicators and creatinine excretion in children. Am. J. Clin. Nutr. 1982, 36, 691–696. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, H.D.; Samani-Radia, D.; Jebb, S.A.; Prentice, A.M. Skeletal muscle mass reference curves for children and adolescents. Pediatr. Obes. 2013, 9, 249–259. [Google Scholar] [CrossRef]
- Jacobs, J.; Jansen, M.; Janssen, H.; Raijmann, W.; Van Alfen, N.; Pillen, S. Quantitative muscle ultrasound and muscle force in healthy children: A 4-year follow-up study. Muscle Nerve 2013, 47, 856–863. [Google Scholar] [CrossRef]
- Clark, R.V.; Walker, A.C.; Miller, R.R.; O’connor-Semmes, R.L.; Ravussin, E.; Cefalu, W.T. Creatine (methyl-d3) dilution in urine for estimation of total body skeletal muscle mass: Accuracy and variability vs. MRI and DXA. J. Appl. Physiol. 2018, 124, 1–9. [Google Scholar] [CrossRef]
- Wang, Z.; Heshka, S.; Pietrobelli, A.; Chen, Z.; Silva, A.M.; Sardinha, L.B.; Wang, J.; Gallager, D.; Heymsfield, S.B. A New Total Body Potassium Method to Estimate Total Body Skeletal Muscle Mass in Children. J. Nutr. 2007, 137, 1988–1991. [Google Scholar] [CrossRef]
- Arrowsmith, F.E.; Allen, J.R.; Gaskin, K.J.; A Gruca, M.; Clarke, S.L.; Briody, J.N.; Howman-Giles, R.B.; Somerville, H.; O’loughlin, E.V. Reduced body protein in children with spastic quadriplegic cerebral palsy. Am. J. Clin. Nutr. 2006, 83, 613–618. [Google Scholar] [CrossRef]
- Guo, M.; Zemel, B.S.; Hawkes, C.P.; Long, J.; Kelly, A.; Leonard, M.B.; Jaramillo, D.; Mostoufi-Moab, S. Sarcopenia and preserved bone mineral density in paediatric survivors of high-risk neuroblastoma with growth failure. J. Cachex- Sarcopenia Muscle 2021, 12, 1024–1033. [Google Scholar] [CrossRef]
- Lurz, E.; Patel, H.; Frimpong, R.G.; Ricciuto, A.; Kehar, M.; Wales, P.W.; Towbin, A.; Chavhan, G.; Kamath, B.M.; Ng, V.L. Sarcopenia in Children with End-Stage Liver Disease. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Mazahery, H.; Von Hurst, P.R.; McKinlay, C.J.D.; Cormack, B.E.; Conlon, C.A. Air displacement plethysmography (pea pod) in full-term and pre-term infants: A comprehensive review of accuracy, reproducibility, and practical challenges. Matern. Health Neonatol. Perinatol. 2018, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.C.K.; Fuller, N.J.; Wright, A.; Fewtrell, M.S.; Cole, T.J. Evaluation of air-displacement plethysmography in children aged 5–7 years using a three-component model, of body composition. Br. J. Nutr. 2003, 90, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Butte, N.F.; Hopkinson, J.M.; Wong, W.W.; Smith, E.O.; Ellis, K.J. Body composition during the first 2 years of life: An updated reference. Pediatr. Res. 2000, 47, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Cortés, L.; Tapia-Rojas, M.; López-Aguilar, E.; Mejía-Aranguré, J.M.; Rivera-Márquez, H. Body composi-tion by dilution of deuterium oxide in Mexican children with lymphoma and solid tumors. Nutrition 2007, 23, 739–744. [Google Scholar] [CrossRef]
- Ritz, A.; Kolorz, J.; Hubertus, J.; Ley-Zaporozhan, J.; von Schweinitz, D.; Koletzko, S.; Häberle, B.; Schmid, I.; Kappler, R.; Berger, M.; et al. Sarcopenia is a prognostic outcome marker in children with high-risk hepatoblas-toma. Pediatr. Blood Cancer 2021, 4, e28862. [Google Scholar] [CrossRef]
- Ritz, A.; Froeba-Pohl, A.; Kolorz, J.; Vigodski, V.; Hubertus, J.; Ley-Zaporozhan, J.; von Schweinitz, D.; Häberle, B.; Schmid, I.; Kappler, R.; et al. Total Psoas Muscle Area as a Marker for Sarcopenia Is Related to Outcome in Children with Neuroblastoma. Front. Surg. 2021, 8, 333. [Google Scholar] [CrossRef]
- Brambilla, P.; Bedogni, G.; Moreno, L.A.; Goran, M.I.; Gutin, B.; Fox, K.R.; Peters, D.M.; Barbeau, P.; De Simone, M.; Pietrobelli, A. Crossvalidation of anthropometry; against magnetic resonance imaging for the assessment of visceral and subcutaneous adipose tissue in children. Int. J. Obes. 2006, 30, 23–30. [Google Scholar] [CrossRef]
- Baumgartner, R.N.; Koehler, K.M.; Gallagher, D.; Romero, L.; Heymsfield, S.B.; Ross, R.R.; Garry, P.J.; Lindeman, R.D. Epidemiology of Sarcopenia among the Elderly in New Mexico. Am. J. Epidemiol. 1998, 147, 755–763. [Google Scholar] [CrossRef]
- Duren, D.L.; Sherwood, R.J.; Czerwinski, S.A.; Lee, M.; Choh, A.C.; Siervogel, R.M.; Chumlea, W.C. Body Composition Methods: Comparisons and Interpretation. J. Diabetes Sci. Technol. 2008, 2, 1139–1146. [Google Scholar] [CrossRef]
- Carlson, B. (Ed.) Chapter 3: Normal Muscle Growth. In Muscle Biology: The Life History of a Muscle; Academic Press: Cambridge, MA, USA, 2021; pp. 57–75. [Google Scholar]
- Lexell, J.; Sjöström, M.; Nordlund, A.-S.; Taylor, C. Growth and development of human muscle: A quantitative morphological study of whole vastus lateralis from childhood to adult age. Muscle Nerve 1992, 15, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Carlson, B. (Ed.) Chapter 4—Muscle adaptation to increased use. In Muscle Biology, The Life History of a Muscle; Academic Press: Cambridge, MA, USA, 2021; pp. 77–92. [Google Scholar]
- Sousa-Victor, P.; García-Prat, L.; Muñoz-Cánoves, P. Control of satellite cell function in muscle regeneration and its disruption in ageing. Nat. Rev. Mol. Cell Biol. 2021, 23, 204–226. [Google Scholar] [CrossRef] [PubMed]
- Woldt, E.; Sebti, Y.; Solt, L.A.; Duhem, C.; Lancel, S.; Eeckhoute, J.; Hesselink, M.K.C.; Paquet, C.; Delhaye, S.; Shin, Y.; et al. Reverb-α modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy. Nat. Med. 2013, 19, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Poole, D.C.; Edward, F. Adolph Distinguished Lecture. Contemporary model of muscle microcirculation: Gateway to function and dysfunction. J. Appl. Physiol. 2019, 127, 1012–1033. [Google Scholar] [CrossRef]
- Carlson, B. (Ed.) Chapter 5—Muscle adaptation to decreased use. In Muscle Biology, The Life History of a Muscle; Academic Press: Cambridge, MA, USA, 2021; pp. 95–116. [Google Scholar]
- Gram, M.; Dahl, R.; Dela, F. Physical inactivity and muscle oxidative capacity in humans. Eur. J. Sport Sci. 2013, 14, 376–383. [Google Scholar] [CrossRef]
- Bogdanis, G.C. Effects of Physical Activity and Inactivity on Muscle Fatigue. Front. Physiol. 2012, 3, 142. [Google Scholar] [CrossRef]
- Fink, J.; Schoenfeld, B.J.; Nakazato, K. The role of hormones in muscle hypertrophy. Physician Sportsmed. 2017, 46, 129–134. [Google Scholar] [CrossRef]
- Kadi, F. Cellular and molecular mechanisms responsible for the action of testosterone on human skeletal muscle. A basis for illegal performance enhancement. Br. J. Pharmacol. 2008, 154, 522–528. [Google Scholar] [CrossRef]
- Russell-Jones, D.L.; Weissberger, A.J.; Bowes, S.B.; Kelly, J.M.; Thomason, M.; Umpleby, A.M.; Jones, R.H.; Sonksen, P.H. Protein metabolism in growth hormone deficiency, and effects of growth hormone replacement therapy. Acta Endocrinol. 1993, 128 (Suppl. S2), 44–47. [Google Scholar]
- Yarasheski, K.E.; Zachweija, J.J.; Angelopoulos, T.J.; Bier, D.M. Short-term growth hormone treatment does not increase muscle protein synthesis in experienced weight lifters. J. Appl. Physiol. 1993, 74, 3073–3076. [Google Scholar] [CrossRef]
- Cuneo, R.C.; Salomon, F.; Wiles, C.M.; Sonksen, P.H. Skeletal muscle performance in adults with growth hor-mone deficiency. Horm. Res. 1990, 33 (Suppl. S4), 55–60. [Google Scholar] [CrossRef]
- Chikani, V.; Ho, K.K.Y. Action of GH on skeletal muscle function: Molecular and metabolic mechanisms. J. Mol. Endocrinol. 2014, 52, R107–R123. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.D.; Powell, C.; Kaufman, K.; Sun, P.C.; Bahn, R.S.; Nair, K.S. The Impact of Overt and Subclinical Hyperthyroidism on Skeletal Muscle. Thyroid 2006, 16, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.K.; Lee, Y.J.; Choi, S.H.; Lim, S.; Yang, E.J.; Lim, J.-Y.; Paik, N.-J.; Kim, K.W.; Park, K.S.; Jang, H.C.; et al. Subclinical Hypothyroidism has Little Influences on Muscle Mass or Strength in Elderly People. J. Korean Med. Sci. 2010, 25, 1176–1181. [Google Scholar] [CrossRef] [PubMed]
- Sambasivan, R.; Tajbakhsh, S. Skeletal muscle stem cell birth and properties. Semin. Cell Dev. Biol. 2007, 18, 870–882. [Google Scholar] [CrossRef] [PubMed]
- Rowe, R.W.; Goldspink, G. Muscle fibre growth in five different muscles in both sexes of mice. J. Anat. 1969, 104 Pt 3, 519–530. [Google Scholar]
- Saltin, B. Metabolic fundamentals in exercise. Med. Sci. Sport. Exerc. 1973, 5, 137–146. [Google Scholar] [CrossRef]
- Karasik, D.; Kiel, D.P. Genetics of the musculoskeletal system: A pleiotropic approach. J. Bone Miner Res. 2008, 23, 788–802. [Google Scholar] [CrossRef]
- Harvey, N.C.; Moon, R.J.; Sayer, A.A.; Ntani, G.; Davies, J.H.; Javaid, M.K.; Robinson, S.M.; Godfrey, K.M.; Inskip, H.M.; Cooper, C.; et al. Maternal antenatal vitamin D status and offspring muscle development: Findings from the Southampton Women’s Survey. J. Clin. Endocrinol. Metab. 2014, 99, 330–337. [Google Scholar] [CrossRef]
- Latouche, C.; Heywood, S.E.; Henry, S.L.; Ziemann, M.; Lazarus, R.; El-Osta, A.; Armitage, J.A.; Kingwell, B.A. Maternal Overnutrition Programs Changes in the Expression of Skeletal Muscle Genes That Are Associated with Insulin Resistance and Defects of Oxidative Phosphorylation in Adult Male Rat Offspring. J. Nutr. 2014, 144, 237–244. [Google Scholar] [CrossRef]
- Muralimanoharan, S.; Guo, C.; Myatt, L.; Maloyan, A. Sexual dimorphism in miR-210 expression and mitochondrial dysfunction in the placenta with maternal obesity. Int. J. Obes. 2015, 39, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Sayer, A.A.; Syddall, H.; Martin, H.; Patel, H.; Baylis, D.; Cooper, C. The developmental origins of sarcopenia. J. Nutr. Health Aging 2008, 12, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Dodds, R.; Denison, H.; Ntani, G.; Cooper, R.; Cooper, C.; Sayer, A.A.; Baird, J. Birth weight and muscle strength: A systematic review and meta-analysis. J. Nutr. Health Aging 2012, 16, 609–615. [Google Scholar] [CrossRef]
- Hediger, M.L.; Overpeck, M.D.; Kuczmarski, R.J.; McGlynn, A.; Maurer, K.R.; Davis, W.W. Muscularity and Fatness of Infants and Young Children Born Small- or Large-for-Gestational-Age. Pediatrics 1998, 102, e60. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A.; Wells, J.; Cole, T.J.; Fewtrell, M.; Lucas, A. Programming of lean body mass: A link between birth weight, obesity, and cardiovascular disease? Am. J. Clin. Nutr. 2003, 77, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Kavazis, A.N.; McClung, J.M. Oxidative stress and disuse muscle atrophy. J. Appl. Physiol. 2007, 102, 2389–2397. [Google Scholar] [CrossRef] [PubMed]
- Thomason, D.B.; Biggs, R.B.; Booth, F.W. Protein metabolism and beta-myosin heavy-chain mRNA in unweighted soleus muscle. Am. J. Physiol. Content 1989, 257, R300–R305. [Google Scholar] [CrossRef] [PubMed]
- Hyatt, H.; Deminice, R.; Yoshihara, T.; Powers, S.K. Mitochondrial dysfunction induces muscle atrophy during prolonged inactivity: A review of the causes and effects. Arch. Biochem. Biophys. 2018, 662, 49–60. [Google Scholar] [CrossRef]
- Allen, D.L.; Linderman, J.K.; Roy, R.R.; Bigbee, A.J.; Grindeland, R.E.; Mukku, V.; Edgerton, V.R. Apoptosis: A mechanism contributing to remodeling of skeletal muscle in response to hindlimb unweighting. Am. J. Physiol. Cell Physiol. 1997, 273, C579–C587. [Google Scholar] [CrossRef]
- Sandri, M. Apoptotic signaling in skeletal muscle fibers during atrophy. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 249–253. [Google Scholar] [CrossRef]
- Valente, V.B.; Verza, F.A.; Lopes, F.Y.K.; Ferreira, J.Z.; dos Santos, P.S.P.; Sundefeld, M.L.M.M.; Biasoli, R.; Miyahara, G.I.; Soubhia, A.M.P.; de Andrade, M.; et al. Stress hormones concentrations in the normal microenvironment predict risk for chemically induced cancer in rats. Psychoneuroendocrinology 2018, 89, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Baracos, V.E.; Mazurak, V.C.; Bhullar, A.S. Cancer cachexia is defined by an ongoing loss of skeletal muscle mass. Ann. Palliat. Med. 2019, 8, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Bindels, L.B.; Beck, R.; Schakman, O.; Martin, J.C.; De Backer, F.; Sohet, F.M.; Dewulf, E.M.; Pachikian, B.D.; Neyrinck, A.M.; Thissen, J.-P.; et al. Restoring Specific Lactobacilli Levels Decreases Inflammation and Muscle Atrophy Markers in an Acute Leukemia Mouse Model. PLoS ONE 2012, 7, e37971. [Google Scholar] [CrossRef]
- Varian, B.J.; Goureshetti, S.; Poutahidis, T.; Lakritz, J.R.; Levkovich, T.; Kwok, C.; Teliousis, K.; Ibrahim, Y.M.; Mirabal, S.; Erdman, S.E. Beneficial bacteria inhibit cachexia. Oncotarget 2016, 7, 11803–11816. [Google Scholar] [CrossRef] [PubMed]
- Bateman, L.S.; McSwain, R.T.; Lott, T.; Brown, T.M.; Cemenja, S.L.; Jenkins, J.M.; Tapper, A.M.; Parr, J.J.; Dolbow, D.R. Effects of Ibuprofen on Muscle Hypertrophy and Inflammation: A Review of Literature. Curr. Phys. Med. Rehabil. Rep. 2023, 11, 43–50. [Google Scholar] [CrossRef]
- Bailey, P.; Holowacz, T.; Lassar, A.B. The origin of skeletal muscle stem cells in the embryo and the adult. Curr. Opin. Cell Biol. 2001, 13, 679–689. [Google Scholar] [CrossRef]
- Yan, X.; Zhu, M.-J.; Dodson, M.V.; Du, M. Developmental Programming of Fetal Skeletal Muscle and Adipose Tissue Development. J. Genom. 2013, 1, 29–38. [Google Scholar] [CrossRef]
- Carbone, J.W.; Pasiakos, S.M. Dietary Protein and Muscle Mass: Translating Science to Application and Health Benefit. Nutrients 2019, 11, 1136. [Google Scholar] [CrossRef]
- Garcia, M.; Seelaender, M.; Sotiropoulos, A.; Coletti, D.; Lancha, A.H. Vitamin D, muscle recovery, sarcopenia, cachexia, and muscle atrophy. Nutrition 2019, 60, 66–69. [Google Scholar] [CrossRef]
- Blakeley, C.E.; Van Rompay, M.I.; Schultz, N.S.; Sacheck, J.M. Relationship between muscle strength and dyslipidemia, serum 25(OH)D, and weight status among diverse schoolchildren: A cross-sectional analysis. BMC Pediatr. 2018, 18, 23. [Google Scholar] [CrossRef]
- Artero, E.G.; Ruiz, J.R.; Ortega, F.B.; España-Romero, V.; Vicente-Rodríguez, G.; Molnar, D.; Gottrand, F.; González-Gross, M.; Breidenassel, C.; Moreno, L.A.; et al. Muscular and cardiorespiratory fitness are independently associated with metabolic risk in adolescents: The HELENA study. Pediatr. Diabetes 2011, 12, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Grøntved, A.; Ried-Larsen, M.; Møller, N.C.; Kristensen, P.L.; Froberg, K.; Brage, S.; Andersen, L.B. Muscle strength in youth and cardiovascular risk in young adulthood (the European Youth Heart Study). Br. J. Sport. Med. 2013, 49, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Zembura, M.; Matusik, P. Sarcopenic Obesity in Children and Adolescents: A Systematic Review. Front. Endocrinol. 2022, 13, 914740. [Google Scholar] [CrossRef] [PubMed]
- Ruschkewitz, Y.; Gefen, A. Cellular-scale transport in deformed skeletal muscle following spinal cord injury. Comput. Methods Biomech. Biomed. Eng. 2011, 14, 411–424. [Google Scholar] [CrossRef]
- Gash, M.C.; Kandle, P.F.; Murray, I.; Varacallo, M. Physiology, Muscle Contraction. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Tonson, A.; Ratel, S.; LE Fur, Y.; Cozzone, P.; Bendahan, D. Effect of Maturation on the Relationship between Muscle Size and Force Production. Med. Sci. Sport. Exerc. 2008, 40, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Uchitel, O.D.; Dubrovsky, A.L. Electrophysiologic denervation changes of human muscle fibers in motoneuron diseases. Muscle Nerve 1986, 9, 748–755. [Google Scholar] [CrossRef]
- Goodman, C.A.; Hornberger, T.A.; Robling, A.G. Bone and skeletal muscle: Key players in mechanotransduction and potential overlapping mechanisms. Bone 2015, 80, 24–36. [Google Scholar] [CrossRef]
- Sui, S.X.; Williams, L.J.; Holloway-Kew, K.L.; Hyde, N.K.; Pasco, J.A. Skeletal Muscle Health and Cognitive Function: A Narrative Review. Int. J. Mol. Sci. 2020, 22, 255. [Google Scholar] [CrossRef]
- Wang, D.X.; Yao, J.; Zirek, Y.; Reijnierse, E.M.; Maier, A.B. Muscle mass, strength, and physical performance predicting activities of daily living: A meta-analysis. J. Cachex- Sarcopenia Muscle 2019, 11, 3–25. [Google Scholar] [CrossRef]
- Wolfe, R.R. The underappreciated role of muscle in health and disease 1–3. Am. J. Clin. Nutr. 2006, 84, 475–482. [Google Scholar] [CrossRef]
- de Almeida-Neto, P.F.; Medeiros, R.C.D.S.C.D.; de Matos, D.G.; Baxter-Jones, A.D.G.; Aidar, F.J.; de Assis, G.G.; Dantas, P.M.S.; Cabral, B.G.D.A.T. Lean mass and biological maturation as predictors of muscle power and strength performance in young athletes. PLoS ONE 2021, 16, e0254552. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Bruton, A.; Gonzalez-Aguero, A.; Matute-Llorente, A.; Lozano-Berges, G.; Gomez-Cabello, A.; Moreno, L.; Casajus, J.; Vicente-Rodríguez, G. The muscle-bone unit in adolescent swimmers. Osteoporos. Int. 2019, 30, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Sanchis-Moysi, J.; Dorado, C.; Ortiz, R.A.; Serrano-Sanchez, A.J.; Calbet, J.A. Effects of training frequency on physical fitness in male prepubertal tennis players. J. Sport. Med. Phys. Fit. 2011, 51, 409–416. [Google Scholar]
- Avitabile, C.M.; McBride, M.G.; Harris, M.A.; Whitehead, K.K.; Fogel, M.A.; Paridon, S.M.; Zemel, B.S. Skeletal muscle deficits are associated with worse exercise performance in pediatric pulmonary hypertension. Front. Pediatr. 2022, 10, 1025420. [Google Scholar] [CrossRef] [PubMed]
- Gusso, S.; Munns, C.F.; Colle, P.; Derraik, J.G.B.; Biggs, J.B.; Cutfield, W.S.; Hofman, P.L. Effects of whole-body vibration training on physical function, bone and muscle mass in adolescents and young adults with cerebral palsy. Sci. Rep. 2016, 6, 22518. [Google Scholar] [CrossRef]
- Molenaar, H.; Zuidam, J.M.; Selles, R.W.; Stam, H.J.; Hovius, S.E. Age-Specific Reliability of Two Grip-Strength Dynamometers When Used by Children. J. Bone Jt. Surg. 2008, 90, 1053–1059. [Google Scholar] [CrossRef]
- Liusuwan, R.A.; Widman, L.M.; Abresch, R.T.; Johnson, A.J.; McDonald, C.M. Behavioral Intervention, Exercise, and Nutrition Education to Improve Health and Fitness (BENEfit) in Adolescents with Mobility Impairment Due to Spinal Cord Dysfunction. J. Spinal Cord Med. 2007, 30, S119–S126. [Google Scholar] [CrossRef]
- Liusuwan, R.A.; Widman, L.M.; Abresch, R.T.; Styne, D.M.; McDonald, C.M. Body Composition and Resting Energy Expenditure in Patients Aged 11 to 21 Years with Spinal Cord Dysfunction Compared to Controls: Comparisons and Relationships Among the Groups. J. Spinal Cord Med. 2007, 30, S105–S111. [Google Scholar] [CrossRef]
- Liu, A.J.W.; Briody, J.N.; Munns, C.F.; Waugh, M.-C.A. Regional changes in bone mineral density following spinal cord injury in children. Dev. Neurorehabilit. 2008, 11, 51–59. [Google Scholar] [CrossRef]
- Johnston, T.E.; Modlesky, C.M.; Betz, R.R.; Lauer, R.T. Muscle Changes Following Cycling and/or Electrical Stimulation in Pediatric Spinal Cord Injury. Arch. Phys. Med. Rehabil. 2011, 92, 1937–1943. [Google Scholar] [CrossRef]
- Biggin, A.; Briody, J.N.; Ramjan, K.A.; Middleton, A.; Waugh, M.-C.A.; Munns, C.F. Evaluation of bone mineral density and morphology using pQCT in children after spinal cord injury. Dev. Neurorehabilit. 2013, 16, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Curley, N.; Yang, Y.; Dean, J.; Salorio, C.; Sadowsky, C. Description of Bone Health Changes in a Cohort of Children with Acute Flaccid Myelitis (AFM). Top. Spinal Cord Inj. Rehabil. 2022, 28, 42–52. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention; How Much Physical Activity Do Children Need. Available online: https://www.cdc.gov/physicalactivity/basics/children/index.htm#:~:text=60%20minutes%20or%20more%20of,should%20include%20vigorous-intensity%20activities (accessed on 2 April 2023).
- National Survey of Children’s Health (NSCH), HRSA/MCHB. Available online: https://health.gov/healthypeople/objectives-and-data/browse-objectives/physical-activity/increase-proportion-children-who-do-enough-aerobic-physical-activity-pa-09 (accessed on 2 April 2023).
- Shavelle, R.M.; DeVivo, M.J.; Paculdo, D.R.; Vogel, L.C.; Strauss, D.J. Long-Term Survival After Childhood Spinal Cord Injury. J. Spinal Cord Med. 2007, 30, S48–S54. [Google Scholar] [CrossRef] [PubMed]
- Krzysztofik, M.; Wilk, M.; Wojdała, G.; Gołaś, A. Maximizing Muscle Hypertrophy: A Systematic Review of Advanced Resistance Training Techniques and Methods. Int. J. Environ. Res. Public Health 2019, 16, 4897. [Google Scholar] [CrossRef]
- Blimkie, C.J. Resistance training during pre- and early puberty: Efficacy, trainability, mechanisms, and persistence. Can. J. Sport Sci. 1992, 17, 264–279. [Google Scholar] [PubMed]
- Falk, B.; Eliakim, A. Resistance training, skeletal muscle and growth. Pediatr. Endocrinol. Rev. 2003, 1, 120–127. [Google Scholar]
- Vingren, J.L.; Kraemer, W.J.; Ratamess, N.A.; Anderson, J.M.; Volek, J.S.; Maresh, C.M. Testosterone Physiology in Resistance Exercise and Training. Sport. Med. 2010, 40, 1037–1053. [Google Scholar] [CrossRef]
- Walters, B.K.; Read, C.R.; Estes, A.R. The effects of resistance training, overtraining, and early specialization on youth athlete injury and development. J. Sport. Med. Phys. Fit. 2018, 58, 1339–1348. [Google Scholar] [CrossRef]
- Legerlotz, K. The Effects of Resistance Training on Health of Children and Adolescents with Disabilities. Am. J. Lifestyle Med. 2018, 14, 382–396. [Google Scholar] [CrossRef]
- Castelli, F.; Valero-Breton, M.; Hernandez, M.; Guarda, F.; Cornejo, J.; Cabello-Verrugio, C.; Cabrera, D. Regulatory Mechanisms of Muscle Mass: The Critical Role of Resistance Training in Children and Adolescent. Adv. Exp. Med. Biol. 2023, 1410, 21–34. [Google Scholar] [CrossRef]
- Dolbow, D.R.; Gorgey, A.S.; Recio, A.C.; Stiens, S.A.; Curry, A.C.; Sadowsky, C.L.; Gater, D.R.; Martin, R.; McDonald, J.W. Activity-Based Restorative Therapies after Spinal Cord Injury: Inter-institutional conceptions and perceptions. Aging Dis. 2015, 6, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, W.J.; Ratamess, N.A. Hormonal Responses and Adaptations to Resistance Exercise and Training. Sport. Med. 2005, 35, 339–361. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Sadowsky, C.; Obst, K.; Meyer, B.; McDonald, J. Functional Electrical Stimulation in Spinal Cord Injury: From Theory to Practice. Top. Spinal Cord Inj. Rehabil. 2012, 18, 28–33. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Dolbow, D.R.; Cifu, D.X.; Gater, D.R. Neuromuscular electrical stimulation attenuates thigh skeletal muscles atrophy but not trunk muscles after spinal cord injury. J. Electromyogr. Kinesiol. 2013, 23, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Johnston, T.E.; Smith, B.T.; Betz, R.R. Strengthening of Partially Denervated Knee Extensors Using Percutaneous Electric Stimulation in a Young Man with Spinal Cord Injury. Arch. Phys. Med. Rehabil. 2005, 86, 1037–1042. [Google Scholar] [CrossRef]
- Sadowsky, C.L.; Hammond, E.R.; Strohl, A.B.; Commean, P.K.; Eby, S.A.; Damiano, D.L.; Wingert, J.R.; Bae, K.T.; McDonald, J.W. Lower extremity functional electrical stimulation cycling promotes physical and functional recovery in chronic spinal cord injury. J. Spinal Cord Med. 2013, 36, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Bosques, G.; Martin, R.; McGee, L.; Sadowsky, C. Does therapeutic electrical stimulation improve function in children with disabilities? A comprehensive literature review. J. Pediatr. Rehabil. Med. 2016, 9, 83–99. [Google Scholar] [CrossRef]
- Millward, D.J. Interactions between Growth of Muscle and Stature: Mechanisms Involved and Their Nutritional Sensitivity to Dietary Protein: The Protein-Stat Revisited. Nutrients 2021, 13, 729. [Google Scholar] [CrossRef]
- Koletzko, B.; Demmelmair, H.; Grote, V.; Totzauer, M. Optimized protein intakes in term infants support physiological growth and promote long-term health. Semin. Perinatol. 2019, 43, 151153. [Google Scholar] [CrossRef]
- US Department of Health and Human Services. Dietary Reference Intake. Available online: https://health.gov/our-work/nutrition-physical-activity/dietary-guidelines/dietary-reference-intakes (accessed on 2 April 2023).
- Bigford, G.; Nash, M.S. Nutritional Health Considerations for Persons with Spinal Cord Injury. Top. Spinal Cord Inj. Rehabil. 2017, 23, 188–206. [Google Scholar] [CrossRef]
- Bell, K.L.; Samson-Fang, L. Nutritional management of children with cerebral palsy. Eur. J. Clin. Nutr. 2013, 67, S13–S16. [Google Scholar] [CrossRef] [PubMed]
- Harmon, K.K.; Stout, J.R.; Fukuda, D.H.; Pabian, P.S.; Rawson, E.S.; Stock, M.S. The Application of Creatine Supplementation in Medical Rehabilitation. Nutrients 2021, 13, 1825. [Google Scholar] [CrossRef] [PubMed]
- Pinder, M.A.; Myrie, S.B. Creatine Supplementation and Skeletal Muscle Metabolism for Building Muscle Mass- Review of the Potential Mechanisms of Action. Curr. Protein Pept. Sci. 2017, 18, 1273–1287. [Google Scholar] [CrossRef]
- Jacobs, P.L.; Mahoney, E.T.; Cohn, K.A.; Sheradsky, L.F.; Green, B.A. Oral creatine supplementation enhances upper extremity work capacity in persons with cervical-level spinal cord injury. Arch. Phys. Med. Rehabil. 2002, 83, 19–23. [Google Scholar] [CrossRef]
- Amorim, S.; Teixeira, V.H.; Corredeira, R.; Cunha, M.; Maia, B.; Margalho, P.; Pires, J. Creatine or vitamin D supplementation in individuals with a spinal cord injury undergoing resistance training: A double-blinded, randomized pilot trial. J. Spinal Cord Med. 2017, 41, 471–478. [Google Scholar] [CrossRef]
- Jagim, A.; Kerksick, C. Creatine Supplementation in Children and Adolescents. Nutrients 2021, 13, 664. [Google Scholar] [CrossRef] [PubMed]
- Canto, C.; Houtkooper, R.H.; Pirinen, E.; Youn, D.Y.; Oosterveer, M.H.; Cen, Y.; Fernandez-Marcos, P.J.; Yamamoto, H.; Andreux, P.A.; Cettour-Rose, P.; et al. The NAD+ Precursor Nicotinamide Riboside Enhances Oxidative Metabolism and Protects against High-Fat Diet-Induced Obesity. Cell Metab. 2012, 15, 838–847. [Google Scholar] [CrossRef]
- Zhang, H.; Ryu, D.; Wu, Y.; Gariani, K.; Wang, X.; Luan, P.; D’Amico, D.; Ropelle, E.R.; Lutolf, M.P.; Aebersold, R.; et al. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science 2016, 352, 1436–1443. [Google Scholar] [CrossRef]
- Mehmel, M.; Jovanović, N.; Spitz, U. Nicotinamide Riboside—The Current State of Research and Therapeutic Uses. Nutrients 2020, 12, 1616. [Google Scholar] [CrossRef]
- Clinical Trials.Gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=Nicotinamide+riboside&cntry=&state=&city=&dist= (accessed on 1 April 2023).
- Song, M.; Armenian, S.H.; Bhandari, R.; Lee, K.; Ness, K.; Putt, M.; Lindenfeld, L.; Manoukian, S.; Wade, K.; Dedio, A.; et al. Exercise training and NR supplementation to improve muscle mass and fitness in adolescent and young adult hematopoietic cell transplant survivors: A randomized controlled trial {1}. BMC Cancer 2022, 22, 795. [Google Scholar] [CrossRef]
- Gilligan, L.A.; Towbin, A.J.; Dillman, J.R.; Somasundaram, E.; Trout, A.T. Quantification of skeletal muscle mass: Sarcopenia as a marker of overall health in children and adults. Pediatr. Radiol. 2019, 50, 455–464. [Google Scholar] [CrossRef] [PubMed]
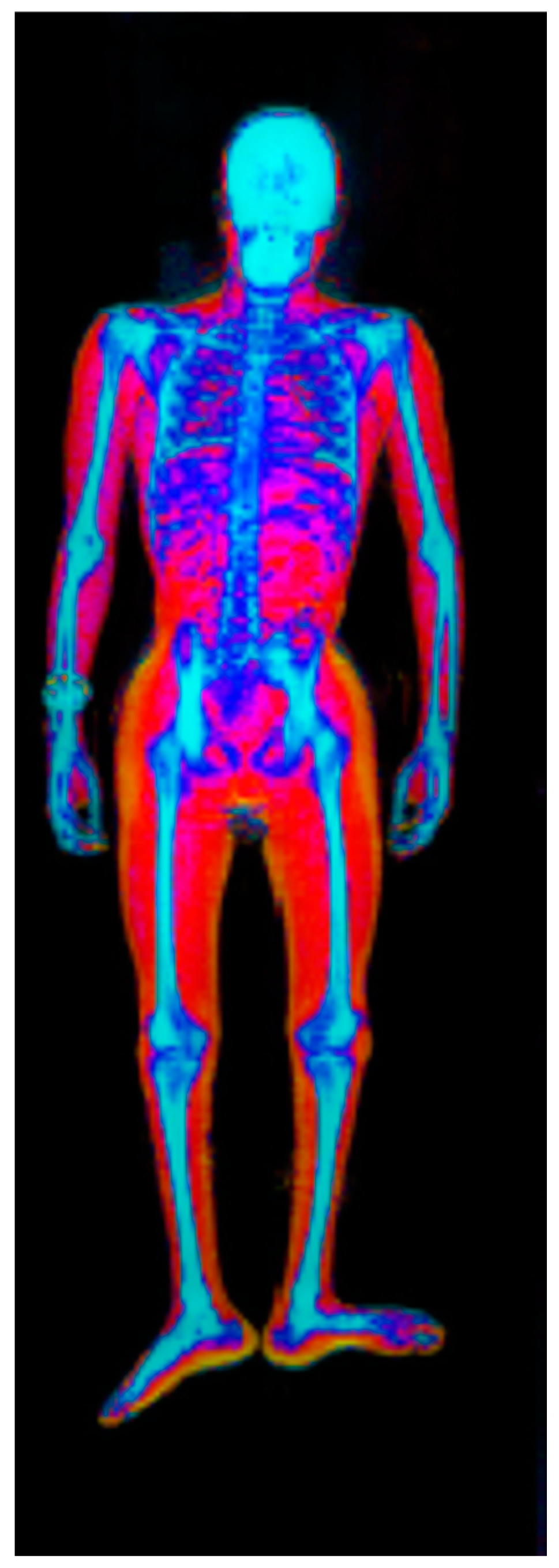
| Method | Cost | Availability | Pros | Cons |
|---|---|---|---|---|
| Anthropometrics (SF, BMI, WC) | + | ++++ | Noninvasive Can be used for screening | Poor precision in obese Some operator training required |
| BIA | ++ | ++ | Easy to use Reproducible | Subject dependent (hydration status, recent food intake, body/air temperature, recent physical activity status) |
| US | ++ | +++ | Safe Noninvasive Radiation free | Operator dependent technique Muscle- fat interface hard to determine |
| Biochemical markers (K, Cr) | ++ | ++ | Safe | Requires appropriate 24-h urine collection |
| DXA | ++ | ++ | Safe Can provide regional estimates (trunk, legs, arms) | Center-based (not portable) Subject size limitations Variations between manufacturer’s software do not allow for comparisons Water-bone free interface hard to determine |
| CT | ++++ | ++ | Highly reliable | Radiation exposure Center-based |
| MRI | ++++ | ++ | Highly reliable No radiation involved | Center-based Variations between manufacturer’s software do not allow for comparisons Subject size limitations |
| Author, Year, Study Design | Sample, Methods | Results | Conclusions |
|---|---|---|---|
| Liusuwan 2007 [91], USA Prospective study with intervention | N = 20 adolescents with spinal cord dysfunction (SCD) related to spinal cord injury or spina bifida (7 females, length of injury: 2 18 years from injury onset) Age: 11–18 years (mean 15.4 ± 2.2 years) Intervention: 16 weeks program consisting of exercise, education, and behavioral modification for nutritional intake Lean muscle body mass measured by DXA | N = 14 adolescents completed the study Whole body lean tissue and muscle work efficiency increased; some muscle strength (shoulder extensors) also increased. | A multidisciplinary program involving exercise, education, and nutrition can increase function and lean muscle body mass in adolescents with paralysis related to SCD |
| Liusuwan, 2007 [92], USA Retrospective cross-sectional study, no intervention | N = 215 children Age: 11–21 33 with SCI (12 females), 66 with spinal bifida SB (30 females), 31 overweight able body (12 females), 85 able body controls with normal BMI (44 females) Lean muscle body mass measured by DXA | Gender differences were observed in all groups; SCI and SB adolescents had significantly lower lean muscle body mass than able body ones (controls and overweight) Resting energy expenditure was lower in adolescents with SCI and SB | Gender differences in muscle mass are present in children with and without paralysis. Lean muscle body mass was lower in children with SC-related paralysis Resting energy expenditure directly correlated with lean muscle body mass |
| Liu, 2008 [93], Australia Retrospective cross-sectional study, no intervention | N = 18 children with pediatric-onset SCI (9 females, 0.1–7.2 years from injury onset) Age: 5.3–17.1 Follow-up time: 5.0 ± 3.6 years (range 0.4–12.4 years) Lean muscle body mass measured using DXA. | Lean muscle body mass was decreased below injury level. Lean muscle body mass loss occurred mostly in the first year after onset of neurologic deficit. After first year of paralysis, age-appropriate increase in lean body muscle mass occurred | Lean body muscle mass loss occurs rapidly following neurologic injury onset in children with pediatric-onset SCI |
| Johnston, 2011 [94], USA Prospective randomized controlled study, with intervention | N = 30 children with chronic SCI (>12 months from injury onset) Age: 5–13 years Interventions: 6 months of exercise-cycling (functional electrically stimulated FESC and passive PC and electrically stimulated strengthening (ES) Muscle mass measured with MRI | ES group had greatest increase in volume of the stimulated muscle; FES group had greatest increase in strength in stimulated muscles | ES and FES increase both muscle volume and strength in children with chronic SCI |
| Biggin, 2013 [95], Australia Retrospective cross-sectional cohort study, no intervention | N = 19 children with SCI (9 females, 9 with paraplegia and 10 with tetraplegia). Age: 1.9–18.8 Muscle mass measured by Cross-sectional computer tomography pQCT | Children that had the ability to stand had greater cross-sectional calf muscle area compared with those unable to stand. | Standing helps preserve calf muscle mass |
| Curley, 2022 [96], USA Retrospective cross-sectional study, no intervention | N = 41 children with acute flaccid myelitis (AFM) (24 females, time from injury onset 3–57 months) Age: 4 months–21 years old Lean muscle body mass measured using DXA | Functional performance, as assessed by the validated functional score Physical Abilities and Mobility Scale (PAMS), directly correlates with lean muscle mass as measured by DXA but not with muscle strength in children with AFM | Lean muscle mass correlates with functional performance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadowsky, C.L. Targeting Sarcopenia as an Objective Clinical Outcome in the Care of Children with Spinal Cord-Related Paralysis: A Clinician’s View. Children 2023, 10, 837. https://doi.org/10.3390/children10050837
Sadowsky CL. Targeting Sarcopenia as an Objective Clinical Outcome in the Care of Children with Spinal Cord-Related Paralysis: A Clinician’s View. Children. 2023; 10(5):837. https://doi.org/10.3390/children10050837
Chicago/Turabian StyleSadowsky, Cristina L. 2023. "Targeting Sarcopenia as an Objective Clinical Outcome in the Care of Children with Spinal Cord-Related Paralysis: A Clinician’s View" Children 10, no. 5: 837. https://doi.org/10.3390/children10050837





