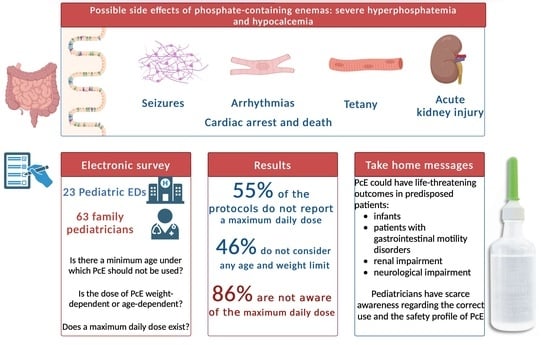
The Risks of Phosphate Enemas in Toddlers: A Life-Threatening Unawareness
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Setting and Population
2.3. Data Collection Procedures
2.4. Data Analysis
2.5. Ethics
3. Results
4. Discussion
4.1. Use of PcEs within the Clinical Practice
4.2. Pathogenesis of Damage Related to Phosphate Enemas
4.3. Clinical Manifestations
4.4. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Country | Name |
|---|---|
| United Arab Emirates | Fleet Saline Enema Relief |
| Argentina | Novonil Prontonema |
| Austria | Clysmol |
| Australia | Travad |
| Bangladesh | Anema |
| Belgium | Cleen Colowash Fleet |
| Bulgaria | Enema Balkan |
| Brazil | Enema jp Enemaplex Enemed Fleet L-enema Phosphoenema |
| Canada | Fleet Enema |
| Chile | Casen Enema Adultos Fleet Forflow Phosfoenema |
| China | Dibasic sodium phosphate Evac Fleet Sodium phosphates rectal |
| Colombia | Enematrol Forflow Travad |
| Denmark | Cleenema |
| Dominican Republic | Enema Fleet |
| Ecuador | Fleet |
| France | Normacol lavement enfants |
| United Kingdom | Sodium phosphate |
| Germany | Klistier Fresenius Kabi Klysma |
| Greece | Bioklism Klysmol |
| Hong Kong | Fleet |
| Indonesia | Fosen Purgatix |
| Ireland | Cleenema ready to use Fleet |
| Israel | Fleet |
| India | Practo Clyss Royal |
| Italy | Clisflex Clisma fleet |
| Jordan | Fletchers Phosphat Klysmol Phosphate |
| Kenya | Enemax |
| Republic of Korea | Fleet |
| Lebanon | Alfa Clyss Fleet PractoClyss |
| Luxembourg | Cleen Fleet |
| Mexico | Fleet fosf-sodio |
| Malaysia | Fleet |
| Nigeria | Enemax Enemax(p) |
| The Netherlands | Colex enema Fleet |
| Peru | Adulax Lainema Laxoven Laxoven Pediatrico Movilax |
| Philippines | Fleet |
| Pakistan | Fast Rapida |
| Poland | Fleet PROCTANAL |
| Portugal | Enema fleet Fleet |
| Paraguay | Fleet enemas adultos Fleet enemas ninos Fosfocol |
| Russia | Enema clean |
| Spain | Enema Casen Infantil Lainema |
| Singapore | Fleet |
| Thailand | Fleet |
| Tunisia | Normacol lavament |
| Turkey | B.T. Enema Fleet |
| Taiwan | Evac Fleet |
| Ukraine | Normacol |
| Uruguay | Fosfocol |
| USA | Fleet Enema Extra Fleet Enema Fleet Pedia-Lax Enema GoodSense Enema LaCrosse Complete |
| Bolivarian Republic of Venezuela | Clismalax Fleet Fleet enema Fosfolit |
| South Africa | Lenolax Lenolax Paediatric |
| Zimbabwe | Fosenema |
References
- Mugie, S.M.; Benninga, M.A.; Di Lorenzo, C. Epidemiology of constipation in children and adults: A systematic review. Best Pract. Res. Clin. Gastroenterol. 2011, 25, 3–18. [Google Scholar] [CrossRef]
- Koppen, I.J.N.; Vriesman, M.H.; Tabbers, M.M.; Di Lorenzo, C.; Benninga, M.A. Awareness and Implementation of the 2014 ESPGHAN/NASPGHAN Guideline for Childhood Functional Constipation. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Bongers, M.E.J.; Benninga, M.A.; Maurice-Stam, H.; Grootenhuis, M.A. Health-related quality of life in young adults with symptoms of constipation continuing from childhood into adulthood. Health Qual. Life Outcomes 2009, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Vriesman, M.H.; Rajindrajith, S.; Koppen, I.J.N.; van Etten-Jamaludin, F.S.; van Dijk, M.; Devanarayana, N.M.; Tabbers, M.M.; Benninga, M.A. Quality of Life in Children with Functional Constipation: A Systematic Review and Meta-Analysis. J. Pediatr. 2019, 214, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Van Tilburg, M.A.; Squires, M.; Blois-Martin, N.; Williams, C.; Benninga, M.A.; Peeters, B.; Ulshen, M. Parental knowledge of fecal incontinence in children. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Fishman, L.; Rappaport, L.; Schonwald, A.; Nurko, S. Trends in referral to a single encopresis clinic over 20 years. Pediatrics 2003, 111, e604–e607. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, M.M.; van Rossum, C.H.; de Lorijn, F.; Reitsma, J.B.; Di Lorenzo, C.; Benninga, M.A. Functional constipation in infants: A follow-up study. J. Pediatr. 2005, 147, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Penner, M.; Xie, W.Y.; Binepal, N.; Switzer, L.; Fehlings, D. Characteristics of pain in children and youth with cerebral palsy. Pediatrics 2013, 132, e407–e413. [Google Scholar] [CrossRef] [PubMed]
- Loening-Baucke, V. Constipation in children. N. Engl. J. Med. 1998, 339, 1155–1156. [Google Scholar] [CrossRef]
- Veugelers, R.; Benninga, M.A.; Calis, E.A.; Willemsen, S.P.; Evenhuis, H.; Tibboel, D.; Penning, C. Prevalence and clinical presentation of constipation in children with severe generalized cerebral palsy. Dev. Med. Child. Neurol. 2010, 52, e216–e221. [Google Scholar] [CrossRef]
- Bekkali, N.L.; van den Berg, M.M.; Dijkgraaf, M.G.; van Wijk, M.P.; Bongers, M.E.; Liem, O.; Benninga, M.A. Rectal fecal impaction treatment in childhood constipation: Enemas versus high doses oral PEG. Pediatrics 2009, 124, e1108–e1115. [Google Scholar] [CrossRef]
- Loening-Baucke, V. Prevalence, symptoms and outcome of constipation in infants and toddlers. J. Pediatr. 2005, 146, 359–363. [Google Scholar] [CrossRef]
- Norbedo, S.; Bassanese, G.; Barbieri, F.; Barbi, E. Acute Abdominal Pain: Recognition and Management of Constipation in the Emergency Department. Pediatr. Emerg. Care 2017, 33, e75–e78. [Google Scholar] [CrossRef]
- Van den Heuvel, R.; Hertel, M.; Gallagher, J.; Naidoo, V. A toddler who refused to stand or walk: Lumbar spondylodiscitis. BMJ Case Rep. 2012, 2012, bcr2012007007. [Google Scholar] [CrossRef]
- Bokova, E.; Svetanoff, W.J.; Rosen, J.M.; Levitt, M.A.; Rentea, R.M. State of the Art Bowel Management for Pediatric Colorectal Problems: Functional Constipation. Children 2023, 10, 1078. [Google Scholar] [CrossRef]
- Mueller, J.L.; Goldstein, A.M. The science of Hirschsprung disease: What we know and where we are headed. Semin. Pediatr. Surg. 2022, 31, 151157. [Google Scholar] [CrossRef]
- Urdaneta-Carruyo, E.; Suranyi, A.; Milano, M. Infantile botulism: Clinical and laboratory observations of a rare neuroparalytic disease. J. Paediatr. Child Health 2000, 36, 193–195. [Google Scholar] [CrossRef]
- Rosen, R.; Buonomo, C.; Andrade, R.; Nurko, S. Incidence of spinal cord lesions in patients with intractable constipation. J. Pediatr. 2004, 145, 409–411. [Google Scholar] [CrossRef]
- Suffia, C.; Sorrentino, S.; Vetrella, S.; Bifano, D.; Nantron, M.; De Bernardi, B.; Gandolfo, C. Neuroblastoma presenting with symptoms of epidural compression at birth: A case report. Ital. J. Pediatr. 2016, 42, 52. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tabbers, M.M.; Di Lorenzo, C.; Berger, M.Y.; Faure, C.; Langendam, M.W.; Nurko, S.; Staiano, A.; Vandenplas, Y.; Benninga, M.A. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition; North American Society for Pediatric Gastroenterology. Evaluation and treatment of functional constipation in infants and children: Evidence-based recommendations from ESPGHAN and NASPGHAN. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 258–274. [Google Scholar] [CrossRef] [PubMed]
- Candy, D.; Belsey, J. Macrogol (polyethylene glycol) laxatives in children with functional constipation and faecal impaction: A systematic review. Arch. Dis. Child. 2009, 94, 156–160. [Google Scholar] [CrossRef]
- Miller, M.K.; Dowd, M.D.; Friesen, C.A.; Walsh-Kelly, C.M. A randomized trial of enema versus polyethylene glycol 3350 for fecal disimpaction in children presenting to an emergency department. Pediatr. Emerg. Care 2012, 28, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, N.; Devanarayana, N.M.; Benninga, M.A.; van Dijk, M.; Rajindrajith, S. Psychological maladjustment and quality of life in adolescents with constipation. Arch. Dis. Child. 2017, 102, 268–273. [Google Scholar] [CrossRef]
- Devanarayana, N.M.; Rajindrajith, S. Association between constipation and stressful life events in a cohort of Sri Lankan children and adolescents. J. Trop. Pediatr. 2010, 56, 144–148. [Google Scholar] [CrossRef]
- Santucci, N.R.; Rein, L.E.; van Tilburg, M.A.; Karpinski, A.; Rosenberg, A.; Amado-Feeley, A.; Stoops, E.; Herdes, R.E.; Hyman, P.E. Self-Efficacy in Children with Functional Constipation Is Associated with Treatment Success. J. Pediatr. 2020, 216, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Borowitz, S.M.; Cox, D.J.; Kovatchev, B.; Ritterband, L.M.; Sheen, J.; Sutphen, J. Treatment of childhood constipation by primary care physicians: Efficacy and predictors of outcome. Pediatrics 2005, 115, 873–877. [Google Scholar] [CrossRef]
- Freedman, S.B.; Thull-Freedman, J.; Rumantir, M.; Eltorki, M.; Schuh, S. Pediatric constipation in the emergency department: Evaluation, treatment, and outcomes. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 327–333. [Google Scholar] [CrossRef]
- Anderson, J.; Furnival, R.A.; Zhang, L.; Lunos, S.A.; Sadiq, Z.; Strutt, J.R.; Kaila, R.; Hendrickson, M.A. A Comparison of the Efficacy of Enema Solutions in Pediatric Emergency Department Patients. J. Emerg. Med. 2019, 57, 461–468. [Google Scholar] [CrossRef]
- Mendoza, J.; Legido, J.; Rubio, S.; Gisbert, J.P. Systematic review: The adverse effects of sodium phosphate enema. Aliment. Pharmacol. Ther. 2007, 26, 9–20. [Google Scholar] [CrossRef]
- Ladenhauf, H.N.; Stundner, O.; Spreitzhofer, F.; Deluggi, S. Severe hyperphosphatemia after administration of sodium-phosphate containing laxatives in children: Case series and systematic review of literature. Pediatr. Surg. Int. 2012, 28, 805–814. [Google Scholar] [CrossRef]
- Biebl, A.; Grillenberger, A.; Schmitt, K. Enema-induced severe hyperphosphatemia in children. Eur. J. Pediatr. 2009, 168, 111–112. [Google Scholar] [CrossRef]
- Becknell, B.; Smoyer, W.E.; O’Brien, N.F. Hemodialysis for near-fatal sodium phosphate toxicity in a child receiving sodium phosphate enemas. Pediatr. Emerg. Care 2014, 30, 814–817. [Google Scholar] [CrossRef]
- Helikson, M.A.; Parham, W.A.; Tobias, J.D. Hypocalcemia and hyperphosphatemia after phosphate enema use in a child. J. Pediatr. Surg. 1997, 32, 1244–1246. [Google Scholar] [CrossRef]
- Butani, L. Life-threatening hyperphosphatemia and hypocalcemia from inappropriate use of Fleet enemas. Clin Pediatr. 2005, 44, 93. [Google Scholar] [CrossRef]
- AIFA Website. Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_004062_029319_FI.pdf&sys=m0b1l3 (accessed on 1 January 2023).
- FDA Drug Safety Communication: FDA Warns of Possible Harm from Exceeding Recommended Dose of over-the-Counter Sodium Phosphate Products to Treat Constipation FDA. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-warns-possible-harm-exceeding-recommended-dose-over-counter-sodium (accessed on 1 January 2023).
- Jacobson, R.M.; Peery, J.; Thompson, W.O.; Kanapka, J.A.; Caswell, M. Serum electrolyte shifts following administration of sodium phosphates enema. Gastroenterol. Nurs. 2010, 33, 191–201. [Google Scholar] [CrossRef]
- Ainley, E.J.; Winwood, P.J.; Begley, J.P. Measurement of serum electrolytes and phosphate after sodium phosphate colonoscopy bowel preparation: An evaluation. Dig. Dis. Sci. 2005, 50, 1319–1323. [Google Scholar] [CrossRef]
- Domico, M.B.; Huynh, V.; Anand, S.K.; Mink, R. Severe hyperphosphatemia and hypocalcemic tetany after oral laxative administration in a 3-month-old infant. Pediatrics 2006, 118, e1580–e1583. [Google Scholar] [CrossRef]
- Hamilton Smith, R.; Eddleston, M.; Bateman, D.N. Nicholas Bateman Toxicity of phosphate enemas—An updated review. Clin. Toxicol. 2022, 60, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Ismail, E.A.; Al-Mutairi, G.; Al-Anzy, H. A fatal small dose of phosphate enema in a young child with no renal or gastrointestinal abnormality. J. Pediatr. Gastroenterol. Nutr. 2000, 30, 220–221. [Google Scholar] [CrossRef] [PubMed]
- Marraffa, J.M.; Hui, A.; Stork, C.M. Severe hyperphosphatemia and hypocalcemia following the rectal administration of a phosphate-containing Fleet pediatric enema. Pediatr. Emerg. Care 2004, 20, 453–456. [Google Scholar] [CrossRef]


| PED | FP | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Question | Answer | % | Number of Centers | Cumulative % | Total | % | Number | Total | Cumulative % |
| Do you perform enemas in children with abdominal pain and suspicion of functional constipation? | yes | 95 | 22 | 23 | 36 | 23 | 63 | ||
| no | 5 | 1 | 64 | 40 | |||||
| Do you use phosphate-containing enemas? | yes | 50 | 11 | 22 | 32 | 20 | 63 | 32 | |
| no | 50 | 11 | 68 | 43 | |||||
| Is there a minimum age under which phosphate-containing enemas should not be used? | 1 yo | 36 | 4 | 64 | 11 | 24 | 15 | 63 | 49 |
| 2 yo | 36 | 4 | 29 | 20 | |||||
| 3 yo | 18 | 2 | 23 | 14 | |||||
| no | 10 | 1 | 11 | 7 | |||||
| I don’t know | 11 | 7 | |||||||
| Is the dose of phosphate-containing enemas weight-dependent or age-dependent? | yes | 54 | 6 | 46 | 11 | ||||
| no | 46 | 5 | |||||||
| Does a maximum daily dose exist for phosphate-containing enemas? | 1 | 45 | 5 | 55 | 11 | 14 | 9 | 63 | 54 |
| 2 | 27 | 3 | 23 | 15 | |||||
| 3 | 10 | 1 | 20 | 12 | |||||
| no | 18 | 2 | 24 | 15 | |||||
| I don’t know | 19 | 12 | |||||||
| Have you ever experienced substantial adverse reactions using phosphate-containing enemas, like drowsiness or hypocalcemia? | yes | ||||||||
| no | 100 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zago, A.; Occhipinti, A.A.; Bramuzzo, M.; Ceconi, V.; Colacino, V.; Barbi, E.; Poropat, F. The Risks of Phosphate Enemas in Toddlers: A Life-Threatening Unawareness. Children 2024, 11, 349. https://doi.org/10.3390/children11030349
Zago A, Occhipinti AA, Bramuzzo M, Ceconi V, Colacino V, Barbi E, Poropat F. The Risks of Phosphate Enemas in Toddlers: A Life-Threatening Unawareness. Children. 2024; 11(3):349. https://doi.org/10.3390/children11030349
Chicago/Turabian StyleZago, Alessandro, Alessandro Agostino Occhipinti, Matteo Bramuzzo, Viola Ceconi, Vincenzo Colacino, Egidio Barbi, and Federico Poropat. 2024. "The Risks of Phosphate Enemas in Toddlers: A Life-Threatening Unawareness" Children 11, no. 3: 349. https://doi.org/10.3390/children11030349
APA StyleZago, A., Occhipinti, A. A., Bramuzzo, M., Ceconi, V., Colacino, V., Barbi, E., & Poropat, F. (2024). The Risks of Phosphate Enemas in Toddlers: A Life-Threatening Unawareness. Children, 11(3), 349. https://doi.org/10.3390/children11030349