Intellectual Development in Mexican Preterm Children at Risk of Perinatal Brain Damage: A Longitudinal Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Instruments and Procedure
2.3. Data Analysis
2.4. Ethical Considerations
3. Results
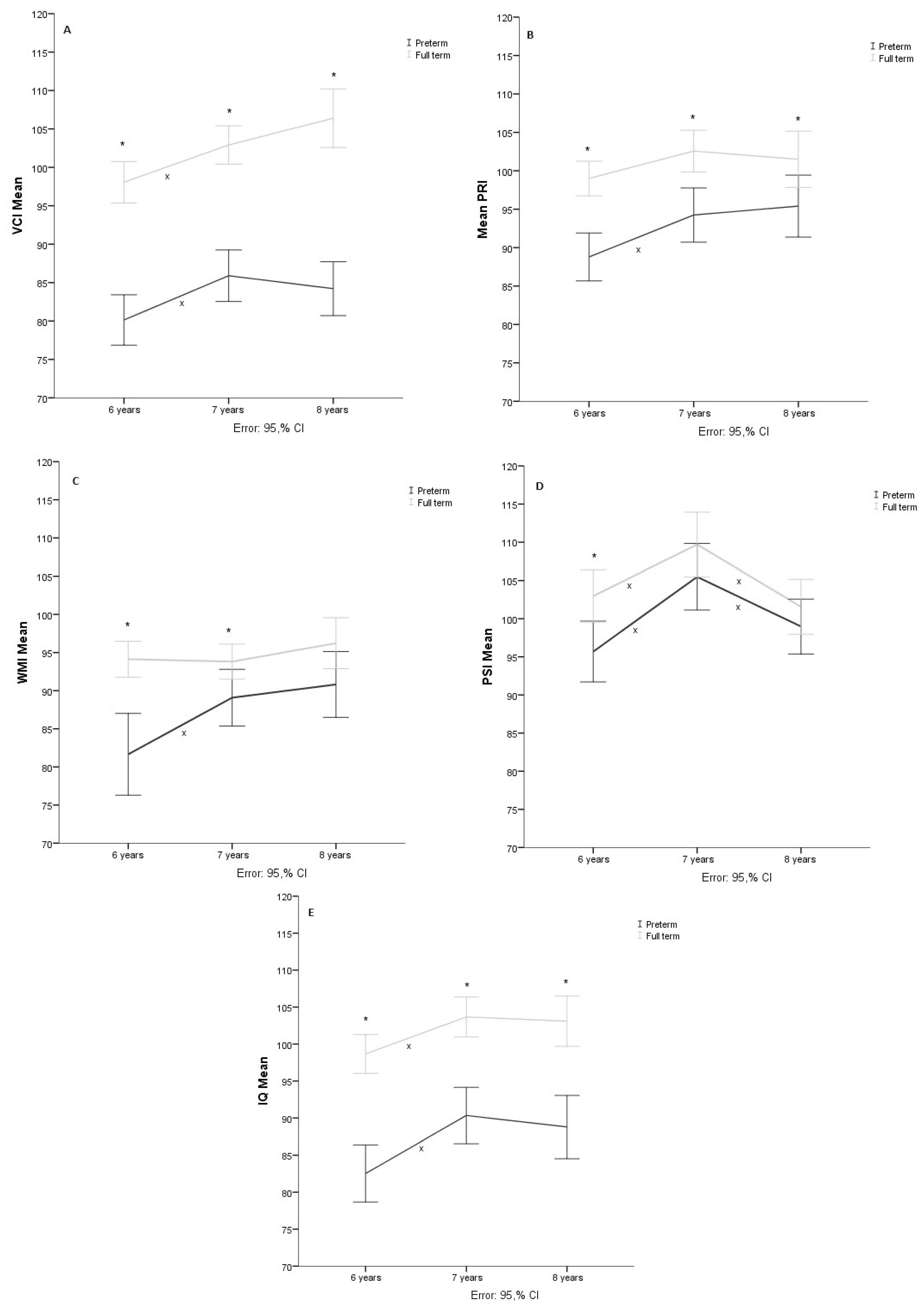
3.1. Verbal Comprehension
3.2. Perceptual Reasoning
3.3. Working Memory
3.4. Processing Speed
3.5. Total Intelligence Quotient
4. Discussion
5. Limitations and Future Considerations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Born too Soon: Decade of Action on Preterm Birth; World Health Organization: Geneva, Switzerland, 2023; Available online: https://www.who.int/publications/i/item/9789240073890 (accessed on 27 April 2024).
- Vogel, J.P.; Chawanpaiboon, S.; Moller, A.B.; Watananirun, K.; Bonet, M.; Lumbiganon, P. The global epidemiology of preterm birth. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 52, 3–12. [Google Scholar] [CrossRef]
- Sansavini, A.; Guarini, A.; Caselli, M.C. Preterm birth: Neuropsychological profiles and atypical developmental pathways. Dev. Disabil. Res. Rev. 2011, 17, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Villar, J.; Papageorghiou, A.T.; Knight, H.E.; Gravett, M.G.; Iams, J.; Waller, S.A.; Kramer, M.; Culhane, J.F.; Barros, F.C.; Conde-Agudelo, A.; et al. The preterm birth syndrome: A prototype phenotypic classification. Am. J. Obstet. Gynecol. 2012, 206, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Rathod, P.; Patel, T.; Desai, A.; Chandel, D. Socioeconomic, biological and genetic factors influencing preterm birth. Asian Pac. J. Reprod. 2020, 9, 215–222. [Google Scholar]
- Purisch, S.E.; Gyamfi-Bannerman, C. Epidemiology of preterm birth. In Seminars in Perinatology; WB Saunders: Philadelphia, PA, USA, 2017; Volume 41, pp. 387–391. [Google Scholar] [CrossRef]
- Ohuma, E.O.; Moller, A.B.; Bradley, E.; Chakwera, S.; Hussain-Alkhateeb, L.; Lewin, A.; Okwaraji, Y.B.; Mahanani, W.R.; Johansson, E.W.; Lavin, T.; et al. National, regional, and global estimates of preterm birth in 2020, with trends from 2010: A systematic analysis. Lancet 2023, 402, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Beck, S.; Wojdyla, D.; Say, L.; Betran, A.P.; Merialdi, M.; Requejo, J.H.; Rubens, C.; Menon, R.; Van Look, P.F.A. The worldwide incidence of preterm birth: A systematic review of maternal mortality and morbidity. Bull. World Health Organ. 2010, 88, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Blencowe, H.; Cousens, S.; Oestergaard, M.Z.; Chou, D.; Moller, A.B.; Narwal, R.; Adler, A.; Garcia, C.V.; Rohde, S.; Say, L.; et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: A systematic analysis and implications. Lancet 2012, 379, 2162–2172. [Google Scholar] [CrossRef] [PubMed]
- Chawanpaiboon, S.; Vogel, J.P.; Moller, A.B.; Lumbiganon, P.; Petzold, M.; Hogan, D.; Landoulsi, S.; Jampathong, N.; Kongwattanakul, K.; Laopaiboon, M.; et al. Global, regional, and national estimates of levels of preterm birth in 2014: A systematic review and modelling analysis. Lancet. Glob. Health 2019, 7, e37–e46. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Preterm Birth Estimates. 2018. Available online: https://ptb.srhr.org/ (accessed on 2 June 2021).
- Harmony, T. Factores que inciden en el desarrollo del sistema nervioso del niño. In Aproximaciones de las Neurociencias a la Conducta; Manual Moderno: Ciudad de México, México, 2004; pp. 147–162. [Google Scholar]
- Leonard, H.; Montgomery, A.; Wolff, B.; Strumpher, E.; Masi, A.; Woolfenden, S.; Williams, K.; Eapen, V.; Finlay-Jones, A.; Whitehouse, A.; et al. A systematic review of the biological, social, and environmental determinants of intellectual disability in children and adolescents. Front. Psychiatry 2022, 13, 926681. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.d.C.; Alves, C.R.L.; Guimarães, M.A.P.; Menezes, K.K.P.d.; Magalhães, L.d.C. Effects of early interventions focused on the family in the development of children born preterm and/or at social risk: A meta-analysis. J. Pediatr. 2020, 96, 20–38. [Google Scholar] [CrossRef] [PubMed]
- Forigua, J.C. Aproximaciones de las neurociencias a la conducta. Rev. Latinoam. Psicol. 2007, 39, 627–628. [Google Scholar]
- Montaño-Pérez, C.M.; Cázarez-Ortiz, M.; Juárez-Astorga, A.; Ramírez-Moreno, M.A. Morbilidad y mortalidad en recién nacidos menores de 1000 gramos en una institución pública de tercer nivel en México. Rev. Mex. Pediatr. 2019, 86, 108–111. [Google Scholar]
- Jin, C.; Londono, I.; Mallard, C.; Lodygensky, G.A. New means to assess neonatal inflammatory brain injury. J. Neuroinflammation 2015, 12, 180. [Google Scholar] [CrossRef]
- Aarnoudse-Moens, C.S.H.; Duivenvoorden, H.J.; Weisglas-Kuperus, N.; Van Goudoever, J.B.; Oosterlaan, J. The profile of executive function in very preterm children at 4 to 12 years. Dev. Med. Child Neurol. 2012, 54, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Gnigler, M.; Neubauer, V.; Griesmaier, E.; Zotter, S.; Kager, K.; Kiechl-Kohlendorfer, U. Very preterm children are at increased risk of reduced processing speed at 5 years of age, predicted by typical complications of prematurity and prenatal smoking. Acta Paediatr. 2015, 104, e124–e129. [Google Scholar] [CrossRef] [PubMed]
- Linsell, L.; Malouf, R.; Morris, J.; Kurinczuk, J.J.; Marlow, N. Prognostic Factors for Poor Cognitive Development in Children Born Very Preterm or With Very Low Birth Weight: A Systematic Review. JAMA Pediatr. 2015, 169, 1162–1172. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pereira, M.; Fernández, M.P.; Gómez-Taibo, M.L.; Martínez-López, Z.; Arce, C. A Follow-Up Study of Cognitive Development in Low Risk Preterm Children. Int. J. Environ. Res. Public Health 2020, 17, 2380. [Google Scholar] [CrossRef]
- Stålnacke, J.; Lundequist, A.; Böhm, B.; Forssberg, H.; Smedler, A.C. Individual cognitive patterns and developmental trajectories after preterm birth. Child Neuropsychol. 2015, 21, 648–667. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.W.; Chu, C.H.; Lin, Y.C.; Huang, C.C. Trends in Gestational Age-Related Intelligence Outcomes of School-Age Children Born Very Preterm from 2001 to 2015 in Taiwan. J. Pediatr. 2023, 261, 113584. [Google Scholar] [CrossRef] [PubMed]
- Qasemzadeh, M.; Pirnia, S.; Mohebi, S.; Matin, S.; Ebrahimi, H.; Ebrahimi, H.; Jangholi, E.; Gharehbeglou, M. Correlation of intelligence quotient (IQ) of children younger than 12 years old with history of preterm birth. Galen Med. J. 2013, 3, 120–125. [Google Scholar]
- Heuvelman, H.; Abel, K.; Wicks, S.; Gardner, R.; Johnstone, E.; Lee, B.; Magnusson, C.; Dalman, C.; Rai, D. Gestational age at birth and risk of intellectual disability without a common genetic cause. Eur. J. Epidemiol. 2018, 33, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Twilhaar, E.S.; Wade, R.M.; de Kieviet, J.F.; van Goudoever, J.B.; van Elburg, R.M.; Oosterlaan, J. Cognitive Outcomes of Children Born Extremely or Very Preterm Since the 1990s and Associated Risk Factors: A Meta-analysis and Meta-regression. JAMA Pediatr. 2018, 172, 361–367. [Google Scholar] [CrossRef] [PubMed]
- van Veen, S.; van Wassenaer-Leemhuis, A.G.; Oosterlaan, J.; van Kaam, A.H.; Aarnoudse-Moens, C.S.H. Eight-year-old very and extremely preterm children showed more difficulties in performance intelligence than verbal intelligence. Acta Paediatr. 2020, 109, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.W.; Wang, S.T.; Wang, L.W.; Kao, Y.C.; Chu, C.L.; Wu, C.C.; Hsieh, Y.T.; Chiang, C.H.; Huang, C.C. Behavioral characteristics of autism spectrum disorder in very preterm birth children. Mol. Autism 2019, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Linsell, L.; Malouf, R.; Johnson, S.; Morris, J.; Kurinczuk, J.J.; Marlow, N. Prognostic Factors for Behavioral Problems and Psychiatric Disorders in Children Born Very Preterm or Very Low Birth Weight: A Systematic Review. J. Dev. Behav. Pediatr. JDBP 2016, 37, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Buys, N. Early executive function deficit in preterm children and its association with neurodevelopmental disorders in childhood: A literature review. Int. J. Adolesc. Med. Health 2012, 24, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Twilhaar, E.S.; Pierrat, V.; Marchand-Martin, L.; Benhammou, V.; Kaminski, M.; Ancel, P.Y. Profiles of Functioning in 5.5-Year-Old Very Preterm Born Children in France: The EPIPAGE-2 Study. J. Am. Acad. Child Adolesc. Psychiatry 2022, 61, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, D. Wechsler Intelligence Scale for Children (WISC-IV); Manual Moderno: Mexico City, Mexico, 2007. [Google Scholar]
- Fitzpatrick, A.; Carter, J.; Quigley, M.A. Association of Gestational Age With Verbal Ability and Spatial Working Memory at Age 11. Pediatrics 2016, 138. [Google Scholar] [CrossRef] [PubMed]
- Korpela, S.; Nyman, A.; Munck, P.; Ahtola, A.; Matomäki, J.; Korhonen, T.; Parkkola, R.; Haataja, L. Working memory in very-low-birthweight children at the age of 11 years. Child Neuropsychol. J. Norm. Abnorm. Dev. Child. Adolesc. 2018, 24, 338–353. [Google Scholar] [CrossRef]
- Ford, R.M.; Griffiths, S.; Neulinger, K.; Andrews, G.; Shum, D.H.K.; Gray, P.H. Impaired prospective memory but intact episodic memory in intellectually average 7- to 9-year-olds born very preterm and/or very low birth weight. Child Neuropsychol. 2017, 23, 954–979. [Google Scholar] [CrossRef] [PubMed]
- Kaul, Y.F.; Johansson, M.; Månsson, J.; Stjernqvist, K.; Farooqi, A.; Serenius, F.; Thorell, L.B. Cognitive profiles of extremely preterm children: Full-Scale IQ hides strengths and weaknesses. Acta Paediatr. 2021, 110, 1817–1826. [Google Scholar] [CrossRef] [PubMed]
- Arpi, E.; D’Amico, R.; Lucaccioni, L.; Bedetti, L.; Berardi, A.; Ferrari, F. Worse global intellectual and worse neuropsychological functioning in preterm-born children at preschool age: A meta-analysis. Acta Paediatr. 2019, 108, 1567–1579. [Google Scholar] [CrossRef] [PubMed]
- Begega, A.; Méndez-López, M.; de Iscar, M.J.; Cuesta-Izquierdo, M.; Solís, G.; Fernández-Colomer, B.; Álvarez, L.; Méndez, M.; Arias, J.L. Assessment of the global intelligence and selective cognitive capacities in preterm 8-year-old children. Psicothema 2010, 22, 648–653. [Google Scholar] [PubMed]
- Loe, I.M.; Lee, E.S.; Luna, B.; Feldman, H.M. Executive function skills are associated with reading and parent-rated child function in children born prematurely. Early Hum. Dev. 2012, 88, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Sejer, E.P.F.; Bruun, F.J.; Slavensky, J.A.; Mortensen, E.L.; Schiøler Kesmodel, U. Impact of gestational age on child intelligence, attention and executive function at age 5: A cohort study. BMJ Open 2019, 9, e028982. [Google Scholar] [CrossRef] [PubMed]
- Hollanders, J.J.; Schaëfer, N.; van der Pal, S.M.; Oosterlaan, J.; Rotteveel, J.; Finken, M.J.J. Long-Term Neurodevelopmental and Functional Outcomes of Infants Born Very Preterm and/or with a Very Low Birth Weight. Neonatology 2019, 115, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Jaekel, J.; Sorg, C.; Baeuml, J.; Bartmann, P.; Wolke, D. Head Growth and Intelligence from Birth to Adulthood in Very Preterm and Term Born Individuals. J. Int. Neuropsychol. Soc. JINS 2019, 25, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Soria-Pastor, S.; Gimenez, M.; Narberhaus, A.; Falcon, C.; Botet, F.; Bargallo, N.; Mercader, J.M.; Junque, C. Patterns of cerebral white matter damage and cognitive impairment in adolescents born very preterm. Int. J. Dev. Neurosci. Off. J. Int. Soc. Dev. Neurosci. 2008, 26, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Zubiaurre-Elorza, L.; Soria-Pastor, S.; Junqué, C.; Fernandez-Espejo, D.; Segarra, D.; Bargalló, N.; Romano-Berindoague, C.; Macaya, A. Thalamic changes in a preterm sample with periventricular leukomalacia: Correlation with white-matter integrity and cognitive outcome at school age. Pediatr. Res. 2012, 71, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Guillot, M.; Miller, S.P. The dimensions of white matter injury in preterm neonates. Semin. Perinatol. 2021, 45, 151469. [Google Scholar] [CrossRef] [PubMed]
- Volpe, J.J. Dysmaturation of Premature Brain: Importance, Cellular Mechanisms, and Potential Interventions. Pediatr. Neurol. 2019, 95, 42–66. [Google Scholar] [CrossRef] [PubMed]
- Goddings, A.L.; Roalf, D.; Lebel, C.; Tamnes, C.K. Development of white matter microstructure and executive functions during childhood and adolescence: A review of diffusion MRI studies. Dev. Cogn. Neurosci. 2021, 51, 101008. [Google Scholar] [CrossRef] [PubMed]
- Graff, K.; Tansey, R.; Ip, A.; Rohr, C.; Dimond, D.; Dewey, D.; Bray, S. Benchmarking common preprocessing strategies in early childhood functional connectivity and intersubject correlation fMRI. Dev. Cogn. Neurosci. 2022, 54, 101087. [Google Scholar] [CrossRef] [PubMed]
- Cepeda, N.J.; Blackwell, K.A.; Munakata, Y. Speed isn’t everything: Complex processing speed measures mask individual differences and developmental changes in executive control. Dev. Sci. 2013, 16, 269–286. [Google Scholar] [CrossRef] [PubMed]
- Wehrle, F.M.; Kaufmann, L.; Benz, L.D.; Huber, R.; O’Gorman, R.L.; Latal, B.; Hagmann, C.F. Very preterm adolescents show impaired performance with increasing demands in executive function tasks. Early Hum. Dev. 2016, 92, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Bruggink, J.L.M.; Van Braeckel, K.N.; Bos, A.F. The early motor repertoire of children born preterm is associated with intelligence at school age. Pediatrics 2010, 125, e1356–e1363. [Google Scholar] [CrossRef] [PubMed]
- Eryigit Madzwamuse, S.; Baumann, N.; Jaekel, J.; Bartmann, P.; Wolke, D. Neuro-cognitive performance of very preterm or very low birth weight adults at 26 years. J. Child Psychol. Psychiatry 2015, 56, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Roze, E.; Reijneveld, S.A.; Stewart, R.E.; Bos, A.F. Multi-domain cognitive impairments at school age in very preterm-born children compared to term-born peers. BMC Pediatr. 2021, 21, 169. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, E.K.; Song, H.; Cheon, J.E.; Kim, B.N.; Kim, H.S.; Shin, S.H. Association of Brain Microstructure and Functional Connectivity With Cognitive Outcomes and Postnatal Growth Among Early School-Aged Children Born with Extremely Low Birth Weight. JAMA Netw. Open 2023, 6, e230198. [Google Scholar] [CrossRef] [PubMed]
- Hinojosa-Rodríguez, M.; Jiménez, J.O.D.L.; Colín, M.E.J.; Moreira, E.G.; Bautista, C.S.F.; Harmony, T. Long-term therapeutic effects of Katona therapy in moderate-to-severe perinatal brain damage. Neurosci. Lett. 2020, 738, 135345. [Google Scholar] [CrossRef] [PubMed]
- Harmony, T. Outcome of infants at risk of brain damage after Katona neurohabilitation therapy. Int. J. Neurorehabilit. 2017, 4, 2376-0281. [Google Scholar] [CrossRef]
- García-Bermúdez, O.; Cruz-Quintana, F.; Pérez-García, M.; Hidalgo-Ruzzante, N.; Fernández-Alcántara, M.; Pérez-Marfil, M.N. Improvement of executive functions after the application of a neuropsychological intervention program (PEFEN) in pre-term children. Child. Youth Serv. Rev. 2019, 98, 328–336. [Google Scholar] [CrossRef]
| Preterm (n = 39) | Full Term (n = 35) | X2 | |||
|---|---|---|---|---|---|
| Variable | n | % | n | % | |
| Sex | 0.525 | ||||
| Girls | 20 | 51.3 | 15 | 42.9 | |
| Boys | 19 | 48.7 | 20 | 57.1 | |
| Dominance | 1.79 | ||||
| Right-handed | 33 | 84.6 | 33 | 94.3 | |
| Left-handed | 2.29 | 0.970 | 2.62 | 0.651 | |
| Schooling at the start of the study | 12.7 * | ||||
| 3rd Preschool | 25 | 64 | 23 | 8 | |
| 1st Elementary | 14 | 36 | 77 | 27 | |
| Mean | SD | Mean | SD | Mann–Whitney’sU | |
| Gestational age | 31.7 | 2.8 | 38.7 | 0.85 | 12.0 * |
| Birth weight (grams) | 1645.77 | 463 | 3204.4 | 553 | 0.0 * |
| Maternal education (yrs) | 15.1 | 4.1 | 15.3 | 4 | 675.5 |
| Risk factors (frequency) | 6 | 3 | - | - | - |
| Age of assessment (months) | |||||
| 1st Assessment | 74.7 | 4.3 | 76.3 | 5.5 | 546.5 |
| 2nd Assessment | 87 | 3.7 | 87.8 | 4.4 | 595.5 |
| 3rd Assessment | 100.1 | 3.4 | 100.1 | 3.5 | 671.5 |
| Preterm (n = 39) | Full Term (n = 35) | |||||||
|---|---|---|---|---|---|---|---|---|
| Index | Age | Mean | SD | CI %95 | Mean | SD | CI %95 | p |
| VCI | ||||||||
| 6 years | 80.9 | 10.9 | 77.3–84.4 | 98.1 | 7.4 | 95.5–100.6 | 0.000 * | |
| 7 years | 86.2 | 10.2 | 82.9–89.5 | 103.2 | 7.9 | 100.5–105.9 | 0.000 * | |
| 8 years | 84.5 | 10.7 | 81–88 | 105.7 | 11.4 | 101.5–109.4 | 0.000 * | |
| PRI | ||||||||
| 6 years | 87.3 | 11.1 | 83.6–90.9 | 99.3 | 7.8 | 96.6–102 | 0.000 * | |
| 7 years | 93.0 | 11.7 | 89.1–96.8 | 104.3 | 9.5 | 101.1–107.6 | 0.001 * | |
| 8 years | 93.6 | 13.9 | 89.1–98.2 | 103.8 | 11.5 | 99.9–107.8 | 0.029 * | |
| WMI | ||||||||
| 6 years | 81.3 | 16.2 | 76–86.5 | 94.4 | 6.3 | 92.2–96.6 | 0.000 * | |
| 7 years | 88.1 | 12.5 | 84.1– 92.2 | 96.2 | 9.0 | 93.1–99.3 | 0.041 * | |
| 8 years | 89.8 | 14.2 | 85.2–94.4 | 97.2 | 9.0 | 94.1–100.3 | 0.056 | |
| PSI | ||||||||
| 6 years | 93.3 | 13.4 | 88.9–97.6 | 103.9 | 11.1 | 100–107.7 | 0.006 | |
| 7 years | 101.8 | 16.5 | 96.4–107.2 | 110 | 12.1 | 105.9–102.7 | 0.163 | |
| 8 years | 95.2 | 15.0 | 90.3–100.1 | 101.5 | 10.1 | 98–105 | 0.305 | |
| IQ | ||||||||
| 6 years | 81.8 | 12.4 | 77.8–85.8 | 98.77 | 7.1 | 96.3–101.2 | 0.000 * | |
| 7 years | 89.4 | 12.5 | 85.4–93.5 | 104.9 | 9.0 | 101.8–108.0 | 0.002 * | |
| 8 years | 87.9 | 13.8 | 83.4–92.4 | 103.6 | 9.5 | 100.3–106.9 | 0.001 * |
| Index | Multivariate Effects | Sphericity | Intra-Subjects Effects Test | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wilks Age*Group | F | n | W (p) | Statistic | Effect | DF | Sum Square | Mean Square | F | ||
| VCI | 0.922 | 2.8 | 71 | 0.995 (0.838) | Sphericity assumed | Age | 2 | 1589.0 | 794.5 | 17.7 ** | 0.20 |
| Age*group | 2 | 268.7 | 134.3 | 2.9 | 0.04 | ||||||
| PRI | 0.955 | 1.5 | 67 | 0.838 (0.003) | Huynh-Feldt | Age | 1.79 | 909.4 | 507.9 | 11.6 ** | 0.15 |
| Age*group | 1.79 | 141.0 | 78.7 | 1.80 | 0.02 | ||||||
| WMI | 0.871 | 4.8 * | 69 | 0.918 (0.060) | Sphericity assumed | Age | 2 | 1105.8 | 552.9 | 11.1 ** | 0.14 |
| Age*group | 2 | 627.6 | 313.8 | 6.3 ** | 0.08 | ||||||
| PSI | 0.959 | 1.4 | 69 | 0.958 (0.240) | Sphericity assumed | Age | 2 | 2827.7 | 1413.8 | 19.4 ** | 0.22 |
| Age*group | 2 | 196.1 | 98.0 | 1.3 | 0.02 | ||||||
| IQ | 0.972 | 0.977 | 71 | 0.902 (0.030) | Huynh-Feldt | Age | 1.89 | 1666.0 | 878.8 | 25.5 ** | 0.27 |
| Age*group | 1.89 | 72.2 | 38.0 | 1.1 | 0.01 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-González, C.; Ricardo-Garcell, J.; Alvarez-Núñez, D.; Galindo-Aldana, G. Intellectual Development in Mexican Preterm Children at Risk of Perinatal Brain Damage: A Longitudinal Study. Children 2024, 11, 652. https://doi.org/10.3390/children11060652
Torres-González C, Ricardo-Garcell J, Alvarez-Núñez D, Galindo-Aldana G. Intellectual Development in Mexican Preterm Children at Risk of Perinatal Brain Damage: A Longitudinal Study. Children. 2024; 11(6):652. https://doi.org/10.3390/children11060652
Chicago/Turabian StyleTorres-González, Cynthia, Josefina Ricardo-Garcell, Daniel Alvarez-Núñez, and Gilberto Galindo-Aldana. 2024. "Intellectual Development in Mexican Preterm Children at Risk of Perinatal Brain Damage: A Longitudinal Study" Children 11, no. 6: 652. https://doi.org/10.3390/children11060652
APA StyleTorres-González, C., Ricardo-Garcell, J., Alvarez-Núñez, D., & Galindo-Aldana, G. (2024). Intellectual Development in Mexican Preterm Children at Risk of Perinatal Brain Damage: A Longitudinal Study. Children, 11(6), 652. https://doi.org/10.3390/children11060652







