Sex Differences in White Matter Diffusivity in Children with Developmental Dyslexia
Abstract
1. Introduction
1.1. The Reading Network: The Healthy Brain
1.1.1. White Matter Tracts Supporting the Reading Network
Superior Longitudinal Fasciculus
Inferior Longitudinal Fasciculus
Uncinate Fasciculus
Corpus Callosum
1.1.2. White Matter Diffusivity in Typical Readers
1.2. Neurobehavioral Abnormalities in the Reading Network in Children with DD
1.3. Sex Differences
1.3.1. Sex Differences in Children with Typical Reading Skills
1.3.2. Sex Differences in Children with DD
1.4. Current Study
2. Materials and Methods
2.1. Participants
2.2. Identifying Dyslexic (DD) and Typically Developing (TD) Readers
2.3. Scanning and DWI Imaging
2.4. White Matter Tract Reconstruction
2.5. Analysis
3. Results
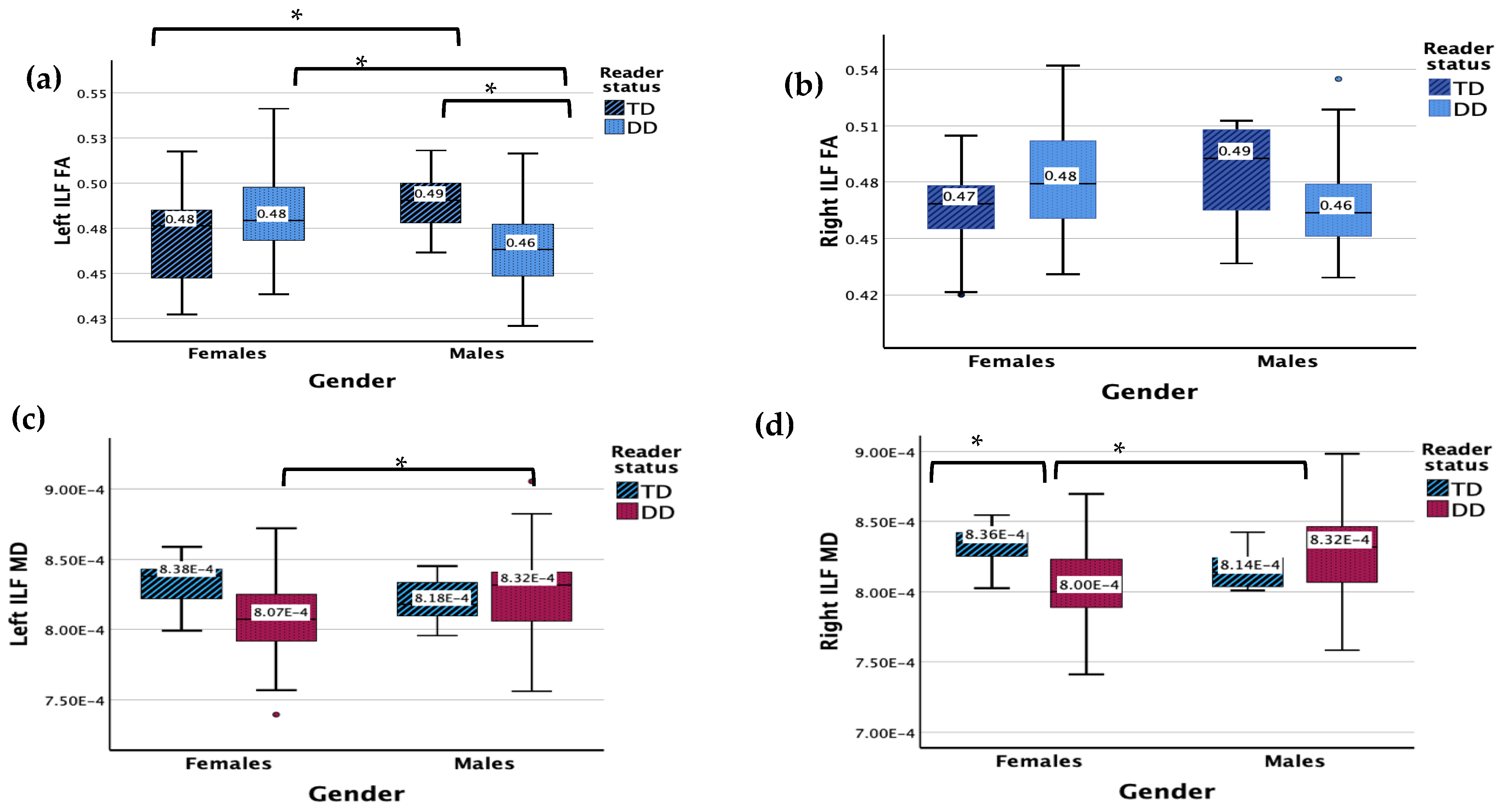
3.1. Inferior Longitudinal Fasciculus (ILF)
3.2. Superior Longitudinal Fasciculus (SLF) Parietal
3.3. Superior Longitudinal Fasciculus (SLF) Temporal
3.4. Uncinate Fasciculus (UF)
3.5. Corpus Callosum (CC)
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Katusic, S.K.; Colligan, R.C.; Barbaresi, W.J.; Schaid, D.J.; Jacobsen, S.J. Incidence of Reading Disability in a Population-Based Birth Cohort, 1976–1982, Rochester, Minn. Mayo Clin. Proc. 2001, 76, 1081–1092. [Google Scholar] [CrossRef]
- Norton, E.S.; Beach, S.D.; Gabrieli, J.D. Neurobiology of Dyslexia. Curr. Opin. Neurobiol. 2015, 30, 73–78. [Google Scholar] [CrossRef]
- Gutierrez Fresneda, R.; Planelles, M. Effects of the Development of Reading Comprehension Questions on Learning Improvement. Rev. Leng. Mod. 2022, 28, 61–74. [Google Scholar] [CrossRef]
- Vandermosten, M.; Boets, B.; Wouters, J.; Ghesquière, P. A Qualitative and Quantitative Review of Diffusion Tensor Imaging Studies in Reading and Dyslexia. Neurosci. Biobehav. Rev. 2012, 36, 1532–1552. [Google Scholar] [CrossRef]
- Fields, R.D. Change in the Brain’s White Matter. Science 2010, 330, 768–769. [Google Scholar] [CrossRef]
- Michel, L.C.; McCormick, E.M.; Kievit, R.A. Grey and White Matter Metrics Demonstrate Distinct and Complementary Prediction of Differences in Cognitive Performance in Children: Findings from ABCD (N = 11,876). bioRxiv 2023. [Google Scholar] [CrossRef]
- Wise Younger, J.; Tucker-Drob, E.; Booth, J.R. Longitudinal Changes in Reading Network Connectivity Related to Skill Improvement. NeuroImage 2017, 158, 90–98. [Google Scholar] [CrossRef]
- Beaulieu, C.; Plewes, C.; Paulson, L.A.; Roy, D.; Snook, L.; Concha, L.; Phillips, L. Imaging Brain Connectivity in Children with Diverse Reading Ability. NeuroImage 2005, 25, 1266–1271. [Google Scholar] [CrossRef]
- Pugh, K.R.; Mencl, W.E.; Jenner, A.R.; Katz, L.; Frost, S.J.; Lee, J.R.; Shaywitz, S.E.; Shaywitz, B.A. Neurobiological Studies of Reading and Reading Disability. J. Commun. Disord. 2001, 34, 479–492. [Google Scholar] [CrossRef]
- Sandak, R.; Mencl, W.E.; Frost, S.J.; Rueckl, J.G.; Katz, L.; Moore, D.L.; Mason, S.A.; Fulbright, R.K.; Constable, R.T.; Pugh, K.R. The Neurobiology of Adaptive Learning in Reading: A Contrast of Different Training Conditions. Cogn. Affect. Behav. Neurosci. 2004, 4, 67–88. [Google Scholar] [CrossRef]
- Yu, X.; Raney, T.; Perdue, M.V.; Zuk, J.; Ozernov-Palchik, O.; Becker, B.L.C.; Raschle, N.M.; Gaab, N. Emergence of the Neural Network Underlying Phonological Processing from the Prereading to the Emergent Reading Stage: A Longitudinal Study. Hum. Brain Mapp. 2018, 39, 2047–2063. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kanjlia, S.; Merabet, L.B.; Bedny, M. Development of the Visual Word Form Area Requires Visual Experience: Evidence from Blind Braille Readers. J. Neurosci. 2017, 37, 11495–11504. [Google Scholar] [CrossRef] [PubMed]
- Glezer, L.S.; Jiang, X.; Riesenhuber, M. Evidence for Highly Selective Neuronal Tuning to Whole Words in the “Visual Word Form Area”. Neuron 2009, 62, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Richlan, F.; Kronbichler, M.; Wimmer, H. Meta-Analyzing Brain Dysfunctions in Dyslexic Children and Adults. NeuroImage 2011, 56, 1735–1742. [Google Scholar] [CrossRef] [PubMed]
- Simos, P.G.; Fletcher, J.M.; Sarkari, S.; Billingsley-Marshall, R.; Denton, C.A.; Papanicolaou, A.C. Intensive Instruction Affects Brain Magnetic Activity Associated with Oral Word Reading in Children with Persistent Reading Disabilities. J. Learn. Disabil. 2007, 40, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Shaywitz, B.A.; Shaywitz, S.E.; Pugh, K.R.; Mencl, W.E.; Fulbright, R.K.; Skudlarski, P.; Constable, R.T.; Marchione, K.E.; Fletcher, J.M.; Lyon, G.R.; et al. Disruption of Posterior Brain Systems for Reading in Children with Developmental Dyslexia. Biol. Psychiatry 2002, 10, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, K.M.; McQueeny, T.; Howe, S.R.; Shear, P.; Szaflarski, J. Superior Longitudinal Fasciculus and Language Functioning in Healthy Aging. Brain Res. 2014, 1562, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Porto de Oliveira, J.V.M.; Raquelo-Menegassio, A.F.; Maldonado, I.L. What’s Your Name Again? A Review of the Superior Longitudinal and Arcuate Fasciculus Evolving Nomenclature. Clin. Anat. 2021, 34, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Janelle, F.; Iorio-Morin, C.; D’amour, S.; Fortin, D. Superior Longitudinal Fasciculus: A Review of the Anatomical Descriptions with Functional Correlates. Front. Neurol. 2022, 13, 794618. [Google Scholar] [CrossRef]
- Rolheiser, T.; Stamatakis, E.A.; Tyler, L.K. Dynamic Processing in the Human Language System: Synergy between the Arcuate Fascicle and Extreme Capsule. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 16949–16957. [Google Scholar] [CrossRef]
- Rauschecker, A.M.; Deutsch, G.K.; Ben-Shachar, M.; Schwartzman, A.; Perry, L.M.; Dougherty, R.F. Reading Impairment in a Patient with Missing Arcuate Fasciculus. Neuropsychologia 2009, 47, 180–194. [Google Scholar] [CrossRef]
- Ffytche, D.H. The Hodology of Hallucinations. Cortex J. Devoted Study Nerv. Syst. Behav. 2008, 44, 1067–1083. [Google Scholar] [CrossRef]
- Ross, E.D. Sensory-Specific Amnesia and Hypoemotionality in Humans and Monkeys: Gateway for Developing a Hodology of Memory. Cortex J. Devoted Study Nerv. Syst. Behav. 2008, 44, 1010–1022. [Google Scholar] [CrossRef]
- Papagno, C. Naming and the Role of the Uncinate Fasciculus in Language Function. Curr. Neurol. Neurosci. Rep. 2011, 11, 553–559. [Google Scholar] [CrossRef]
- Catani, M.; Mesulam, M. What Is a Disconnection Syndrome? Cortex J. Devoted Study Nerv. Syst. Behav. 2008, 44, 911–913. [Google Scholar] [CrossRef] [PubMed]
- Grossman, M.; McMillan, C.; Moore, P.; Ding, L.; Glosser, G.; Work, M.; Gee, J. What’s in a Name: Voxel-Based Morphometric Analyses of MRI and Naming Difficulty in Alzheimer’s Disease, Frontotemporal Dementia and Corticobasal Degeneration. Brain J. Neurol. 2004, 127, 628–649. [Google Scholar] [CrossRef]
- Von Der Heide, R.J.; Skipper, L.M.; Klobusicky, E.; Olson, I.R. Dissecting the Uncinate Fasciculus: Disorders, Controversies and a Hypothesis. Brain 2013, 136, 1692–1707. [Google Scholar] [CrossRef] [PubMed]
- Olivé, G.; Peñaloza, C.; Vaquero, L.; Laine, M.; Martin, N.; Rodriguez-Fornells, A. The Right Uncinate Fasciculus Supports Verbal Short-Term Memory in Aphasia. Brain Struct. Funct. 2023, 228, 875–893. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, D.J.; Krafft, C.E.; Schwarz, N.F.; Chi, L.; Rodrigue, A.L.; Pierce, J.E.; Allison, J.D.; Yanasak, N.E.; Liu, T.; Davis, C.L.; et al. The Relationship between Uncinate Fasciculus White Matter Integrity and Verbal Memory Proficiency in Children. Neuroreport 2014, 25, 921–925. [Google Scholar] [CrossRef]
- Arrington, C.N.; Kulesz, P.A.; Juranek, J.; Cirino, P.T.; Fletcher, J.M. White Matter Microstructure Integrity in Relation to Reading Proficiency. Brain Lang. 2017, 174, 103–111. [Google Scholar] [CrossRef]
- Frye, R.E.; Hasan, K.; Xue, L.; Strickland, D.; Malmberg, B.; Liederman, J.; Papanicolaou, A. Splenium Microstructure Is Related to Two Dimensions of Reading Skill. NeuroReport 2008, 19, 1627–1631. [Google Scholar] [CrossRef] [PubMed]
- Bartha-Doering, L.; Schwartz, E.; Kollndorfer, K.; Fischmeister, F.P.S.; Novak, A.; Langs, G.; Werneck, H.; Prayer, D.; Seidl, R.; Kasprian, G. Effect of Corpus Callosum Agenesis on the Language Network in Children and Adolescents. Brain Struct. Funct. 2021, 226, 701–713. [Google Scholar] [CrossRef]
- Dougherty, R.F.; Ben-Shachar, M.; Deutsch, G.K.; Hernandez, A.; Fox, G.R.; Wandell, B.A. Temporal-Callosal Pathway Diffusivity Predicts Phonological Skills in Children. Proc. Natl. Acad. Sci. USA 2007, 104, 8556–8561. [Google Scholar] [CrossRef]
- Basser, P.J. Inferring Microstructural Features and the Physiological State of Tissues from Diffusion-Weighted Images. NMR Biomed. 1995, 8, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Ment, L.R.; Scheinost, D.; Constable, T. 14-Microstructural and Functional Connectivity in the Developing Brain. In Swaiman’s Pediatric Neurology, 6th ed.; Swaiman, K.F., Ashwal, S., Ferriero, D.M., Schor, N.F., Finkel, R.S., Gropman, A.L., Pearl, P.L., Shevell, M.I., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 97–106. ISBN 978-0-323-37101-8. [Google Scholar]
- Shin, J.; Rowley, J.; Chowdhury, R.; Jolicoeur, P.; Klein, D.; Grova, C.; Rosa-Neto, P.; Kobayashi, E. Inferior Longitudinal Fasciculus’ Role in Visual Processing and Language Comprehension: A Combined MEG-DTI Study. Front. Neurosci. 2019, 13, 875. [Google Scholar] [CrossRef]
- Barquero, L.A.; Davis, N.; Cutting, L.E. Neuroimaging of Reading Intervention: A Systematic Review and Activation Likelihood Estimate Meta-Analysis. PLoS ONE 2014, 9, e83668. [Google Scholar] [CrossRef] [PubMed]
- Brunswick, N.; McCrory, E.; Price, C.J.; Frith, C.D.; Frith, U. Explicit and Implicit Processing of Words and Pseudowords by Adult Developmental Dyslexics. Brain 1999, 122, 1901–1917. [Google Scholar] [CrossRef]
- Van Der Auwera, S.; Vandermosten, M.; Wouters, J.; Ghesquière, P.; Vanderauwera, J. A Three-Time Point Longitudinal Investigation of the Arcuate Fasciculus throughout Reading Acquisition in Children Developing Dyslexia. NeuroImage 2021, 237, 118087. [Google Scholar] [CrossRef]
- Carter, J.C.; Lanham, D.C.; Cutting, L.E.; Clements-Stephens, A.M.; Chen, X.; Hadzipasic, M.; Kim, J.; Denckla, M.B.; Kaufmann, W.E. A Dual DTI Approach to Analyzing White Matter in Children with Dyslexia. Psychiatry Res. 2009, 172, 215–219. [Google Scholar] [CrossRef]
- Steinbrink, C.; Vogt, K.; Kastrup, A.; Müller, H.-P.; Juengling, F.D.; Kassubek, J.; Riecker, A. The Contribution of White and Gray Matter Differences to Developmental Dyslexia: Insights from DTI and VBM at 3.0T. Neuropsychologia 2008, 46, 3170–3178. [Google Scholar] [CrossRef]
- Su, M.; Zhao, J.; de Schotten, M.T.; Zhou, W.; Gong, G.; Ramus, F.; Shu, H. Alterations in White Matter Pathways Underlying Phonological and Morphological Processing in Chinese Developmental Dyslexia. Dev. Cogn. Neurosci. 2018, 31, 11. [Google Scholar] [CrossRef] [PubMed]
- Yeatman, J.D.; Dougherty, R.F.; Ben-Shachar, M.; Wandell, B.A. Development of White Matter and Reading Skills. Proc. Natl. Acad. Sci. USA 2012, 109, E3045–E3053. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Thiebaut de Schotten, M.; Altarelli, I.; Dubois, J.; Ramus, F. Altered Hemispheric Lateralization of White Matter Pathways in Developmental Dyslexia: Evidence from Spherical Deconvolution Tractography. Cortex J. Devoted Study Nerv. Syst. Behav. 2016, 76, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Lebel, C.; Benischek, A.; Geeraert, B.; Holahan, J.; Shaywitz, S.; Bakhshi, K.; Shaywitz, B. Developmental Trajectories of White Matter Structure in Children with and without Reading Impairments. Dev. Cogn. Neurosci. 2019, 36, 100633. [Google Scholar] [CrossRef] [PubMed]
- Hynd, G.W.; Hall, J.; Novey, E.S.; Eliopulos, D.; Black, K.; Gonzalez, J.J.; Edmonds, J.E.; Riccio, C.; Cohen, M. Dyslexia and Corpus Callosum Morphology. Arch. Neurol. 1995, 52, 32–38. [Google Scholar] [CrossRef] [PubMed]
- von Plessen, K.; Lundervold, A.; Duta, N.; Heiervang, E.; Klauschen, F.; Smievoll, A.I.; Ersland, L.; Hugdahl, K. Less Developed Corpus Callosum in Dyslexic Subjects—A Structural MRI Study. Neuropsychologia 2002, 40, 1035–1044. [Google Scholar] [CrossRef]
- Burman, D.D.; Bitan, T.; Booth, J.R. Sex Differences in Neural Processing of Language among Children. Neuropsychologia 2008, 46, 1349–1362. [Google Scholar] [CrossRef] [PubMed]
- Lynn, R.; Mikk, J. Sex Differences in Reading Achievement. Trames J. Humanit. Soc. Sci. 2009, 13, 3. [Google Scholar] [CrossRef]
- Reilly, D.; Neumann, D.L.; Andrews, G. Gender Differences in Reading and Writing Achievement: Evidence from the National Assessment of Educational Progress (NAEP). Am. Psychol. 2019, 74, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Spencer, M.; Cutting, L.E. Relations among Executive Function, Decoding, and Reading Comprehension: An Investigation of Sex Differences. Discourse Process. 2021, 58, 42–59. [Google Scholar] [CrossRef]
- Adani, S.; Cepanec, M. Sex Differences in Early Communication Development: Behavioral and Neurobiological Indicators of More Vulnerable Communication System Development in Boys. Croat. Med. J. 2019, 60, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Liang, X.; Ou, J.; Li, H.; Luo, Y.; Tan, L.H. Sex Differences in Functional Brain Networks for Language. Cereb. Cortex 2020, 30, 1528–1537. [Google Scholar] [CrossRef] [PubMed]
- Sommer, I.E.C. Do Women Really Have More Bilateral Language Representation than Men? A Meta-Analysis of Functional Imaging Studies. Brain 2004, 127, 1845–1852. [Google Scholar] [CrossRef] [PubMed]
- Kanaan, R.A.; Allin, M.; Picchioni, M.; Barker, G.J.; Daly, E.; Shergill, S.S.; Woolley, J.; McGuire, P.K. Gender Differences in White Matter Microstructure. PLoS ONE 2012, 7, e38272. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulou, G.; Meier, E.L.; Kasselimis, D.; Pan, Y.; Tsolakopoulos, D.; Velonakis, G.; Karavasilis, E.; Kelekis, N.L.; Goutsos, D.; Potagas, C.; et al. Investigating Gray and White Matter Structural Substrates of Sex Differences in the Narrative Abilities of Healthy Adults. Front. Neurosci. 2020, 13, 1424. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Mody, M.; Fujioka, T.; Kimura, Y.; Okazawa, H.; Kosaka, H. Sex Differences in White Matter Pathways Related to Language Ability. Front. Neurosci. 2019, 13, 898. [Google Scholar] [CrossRef]
- Zhao, J.; Song, Z.; Zhao, Y.; Thiebaut de Schotten, M.; Altarelli, I.; Ramus, F. White Matter Connectivity in Uncinate Fasciculus Accounts for Visual Attention Span in Developmental Dyslexia. Neuropsychologia 2022, 177, 108414. [Google Scholar] [CrossRef] [PubMed]
- Berninger, V.W.; Nielsen, K.H.; Abbott, R.D.; Wijsman, E.; Raskind, W. Gender Differences in Severity of Writing and Reading Disabilities. J. Sch. Psychol. 2008, 46, 151–172. [Google Scholar] [CrossRef]
- Krafnick, A.J.; Evans, T.M. Neurobiological Sex Differences in Developmental Dyslexia. Front. Psychol. 2019, 9, 2669. [Google Scholar] [CrossRef]
- Giofrè, D.; Allen, K.; Toffalini, E.; Mammarella, I.C.; Caviola, S. Decoding Gender Differences: Intellectual Profiles of Children with Specific Learning Disabilities. Intelligence 2022, 90, 101615. [Google Scholar] [CrossRef]
- Altarelli, I.; Monzalvo, K.; Iannuzzi, S.; Fluss, J.; Billard, C.; Ramus, F.; Dehaene-Lambertz, G. A Functionally Guided Approach to the Morphometry of Occipitotemporal Regions in Developmental Dyslexia: Evidence for Differential Effects in Boys and Girls. J. Neurosci. 2013, 33, 11296–11301. [Google Scholar] [CrossRef] [PubMed]
- Rumsey, J.M.; Nace, K.; Donohue, B.; Wise, D.; Maisog, J.M.; Andreason, P. A Positron Emission Tomographic Study of Impaired Word Recognition and Phonological Processing in Dyslexic Men. Arch. Neurol. 1997, 54, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Lambe, E.K. Dyslexia, Gender, and Brain Imaging. Neuropsychologia 1999, 37, 521–536. [Google Scholar] [CrossRef] [PubMed]
- Wechsler Abbreviated Scale of Intelligence|Second Edition. Available online: https://www.pearsonassessments.com/store/usassessments/en/Store/Professional-Assessments/Cognition-%26-Neuro/Wechsler-Abbreviated-Scale-of-Intelligence-%7C-Second-Edition/p/100000593.html (accessed on 21 January 2024).
- Blackwell, T.L. Test Review: Woodcock, R. W., McGrew, K. S., & Mather, N. Woodcock-Johnson® III Test. Riverside Publishing Company. Itasca, IL. Rehabil. Couns. Bull. 2001, 44, 232–235. [Google Scholar] [CrossRef]
- Tarar, J.M.; Meisinger, E.B.; Dickens, R.H. Test Review: Test of Word Reading Efficiency–Second Edition (TOWRE-2) by Torgesen, J. K., Wagner, R. K., & Rashotte, C. A. Can. J. Sch. Psychol. 2015, 30, 320–326. [Google Scholar] [CrossRef]
- Newcomer: Standardized Reading Inventory—Google Scholar. Available online: https://scholar.google.com/scholar_lookup?title=Standardized+Reading+Inventory&author=PL+Newcomer&publication_year=1999& (accessed on 21 January 2024).
- Grizzle, R.; Davis, A. Woodcock-Johnson Psychoeducational Test Battery; American Psychological Association (APA): Washington, DC, USA, 2011; pp. 2723–2725. [Google Scholar]
- Parker, D.C.; Zaslofsky, A.F.; Burns, M.K.; Kanive, R.; Hodgson, J.; Scholin, S.E.; Klingbeil, D.A. A Brief Report of the Diagnostic Accuracy of Oral Reading Fluency and Reading Inventory Levels for Reading Failure Risk Among Second- and Third-Grade Students. Read. Writ. Q. 2015, 31, 56–67. [Google Scholar] [CrossRef]
- Doty, S.J.; Hixson, M.D.; Decker, D.M.; Reynolds, J.L.; Drevon, D.D. Reliability and Validity of Advanced Phonics Measures. J. Psychoeduc. Assess. 2015, 33, 503–521. [Google Scholar] [CrossRef]
- Dean, R.S. Woodcock-Johnson III Tests of Achievement. In Encyclopedia of Child Behavior and Development; Goldstein, S., Naglieri, J.A., Eds.; Springer: Boston, MA, USA, 2011; pp. 1575–1576. ISBN 978-0-387-79061-9. [Google Scholar]
- Hong, G.-S.; Lee, C.W.; Kim, M.; Jang, S.W.; Chung, S.R.; Yoon, G.Y.; Kim, J.K. Diffusion-Weighted Imaging with Reverse Phase-Encoding Polarity: The Added Value to the Conventional Diffusion-Weighted Imaging in Differentiating Acute Infarctions from Hyperintense Brainstem Artifacts. Eur. Radiol. 2017, 27, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Irfanoglu, M.O.; Nayak, A.; Jenkins, J.; Hutchinson, E.B.; Sadeghi, N.; Thomas, C.P.; Pierpaoli, C. DR-TAMAS: Diffeomorphic Registration for Tensor Accurate Alignment of Anatomical Structures. NeuroImage 2016, 132, 439–454. [Google Scholar] [CrossRef]
- Beer, J.C.; Tustison, N.J.; Cook, P.A.; Davatzikos, C.; Sheline, Y.I.; Shinohara, R.T.; Linn, K.A. Longitudinal ComBat: A Method for Harmonizing Longitudinal Multi-Scanner Imaging Data. NeuroImage 2020, 220, 117129. [Google Scholar] [CrossRef]
- Fortin, J.-P.; Cullen, N.; Sheline, Y.I.; Taylor, W.D.; Aselcioglu, I.; Cook, P.A.; Adams, P.; Cooper, C.; Fava, M.; McGrath, P.J.; et al. Harmonization of Cortical Thickness Measurements across Scanners and Sites. NeuroImage 2018, 167, 104–120. [Google Scholar] [CrossRef] [PubMed]
- Yendiki, A. Automated Probabilistic Reconstruction of White-Matter Pathways in Health and Disease Using an Atlas of the Underlying Anatomy. Front. Neuroinformatics 2011, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Fischl, B. FreeSurfer. NeuroImage 2012, 62, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Figley, C.R.; Uddin, M.N.; Wong, K.; Kornelsen, J.; Puig, J.; Figley, T.D. Potential Pitfalls of Using Fractional Anisotropy, Axial Diffusivity, and Radial Diffusivity as Biomarkers of Cerebral White Matter Microstructure. Front. Neurosci. 2022, 15, 799576. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, K.E.; Abaryan, Z.; Laltoo, E.; Hernandez, L.M.; Gandal, M.J.; McCracken, J.T.; Thompson, P.M. White Matter Microstructure Shows Sex Differences in Late Childhood: Evidence from 6797 Children. Hum. Brain Mapp. 2023, 44, 535–548. [Google Scholar] [CrossRef]
- Roy, E.; Richie-Halford, A.; Kruper, J.; Narayan, M.; Bloom, D.; Nedelec, P.; Rauschecker, A.M.; Sugrue, L.P.; Brown, T.T.; Jernigan, T.L.; et al. White Matter and Literacy: A Dynamic System in Flux. Dev. Cogn. Neurosci. 2024, 65, 101341. [Google Scholar] [CrossRef] [PubMed]
- Aylward, E.H.; Richards, T.L.; Berninger, V.W.; Nagy, W.E.; Field, K.M.; Grimme, A.C.; Richards, A.L.; Thomson, J.B.; Cramer, S.C. Instructional Treatment Associated with Changes in Brain Activation in Children with Dyslexia. Neurology 2003, 61, 212–219. [Google Scholar] [CrossRef]
- Meisler, S.L.; Gabrieli, J.D.E. A Large-Scale Investigation of White Matter Microstructural Associations with Reading Ability. NeuroImage 2022, 249, 118909. [Google Scholar] [CrossRef]
- Smith, E.; Reimer, D. Understanding Gender Inequality in Children’s Reading Behavior: New Insights from Digital Behavioral Data. Child Dev. 2024, 95, 625–635. [Google Scholar] [CrossRef]
- Yeatman, J.D.; Huber, E. Sensitive periods for white matter plasticity and reading intervention. bioRxiv 2019. [Google Scholar] [CrossRef]
- Gabrieli, J.D. Dyslexia: A new synergy between education and cognitive neuroscience. Science 2009, 325, 280–283. [Google Scholar] [CrossRef]
- Ambinakudige, S.; Parisi, D.; Cappello, G.C.; Lotfata, A. Diversity or Segregation? A Multi-Decadal Spatial Analysis of Demographics of Atlanta Neighborhoods. Spat. Demogr. 2017, 5, 123–144. [Google Scholar] [CrossRef]
- Yoshida, S.; Oishi, K.; Faria, A.V.; Mori, S. Diffusion Tensor Imaging of Normal Brain Development. Pediatr. Radiol. 2013, 43, 15–27. [Google Scholar] [CrossRef]
- Tournier, J.-D.; Mori, S.; Leemans, A. Diffusion Tensor Imaging and Beyond. Magn. Reson. Med. 2011, 65, 1532–1556. [Google Scholar] [CrossRef]
- Mukherjee, P.; Miller, J.H.; Shimony, J.S.; Conturo, T.E.; Lee, B.C.P.; Almli, C.R.; McKinstry, R.C. Normal Brain Maturation during Childhood: Developmental Trends Characterized with Diffusion-Tensor MR Imaging. Radiology 2001, 221, 349–358. [Google Scholar] [CrossRef]
- Neil, J.J.; Shiran, S.I.; McKinstry, R.C.; Schefft, G.L.; Snyder, A.Z.; Almli, C.R.; Akbudak, E.; Aronovitz, J.A.; Miller, J.P.; Lee, B.C.; et al. Normal Brain in Human Newborns: Apparent Diffusion Coefficient and Diffusion Anisotropy Measured by Using Diffusion Tensor MR Imaging. Radiology 1998, 209, 57–66. [Google Scholar] [CrossRef]


| DD | TD | |||
|---|---|---|---|---|
| Males (n = 31) | Females (n = 36) | Males (n = 11) | Females (n = 9) | |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Age | 8.97 (0.84) | 8.80 (0.71) | 8.73 (0.79) | 8.78 (0.44) |
| WASI-2 FSIQ | 92.30 (7.35) | 91.92 (9.45) | 114.10 (11.74) | 110.00 (14.49) |
| WJ3 Basic | 82.68 (8.52) | 87.72 (7.17) | 112.73 (6.54) | 110.78 (6.24) |
| TOWRE-2 | 69.10 (8.94) | 73.31 (8.63) | 107.45 (10.45) | 103.00 (12.42) |
| SRI-2 | 73.78 (12.17) | 77.75 (9.82) | 105.18 (15.65) | 109.33 (15.18) |
| % With ADHD | 51.61% | 19.45% | 0% | 0% |
| % With SLI | 22.58% | 27.78% | 0% | 0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, G.; Arrington, C.N.; Morris, R. Sex Differences in White Matter Diffusivity in Children with Developmental Dyslexia. Children 2024, 11, 721. https://doi.org/10.3390/children11060721
Gupta G, Arrington CN, Morris R. Sex Differences in White Matter Diffusivity in Children with Developmental Dyslexia. Children. 2024; 11(6):721. https://doi.org/10.3390/children11060721
Chicago/Turabian StyleGupta, Gehna, C. Nikki Arrington, and Robin Morris. 2024. "Sex Differences in White Matter Diffusivity in Children with Developmental Dyslexia" Children 11, no. 6: 721. https://doi.org/10.3390/children11060721
APA StyleGupta, G., Arrington, C. N., & Morris, R. (2024). Sex Differences in White Matter Diffusivity in Children with Developmental Dyslexia. Children, 11(6), 721. https://doi.org/10.3390/children11060721






