Removal of Ciprofloxacin from Wastewater by Ultrasound/Electric Field/Sodium Persulfate (US/E/PS)
Abstract
:1. Introduction
2. Experimental Materials and Methods
2.1. The Main Experimental Instruments and Equipment Are as Follows
2.2. Major Chemical Reagents and Pharmaceuticals
2.3. Experimental Methods
2.4. Solution Preparation and Measurement Method
2.4.1. Solution Preparation and Storage Method
2.4.2. Analytical Method
2.4.3. Index Calculation Formula
Removal Rate
First Order Reaction Rate Constant
3. Results and Discussion
3.1. Validation of Ciprofloxacin Removal by Different Reaction Systems
3.2. Effect of Ultrasonic Power on CIP Removal Efficiency
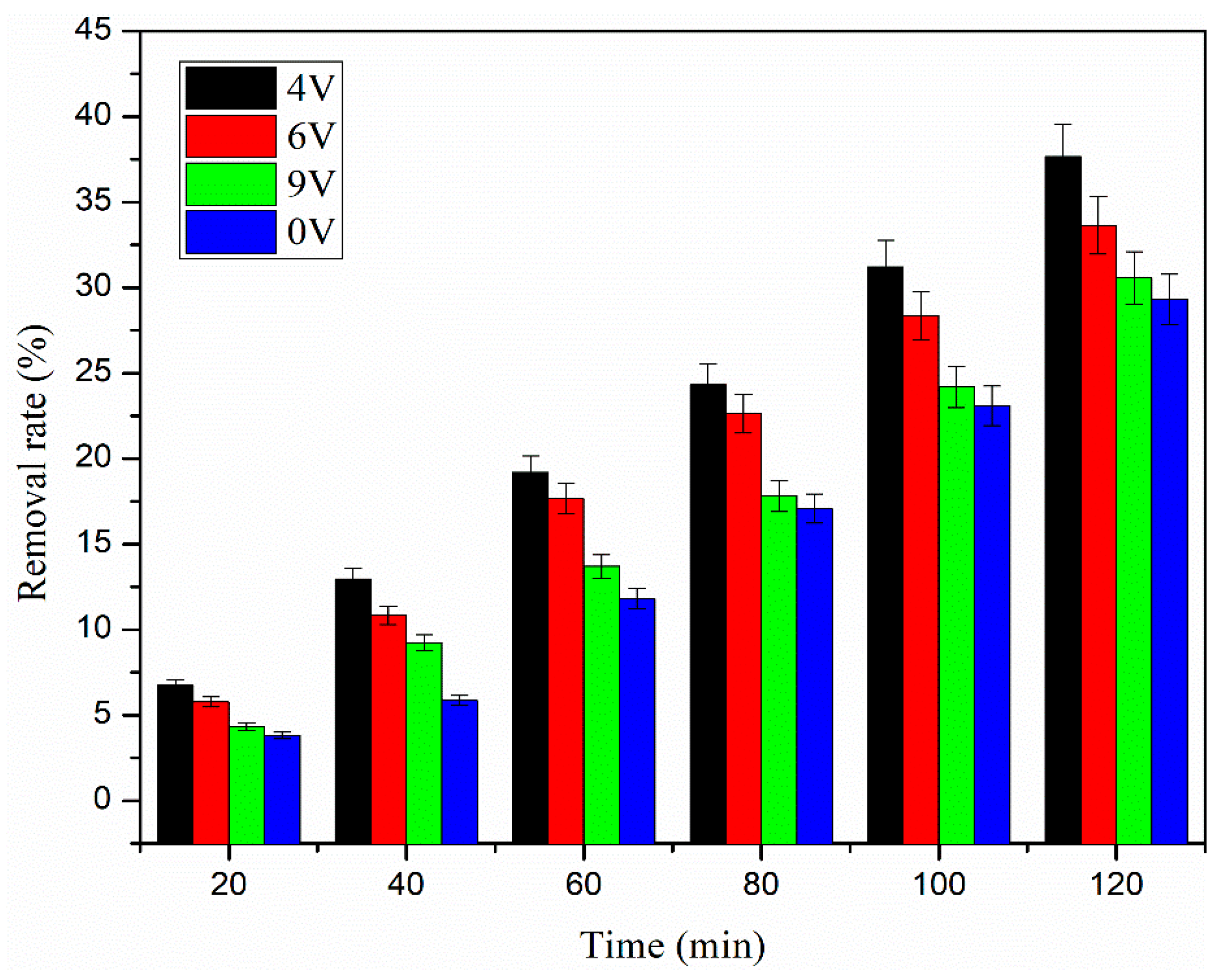
3.3. Effect of Electrode Potential on CIP Removal Efficiency
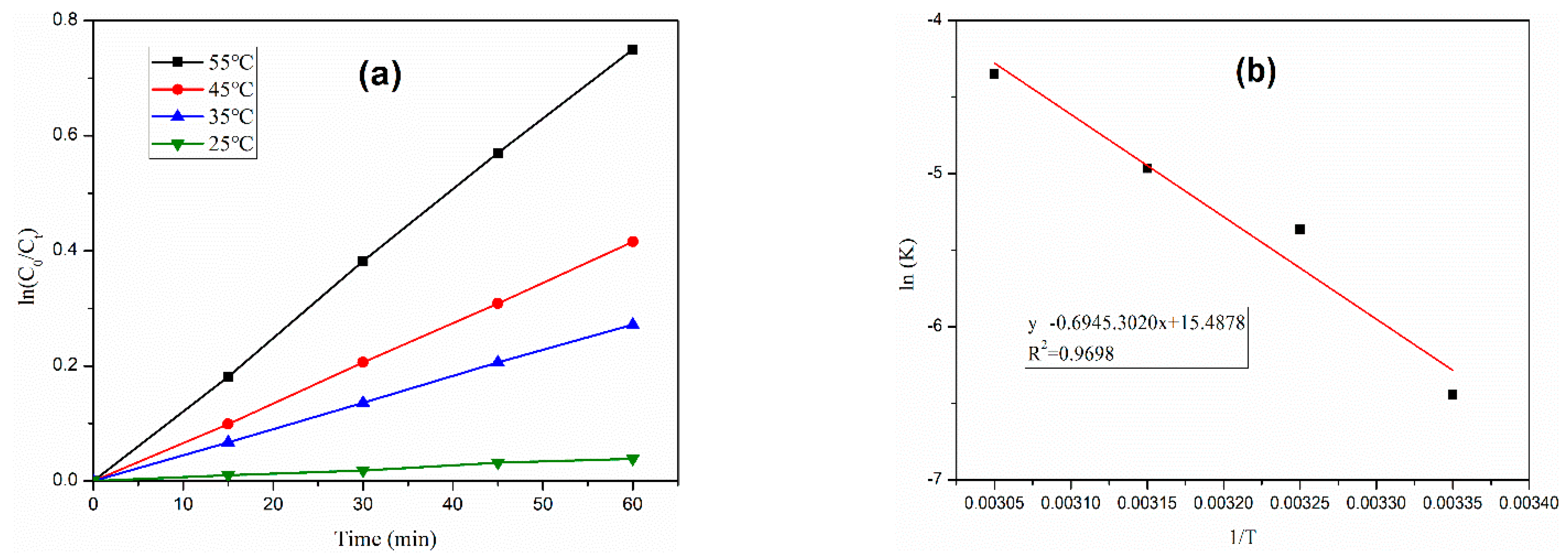
3.4. Effect of Reaction Temperature on CIP Removal Efficiency
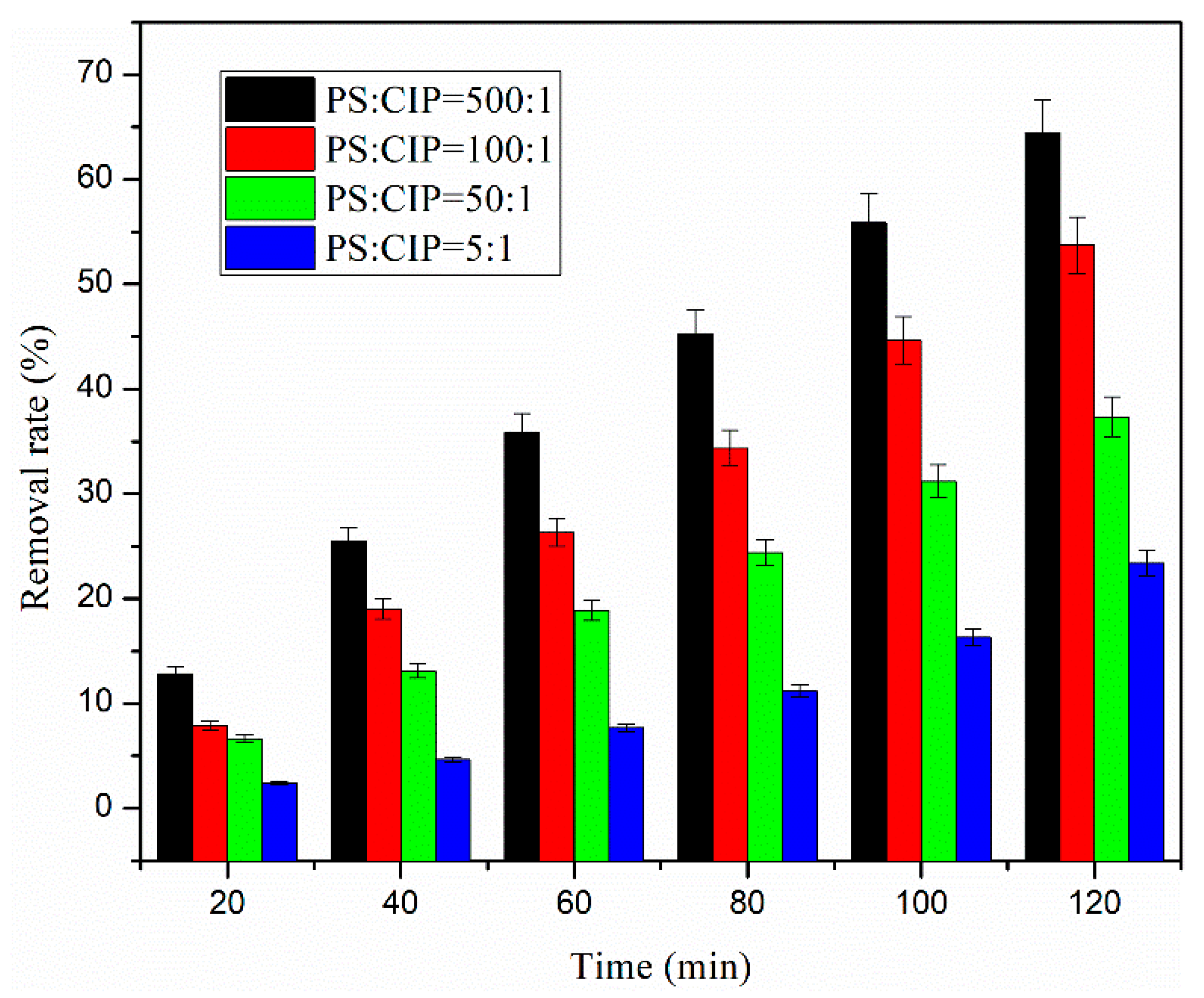
3.5. Effect of the Ratio of PS to Initial Molar Concentration of Ciprofloxacin on CIP Removal Efficiency
3.6. Effect of Initial pH on CIP Removal Efficiency
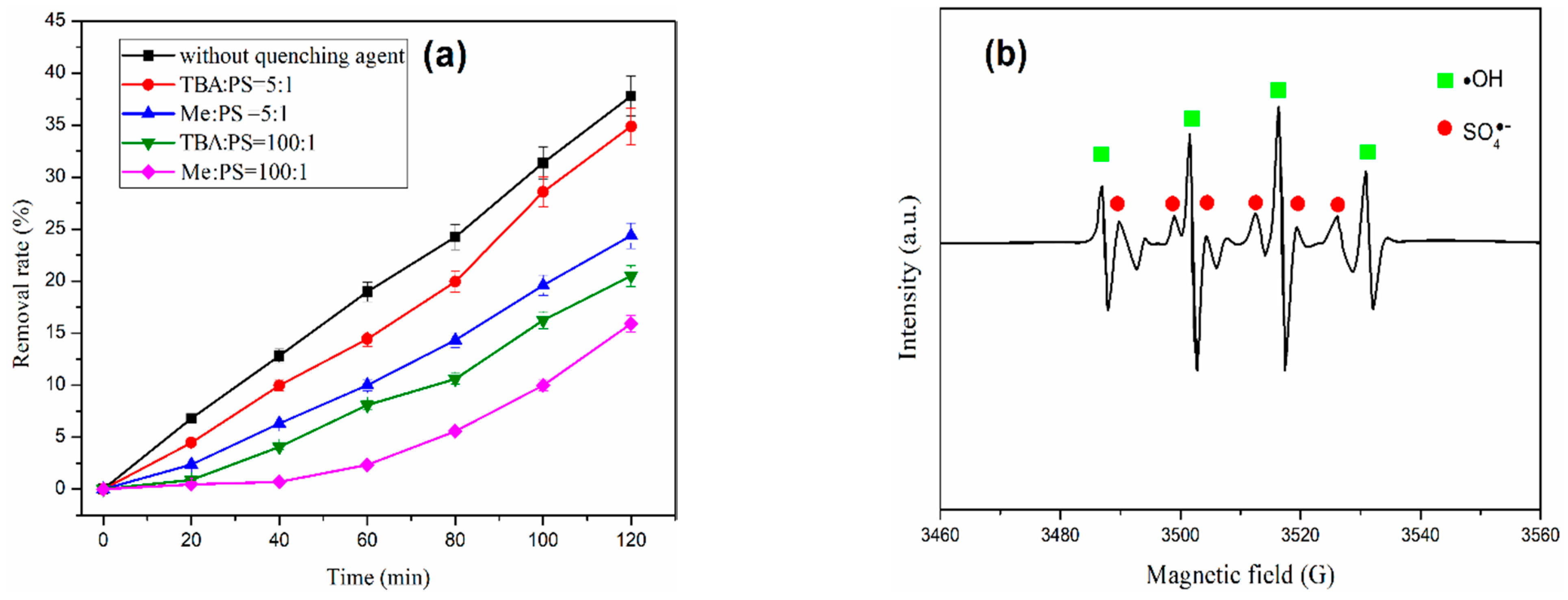
3.7. Effect of Free Radical Quenching Agents on CIP Removal Efficiency
4. Mechanism Analysis
5. Conclusions
- (1)
- The reaction of ciprofloxacin by US/E/PS system is consistent with quasi-primary reaction kinetics, with a reaction rate constant of 0.0046 min−1, which is about 2.7 times that of peroxynitrite oxidation alone (0.0017 min−1). The combination of ultrasonic oxidation, electro-oxidation, and persulfate oxidation has a strong synergistic effect, with a synergistic coefficient of S = 2.10. Both ultrasonic oxidation and electro-oxidation can activate PS to continuously produce and •OH, which can effectively remove and degrade CIP.
- (2)
- Ultrasound can effectively increase the removal rate of ciprofloxacin. Excessive ultrasound power leads to excessive growth of cavitation bubbles, which affects the reaction and deteriorates the removal effect. The removal rates at 50 W, 100 W, and 200 W are 13.2%, 37.4%, and 36.9%, respectively.
- (3)
- It is helpful to remove ciprofloxacin by electrifying the reaction system, but the electrode potential increases excessively, the current efficiency decreases, and the removal effect of ciprofloxacin shows a downward trend. The removal rates of ciprofloxacin were 29.3%, 37.4%, 33.5%, and 30.5%, respectively, when the electrode potentials were 0 V, 4 V, 6 V, and 9 V.
- (4)
- The apparent activation energy of ciprofloxacin is 54.00 kJ/mol, and the removal efficiency of ciprofloxacin increases obviously with the increase in reaction temperature. The removal rate at 55 °C is more than three times that at 25 °C.
- (5)
- The removal efficiency of ciprofloxacin increases with the increase in the initial molar concentration ratio of persulfate to ciprofloxacin. The ratio of PS to ciprofloxacin increases 100 times from 5:1 to 500:1, and the removal efficiency of ciprofloxacin correspondingly increases from 23.1% to 64.0%, an increase of nearly three times. The quasi-stage reaction rate constant increased from 0.0019 min−1 to 0.0099 min−1, a 5.2-fold acceleration.
- (6)
- The pH of the solution has multiple effects on the removal of ciprofloxacin in the reaction system, such as the morphology of substrate, the type and activity of free radicals, etc. The removal rate is the highest when pH = 11.0. The removal rates of ciprofloxacin were 37.4%, 39.1%, and 42.3%, respectively, after reaction for 2 h at initial pH = 5.0, 7.0, and 11.0.
- (7)
- The sulfate radicals and hydroxyl radicals were detected in the US/E/PS system for the removal of ciprofloxacin from water, and the sulfate radicals were dominant. Therefore, strategies and methods to increase the number of sulfate radicals are beneficial to improve degradation and removal of CIP.
- (8)
- The piperazine ring in the structure of ciprofloxacin is easy to cleave, the piperazine ring (P.1) is attacked by and •OH, and the ring-opening of piperazine ring causes C2H2O generation. and •OH are likely to completely dealkylate the piperazine ring after further action, and the product (P.3) is generated. The product (P.2) comes from hydroxylation of the aromatic ring, and the substitution reaction may take place at two positions. Free radicals of and •OH are the most direct driving force for CIP decomposition, degradation, and removal.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, F.; Huang, J.; Wang, M.; Chen, L.; Xiao, Y. Sources, distribution and dynamics of antibiotics in Litopenaeus vannamei farming environment. Aquaculture 2021, 545, 737200. [Google Scholar] [CrossRef]
- Zheng, D.; Yin, G.; Liu, M.; Chen, C.; Jiang, Y.; Hou, L.; Zheng, Y. A systematic review of antibiotics and antibiotic resistance genes in estuarine and coastal environments. Sci. Total. Environ. 2021, 777, 146009. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, X.; Yan, K. Facile synthesis of cobalt-iron layered double hydroxides nanosheets for direct activation of peroxymonosulfate (PMS) during degradation of fluoroquinolones antibiotics. J. Clean. Prod. 2021, 310, 127584. [Google Scholar] [CrossRef]
- Sordello, F.; Fabbri, D.; Rapa, L.; Minero, C.; Minella, M.; Vione, D. Electrochemical abatement of cefazolin: Towards a viable treatment for antibiotic-containing urine. J. Clean. Prod. 2021, 289, 125722. [Google Scholar] [CrossRef]
- Zhao, L.; Ji, Y.; Yao, J.; Long, S.; Li, D.; Yang, Y. Quantifying the fate and risk assessment of different antibiotics during wastewater treatment using a Monte Carlo simulation. J. Clean. Prod. 2017, 168, 626–631. [Google Scholar] [CrossRef]
- He, F.; Cai, W.; Lin, J.; Yu, B.; Owens, G.; Chen, Z. Reducing the impact of antibiotics in wastewaters: Increased removal of mitoxantrone from wastewater by biosynthesized manganese nanoparticles. J. Clean. Prod. 2021, 293, 126207. [Google Scholar] [CrossRef]
- Zhang, Q.; Fan, D.; Pang, X.; Zhu, W.; Zhao, J.; Xu, J. Effects of polyethylene microplastics on the fate of antibiotic resistance genes and microbial communities in anaerobic digestion of dairy wastes. J. Clean. Prod. 2021, 292, 125909. [Google Scholar] [CrossRef]
- Lu, D.; Xu, S.; Ma, J. Adsorption and desorption behaviors of antibiotic CIP on functionalized spherical MCM-41 for water treatment. J. Clean. Prod. 2020, 264, 121644. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, Z.; Zhu, Y. Changes in abiotic dissipation rates and bound fractions of antibiotics in biochar-amended soil. J. Clean. Prod. 2020, 256, 120314. [Google Scholar] [CrossRef]
- Hu, Y.; Shen, Y.; Wang, J. Pretreatment of antibiotic fermentation residues by combined ultrasound and alkali for enhancing biohydrogen production. J. Clean. Prod. 2020, 268, 122190. [Google Scholar] [CrossRef]
- Singh, R.; Singh, A.P.; Kumar, S.; Giri, B.S.; Kim, K.-H. Antibiotic resistance in major rivers in the world: A systematic review on occurrence, emergence, and management strategies. J. Clean. Prod. 2019, 234, 1484–1505. [Google Scholar] [CrossRef]
- Zhang, X.; Dai, Y.; Chen, C.; Yang, Y. Occurrence and removal of quinolone, tetracycline, and macrolide antibiotics from urban wastewater in constructed wetlands. J. Clean. Prod. 2020, 252, 119677. [Google Scholar] [CrossRef]
- Lin, Z.; Wu, Z.-L.; Sun, Z.-Y.; Gou, M.; Xia, Z.-Y.; Tang, Y.-Q. Aerobic post-treatment of anaerobic digested sludge with a focus on organic matter stability and the fate of antibiotic resistance genes. J. Clean. Prod. 2021, 289, 125798. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, Y.; Xiong, W.; Sun, W.; Fan, Q.; Yang, Z. Metagenomic approach reveals the fate of antibiotic resistance genes in a temperature-raising anaerobic digester treating municipal sewage sludge. J. Clean. Prod. 2020, 277, 123504. [Google Scholar] [CrossRef]
- Nannapaneni, R.; Story, R.; Wiggins, K.C.; Johnson, M.G. Concurrent Quantitation of Total Campylobacter and Total Ciprofloxacin-Resistant Campylobacter Loads in Rinses from Retail Raw Chicken Carcasses from 2001 to 2003 by Direct Plating at 42 °C. Appl. Environ. Microbiol. 2005, 71, 4510–4515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Gao, S.; Zhu, J.; Xia, X.; Wang, M.; Xiong, Y. Enhanced activation of peroxydisulfate by strontium modified BiFeO3perovskite for ciprofloxacin degradation. J. Environ. Sci. 2021, 99, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Giri, A.S.; Golder, A.K. Ciprofloxacin degradation in photo-Fenton and photo-catalytic processes: Degradation mechanisms and iron chelation. J. Environ. Sci. 2019, 80, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; He, J. Degradation of ciprofloxacin by the Mn cycle system (MnCS): Construction, characterization and bacterial analysis. Environ. Res. 2021, 195, 110860. [Google Scholar] [CrossRef]
- Carabineiro, S.; Thavorn-Amornsri, T.; Pereira, M.; Figueiredo, J. Adsorption of ciprofloxacin on surface-modified carbon materials. Water Res. 2011, 45, 4583–4591. [Google Scholar] [CrossRef] [PubMed]
- Carabineiro, S.; Thavorn-Amornsri, T.; Pereira, M.; Serp, P.; Figueiredo, J. Comparison between activated carbon, carbon xerogel and carbon nanotubes for the adsorption of the antibiotic ciprofloxacin. Catal. Today 2012, 186, 29–34. [Google Scholar] [CrossRef]
- Mirzaei, A.; Haghighat, F.; Chen, Z.; Yerushalmi, L. Sonocatalytic removal of ampicillin by Zn(OH)F: Effect of operating parameters, toxicological evaluation and by-products identification. J. Hazard. Mater. 2019, 375, 86–95. [Google Scholar] [CrossRef]
- Gholami, P.; Dinpazhoh, L.; Khataee, A.; Hassani, A.; Bhatnagar, A. Facile hydrothermal synthesis of novel Fe-Cu layered double hydroxide/biochar nanocomposite with enhanced sonocatalytic activity for degradation of cefazolin sodium. J. Hazard. Mater. 2020, 381, 120742. [Google Scholar] [CrossRef]
- Gholami, P.; Dinpazhoh, L.; Khataee, A.; Orooji, Y. Sonocatalytic activity of biochar-supported ZnO nanorods in degradation of gemifloxacin: Synergy study, effect of parameters and phytotoxicity evaluation. Ultrason. Sonochem. 2019, 55, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Soltani, R.D.C.; Mashayekhi, M.; Naderi, M.; Boczkaj, G.; Jorfi, S.; Safari, M. Sonocatalytic degradation of tetracycline antibiotic using zinc oxide nanostructures loaded on nano-cellulose from waste straw as nanosonocatalyst. Ultrason. Sonochem. 2019, 55, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Khataee, A.; Gholami, P.; Kayan, B.; Kalderis, D.; Dinpazhoh, L.; Akay, S. Synthesis of ZrO2 nanoparticles on pumice and tuff for sonocatalytic degradation of rifampin. Ultrason. Sonochem. 2018, 48, 349–361. [Google Scholar] [CrossRef]
- Qiao, J.; Lv, M.; Qu, Z.; Zhang, M.; Cui, X.; Wang, D.; Piao, C.; Liu, Z.; Wang, J.; Song, Y. Preparation of a novel Z-scheme KTaO3/FeVO4/Bi2O3 nanocomposite for efficient sonocatalytic degradation of ceftriaxone sodium. Sci. Total Environ. 2019, 689, 178–192. [Google Scholar] [CrossRef] [PubMed]
- Harrabi, M.; Ammar, H.B.; Mbarki, K.; Naifar, I.; Yaiche, C.; Aloulou, F.; Elleuch, B. Ultrasonic power improvement of flumequine degradation effectiveness in aqueous solution via direct and indirect action of mechanical acoustic wave. Ultrason. Sonochem. 2018, 48, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Soltani, R.D.C.; Mashayekhi, M.; Jorfi, S.; Khataee, A.; Ghanadzadeh, M.J.; Sillanp, M. Implementation of martite nanoparticles prepared through planetary ball milling as a heterogeneous activator of oxone for degradation of tetracycline antibiotic: Ultrasound and peroxy-enhancement. Chemosphere 2018, 210, 699–708. [Google Scholar] [CrossRef]
- Vinesh, V.; Shaheer, A.R.M.; Neppolian, B. Reduced graphene oxide (rGO) supported electron deficient B-doped TiO2 (Au/B-TiO2/rGO) nanocomposite: An efficient visible light sonophotocatalyst for the degradation of Tetracycline (TC). Ultrason. Sonochem. 2019, 50, 302–310. [Google Scholar] [CrossRef]
- Pirsaheb, M.; Moradi, N. Sonochemical degradation of pesticides in aqueous solution: Investigation on the influence of operating parameters and degradation pathway—A systematic review. RSC Adv. 2020, 10, 7396–7423. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Vieira, C.L.Z.; Wolfson, J.M.; Pingtian, G.; Huang, S. Review on the treatment of organic pollutants in water by ultrasonic technology. Ultrason. Sonochem. 2019, 55, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Q.; Peng, C.; Wu, B.; Xu, J.; Ma, F.; Gu, Q. Sustained-Release of Sodium Persulfate Composite and Degradation of 2, 4-Dinitrotoluene. Res. Environ. Sci. 2020, 26. [Google Scholar] [CrossRef]
- Kuppa, R.; Moholkar, V.S. Physical features of ultrasound-enhanced heterogeneous permanganate oxidation. Ultrason. Sonochem. 2010, 17, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Guo, Y.; Dong, H.; Guan, X.; Zhou, G.; Sun, Y. Activated peroxydisulfate by sulfidated zero-valent iron for enhanced organic micropollutants removal from water. Chem. Eng. J. 2020, 396, 125301. [Google Scholar] [CrossRef]
- Khataee, A.; Rad, T.S.; Nikzat, S.; Hassani, A.; Aslan, M.H.; Kobya, M.; Demirbas, E. Fabrication of NiFe layered double hydroxide/reduced graphene oxide (NiFe- LDH/rGO) nanocomposite with enhanced sonophotocatalytic activity for the degradation of moxifloxacin. Chem. Eng. J. 2019, 375, 122102. [Google Scholar] [CrossRef]
- Hosseini, M.; Kahkha, M.R.R.; Fakhri, A.; Tahami, S.; Lariche, M.J. Degradation of macrolide antibiotics via sono or photo coupled with Fenton methods in the presence of ZnS quantum dots decorated SnO2 nanosheets. J. Photochem. Photobiol. B Biol. 2018, 185, 24–31. [Google Scholar] [CrossRef]
- Huang, Y.H.; Su, H.T.; Lin, L.W. Degradation of furfural in aqueous solution using activated persulfate and peroxymonosulfate by ultrasound irradiation. J. Environ. Sci. 2020, 266, 110616. [Google Scholar]
- Serna-Galvis, E.A.; Montoya-Rodríguez, D.; Isaza-Pineda, L.; Ibáñez, M.; Hernández, F.; Moncayo-Lasso, A.; Torres-Palma, R.A. Sonochemical degradation of antibiotics from representative classes-Considerations on structural effects, initial transformation products, antimicrobial activity and matrix. Ultrason. Sonochem. 2019, 50, 157–165. [Google Scholar] [CrossRef]
- Qiao, J.; Zhang, H.; Li, G.; Li, S.; Qu, Z.; Zhang, M.; Wang, J.; Song, Y. Fabrication of a novel Z-scheme SrTiO3/Ag2S/CoWO4 composite and its application in sonocatalytic degradation of tetracyclines. Sep. Purif. Technol. 2019, 211, 843–856. [Google Scholar] [CrossRef]
- Rahdar, S.; Igwegbe, C.A.; Rahdar, A.; Ahmadi, S. Efficiency of sono-nano-catalytic process of magnesium oxide nano particle in removal of penicillin G from aqueous solution. Desalin. Water Treat. 2018, 106, 330–335. [Google Scholar] [CrossRef]
- Yazdani, A.; Sayadi, M.H. Sonochemical degradation of azithromycin in aqueous solution. Environ. Heal. Eng. Manag. 2018, 5, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Nasseri, S.; Mahvi, A.H.; Seyedsalehi, M.; Yaghmaeian, K.; Nabizadeh, R.; Alimohammadi, M.; Safari, G.H. Degradation kinetics of tetracycline in aqueous solutions using peroxydisulfate activated by ultrasound irradiation: Effect of radical scavenger and water matrix. J. Mol. Liq. 2017, 241, 704–714. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, Y.; Zhou, M.; Cai, J.; Li, X.; Tian, Y. Synergistic degradation of antibiotic sulfamethazine by novel pre-magnetized Fe0/PS process enhanced by ultrasound. Chem. Eng. J. 2018, 354, 777–789. [Google Scholar] [CrossRef]
- Malakotian, M.; Asadzadeh, S.N.; Khatami, M.; Ahmadian, M.; Heidari, M.R.; Karimi, P.; Firouzeh, N.; Varma, R.S. Protocol encompassing ultrasound/Fe3O4 nanoparticles/persulfate for the removal of tetracycline antibiotics from aqueous environments. Clean Technol. Environ. Policy 2019, 21, 1665–1674. [Google Scholar] [CrossRef]
- Roy, K.; Agarkoti, C.; Malani, R.S.; Thokchom, B.; Moholkar, V.S. Mechanistic study of sulfadiazine degradation by ultrasound-assisted Fenton-persulfat esystem using yolk-shell Fe3O4@hollow@mSiO2 nanoparticles. Chem. Eng. Sci. 2020, 217, 115522. [Google Scholar] [CrossRef]
- Zeng, L.; Li, S.; Li, X.; Li, J.; Fan, S.; Chen, X.; Yin, Z.; Tade, M.; Liu, S. Visible-light- driven sonophotocatalysis and peroxymonosulfate activation over 3D urchin-like MoS2/C nanoparticles for accelerating levofloxacin elimination: Optimization and kinetic study. Chem. Eng. J. 2019, 378, 122039. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Wang, Z. Removal of Ciprofloxacin from Wastewater by Ultrasound/Electric Field/Sodium Persulfate (US/E/PS). Processes 2022, 10, 124. https://doi.org/10.3390/pr10010124
Ma X, Wang Z. Removal of Ciprofloxacin from Wastewater by Ultrasound/Electric Field/Sodium Persulfate (US/E/PS). Processes. 2022; 10(1):124. https://doi.org/10.3390/pr10010124
Chicago/Turabian StyleMa, Xiao, and Zhenjun Wang. 2022. "Removal of Ciprofloxacin from Wastewater by Ultrasound/Electric Field/Sodium Persulfate (US/E/PS)" Processes 10, no. 1: 124. https://doi.org/10.3390/pr10010124





