Highly Active CuO/KCC−1 Catalysts for Low-Temperature CO Oxidation
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Preparation of Catalysts
2.2. Characterization of Catalysts
2.3. Catalytic and Kinetic Tests
3. Results and Discussion
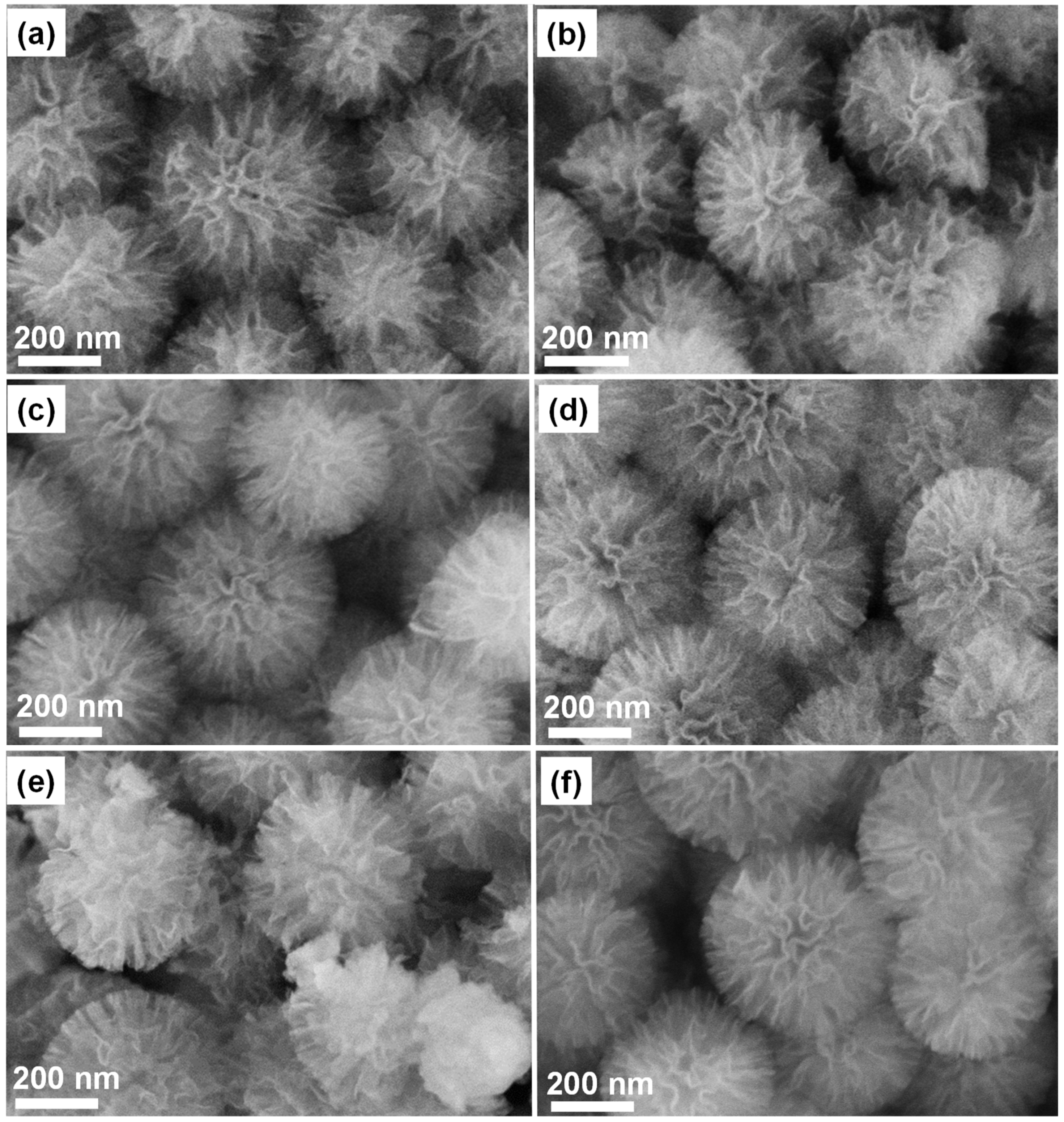
3.1. SEM and TEM Results Analysis
3.2. CO Oxidation Performance, Kinetic and Thermal Stability
3.3. XRD Results Analysis
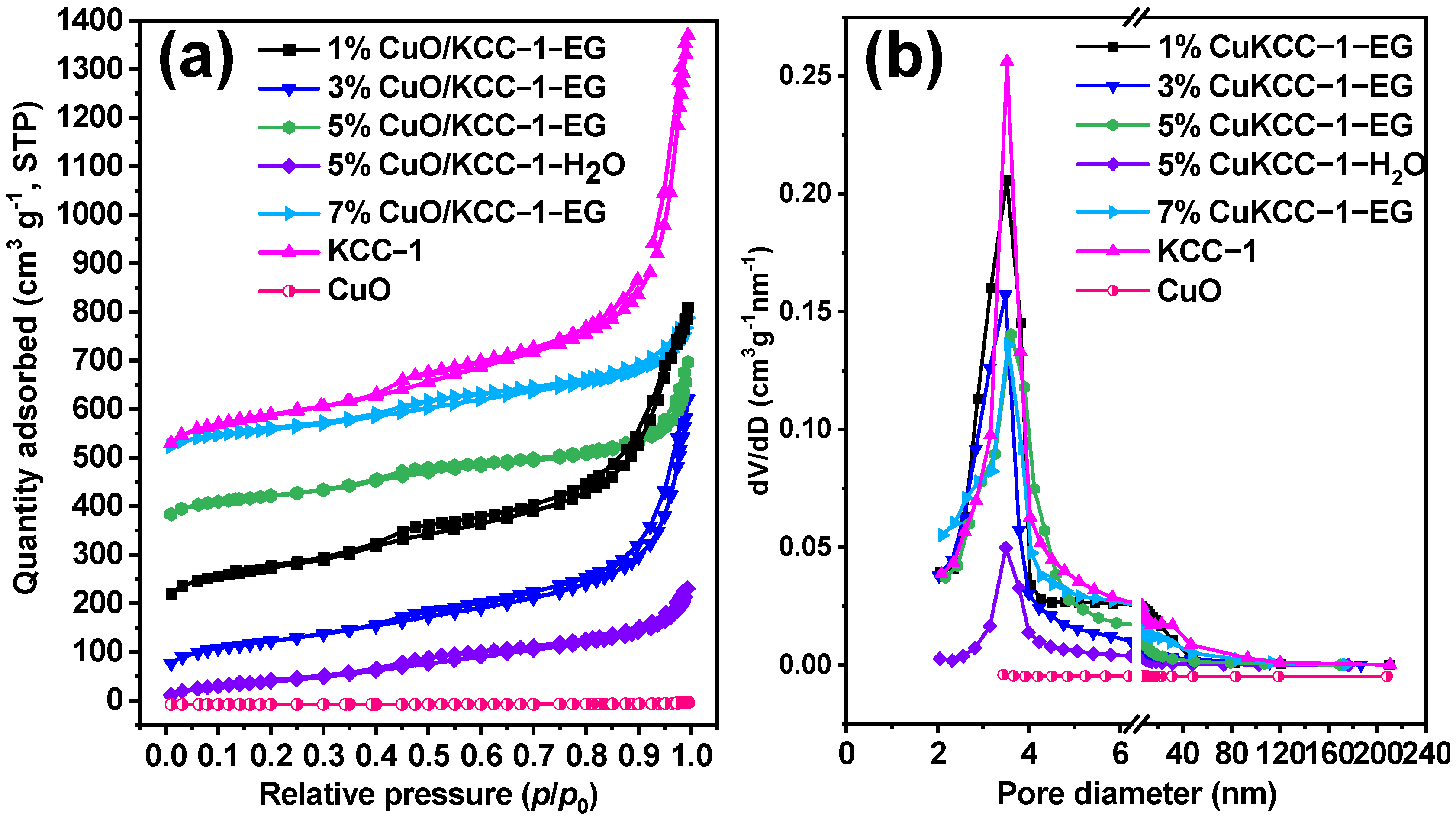
3.4. N2 Adsorption/Desorption Results Analysis
3.5. H2-TPR Result Analysis
3.6. XPS Result Analysis
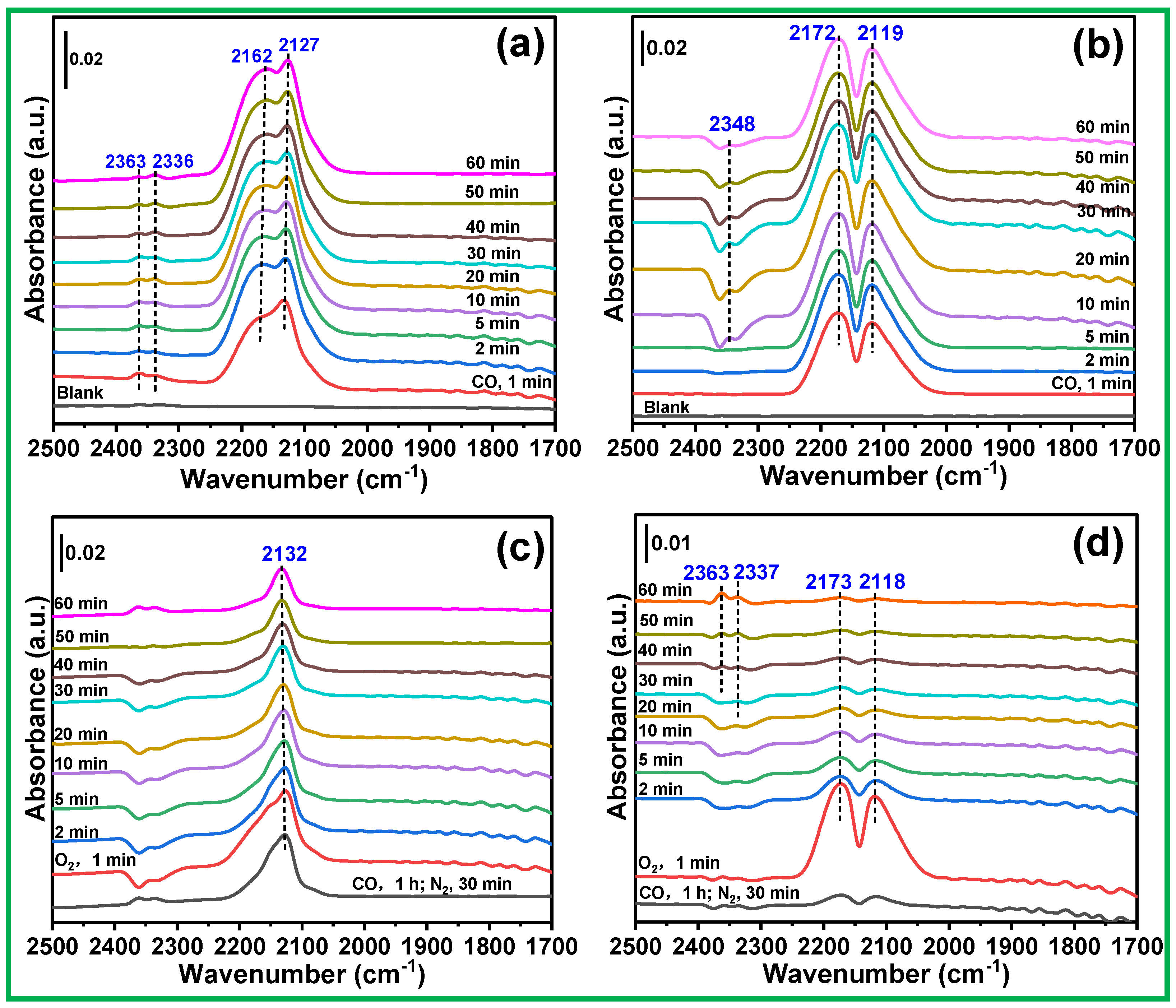
3.7. In Situ DRIFTS Results Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- May, Y.A.; Wang, W.; Yan, H.; Wei, S.; Jia, C. Insights into facet-dependent reactivity of CuO–CeO2 nanocubes and nanorods as catalysts for CO oxidation reaction. Chin. J. Catal. 2020, 41, 1017–1027. [Google Scholar] [CrossRef]
- Wu, C.; Guo, Z.; Chen, X.; Liu, H. Cu/CeO2 as efficient low-temperature CO oxidation catalysts: Effects of morphological structure and Cu content. React. Kinet. Mech. Catal. 2020, 131, 691–706. [Google Scholar] [CrossRef]
- Shi, Y.; Xu, L.; Chen, M.; Yang, B.; Cheng, G.; Wu, C.; Miao, Z.; Wang, N.; Hu, X. Fabricating Cu2O-CuO submicron-cubes for efficient catalytic CO oxidation: The significant effect of heterojunction interface. J. Ind. Eng. Chem. 2022, 105, 324–336. [Google Scholar] [CrossRef]
- May, Y.A.; Wei, S.; Yu, W.; Wang, W.; Jia, C. Highly Efficient CuO/α-MnO2 Catalyst for Low-Temperature CO Oxidation. Langmuir 2020, 36, 11196–11206. [Google Scholar] [CrossRef]
- Feng, C.; Liu, X.; Zhu, T.; Tian, M. Catalytic oxidation of CO on noble metal-based catalysts. Environ. Sci. Pollut. R. 2021, 28, 24847–24871. [Google Scholar] [CrossRef]
- Gao, Y.; Chiang, F.; Li, S.; Zhang, L.; Wang, P.; Hensen, E.J.M. Influence of hematite morphology on the CO oxidation performance of Au/α-Fe2O3. Chin. J. Catal. 2021, 42, 658–665. [Google Scholar] [CrossRef]
- Yang, N.; Pattisson, S.; Douthwaite, M.; Zeng, G.; Zhang, H.; Ma, J.; Hutchings, G.J. Influence of Stabilizers on the Performance of Au/TiO2 Catalysts for CO Oxidation. ACS Catal. 2021, 11, 11607–11615. [Google Scholar] [CrossRef]
- Wang, T.; Xing, J.; Jia, A.; Tang, C.; Wang, Y.; Luo, M.; Lu, J. CO oxidation over Pt/Cr1.3Fe0.7O3 catalysts: Enhanced activity on single Pt atom by H2O promotion. J. Catal. 2020, 382, 192–203. [Google Scholar] [CrossRef]
- Green, I.X.; Tang, W.; Neurock, M.; Yates, J.T. Spectroscopic Observation of Dual Catalytic Sites During Oxidation of CO on a Au/TiO2 Catalyst. Science 2011, 333, 736–739. [Google Scholar] [CrossRef]
- Peng, H.; Peng, Y.; Xu, X.; Fang, X.; Liu, Y.; Cai, J.; Wang, X. SnO2 nano-sheet as an efficient catalyst for CO oxidation. Chin. J. Catal. 2015, 36, 2004–2010. [Google Scholar] [CrossRef]
- Li, Z.; Geng, Y.; Ma, L.; Chen, X.; Li, J.; Chang, H.; Schwank, J.W. Catalytic oxidation of CO over Pt/Fe3O4 catalysts: Tuning O2 activation and CO adsorption. Front. Environ. Sci. Eng. 2020, 14, 65. [Google Scholar] [CrossRef]
- Fujita, T.; Ishida, T.; Shibamoto, K.; Honma, T.; Ohashi, H.; Murayama, T.; Haruta, M. CO Oxidation over Au/ZnO: Unprecedented Change of the Reaction Mechanism at Low Temperature Caused by a Different O2 Activation Process. ACS Catal. 2019, 9, 8364–8372. [Google Scholar] [CrossRef]
- Zhang, N.; Li, L.; Wu, R.; Song, L.; Zheng, L.; Zhang, G.; He, H. Activity enhancement of Pt/MnOx catalyst by novel β-MnO2 for low-temperature CO oxidation: Study of the CO–O2 competitive adsorption and active oxygen species. Catal. Sci. Technol. 2019, 9, 347–354. [Google Scholar] [CrossRef]
- Song, S.; Zhang, C.; Lou, Y.; Wu, Y.; Wang, L.; Guo, Y.; Zhan, W.; Guo, Y. Effects of water on CO catalytic oxidation over Pd/CeO2. J. Rare Earths 2020, 38, 891–898. [Google Scholar] [CrossRef]
- Liu, X.; Jin, Z.; Lu, J.; Wang, X.; Luo, M. Highly active CuO/OMS-2 catalysts for low-temperature CO oxidation. Chem. Eng. J. 2010, 162, 151–157. [Google Scholar] [CrossRef]
- Bi, W.; Hu, Y.; Jiang, H.; Yu, H.; Li, W.; Li, C. In-situ synthesized surface N-doped Pt/TiO2 via flame spray pyrolysis with enhanced thermal stability for CO catalytic oxidation. Appl. Surf. Sci. 2019, 481, 360–368. [Google Scholar] [CrossRef]
- Murthy, P.R.; Munsif, S.; Zhang, J.; Li, W. Influence of CeO2 and ZrO2 on the Thermal Stability and Catalytic Activity of SBA-15-Supported Pd Catalysts for CO Oxidation. Ind. Eng. Chem. Res. 2021, 60, 14424–14433. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, X.; Han, X.; You, X.; Lin, S.; Chen, H.; Liu, W.; Wang, X.; Zhang, N.; Wang, Z.; et al. Catalysts in Coronas: A Surface Spatial Confinement Strategy for High-Performance Catalysts in Methane Dry Reforming. ACS Catal. 2019, 9, 9072–9080. [Google Scholar] [CrossRef]
- Castillejos, E.; Debouttière, P.; Roiban, L.; Solhy, A.; Martinez, V.; Kihn, Y.; Ersen, O.; Philippot, K.; Chaudret, B.; Serp, P. An Efficient Strategy to Drive Nanoparticles into Carbon Nanotubes and the Remarkable Effect of Confinement on Their Catalytic Performance. Angew. Chem. Int. Ed. 2009, 48, 2529–2533. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yang, G.; Ma, Q.; Wu, M.; Tan, Y.; Yoneyama, Y.; Tsubaki, N. Confinement Effect of Carbon Nanotubes: Copper Nanoparticles Filled Carbon Nanotubes for Hydrogenation of Methyl Acetate. ACS Catal. 2012, 2, 1958–1966. [Google Scholar] [CrossRef]
- Deng, D.; Chen, X.; Yu, L.; Wu, X.; Liu, Q.; Liu, Y.; Yang, H.; Tian, H.; Hu, Y.; Du, P.; et al. A single iron site confined in a graphene matrix for the catalytic oxidation of benzene at room temperature. Sci. Adv. 2015, 1, e1500462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baudouin, D.; Szeto, K.C.; Laurent, P.; De Mallmann, A.; Fenet, B.; Veyre, L.; Rodemerck, U.; Copéret, C.; Thieuleux, C. Nickel–Silicide Colloid Prepared under Mild Conditions as a Versatile Ni Precursor for More Efficient CO2 Reforming of CH4 Catalysts. J. Am. Chem. Soc. 2012, 134, 20624–20627. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.; Suryawinata, I.Y.; Kawi, S. Highly carbon resistant multicore-shell catalyst derived from Ni-Mg phyllosilicate nanotubes@silica for dry reforming of methane. Appl. Catal. B-Environ. 2016, 195, 1–8. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Peng, H.; You, X.; Peng, C.; Xu, X.; Liu, W.; Fang, X.; Wang, Z.; Zhang, N.; et al. Nickel nanoparticles embedded in mesopores of AlSBA-15 with a perfect peasecod-like structure: A catalyst with superior sintering resistance and hydrothermal stability for methane dry reforming. Appl. Catal. B-Environ. 2018, 224, 488–499. [Google Scholar] [CrossRef]
- Liu, D.; Quek, X.Y.; Cheo, W.N.E.; Lau, R.; Borgna, A.; Yang, Y. MCM-41 supported nickel-based bimetallic catalysts with superior stability during carbon dioxide reforming of methane: Effect of strong metal–support interaction. J. Catal. 2009, 266, 380–390. [Google Scholar] [CrossRef]
- Dadras, J.; Jimenez-Izal, E.; Alexandrova, A.N. Alloying Pt Sub-nano-clusters with Boron: Sintering Preventative and Coke Antagonist? ACS Catal. 2015, 5, 5719–5727. [Google Scholar] [CrossRef] [Green Version]
- Zhan, W.; He, Q.; Liu, X.; Guo, Y.; Wang, Y.; Wang, L.; Guo, Y.; Borisevich, A.Y.; Zhang, J.; Lu, G.; et al. A Sacrificial Coating Strategy Toward Enhancement of Metal–Support Interaction for Ultrastable Au Nanocatalysts. J. Am. Chem. Soc. 2016, 138, 16130–16139. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Ji, Q.; Imai, T.; Ariga, K.; Abe, H. Sintering-Resistant Nanoparticles in Wide-Mouthed Compartments for Sustained Catalytic Performance. Sci. Rep. 2017, 7, 41773. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Bapat, R.; Qin, L.; Feng, H.; Polshettiwar, V. Atomic Layer Deposited (ALD) TiO2 on Fibrous Nano-Silica (KCC−1) for Photocatalysis: Nanoparticle Formation and Size Quantization Effect. ACS Catal. 2016, 6, 2770–2784. [Google Scholar] [CrossRef]
- Shen, D.; Yang, J.; Li, X.; Zhou, L.; Zhang, R.; Li, W.; Chen, L.; Wang, R.; Zhang, F.; Zhao, D. Biphase Stratification Approach to Three-Dimensional Dendritic Biodegradable Mesoporous Silica Nanospheres. Nano Lett. 2014, 14, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Polshettiwar, V.; Cha, D.; Zhang, X.; Basset, J.M. High-surface-area silica nanospheres (KCC−1) with a fibrous morphology. Angew. Chem. Int. Ed. 2010, 49, 9652–9656. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Meng, H.; Du, Y.; Liu, J.; Hou, B.; Xie, X. Insight into Cu2O/CuO collaboration in the selective catalytic reduction of NO with NH3: Enhanced activity and synergistic mechanism. J. Catal. 2020, 384, 72–87. [Google Scholar] [CrossRef]
- Oyama, S.T.; Zhang, X.; Lu, J.; Gu, Y.; Fujitani, T. Epoxidation of propylene with H2 and O2 in the explosive regime in a packed-bed catalytic membrane reactor. J. Catal. 2008, 257, 1–4. [Google Scholar] [CrossRef]
- Wang, T.; Xing, J.; Zhu, L.; Jia, A.; Wang, Y.; Lu, J.; Luo, M. CO oxidation over supported Pt/CrxFe2-xO3 catalysts and their good tolerance to CO2 and H2O. Appl. Catal. B-Environ. 2019, 245, 314–324. [Google Scholar] [CrossRef]
- Liao, W.; Fang, X.; Cen, B.; Chen, J.; Liu, Y.; Luo, M.; Lu, J. Deep oxidation of propane over WO3-promoted Pt/BN catalysts: The critical role of Pt-WO3 interface. Appl. Catal. B-Environ. 2020, 272, 118858. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Liao, W.; Jia, A.; Wang, Y.; Luo, M.; Lu, J. Highly Active Pt/BN Catalysts for Propane Combustion: The Roles of Support and Reactant-Induced Evolution of Active Sites. ACS Catal. 2019, 9, 1472–1481. [Google Scholar] [CrossRef]
- Hu, Z.; Zhu, Y.; Gao, Z.; Wang, G.; Liu, Y.; Liu, X.; Yuan, Z. CuO catalysts supported on activated red mud for efficient catalytic carbon monoxide oxidation. Chem. Eng. J. 2016, 302, 23–32. [Google Scholar] [CrossRef]
- Tang, X.; Li, J.; Sun, L.; Hao, J. Origination of N2O from NO reduction by NH3 over β-MnO2 and α-Mn2O3. Appl. Catal. B-Environ. 2010, 99, 156–162. [Google Scholar] [CrossRef]
- Arandiyan, H.; Dai, H.; Ji, K.; Sun, H.; Li, J. Pt Nanoparticles Embedded in Colloidal Crystal Template Derived 3D Ordered Macroporous Ce0.6Zr0.3Y0.1O2: Highly Efficient Catalysts for Methane Combustion. ACS Catal. 2015, 5, 1781–1793. [Google Scholar] [CrossRef]
- Cheng, Y.; Song, W.; Liu, J.; Zheng, H.; Zhao, Z.; Xu, C.; Wei, Y.; Hensen, E.J.M. Simultaneous NOx and Particulate Matter Removal from Diesel Exhaust by Hierarchical Fe-Doped Ce–Zr Oxide. ACS Catal. 2017, 7, 3883–3892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, L.; Zhu, S.; Chen, Y.; Wang, Y.; Meng, W.; Xu, S.; Lin, Z.; Li, X.; Sun, M.; Guo, L. Anionic defects engineering of Co3O4 catalyst for toluene oxidation. Fuel, 2021; 122774, in press. [Google Scholar]
- Martínez-Arias, A.; Gamarra, D.; Fernández-García, M.; Hornés, A.; Bera, P.; Koppány, Z.; Schay, Z. Redox-catalytic correlations in oxidised copper-ceria CO-PROX catalysts. Catal. Today 2009, 143, 211–217. [Google Scholar] [CrossRef]






| Catalysts | ||||||
|---|---|---|---|---|---|---|
| 1% CuO/KCC−1 | 1882 | 7.6 × 10−5 | 0.423 | 365 | 3.38 × 10−4 | 3.80 × 10−4 |
| 5% CuO/KCC−1 | 1897 | 2.2 × 10−4 | 0.391 | 358 | 1.09 × 10−3 | 1.22 × 10−3 |
| 7% CuO/KCC−1 | 1926 | 1.4 × 10−4 | 0.403 | 358 | 6.97 × 10−4 | 7.84 × 10−4 |
| CuO | 6114 | 7.5 × 10−5 | 0.412 | 365 | 1.12 × 10−3 | 1.26 × 10−3 |
| Samples | Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Pore Size (nm) |
|---|---|---|---|
| 7%Cu/KCC−1−EG | 309 | 0.76 | 3.5 |
| 5%Cu/KCC−1−EG | 345 | 0.95 | 3.5 |
| 5%Cu/KCC−1−H2O | 264 | 0.87 | 3.4 |
| 3%Cu/KCC−1−EG | 388 | 0.97 | 3.5 |
| 1%Cu/KCC−1−EG | 457 | 1.21 | 3.5 |
| KCC−1 | 638 | 1.45 | 3.5 |
| CuO | 10 | 0.006 | 7.0 |
| Samples | Cu 2p Binding Energy (eV) | ∆E (eV) | O 1s Binding Energy (eV) | Oads/Olat | ||
|---|---|---|---|---|---|---|
| 2p1/2 | 2p3/2 | Oads. | Olat. | |||
| 5% Cu/KCC−1−EG | 952.1 | 932.1 | 20.0 | 531.0 | 530.3 | 1.7 |
| 5% Cu/KCC−1−H2O | 951.5 | 932.1 | 19.4 | 531.0 | 529.1 | 0.9 |
| CuO | 951.9 | 931.8 | 20.1 | 530.9 | 530.3 | 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Li, Y.; Wang, C.; Wang, J.; Liu, W.; Peng, H.; Wu, D. Highly Active CuO/KCC−1 Catalysts for Low-Temperature CO Oxidation. Processes 2022, 10, 145. https://doi.org/10.3390/pr10010145
Luo Y, Li Y, Wang C, Wang J, Liu W, Peng H, Wu D. Highly Active CuO/KCC−1 Catalysts for Low-Temperature CO Oxidation. Processes. 2022; 10(1):145. https://doi.org/10.3390/pr10010145
Chicago/Turabian StyleLuo, Yiwei, Yonglong Li, Conghui Wang, Jing Wang, Wenming Liu, Honggen Peng, and Daishe Wu. 2022. "Highly Active CuO/KCC−1 Catalysts for Low-Temperature CO Oxidation" Processes 10, no. 1: 145. https://doi.org/10.3390/pr10010145






