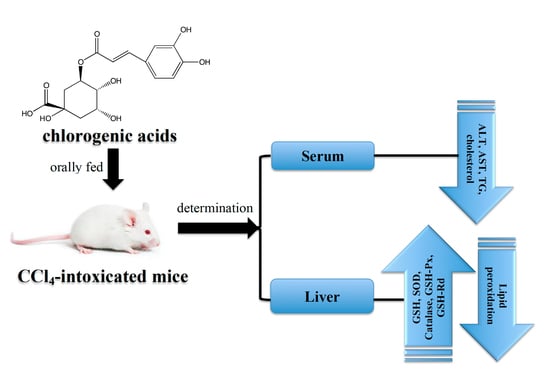
Protective Effects of Chlorogenic Acid against Carbon Tetrachloride-Induced Hepatotoxicity in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. Treatment
2.4. Measurement of Serum AST, ALT, Cholesterol, and TG
2.5. Measurement of GSH, Catalase, SOD, GSH-Rd, and GSH-Px
2.6. Measurement of Lipid Peroxidation
2.7. Histopathological Evaluation
2.8. Statistical Analysis
3. Results
3.1. Effect of CGA in CCl4-Induced Hepatotoxicity
3.2. Hepatic Antioxidant Enzyme Activities
3.3. Lipid Peroxidation and GSH
3.4. Histopathologic Examination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, J.; Danielsson, A.; Zern, M.A. Toxicity of hepatotoxins: New insights into mechanisms and therapy. Expert Opin. Investig. Drugs 1999, 8, 585–607. [Google Scholar] [CrossRef]
- Vitaglione, P.; Morisco, F.; Caporaso, N.; Fogliano, V. Dietary antioxidant compounds and liver health. Crit. Rev. Food Sci. Nutr. 2004, 44, 575–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofer, T. Oxidative stress in human toxicology. Antioxidants 2021, 10, 1159. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.N.; Kim, S.H.; Dey, D.K.; Park, S.M.; Nasif, O.; Bajpai, V.K.; Kang, S.C.; Lee, J.; Park, J.G. 5-O-Demethylnobiletin alleviates CCl4-induced acute liver injury by equilibrating ROS-mediated apoptosis and autophagy induction. Int. J. Mol. Sci. 2021, 22, 1083. [Google Scholar] [CrossRef]
- Geetha, S.; Jayamurthy, P.; Pal, K.; Pandey, S.; Kumar, R.; Sawhney, R.C. Hepatoprotective effects of sea buckthorn (Hippophae rhamnoides L.) against carbon tetrachloride induced liver injury in rats. J. Sci. Food Agric. 2008, 88, 1592–1597. [Google Scholar] [CrossRef]
- Boll, M.; Weber, L.W.; Becker, E.; Stampfl, A. Pathogenesis of carbon tetrachloride-induced hepatocyte injury bioactivation of CCl4 by cytochrome P450 and effects on lipid homeostasis. Z Naturforsch. C J. Biosci. 2001, 56, 111–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhathal, P.S.; Rose, N.R.; Mackay, I.R.; Whittingham, S. Strain differences in mice in carbon tetrachloride-induced liver injury. Br. J. Exp. Pathol. 1983, 64, 524–533. [Google Scholar]
- Al-Amarat, W.; Abukhalil, M.H.; Althunibat, O.Y.; Alfwuaires, M.A.; Alnamshan, M.M.; Alqosaibi, A.I.; Ahmeda, A.F.; Kamel, E.M.; Arab, H.H.; Mahmoud, A.M. Galangin attenuates liver injury, oxidative stress and inflammation, and upregulates Nrf2/HO-1 signaling in streptozotocin-induced diabetic rats. Processes 2021, 9, 1562. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Hirata, Y.; Saito, T.; Kumagai, H. Combined effects of amino acids in garlic and buna-shimeji (Hypsizygus marmoreus) on suppression of CCl4-induced hepatic injury in rats. Foods 2021, 10, 1491. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.K.; Bansal, M.; Soni, G.; Bhatnagar, D. N-nitrosodiethylamine induced oxidative stress in rat liver. Chem. Biol. Interact. 2005, 156, 101–111. [Google Scholar] [CrossRef]
- Stocker, R. Dietary and pharmacological antioxidants in atherosclerosis. Curr. Opin. Lipidol. 1999, 10, 589–597. [Google Scholar] [CrossRef]
- Gonthier, M.P.; Verny, M.A.; Besson, C.; Rémésy, C.; Scalbert, A. Chlorogenic acid bioavailability largely depends on its metabolism by the gut microflora in rats. J. Nutr. 2003, 133, 1853–1859. [Google Scholar] [CrossRef] [Green Version]
- Clifford, M.N. Chlorogenic acids and other cinnamates—nature, occurrence and dietary burden. J. Sci. Food Agric. 1999, 79, 362–372. [Google Scholar] [CrossRef]
- Konishi, Y.; Kobayashi, S. Transepithelial transport of chlorogenic acid, caffeic acid, and their colonic metabolites in intestinal Caco-2 cell monolayers. J. Agric. Food Chem. 2004, 52, 2518–2526. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Lindsay, J.; Laurin, D.; Verreault, R.; Hebert, R.; Helliwell, B.; Hill, G.B.; McDowell, I. Risk factors for Alzheimer’s disease: A prospective analysis from the Canadian Study of Health and Aging. Am. J. Epidemiol. 2002, 156, 445–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salazar-Martinez, E.; Willett, W.C.; Ascherio, A.; Manson, J.E.; Leitzmann, M.F.; Stampfer, M.J.; Hu, F.B. Coffee consumption and risk for type 2 diabetes mellitus. Ann. Intern. Med. 2004, 140, 1–8. [Google Scholar] [CrossRef]
- Ranheim, T.; Halvorsen, B. Coffee consumption and human health: Beneficial or detrimental? Mechanisms for effects of coffee consumption on different risk factors for cardiovascular disease and type 2 diabetes mellitus. Mol. Nutr. Food Res. 2005, 49, 274–284. [Google Scholar] [CrossRef]
- Almeida, A.A.; Farah, A.; Silva, D.A.M.; Nunam, E.A.; Glória, M.B.A. Antibacterial activity of coffee extracts and selected coffee chemical compounds against enterobacteria. J. Agric. Food Chem. 2006, 54, 8738–8743. [Google Scholar] [CrossRef]
- Santos, M.D.; Almeida, M.C.; Lopes, N.P.; Souza, G.E.P. Evaluation of the antiinflamatory, analgesic and antypiretic activity of the natural polyphenol chlorogenic acid. Biol. Pharm. Bull. 2006, 29, 2236–2240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shearer, J.; Farah, A.; de Paulis, T.; Bracy, D.P.; Pencek, R.R.; Graham, T.E.; Wasserman, D.H. Quinides of roasted coffee enhance insulin action in conscious rats. J. Nutr. 2003, 133, 3529–3532. [Google Scholar] [CrossRef]
- Berton, T.R.; Conti, C.J.; Mitchell, D.L.; Aldaz, C.M.; Lubet, R.A.; Fischer, S.M. The effect of vitamin E acetate on ultravioletinduced mouse skin carcinogenesis. Mol. Carcinog. 1998, 23, 175–184. [Google Scholar] [CrossRef]
- Goodla, L.; Manubolu, M.; Pathakoti, K.; Jayakumar, T.; Sheu, J.R.; Fraker, M.; Tchounwou, P.B.; Poondamalli, P.R. Protective effects of Ammannia baccifera against CCl4-induced oxidative stress in rats. Int. J. Environ. Res. Public Health 2019, 16, 1440. [Google Scholar] [CrossRef] [Green Version]
- Brattin, W.J.; Glende, E.A., Jr.; Recknagel, R.O. Pathological mechanisms in carbon tetrachloride hepatotoxicity. J. Free Radic. Biol. Med. 1985, 1, 27–38. [Google Scholar] [CrossRef]
- Hattori, T.; Ito, M.; Suzuki, Y. Studies on antinephritic effects of plant components in rats (1). Effects of saikosaponins originaltype anti-GBM nephritis in rats and its mechanisms. Nippon. Yakurigaku Zasshi 1991, 97, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.R.; Panda, V.S. Antioxidant and hepatoprotective effects of Ginkgo biloba phytosomes in carbon tetrachloride-induced liver injury in rodents. Liver Int. 2007, 27, 393–399. [Google Scholar] [CrossRef]
- Sodergren, E.; Cederberg, J.; Vessby, B.; Basu, S. Vitamin E reduces lipid peroxidation in experimental hepatotoxicity in rats. Eur. J. Nutr. 2001, 40, 10–16. [Google Scholar] [CrossRef]
- Dai, C.; Li, H.; Wang, Y.; Tang, S.; Velkov, T.; Shen, J. Inhibition of oxidative stress and ALOX12 and NF-κB pathways contribute to the protective effect of baicalein on carbon tetrachloride-induced acute liver injury. Antioxidants 2021, 10, 976. [Google Scholar] [CrossRef]
- Tsai, J.C.; Chiu, C.S.; Chen, Y.C.; Lee, M.S.; Hao, X.Y.; Hsieh, M.T.; Kao, C.P.; Peng, W.H. Hepatoprotective effect of Coreopsis tinctoria flowers against carbon tetrachloride-induced liver damage in mice. BMC Complement. Altern. Med. 2017, 17, 139. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Yang, Z.; Zhang, J.; Mu, J.; Zhou, X.; Zhao, X. Liver Injury Induced by Carbon Tetrachloride in Mice Is Prevented by the Antioxidant Capacity of Anji White Tea Polyphenols. Antioxidants 2019, 8, 64. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.F.; Hsu, Y.W.; Ting, H.C.; Huang, C.F.; Yen, C.C. The in vivo antioxidant and antifibrotic properties of green tea (Camellia sinensis, Theaceae). Food Chem. 2013, 136, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Demiroren, K.; Basunlu, M.T.; Erten, R.; Cokluk, E. A comparison of the effects of thymoquinone, silymarin and N-acetylcysteine in an experimental hepatotoxicity. Biomed. Pharmacother. 2018, 106, 1705–1712. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.W.; Tsai, C.F.; Chen, W.K.; Lu, F.J. Protective effects of seabuckthorn (Hippophae rhamnoides L.) seed oil against carbon tetrachloride- induced hepatotoxicity in mice. Food Chem. Toxicol. 2009, 47, 2281–2288. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Role of free radicals and catalytic metal irons in human disease: An overview. Meth. Enzymol. 1990, 186, 59–85. [Google Scholar]
- Wang, B.J.; Liu, C.T.; Tseng, C.Y.; Wu, C.P.; Yu, Z.R. Hepatoprotective and antioxidant effects of Bupleurum kaoi Liu (Chao et Chuang) extract and its fractions fractionated using supercritical CO2 on CCl4-induced liver damage. Food Chem. Toxicol. 2004, 42, 609–617. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.; Osuna, C.; Gitto, E. Actions of melatonin in the reduction of oxidative stress. J. Biomed. Sci. 2000, 7, 444–458. [Google Scholar] [CrossRef]
- Baudrimont, I.; Ahouandjivo, R.; Creppy, E.E. Prevention of lipid peroxidation induced by ochratoxin A in Vero cells in culture by several agents. Chem. Biol. Interact. 1997, 104, 29–40. [Google Scholar] [CrossRef]
- Yang, Y.S.; Ahn, T.H.; Lee, J.C.; Moon, C.J.; Kim, S.H.; Jun, W.; Park, S.C.; Kim, H.C.; Kim, J.C. Protective effects of Pycnogenol® on carbon tetrachloride-induced hepatotoxicity in Sprague–Dawley rats. Food Chem. Toxicol. 2008, 46, 380–387. [Google Scholar] [CrossRef]
- Kadiska, M.B.; Gladen, B.C.; Baird, D.D.; Dikalov, A.E.; Sohal, R.S.; Hatch, G.B.; Jones, D.P.; Mason, R.P.; Barret, J.C. Biomarkers of oxidative stress study: Are plasma antioxidants markers of CCl4 poisoning? J. Free Radic. Biol. Med. 2000, 28, 838–845. [Google Scholar] [CrossRef]
- Dambach, D.M.; Durham, S.K.; Laskin, J.D.; Laskin, D.L. Distinct roles of NFkappaB p50 in the regulation of acetaminophen-induced inflammatory mediator production and hepatotoxicity. Toxicol. Appl. Pharmacol. 2006, 211, 157–165. [Google Scholar] [CrossRef]
- Cantin, A.M.; White, T.B.; Cross, C.E.; Forman, H.J.; Sokol, R.J.; Borowitz, D. Antioxidants in cystic fibrosis conclusions from the CF antioxidant workshop, Bethesda, Maryland, November 11–12, 2003. J. Free Radic. Biol. Med. 2007, 42, 15–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castor, J.A.; Ferrya, G.C.; Castro, C.R.; Sasame, S.; Fenes, O.M.; Gilette, J.R. Prevention of carbon tetrachloride-induced necrosis by inhibitors of drug metabolism. Further studies on the mechanism of their action. Biochem. Pharmacol. 1974, 23, 295–302. [Google Scholar] [CrossRef]
- Halliwell, B. Antioxidants and human disease: A general introduction. Nutr. Rev. 1997, 55, 44–52. [Google Scholar] [CrossRef] [PubMed]


| Design of Treatment | AST (Units/L) | ALT (Units/L) | Cholesterol (mg/dL) | TG (mg/dL) | Total Albumin (mg/dL) |
|---|---|---|---|---|---|
| Normal control | 15.9 ± 3.45 e | 33.1 ± 6.22 e | 223 ± 14.4 b | 162 ± 12.3 c | 38.7 ± 3.52 b |
| CCl4 (3 mL/kg i.p.) | 1230 ± 104 a | 1253 ± 115 a | 313 ± 22.3 a | 261 ± 15.5 a | 22.5 ± 0.77 a |
| Silymarin + CCl4 | 7.37 ± 2.01 f | 16.5 ± 2.60 f | 238 ± 12.9 b | 181 ± 16.0 b | 38.9 ± 1.81 b |
| CGA (60 mg/kg) + CCl4 | 895 ± 29.7 b | 827 ± 71.2 b | 265 ± 20.2 b | 209 ± 11.7 b | 37.2 ± 0.82 b |
| CGA (100 mg/kg) + CCl4 | 390 ± 33.6 c | 317 ± 10.4 c | 249 ± 13.8 b | 188 ± 12.5 b | 38.5 ± 0.67 b |
| CGA (200 mg/kg) + CCl4 | 42.6 ± 4.99 d | 58.5 ± 6.50 d | 197 ± 15.5 b | 155 ± 9.81 c | 41.4 ± 1.12 b |
| p-Value | 0.000 | 0.000 | 0.010 | 0.016 | 0.010 |
| Design of Treatment | Catalase (Units/mg Protein) | SOD (Units/mg Protein) | GSH-Rd (nmole NADPH/min/mg Protein) | GSH-Px (nmole NADPH/min/mg Protein) |
|---|---|---|---|---|
| Normal control | 18.9 ± 1.63 b | 22.1 ± 2.28 d | 99.5 ± 1.22 c | 493 ± 53.4 d |
| CCl4 (3 mL/kg i.p.) | 5.62 ± 0.98 a | 10.9 ± 1.24 a | 72.3 ± 5.09 a | 277 ± 23.2 a |
| Silymarin + CCl4 | 21.0 ± 3.04 b | 15.4 ± 1.11 c | 85.2 ± 2.24 b | 353 ± 30.1 b,c |
| CGA (60 mg/kg) + CCl4 | 15.3 ± 2.58 b | 12.4 ± 1.32 b | 77.5 ± 1.16 a | 309 ± 15.2 b |
| CGA (100 mg/kg) + CCl4 | 19.9 ± 1.18 b | 15.2 ± 1.72 c | 95.9 ± 0.85 c | 453 ± 25.0 d |
| CGA (200 mg/kg) + CCl4 | 21.9 ± 3.37 b | 20.3 ± 1.53 d | 98.1 ± 7.00 c | 544 ± 31.7 e |
| p-Value | 0.008 | 0.020 | 0.041 | 0.011 |
| Design of Treatment | TBARS (nmol/mg Protein) | GSH (μmol/g wet Weight) |
|---|---|---|
| Normal control | 1.28 ± 0.18 d | 4.51 ± 0.58 c |
| CCl4 (3 mL/kg i.p.) | 3.08 ± 0.41 a | 2.60 ± 0.44 a |
| Silymarin + CCl4 | 1.37 ± 0.31 d | 4.47 ± 0.21 c |
| CGA (60 mg/kg) + CCl4 | 2.12 ± 0.14 b | 3.67 ± 0.52 b |
| CGA (100 mg/kg) + CCl4 | 1.75 ± 0.15 c | 4.01 ± 0.22 b,c |
| CGA (200 mg/kg) + CCl4 | 1.29 ± 0.09 d | 4.65 ± 0.19 c |
| p-Value | 0.032 | 0.010 |
| Parameter | Grades */Score ** | Design of Treatment | |||||
|---|---|---|---|---|---|---|---|
| Normal Control | CCl4 Control (3 mL/kg) | Silymarin (200 mg/kg) + CCl4 | CGA (60 mg/kg) + CCl4 | CGA (100 mg/kg) + CCl4 | CGA (200 mg/kg) + CCl4 | ||
| Hepatocyte necrosis | − | 6 | 0 | 0 | 0 | 0 | 0 |
| + | 4 | 0 | 3 | 1 | 4 | 3 | |
| ++ | 0 | 2 | 7 | 4 | 6 | 7 | |
| +++ | 0 | 8 | 0 | 5 | 0 | 0 | |
| Score | 0.40 ± 0.52 b,c | 2.80 ± 0.42 a,c | 1.70 ± 0.48 a,b | 2.4 ± 0.70 a | 1.60 ± 0.52 a,b | 1.70 ± 0.48 a,b | |
| Inflammatory cell infiltration | − | 7 | 0 | 0 | 0 | 0 | 0 |
| + | 3 | 0 | 0 | 0 | 0 | 0 | |
| ++ | 0 | 3 | 9 | 7 | 9 | 10 | |
| +++ | 0 | 7 | 1 | 3 | 1 | 0 | |
| Score | 0.30 ± 0.48 b,c | 2.70 ± 0.48 a,c | 2.10 ± 0.32 a,b | 2.30 ± 0.48 a | 2.10 ± 0.32 a,b | 2.00 ± 0.00 a,b | |
| Ballooning deterioration | − | 9 | 0 | 0 | 0 | 0 | 0 |
| + | 1 | 0 | 5 | 0 | 0 | 2 | |
| ++ | 0 | 2 | 4 | 7 | 8 | 7 | |
| +++ | 0 | 8 | 1 | 3 | 2 | 1 | |
| Score | 0.10 ± 0.32 b,c | 2.80 ± 0.42 a,c | 1.60 ± 0.70 a,b | 2.30 ± 0.48 a | 2.20 ± 0.42 a | 1.90 ± 0.57 a,b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, Y.-W.; Chen, Y.-Y.; Tsai, C.-F. Protective Effects of Chlorogenic Acid against Carbon Tetrachloride-Induced Hepatotoxicity in Mice. Processes 2022, 10, 31. https://doi.org/10.3390/pr10010031
Hsu Y-W, Chen Y-Y, Tsai C-F. Protective Effects of Chlorogenic Acid against Carbon Tetrachloride-Induced Hepatotoxicity in Mice. Processes. 2022; 10(1):31. https://doi.org/10.3390/pr10010031
Chicago/Turabian StyleHsu, Yu-Wen, Ya-Yu Chen, and Chia-Fang Tsai. 2022. "Protective Effects of Chlorogenic Acid against Carbon Tetrachloride-Induced Hepatotoxicity in Mice" Processes 10, no. 1: 31. https://doi.org/10.3390/pr10010031
APA StyleHsu, Y.-W., Chen, Y.-Y., & Tsai, C.-F. (2022). Protective Effects of Chlorogenic Acid against Carbon Tetrachloride-Induced Hepatotoxicity in Mice. Processes, 10(1), 31. https://doi.org/10.3390/pr10010031