The Effect of Electromagnetic Microwave Radiation on Methane Fermentation of Selected Energy Crop Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Materials
2.3. Experimental Setup
2.4. Calculation Methods
2.5. Analytical Methods
2.6. Statistical Methods
3. Results
3.1. Biogas Production and Composition
3.2. Characteristics of Digestate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grifoni, M.; Pedron, F.; Barbafieri, M.; Rosellini, I.; Petruzzelli, G.; Franchi, E. Sustainable Valorization of Biomass: From Assisted Phytoremediation to Green Energy Production. In Handbook on Assisted and Amendments Enhanced Sustainable Remediation Technology; Prasad, M.N.V., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar] [CrossRef]
- Jablonowski, N.D.; Kollmann, T.; Nabel, M.; Damm, T.; Klose, H.; Müller, M.; Bläsing, M.; Seebold, S.; Krafft, S.; Kuperjans, I.; et al. Valorization of Sida (Sida hermaphrodita) biomass for multiple energy purposes. GCB Bioenergy 2017, 9, 202–214. [Google Scholar] [CrossRef]
- Nabel, M.; Temperton, V.M.; Poorter, H.; Lücke, A.; Jablonowski, N.D. Energizing marginal soils—The establishment of the energy crop Sida hermaphrodita as dependent on digestate fertilization, NPK, and legume intercropping. Biomass Bioenergy 2016, 87, 9–16. [Google Scholar] [CrossRef]
- Kazimierowicz, J.; Dzienis, L. Giant miscanthus as a substrate for biogas production. J. Ecol. Eng. 2015, 16, 139–142. [Google Scholar] [CrossRef]
- Kacprzak, A.; Michalska, K.; Romanowska-Duda, Z.; Grzesik, M. Energy crops as a valuable raw material for biogas production. Cosm. Probl. Biol. Sci. 2012, 61, 281–293. [Google Scholar]
- Khan, M.U.; Lee, J.T.E.; Bashir, M.A.; Dissanayake, P.D.; Ok, Y.S.; Tong, Y.W.; Shariati, M.A.; Wu, S.; Ahring, B.K. Current status of biogas upgrading for direct biomethane use: A review. Renew. Sustain. Energy Rev. 2021, 149, 111343. [Google Scholar] [CrossRef]
- Markou, G.; Brule, M.; Balafoutis, A.; Kornaros, M.; Georgakakis, D.; Papadakis, G. Biogas production from energy crops in northern Greece: Economics of electricity generation associated with heat recovery in a greenhouse. Clean Technol. Environ. Policy 2017, 19, 1147–1167. [Google Scholar] [CrossRef]
- Cinar, S.; Cinar, S.O.; Wieczorek, N.; Sohoo, I.; Kuchta, K. Integration of Artificial Intelligence into Biogas Plant Operation. Processes 2021, 9, 85. [Google Scholar] [CrossRef]
- Lombardi, L.; Francini, G. Techno-economic and environmental assessment of the main biogas upgrading technologies. Renew. Energy 2020, 156, 440–458. [Google Scholar] [CrossRef]
- Olugbade, T.O.; Ojo, O.T. Biomass Torrefaction for the Production of High-Grade Solid Biofuels: A Review. Bioenergy Res. 2020, 13, 999–1015. [Google Scholar] [CrossRef]
- Olugbade, T.; Ojo, O.; Mohammed, T. Influence of Binders on Combustion Properties of Biomass Briquettes: A Recent Review. Bioenergy Res. 2019, 12, 241–259. [Google Scholar] [CrossRef]
- Borek, K.; Romaniuk, W.; Roman, K.; Roman, M.; Kuboń, M. The Analysis of a Prototype Installation for Biogas Production from Chosen Agricultural Substrates. Energies 2021, 14, 2132. [Google Scholar] [CrossRef]
- Czekała, W.; Gawrych, K.; Smurzyńska, A.; Mazurkiewicz, J.; Pawlisiak, A.; Chełkowski, D.; Brzoski, M. The possibility of functioning micro-scale biogas plant in selected farm. J. Water Land Dev. 2017, 35, 19–25. [Google Scholar] [CrossRef][Green Version]
- Bourdin, S.; Raulin, F.; Josset, C. On the (un)successful deployment of renewable energies: Territorial context matters. A conceptual framework and an empirical analysis of biogas projects. Energy Stud. Rev. 2020, 24, 4088. [Google Scholar] [CrossRef]
- Ardolino, F.; Cardamone, G.F.; Parrillo, F.; Arena, U. Biogas-to-biomethane upgrading: A comparative review and assessment in a life cycle perspective. Renew. Sustain. Energy Rev. 2021, 139, 110588. [Google Scholar] [CrossRef]
- Verbeeck, K.; Buelens, L.C.; Galvita, V.V.; Marin, G.B.; Van Geem, K.M.; Rabaey, K. Upgrading the value of anaerobic digestion via chemical production from grid injected biomethane. Energy Environ. Sci. 2018, 11, 1788–1802. [Google Scholar] [CrossRef]
- Aryal, N.; Kvist, T. Alternative of Biogas Injection into the Danish Gas Grid System—A Study from Demand Perspective. ChemEngineering 2018, 2, 43. [Google Scholar] [CrossRef]
- Braguglia, C.M.; Gallipoli, A.; Gianico, A.; Pagliaccia, P. Anaerobic bioconversion of food waste into energy: A critical review. Bioresour. Technol. 2018, 248, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Kazimierowicz, J.; Zieliński, M.; Dębowski, M. Influence of the Heating Method on the Efficiency of Biomethane Production from Expired Food Products. Fermentation 2021, 7, 12. [Google Scholar] [CrossRef]
- Kou, X.; Li, R.; Hou, L.; Zhang, L.; Wang, S. Identifying possible non-thermal effects of radio frequency energy on inactivating food microorganisms. Int. J. Food Microbiol. 2018, 269, 89–97. [Google Scholar] [CrossRef]
- Li, H.; Zhao, Z.; Xiouras, C.; Stefanidis, G.D.; Li, X.; Gao, X. Fundamentals and applications of microwave heating to chemicals separation processes. Renew. Sustain. Energy Rev. 2019, 114, 109316. [Google Scholar] [CrossRef]
- Zielińska, M.; Cydzik-Kwiatkowska, A.; Zieliński, M.; Dębowski, M. Impact of temperature, microwave radiation and organic loading rate on methanogenic community and biogas production during fermentation of dairy wastewater. Bioresour. Technol. 2013, 129, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, M.; Ciesielski, S.; Cydzik-Kwiatkowska, A.; Turek, J.; Dębowski, M. Influence of microwave radiation on bacterial community structure in biofilm. Process Biochem. 2007, 42, 1250–1253. [Google Scholar] [CrossRef]
- Dębowski, M.; Kisielewska, M.; Kazimierowicz, J.; Rudnicka, A.; Dudek, M.; Romanowska-Duda, Z.; Zieliński, M. The effects of Microalgae Biomass Co-Substrate on Biogas Production from the Common Agricultural Biogas Plants Feedstock. Energies 2020, 13, 2186. [Google Scholar] [CrossRef]
- Nahm, M.; Morhart, C. Virginia mallow (Sida hermaphrodita (L.) Rusby) as perennial multipurpose crop: Biomass yields, energetic valorization, utilization potentials, and management perspectives. GCB Bioenergy 2018, 10, 393–404. [Google Scholar] [CrossRef]
- Papamatthaiakis, N.; Laine, A.; Haapala, A.; Ikonen, R.; Kuittinen, S.; Pappinen, A.; Kolström, M.; Mola-Yudego, B. New energy crop alternatives for Northern Europe: Yield, chemical and physical properties of Giant knotweed (Fallopia sachalinensis var. ‘Igniscum’) and Virginia mallow (Sida hermaphrodita). Fuel 2021, 304, 121349. [Google Scholar] [CrossRef]
- Nabel, M.; Schrey, S.D.; Poorter, H.; Koller, R.; Jablonowski, N.D. Effects of digestate fertilization on Sida hermaphrodita: Boosting biomass yields on marginal soils by increasing soil fertility. Biomass Bioenergy 2017, 107, 207–213. [Google Scholar] [CrossRef]
- Cumplido-Marin, L.; Graves, A.R.; Burgess, P.J.; Morhart, C.; Paris, P.; Jablonowski, N.D.; Facciotto, G.; Bury, M.; Martens, R.; Nahm, M. Two Novel Energy Crops: Sida hermaphrodita (L.) Rusby and Silphium perfoliatum L.—State of Knowledge. Agronomy 2020, 10, 928. [Google Scholar] [CrossRef]
- Siwek, H.; Włodarczyk, M.; Mozdzer, E.; Bury, M.; Kitczak, T. Chemical composition and biogas formation potential of Sida hermaphrodita and Silphium perfoliatum. Appl. Sci. 2019, 9, 4016. [Google Scholar] [CrossRef]
- Raposo, F.; Borja, R.; Ibelli-Bianco, C. Predictive regression models for biochemical methane potential tests of biomass samples: Pitfalls and challenges of laboratory measurements. Renew. Sustain. Energy Rev. 2020, 127, 109890. [Google Scholar] [CrossRef]
- Das, S.; Das, I.; Ghangrekar, M.M. Role of applied potential on microbial electrosynthesis of organic compounds through carbon dioxide sequestration. J. Environ. Chem. Eng. 2020, 8, 104028. [Google Scholar] [CrossRef]
- Piechota, G. Multi-step biogas quality improving by adsorptive packed column system as application to biomethane upgrading. J. Environ. Chem. Eng. 2021, 9, 105944. [Google Scholar] [CrossRef]
- Munir, M.; Nadeem, M.; Qureshi, T.M.; Leong, T.S.; Gamlath, C.J.; Martin, G.J.; AshokKumar, M. Effects of High Pressure, Microwave and Ultrasound Processing on Proteins and Enzyme Activity in Dairy Systems—A Review. Innov. Food Sci. Emerg. Technol. 2019, 57, 102192. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M.; Kisielewska, M.; Kazimierowicz, J. Evaluation of Anaerobic Digestion of Dairy Wastewater in an Innovative Multi-Section Horizontal Flow Reactor. Energies 2020, 13, 2392. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, Z.; Yang, S.; Zhang, A. Performance of a microwave radiation induced persulfate-hydrogen peroxide binary-oxidant process in treating dinitrodiazophenol wastewater. Sep. Purif. Technol. 2020, 236, 116253. [Google Scholar] [CrossRef]
- Pillet, F.; Gibot, L.; Catrain, A.; Kolosnjaj-Tabi, J.; Courtois, K.; Chretiennot, T.; Bellard, E.; Tarayre, J.; Golzio, M.; Vezinet, R. High power electromagnetic pulse applicators for evaluation of biological effects induced by electromagnetic radiation waves. RSC Adv. 2018, 8, 16319–16329. [Google Scholar] [CrossRef]
- Kulbacka, J.; Choromanska, A.; Rossowska, J.; Wezgowiec, J.; Saczko, J.; Rols, M.P. Cell membrane transport mechanisms: Ion channels and electrical properties of cell membranes. Adv. Anat. Embryol. Cell Biol. 2017, 227, 39–58. [Google Scholar] [CrossRef]
- Mertenes, B.; Knorr, D. Developments of nonthermal processes for food preservation. Food Technol. 1992, 5, 125–133. [Google Scholar]
- Horikoshi, S.; Nakamura, K.; Yashiro, M.; Kadomatsu, K.; Serpone, N. Probing the effect(s) of the microwaves’ electromagnetic fields in enzymatic reactions. Sci. Rep. 2019, 9, 8945. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.-C.; Adams, D.M.; Amelkina, O.; White, K.K.; Amoretti, L.A.; Whitaker, M.G.; Comizzoli, P. Influence of microwave-assisted dehydration on morphological integrity and viability of cat ovarian tissues: First steps toward long-term preservation of complex biomaterials at supra-zero temperatures. PLoS ONE 2019, 14, e0225440. [Google Scholar] [CrossRef]
- Parker, M.C.; Besson, T.; Lamare, S.; Legoy, M.D. Microwave radiation can increase the rate of enzyme catalysed reaction in organic media. Tetrahedron Lett. 1996, 37, 8383–8386. [Google Scholar] [CrossRef]
- Lai, Y.F.; Wang, H.Y.; Peng, R.Y. Establishment of injury models in studies of biological effects induced by microwave radiation. Mil. Med. Res. 2021, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhi, W.J.; Wang, L.F.; Hu, X.J. Recent advances in the effects of microwave radiation on brains. Mil. Med. Res. 2017, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Svidlov, A.; Drobotenko, M.; Basov, A.; Gerasimenko, E.; Malyshko, V.; Elkina, A.; Baryshev, M.; Dzhimak, S. DNA Dynamics under Periodic Force Effects. Int. J. Mol. Sci. 2021, 22, 7873. [Google Scholar] [CrossRef] [PubMed]
- Shamis, Y.; Taube, A.; Mitik-Dineva, N.; Croft, R.; Crawford, R.J.; Ivanova, E.P. Specific electromagnetic effects of microwave radiation on Escherichia coli. Appl. Environ. Microbiol. 2011, 77, 3017–3022. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, M.; Dębowski, M.; Kazimierowicz, J. Microwave Radiation Influence on Dairy Waste Anaerobic Digestion in a Multi-Section Hybrid Anaerobic Reactor (M-SHAR). Processes 2021, 9, 1772. [Google Scholar] [CrossRef]
- Rougier, C.; Prorot, A.; Chazal, P.; Leveque, P.; Leprat, P. Thermal and Nonthermal Effects of Discontinuous Microwave Exposure (2.45 Gigahertz) on the Cell Membrane of Escherichia coli. Appl. Environ. Microbiol. 2014, 80, 4832–4841. [Google Scholar] [CrossRef] [PubMed]

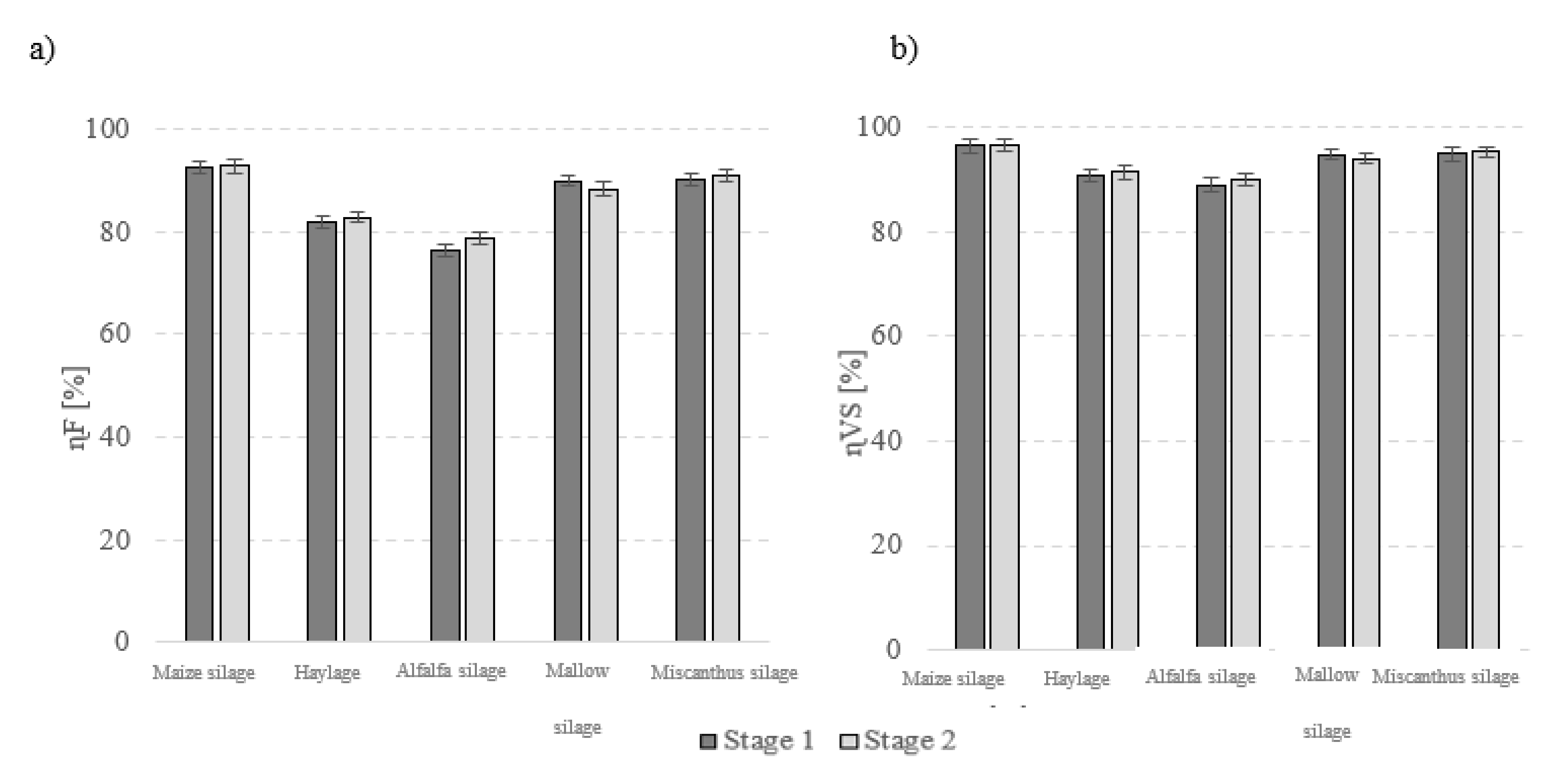
| Raw Material for Fermentation | Moisture (%) | TS (%) | VS (% TS) | TC (mg/g) | TOC (mg/g) | TN (mg/g) | pH | COD (gO2/gTS) |
|---|---|---|---|---|---|---|---|---|
| Maize silage | 73.7 ± 1.1 | 26.3 ± 0.5 | 94.6 ± 1.1 | 442 ± 12 | 417 ± 14 | 12.1 ± 1.0 | 7.88 ± 0.1 | 1.68 ± 0.10 |
| Haylage | 76.5 ± 1.0 | 23.5 ± 0.6 | 89.9 ± 1.2 | 451 ± 10 | 401 ± 12 | 5.3 ± 1.0 | 7.51 ± 0.1 | 1.12 ± 0.21 |
| Alfalfa silage | 81.1 ± 1.0 | 18.9 ± 0.4 | 88.0 ± 1.1 | 409 ± 11 | 352 ± 10 | 17.4 ± 1.2 | 7.61 ± 0.2 | 9.48 ± 0.09 |
| Virginia mallow silage | 69.0 ± 1.2 | 31.0 ± 0.5 | 93.0 ± 1.3 | 410 ± 12 | 380 ± 13 | 6.2 ± 1.0 | 7.67 ± 0.1 | 1.56 ± 0.12 |
| Miscanthus silage | 66.2 ± 1.1 | 33.8 ± 0.4 | 91.1 ± 1.2 | 449 ± 11 | 414 ± 12 | 13.1 ± 1.1 | 7.61 ± 0.2 | 1.70 ± 0.23 |
| Indicator | Unit | Value |
|---|---|---|
| Total solids (TS) | % | 2.01 ± 0.18 |
| Volatile solids (VS) | % TS | 74.42 ± 3.13 |
| Total nitrogen (TN) | mg/g TS | 33.1 ± 2.9 |
| Total phosphorus (TP) | mg/g TS | 2.0 ± 0.2 |
| Total carbon (TC) | mg/g TS | 472 ± 23 |
| Total organic carbon (TOC) | mg/g TS | 343 ± 30 |
| pH | - | 7.03 ± 0.1 |
| Raw Material for Fermentation | Moisture (%) | TS (%) | TC (mg/g) | TOC (mg/g) | TN (mg/g) | pH | COD (gO2/dm3) |
|---|---|---|---|---|---|---|---|
| Maize silage | 97.81 ± 1.2 | 2.19 ± 0.1 | 427 ± 14 | 388 ± 10 | 52 ± 1.3 | 7.48 ± 0.2 | 13.9 ± 3.1 |
| Haylage | 97.29 ± 1.1 | 2.71 ± 0.1 | 434 ± 12 | 389 ± 13 | 51 ± 1.2 | 7.23 ± 0.1 | 13.4 ± 1.3 |
| Alfalfa silage | 97.19 ± 1.1 | 2.81 ± 0.1 | 429 ± 10 | 344 ± 12 | 62 ± 1.4 | 7.42 ± 0.2 | 14.0 ± 1.4 |
| Virginia mallow silage | 97.27 ± 1.1 | 2.73 ± 0.1 | 435 ± 12 | 387 ± 13 | 51 ± 1.2 | 7.48 ± 0.2 | 13.5 ± 1.3 |
| Miscanthus silage | 97.31 ± 1.2 | 2.80 ± 0.1 | 412 ± 11 | 368 ± 12 | 53 ± 1.3 | 7.48 ± 0.3 | 13.7 ± 1.1 |
| STAGE | Average Yield | Average Biogas Composition | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biogas | Methane | |||||||||||
| dm3N/kgFM | dm3N/kgTS | dm3N/kgVS | dm3/kgFM | dm3/kgTS | dm3/kgVS | CH4 (%) | CO2 (%) | O2 (%) | H2S (ppm) | H2 (ppm) | NH3 (ppm) | |
| Maize silage | ||||||||||||
| 1 | 168 ± 8 | 644 ± 21 | 680 ± 28 | 89 ± 5 | 341 ± 11 | 361 ± 12 | 53.0 ± 1.2 | 46.9 ± 1.1 | - | 50 ± 0.9 | 23 ± 0.8 | 473 ± 22 |
| 2 | 178 ± 11 | 681 ± 20 | 720 ± 27 | 105 ± 7 | 403 ± 13 | 426 ± 14 | 59.1 ± 1.6 | 40.8 ± 1.4 | - | 63 ± 1.5 | 31 ± 1.0 | 399 ± 31 |
| Haylage | ||||||||||||
| 1 | 85 ± 6 | 361 ± 19 | 402 ± 11 | 43 ± 4 | 186 ± 9 | 207 ± 7 | 51.6 ± 1.5 | 48.3 ± 1.6 | - | 60 ± 1.8 | 37 ± 1.5 | 72 ± 5 |
| 2 | 94 ± 8 | 402 ± 14 | 448 ± 13 | 50 ± 3 | 213 ± 8 | 237 ± 9 | 53.0 ± 1.4 | 46.9 ± 1.4 | - | 59 ± 2.0 | 43 ± 1.3 | 88 ± 9 |
| Alfalfa silage | ||||||||||||
| 1 | 55 ± 5 | 291 ± 12 | 331 ± 13 | 30 ± 3 | 160 ± 7 | 181 ± 8 | 54.9 ± 1.8 | 45.0 ± 1.5 | - | 60 ± 2.1 | 37 ± 1.4 | 171 ± 12 |
| 2 | 56 ± 7 | 301 ± 14 | 342 ± 18 | 31 ± 2 | 165 ± 9 | 188 ± 9 | 55.0 ± 1.6 | 44.9 ± 1.6 | - | 58 ± 1.9 | 71 ± 2.8 | 108 ± 10 |
| Virginia mallow silage | ||||||||||||
| 1 | 98 ± 9 | 463 ± 16 | 506 ± 16 | 51 ± 4 | 241 ± 9 | 263 ± 10 | 52.0 ± 1.5 | 47.9 ± 1.4 | - | 70 ± 2.8 | 20 ± 0.5 | 470 ± 21 |
| 2 | 102 ± 11 | 471 ± 15 | 519 ± 19 | 52 ± 3 | 244 ± 10 | 269 ± 11 | 51.9 ± 1.7 | 48.0 ± 1.2 | - | 60 ± 1.9 | 26 ± 0.3 | 303 ± 18 |
| Miscanthus silage | ||||||||||||
| 1 | 144 ± 10 | 382 ± 11 | 409 ± 13 | 77 ± 5 | 203 ± 8 | 217 ± 9 | 53.1 ± 1.3 | 46.8 ± 1.1 | - | 47 ± 1.5 | 52 ± 1.9 | 441 ± 22 |
| 2 | 160 ± 11 | 422 ± 15 | 452 ± 12 | 85 ± 7 | 225 ± 9 | 241 ± 10 | 53.3 ± 1.2 | 46.6 ± 1.1 | - | 62 ± 2.1 | 49 ± 1.8 | 399 ± 17 |
| Reaction Kinetics | Raw Material for Fermentation | |||||
|---|---|---|---|---|---|---|
| Maize Silage | Haylage | Alfalfa Silage | Virginia Mallow Silage | Miscanthus Silage | ||
| STAGE 1 | k (1/d) | 0.11 | 0.11 | 0.11 | 0.11 | 0.11 |
| r (dm3N/kgVS·d) | 74.8 | 44.2 | 36.3 | 55.6 | 45.0 | |
| STAGE 2 | k (1/d) | 0.13 | 0.11 | 0.11 | 0.11 | 0.14 |
| r (dm3N/kgVS·d) | 93.6 | 49.3 | 37.4 | 56.3 | 63.0 | |
| Raw Material for Fermentation | Moisture (%) | TS (%) | TC (mg/g) | TOC (mg/g) | TN (mg/g) | pH | COD (gO2/dm3) | |
|---|---|---|---|---|---|---|---|---|
| Maize silage | Stage 1 | 98.82 ± 1.1 | 1.18 ± 0.1 | 325 ± 14 | 205 ± 11 | 32 ± 1.3 | 7.22 ± 0.2 | 10.3 ± 2.2 |
| Stage 2 | 98.77 ± 1.3 | 1.23 ± 0.1 | 282 ± 12 | 171 ± 12 | 41 ± 1.4 | 7.37 ± 0.2 | 9.8 ± 1.8 | |
| Haylage | Stage 1 | 97.59 ± 1.2 | 2.41 ± 0.1 | 327 ± 11 | 219 ± 11 | 49 ± 1.4 | 7.33 ± 0.2 | 11.1 ± 1.1 |
| Stage 2 | 97.43 ± 1.1 | 2.57 ± 0.1 | 318 ± 13 | 206 ± 13 | 48 ± 1.3 | 7.10 ± 0.1 | 10.7 ± 1.2 | |
| Alfalfa silage | Stage 1 | 97.79 ± 1.1 | 2.21 ± 0.1 | 371 ± 14 | 213 ± 12 | 57 ± 1.5 | 7.47 ± 0.2 | 10.7 ± 1.1 |
| Stage 2 | 97.58 ± 1.2 | 2.42 ± 0.1 | 374 ± 12 | 209 ± 14 | 44 ± 1.4 | 7.50 ± 0.3 | 10.1 ± 1.1 | |
| Virginia mallow silage | Stage 1 | 97.67 ± 1.1 | 2.33 ± 0.1 | 327 ± 11 | 202 ± 12 | 41 ± 1.3 | 7.20 ± 0.2 | 10.2 ± 1.0 |
| Stage 2 | 97.99 ± 1.1 | 2.01 ± 0.1 | 325 ± 10 | 207 ± 11 | 43 ± 1.2 | 7.38 ± 0.2 | 10.0 ± 1.2 | |
| Miscanthus silage | Stage 1 | 97.87 ± 1.1 | 2.13 ± 0.1 | 321 ± 12 | 202 ± 13 | 47 ± 1.4 | 7.40 ± 0.2 | 9.7 ± 1.1 |
| Stage 2 | 98.02 ± 1.2 | 1.98 ± 0.1 | 284 ± 10 | 166 ± 12 | 38 ± 1.1 | 7.29 ± 0.2 | 7.7 ± 1.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zieliński, M.; Dębowski, M.; Kazimierowicz, J. The Effect of Electromagnetic Microwave Radiation on Methane Fermentation of Selected Energy Crop Species. Processes 2022, 10, 45. https://doi.org/10.3390/pr10010045
Zieliński M, Dębowski M, Kazimierowicz J. The Effect of Electromagnetic Microwave Radiation on Methane Fermentation of Selected Energy Crop Species. Processes. 2022; 10(1):45. https://doi.org/10.3390/pr10010045
Chicago/Turabian StyleZieliński, Marcin, Marcin Dębowski, and Joanna Kazimierowicz. 2022. "The Effect of Electromagnetic Microwave Radiation on Methane Fermentation of Selected Energy Crop Species" Processes 10, no. 1: 45. https://doi.org/10.3390/pr10010045
APA StyleZieliński, M., Dębowski, M., & Kazimierowicz, J. (2022). The Effect of Electromagnetic Microwave Radiation on Methane Fermentation of Selected Energy Crop Species. Processes, 10(1), 45. https://doi.org/10.3390/pr10010045








