Thermal Behavior of Ceramic Bodies Based on Estonian Clay from the Arumetsa Deposit with Oil Shale Ash and Clinker Dust Additives
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
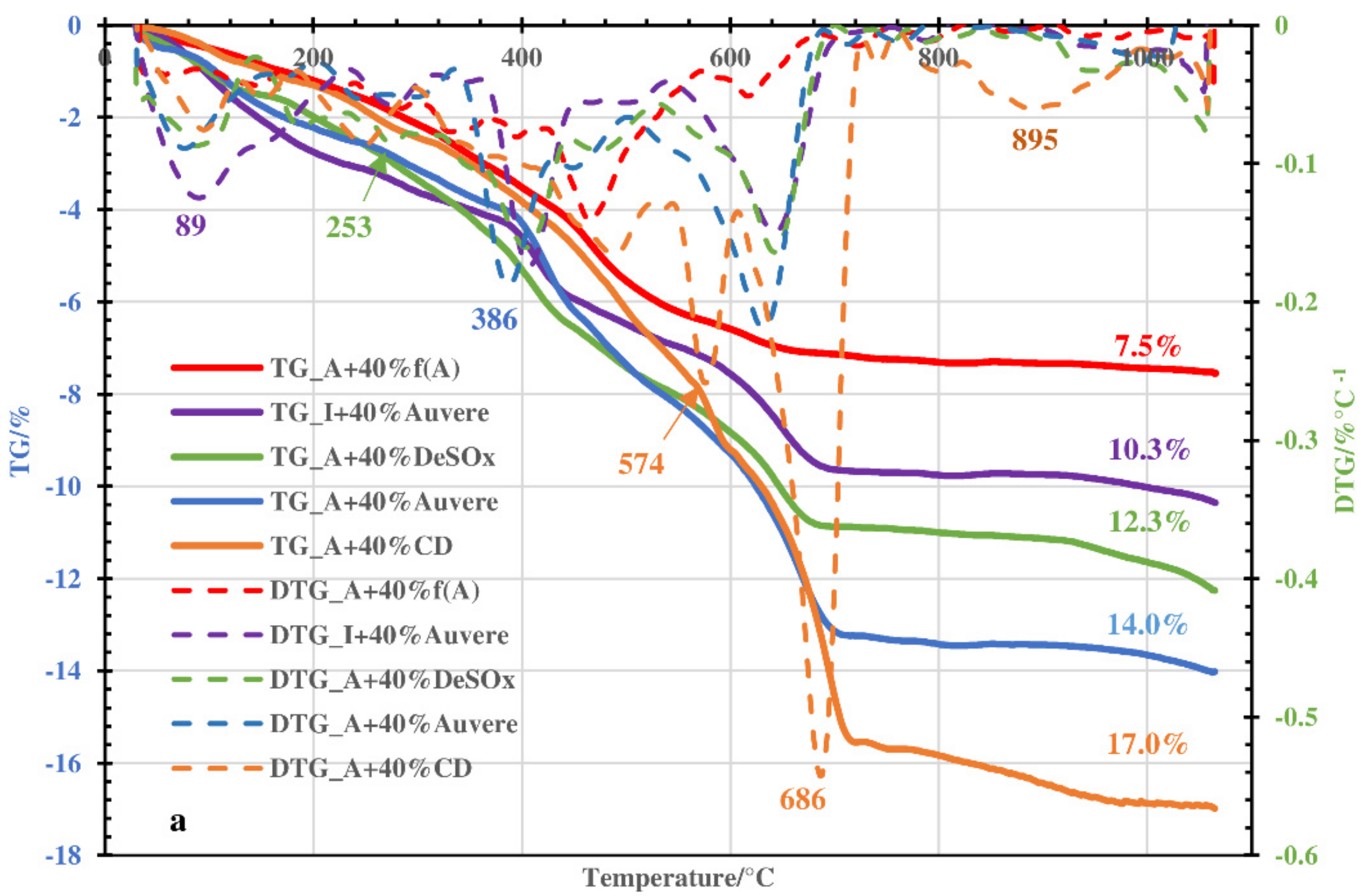
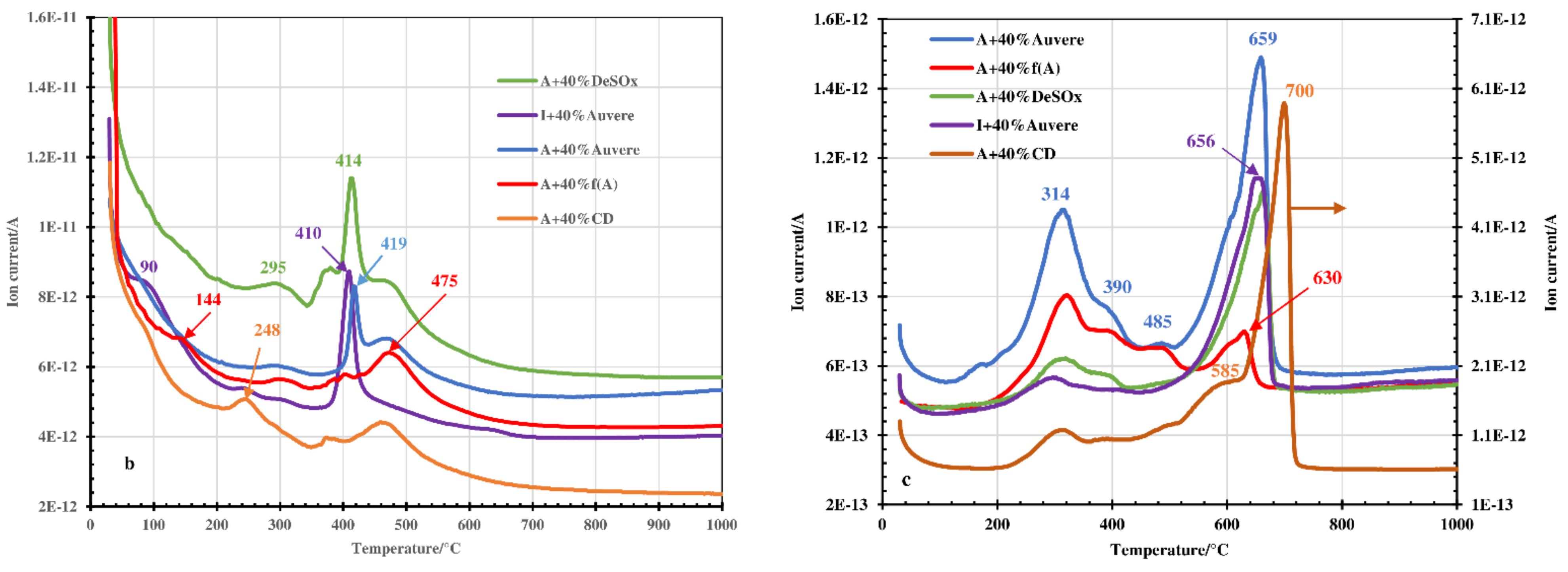
3.1. Thermal and MS Analysis
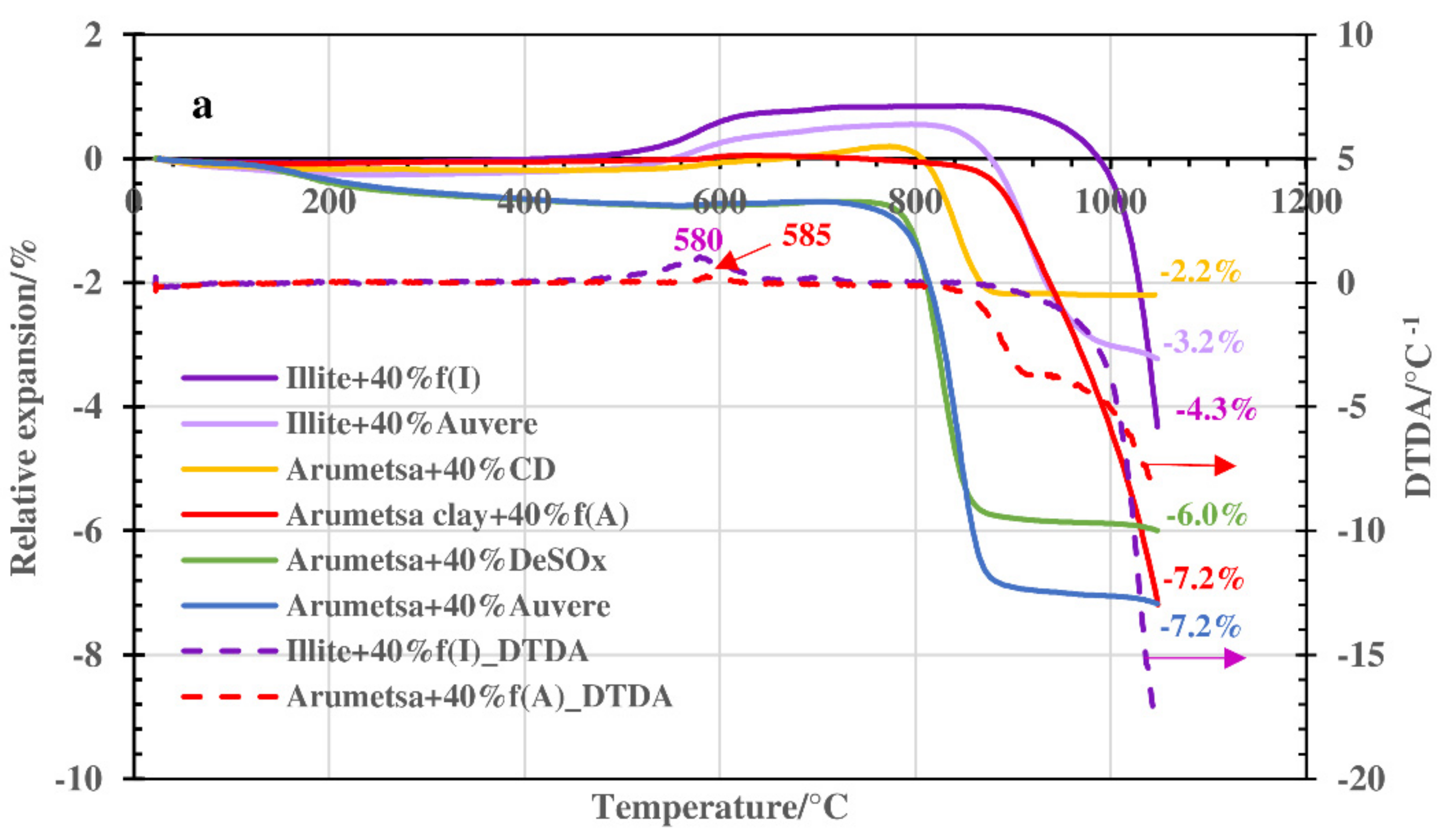
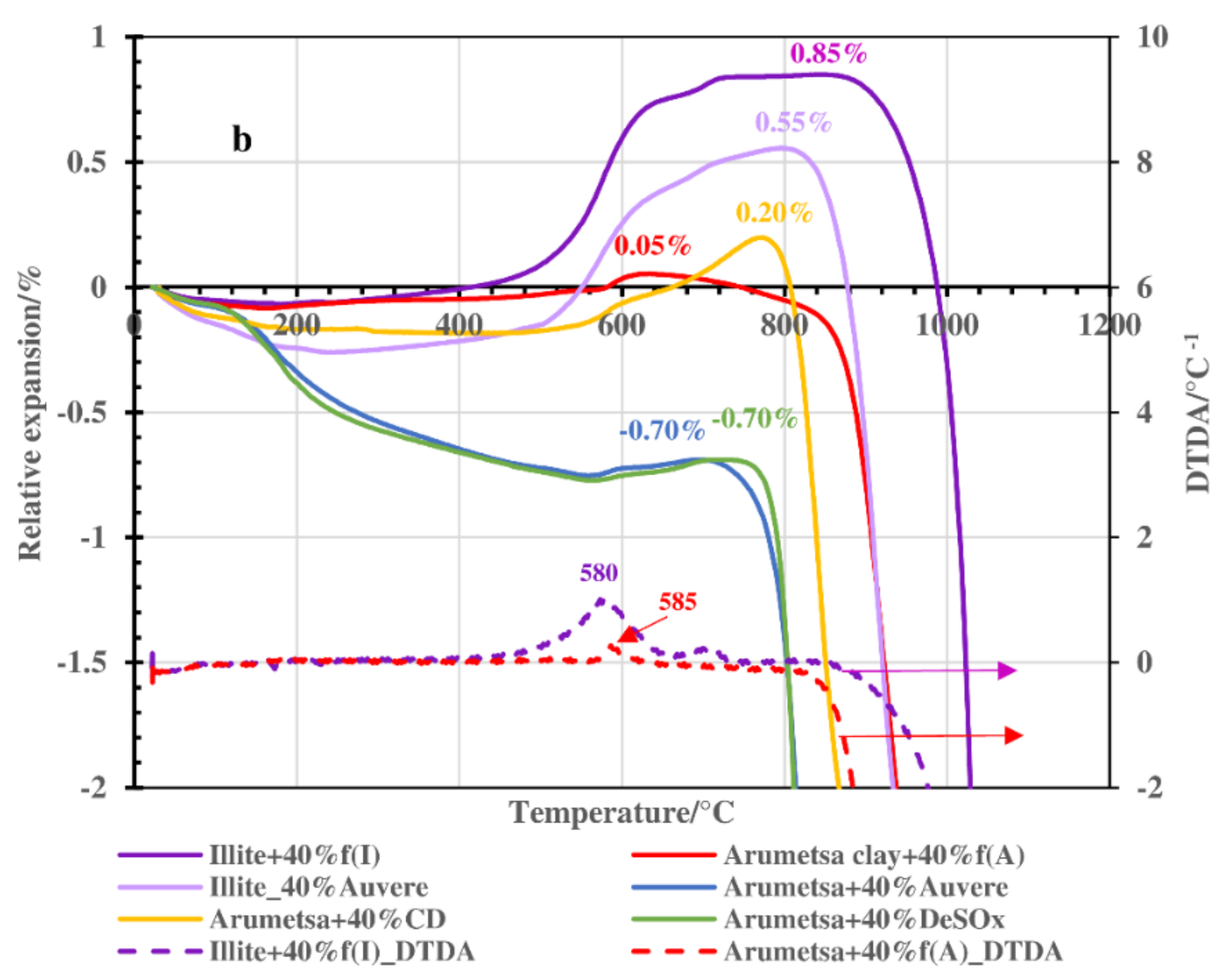
3.2. Thermodilatometry, Physical-Chemical Characteristics and Morphology
3.3. FTIR and XRD Analysis: Acid-Alkali Resistance Test
3.4. Kinetic Calculations
4. Conclusions
- −
- The decomposition of the blends occurred in two steps instead of one, which is characteristic of green clays. In addition to the emission of hygroscopic water, dehydroxylation of clay minerals and thermooxidation of organic matter, the decomposition of portlandite contained in the OS ashes took place at 550–600 °C (step I). During step II (between 550–600 °C and 1050 °C), the dehydroxylation processes continued, decomposition of the carbonates contained in the OS ashes and clinker dust took place, and different solid phase reactions with the formation of secondary silicates occurred.
- −
- Therefore, Arumetsa and illitic clay follow single-step decomposition, but their blends with OS ash or clinker dust follow two-step decomposition, shifting the formation of a liquid phase to higher temperatures.
- −
- These processes are accompanied by contraction and expansion of the ceramic bodies, with the corresponding changes in the specific surface area and porosity values leading to remarkable changes in the structures of the ceramic bodies caused by the formation of a liquid phase. The OS ash and clinker dust additives shifted the formation of a liquid phase to higher temperatures.
- −
- The acid-alkali resistance test of thermally treated ceramic bodies on the leachability of Ca2+, Mg2+, K+ and SO42− showed that in an acidic environment, the relative leachability of all cations increased remarkably. The leachability of the sulfate ions decreased in the case of OS ash additives but increased in the case of clinker dust. In an alkaline environment, the leachability of Ca2+, Mg2+ and especially SO42− decreased, but the leachability of K+ increased, particularly in the case of the clinker dust additive containing notably more arcanite compared with the OS ashes.
- −
- The value of the activation energy E changed in the reaction progress range of 0.1 < α < 0.9 within the large limits for clays, as well as for blends that indicated the complex character of the processes occurring in the thermal treatment of the clays and blends.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CD | Clinker dust |
| CFBC | Circulating fluidized bed combustion |
| DeSOx | Semi-dry desulfurization system |
| DTA | Differential thermal analysis |
| DTG | Differential TG |
| EGA | Evolved gas analysis |
| ESPA | Electrostatic precipitator ash |
| FA | Fly ash |
| FTIR | Fourier transform infrared |
| MS | Mass spectroscopy |
| OS | Oil shale |
| PF | Pulverized firing |
| BET SSA | Brunauer–Emmett–Teller specific surface area |
| TG | Thermomechanical analysis |
| TMA | Thermomechanical analysis |
| XRD | X-ray diffraction |
References
- Kaljuvee, T.; Štubňa, I.; Húlan, T.; Czáki, Š.; Uibu, M.; Jefimova, J. Influence of waste products from electricity and cement industries on the thermal behaviour of Estonian clay from Kunda deposit. J. Therm. Anal. Calorim. 2019, 138, 2635–2650. [Google Scholar] [CrossRef]
- Eesti Energia Annual Report. 2020. Available online: http://www.energia.ee/-/doc/8644186/ettevottest/Aastaaruanne/pdf/AA_2020_EST.pdf (accessed on 30 March 2021).
- Heidelbrg Cement Annual Report. 2018. Available online: https://www.heidelbergcement.com/en/report-and-presentations/annual_report_2018.pdf (accessed on 20 March 2019).
- Brunswick Social Value Review-No 1-2020. Available online: https://www.brunswickgroup.com/media/6645/bsvr_ellenmacarthurfoundation.pdf (accessed on 20 January 2020).
- Realizing the Value of Circular Economy in Real Estate. Available online: https://www.ellenmacarthurfoundation.org/assets/downloads/Realising-the-value-of-circulating-economy-in-real-estate.pdf (accessed on 26 February 2020).
- Bennhammou, A.; El Hafiane, Y.; Abourriche, A.; Abouliatim, Y.; Nibou, L.; Yaacoubi, A.; Tessier-Doyen, N.; Smith, A.; Tanouti, B. Effect of oil shale addition and sintering cycle on the microstructure and mechanical properties of porous cordierite-ceramic. Ceram. Inter. 2014, 40, 8937–8944. [Google Scholar] [CrossRef]
- Cobîrzan, N.; Thalmaier, G.; Balog, A.-A.; Constantinescu, H.; Timiş, I.; Streza, M. Thermophysical properties of fired clay bricks with waste ceramics and paper pulp as pore-forming agent. J. Therm. Anal. Calorim. 2018, 134, 843–851. [Google Scholar] [CrossRef]
- Furlani, E.; Tonello, G.; Maschio, S.; Aneggi, E.; Minichelli, D.; Bruckner, S.; Lucchini, E. Sintering and characterisation of ceramics containing paper sludge, glass cullet and different types of clayey materials. Ceram. Int. 2011, 37, 1293–1299. [Google Scholar] [CrossRef]
- Sutcu, M.; Akkurt, S. The use of recycled paper processing residues in making porous brick with reduced thermal conductivity. Ceram. Int. 2009, 35, 2625–2631. [Google Scholar] [CrossRef] [Green Version]
- Görhan, G.; Şimşek, O. Porous clay bricks manufactured with rice husks. Constr. Build. Mater. 2013, 40, 390–396. [Google Scholar] [CrossRef]
- Faria, K.C.P.; Gurgel, R.F.; Holanda, J.N.F. Recycling of sugarcane bagasse ash waste in the production of clay bricks. J. Environ. Manag. 2012, 101, 7–12. [Google Scholar] [CrossRef]
- Faria, K.C.P.; Holanda, J.N.F. Thermal behavior of ceramic wall tile pastes bearing solid wastes. J. Therm. Anal. Calorim. 2016, 123, 1119–1127. [Google Scholar] [CrossRef]
- Pérez-Villarejo, L.; Eliche-Quesada, D.; Iglesias-Godino, F.J.; Martínez-García, C.; Corpas-Iglesias, F.A. Recycling of ash from biomass incinerator in clay matrix to produce ceramic bricks. J. Environ. Manag. 2012, 95, 5349–5354. [Google Scholar] [CrossRef]
- Eliche-Quesada, D.; Leite-Costa, J. Use of bottom ash from olive pomace combustion in the production of eco-friendly fired clay brick. Waste Manag. 2016, 48, 323–333. [Google Scholar] [CrossRef]
- Fernández-Pereira, C.; de la Casa, J.A.; Gómez-Barea, A.; Arroyo, F.; Leiva, C.; Luna, Y. Application of biomass gasification fly ash for brick manufacturing. Fuel 2011, 90, 220–232. [Google Scholar] [CrossRef]
- Coletti, C.; Maritan, L.; Cultrone, G.; Mazzoli, C. Use of industrial ceramic sludge in brick production: Effect on aesthetic quality and physical properties. Constr. Build. Mater. 2016, 124, 219–227. [Google Scholar] [CrossRef]
- Cultrone, G.; Sebastián, E. Fly ash in clayey materials to improve the quality of solid bricks. Constr. Build. Mater. 2009, 23, 1178–1184. [Google Scholar] [CrossRef]
- Xu, L.; Guo, W.; Wang, T.; Yang, N. Study on fired bricks with replacing clay by fly ash in high volume ratio. Constr. Build. Mater. 2005, 19, 243–247. [Google Scholar]
- Sokolář, R.; Vodová, L. The effect of fluidized fly ash on the properties of dry pressed ceramic tiles based on fly ash—Clay body. Ceram. Int. 2011, 37, 2879–2885. [Google Scholar] [CrossRef]
- Taylor, J.C. Computer programs for standardless quantitative analysis of minerals using the full powder diffraction profile. Powder Diffr. 1991, 6, 2–9. [Google Scholar] [CrossRef] [Green Version]
- Ward, C.R.; Taylor, J.C.; Cohen, D.R. Quantitative mineralogy of sandstones by X-ray diffractrometry and normative analysis. J. Sed. Res. 1999, 69, 1050–1062. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to phenolic plastic. J. Polym. Sci. 1965, 6C, 183–195. [Google Scholar] [CrossRef]
- AKTS. Softwear and Setaram Instruments: A Global Solution for Kinetic Analysis and Determination of the Thermal Stability of Materials; AKTS AG: Siders, Switzerland, 2006; p. 88. [Google Scholar]
- Dweck, J. Qualitative and quantitative characterization of Brazilian natural and organophilic clays by thermal analysis. J. Therm. Anal. Calorim. 2008, 92, 129–135. [Google Scholar] [CrossRef]
- Shvarzman, A.; Kovler, K.; Grader, G.S.; Shter, G.E. The effect of dehydroxylation/amorphization on pozzolanic activity of kaolinite. Cem. Conc. Res. 2003, 33, 405–416. [Google Scholar] [CrossRef]
- Faria, K.C.P.; Holanda, J.N.F. Thermal study of clay ceramic pastes containing sugargrane bagasse ash waste. J. Therm. Anal. Calorim. 2013, 114, 27–32. [Google Scholar] [CrossRef]
- Kaljuvee, T.; Trikkel, A.; Kuusik, R. Decarbonization of natural lime-containing materials and reactivity of calcined products towards SO2 and CO2. J. Therm. Anal. Calorim. 2001, 64, 1229–1240. [Google Scholar] [CrossRef]
- Kaljuvee, T.; Kuusik, R.; Radin, M.; Bender, V. Carbon dioxide binding in the heterogeneous systems formed at combustion of oil shale: 4. Reactivity of ashes towards acid gases in the system fly ash-flue gases. Oil Shale 2004, 21, 13–26. [Google Scholar]
- Štubňa, I.; Trník, A.; Vozár, L. Thermomechanical and thermodilatometric analysis of green alumina porcelain. Ceram. Int. 2009, 35, 1181–1185. [Google Scholar] [CrossRef]
- Ptáček, P.; Frajkorová, F.; Šoukal, F.; Opravil, T. Kinetics and mechanism of three stages of thermal transformation of kaolinite to metakaolinite. Powder Technol. 2014, 264, 439–445. [Google Scholar] [CrossRef]
- Sánches-Soto, P.J.; Ruiz-Conde, A.; Bono, R.; Raigón, M.; Garzón, E. Thermal evolution of a slate. J. Therm. Anal. Calorim. 2007, 90, 133–141. [Google Scholar] [CrossRef]
- Knapek, M.; Húlan, T.; Minárik, P.; Dobroň, P.; Štubňa, I.; Stráská, J.; Chmelík, F. Study of mickrocraking in illite-based ceramics during firing. J. Eur. Ceram. Soc. 2016, 36, 221–226. [Google Scholar] [CrossRef]
- Húlan, T.; Trník, A.; Medved, I. Kinetics of thermal expansion of illite-based ceramics in the dehydroxylation region during heating. J. Therm. Anal. Calorim. 2017, 127, 291–298. [Google Scholar] [CrossRef]
- Cizer, Ö.; Rodrigues-Navarro, C.; Ruiz-Agudo, E.; Elsen, J.; Van Gemert, D.; Van Balen, K. Phase and morphology evolution of carbonate precipitated by carbonation of hydrated lime. J. Mater. Sci. 2012, 47, 6151–6165. [Google Scholar] [CrossRef] [Green Version]
- Eisazadeh, A.; Kassim, K.A.; Nur, H. Solid-state NMR and FTIR studies of lime stabilized montmorillonitic and lateritic clays. Appl. Clay. Sci. 2012, 67–68, 5–10. [Google Scholar] [CrossRef]
- Madejová, J. FTIR techniques in clay mineral studies. Vib. Spectrosc. 2003, 31, 1–10. [Google Scholar] [CrossRef]
- Davarcioğlu, B.; Ҫiftҁi, E. Investigation of Central Anatolian Clays by FTIR Spectroscopy (Arapli-Yesilhisar-Kayseri, Turkey). Int. J. Nat. Eng. Sci. 2009, 3, 167–174. [Google Scholar]
- Shoval, S.; Yadin, E.; Panczer, G. Analysis of thermal phases in calcareous Iron Age pottery using FT-IR and Raman spectroscopy. J. Therm. Anal. Calorim. 2011, 104, 515–525. [Google Scholar] [CrossRef]
- Alver, B.E.; Dikmen, G.; Alver, Ö. Investigation of the influence of heat treatment on the structural properties of illite-rich clay mineral using FT-IR, 29Si MAS NMR, TG and DTA methods. Anadolu Univ. J. Sci. Technol. A Appl. Sci. Eng. 2016, 17, 823–829. [Google Scholar]
- Mozgawa, W.; Król, M.; Dyczek, J.; Deja, J. Investigation of the coal fly ashes using IR spectroscopy. Spectrochim. Acta Part A 2000, 56, 1819–1823. [Google Scholar] [CrossRef] [PubMed]
- Frost, R.L.; Vassallo, A.M. The dehydroxylation of the kaolinite clay minerals using infrared emission spectroscopy. Clays Clay Miner. 1996, 44, 635–651. [Google Scholar] [CrossRef]
- Diko, M.; Ekosse, G.; Ogola, J. Fourier Transform Infrared Spectroscopy and thermal analysis of kaolinite clays from South Africa and Cameroon. Acta Geodyn. Geomater. 2016, 13, 149–158. [Google Scholar]
- Zhirong, L.; Uddin, M.A.; Zhanxue, S. FT-IR and XRD analysis of Natural Na-bentonite and Cu (II)-loaded Na-bentonite. Spectrochim. Acta Part A 2011, 79, 1013–1016. [Google Scholar] [CrossRef] [PubMed]



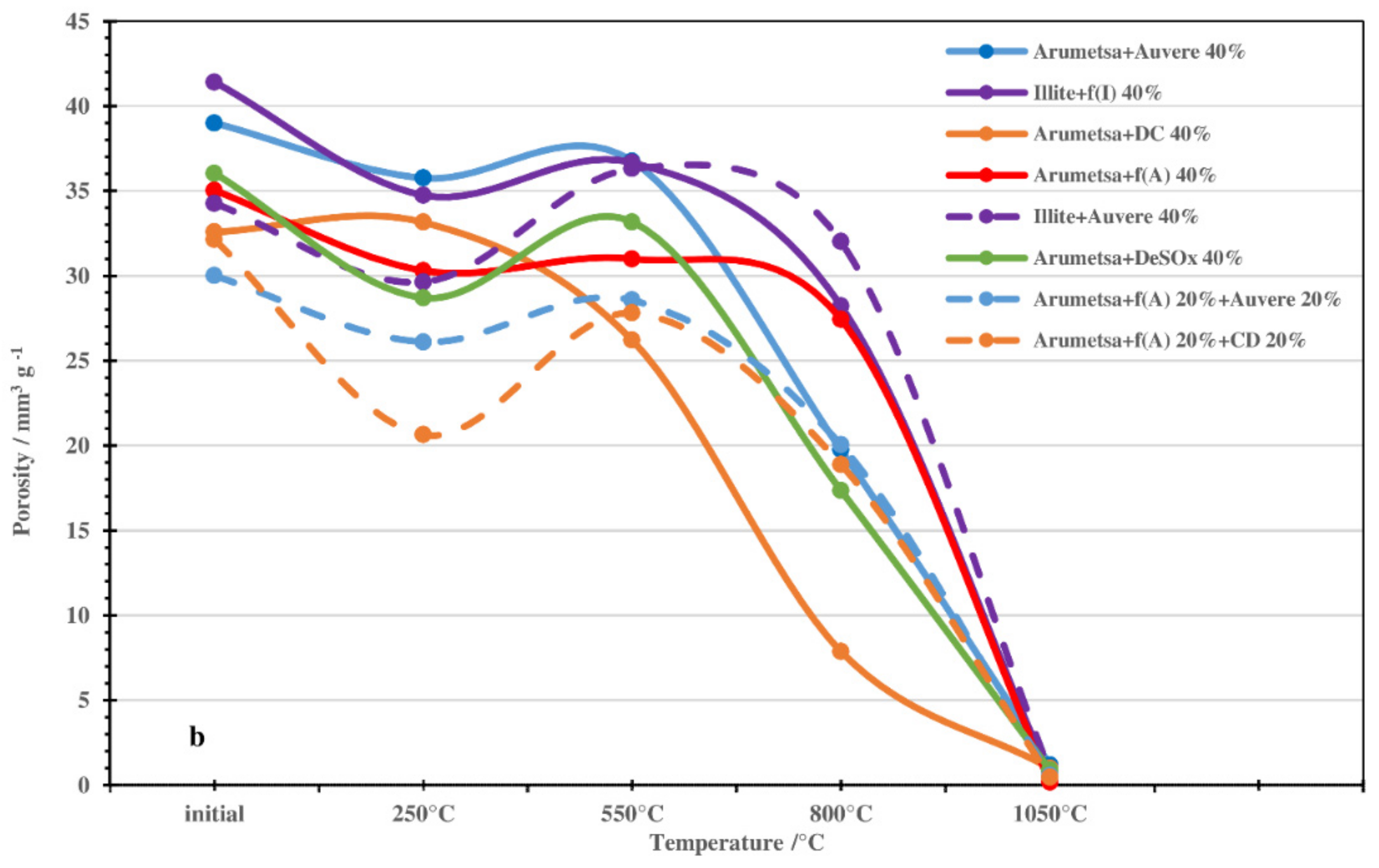

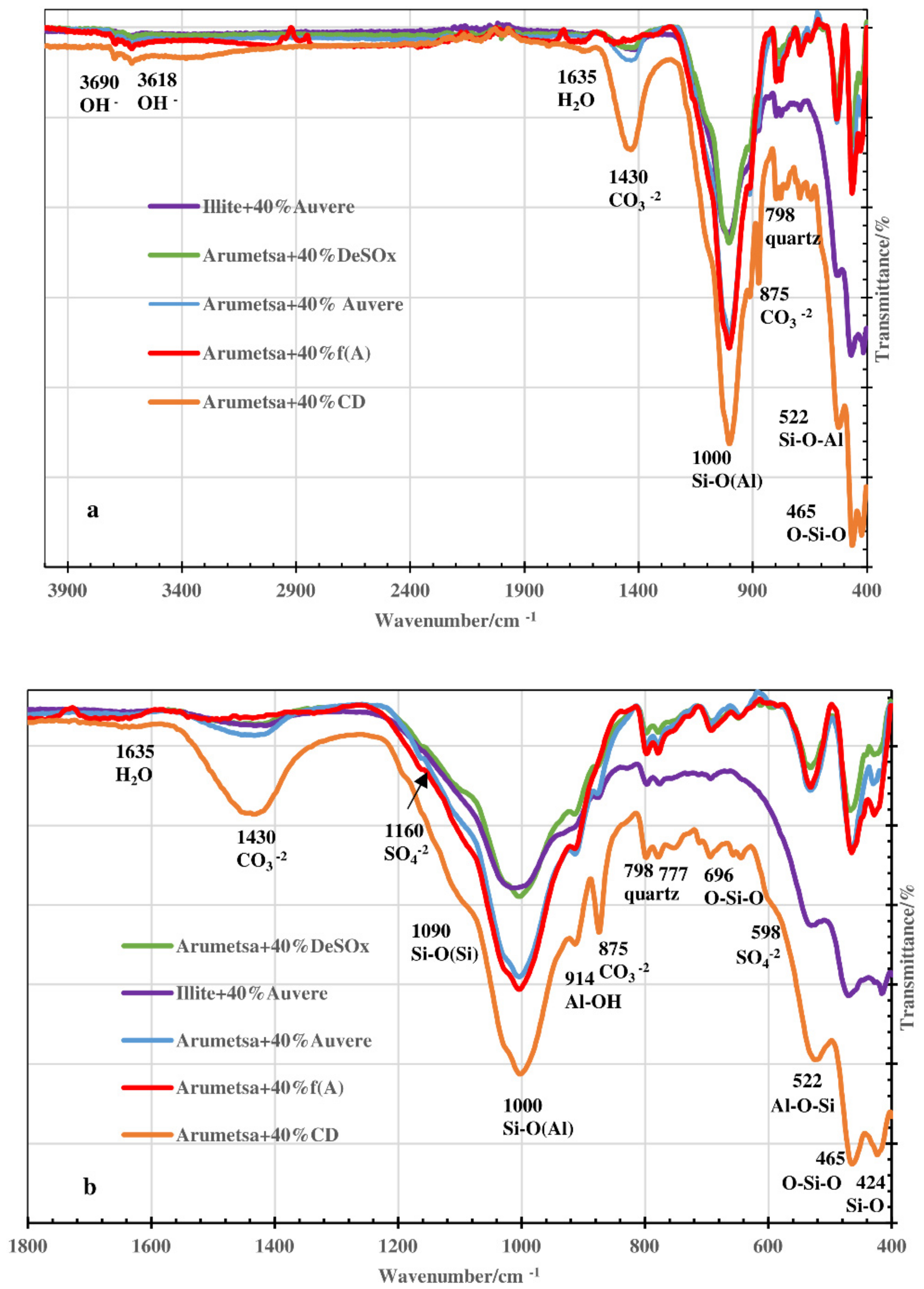
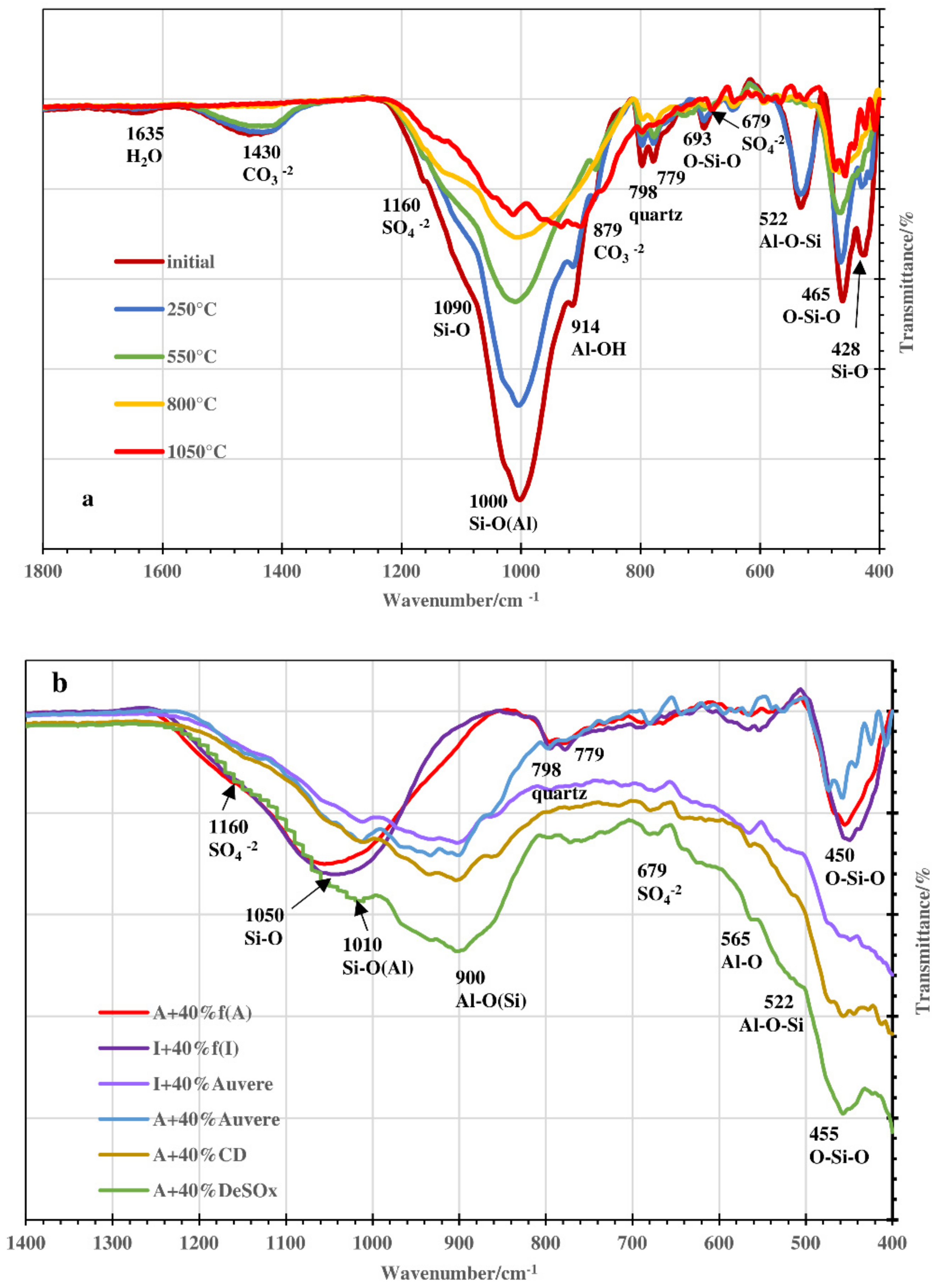

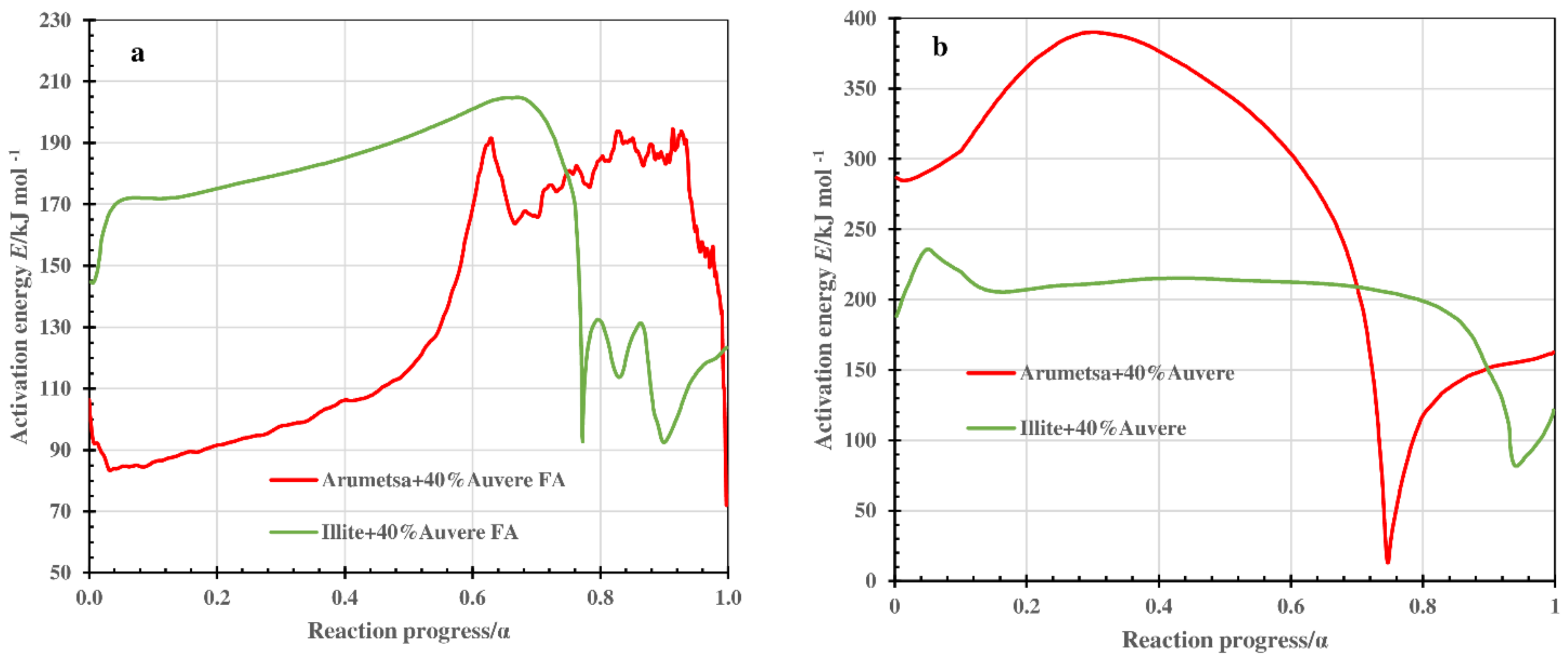
| Compound or Formula | Arumetsa Clay | Illitic Clay | PF/ DeSOx | CFBC/ Auvere FA | Kunda NCF/ CD |
|---|---|---|---|---|---|
| Amorphous phase | - | - | 24.6 | 20.0 | - |
| Kaolin, Al2Si2O5(OH)4 | 11.6 | - | - | - | - |
| Illite, illite-smectite, mica, KAl2(AlSi3O10)(OH)2 | 48.9 | 76.4 | - | - | 7.2 |
| Amphipole, NaCa2(Fe2+,Fe3+)Si6Al2O22(OH)2 | 1.6 | - | - | - | - |
| Lime, CaO | - | - | 6.9 | 8.7 | 0.9 |
| Calcite, CaCO3 | 0.6 | - | 8.8 | 9.8 | 47.3 |
| Dolomite, CaMg(CO3)2 | 2.1 | - | - | - | - |
| Anhydrite, CaSO4 | - | - | 10.7 | 6.0 | 0.4 |
| Quartz, SiO2 | 18.5 | 17.5 | 7.8 | 10.7 | 11.4 |
| Orthoclase, KAlSi3O8 | 3.8 | 5.8 | 5.8 | 13.5 | 1.8 |
| Plagioclase, NaCaAlSi3O6 | 5.3 | - | - | - | - |
| Belite, Ca2SiO4 | - | - | 12.1 | 12.4 | 8.5 |
| Merwinite, Ca3Mg(SiO4)2 | - | - | 4.4 | 1.8 | 3.0 |
| Hematite, Fe2O3 | 1.2 | - | 1.3 | 3.6 | - |
| Periclase, MgO | - | - | 3.5 | 1.6 | 0.6 |
| Arcanite, K2SO4 | - | - | 0.4 | 1.0 | 15.9 |
| Portlandite, Ca(OH)2 | - | - | 10.5 | 7.0 | - |
| Chlorite, (Mg,Al,Fe)6(SiAl)4O10(OH)2 | 4.9 | - | - | - | - |
| Item | Sample or Content (%) | ||||
|---|---|---|---|---|---|
| Arumetsa Clay | Illitic Clay | PF, DeSOx | CFBC, Auvere FA | Kunda NCF, CD | |
| CaOtotal | 1.5 | 0.3 | 36.3 | 36.9 | 41.0 |
| CaOfree | - | - | 7.9 | 11.8 | 3.8 |
| MgO | 2.7 | 1.2 | 4.7 | 2.5 | 6.5 |
| CTC/CTIC Corg * | 0.39/0.07 0.32 | 0.06/ 0.06 | 1.01/1.01 - | 1.47/1.39 0.08 | 5.6/5.6 - |
| SiO2 | 56.5 | 57.0 | 28.5 | 24.2 | 14.6 |
| Al2O3 | 18.4 | 25.0 | 7.3 | 11.2 | 3.5 |
| Fe2O3 | 6.8 | 1.9 | 3.6 | 5.6 | 1.2 |
| SO3total | 0.08 | 0.08 | 9.3 | 3.5 | 7.2 |
| K2O | 4.5 | 8.5 | 3.6 | 3.8 | 8.2 |
| Na2O | 0.58 | 0.02 | 0.32 | 0.10 | - |
| LOI 950 °C | 6.9 | 7.0 | 6.7 | 9.6 | 24.2 |
| BET SSA (m2g−1) | 44.71 | 48.79 | 1.43 | 4.68 | 3.00 |
| Porosity (mm3g−1) | 77.63 | 82.14 | 3.84 | 8.87 | 5.15 |
| dmean (μm) | 12.7 | 17.3 | 49.6 | 21.9 | 41.1 |
| Compound or Formula | Arumetsa(A) 60% + 40%f(A) | A60% + 40% Auvere FA | A60% + 40% DeSOx | A60% + 40%CD | Illite(I) 60% +40%f(I) | I60% + 40% Auvere FA | I60% + 40% DeSOx | I60% + 40% CD |
|---|---|---|---|---|---|---|---|---|
| Amorphous phase | 44.7 | 17.20 | 23.70 | 14.10 | 50.30 | 22.20 | 21.90 | 18.30 |
| Quartz, SiO2 | 18.27 | 8.78 | 5.65 | 5.67 | 16.75 | 10.04 | 7.65 | 6.05 |
| Sillimanite, Al2SiO5 | 0.77 | - | 0.23 | 1.29 | 2.29 | 0.62 | 0.78 | - |
| Diopside, CaMgSi2O6 | - | 5.80 | 5.57 | 9.36 | - | 1.40 | 1.59 | 2.06 |
| Anhydrite, Ca(SO4)2 | - | 1.74 | 0.53 | - | - | 1.48 | 1.33 | 2.37 |
| Wollastonite, CaSiO3 | - | 5.63 | 5.19 | 6.18 | 0.50 | 7.62 | 7.97 | 8.58 |
| Akermanite, Ca2Mg(Si2O7) | - | 14.16 | 11.37 | 18.47 | - | 15.33 | 9.37 | 11.05 |
| Periclase, MgO | 1.05 | 0.58 | 2.44 | 0.09 | - | - | - | - |
| Hematite, Fe2O3 | 5.35 | 5.46 | 5.04 | 1.29 | 0.55 | 2.85 | 3.51 | 3.07 |
| Leucite, K(AlSi2O6) | 0.88 | 6.87 | 9.38 | 12.38 | - | 14.39 | 13.90 | 19.77 |
| Spinel, MgAl2O3 | 10.71 | 5.22 | 6.41 | 9.79 | 5.81 | - | - | - |
| Enstatite, MgSiO3 | - | 5.22 | 4.88 | 6.01 | 1.64 | 5.68 | 5.86 | 5.56 |
| Plagioclase, NaCaAlSi3O6 | - | 0.75 | - | 1.80 | - | - | - | - |
| Mullite, Al6Si2O13 | 11.59 | 15.07 | 5.88 | 1.72 | 3.78 | 2.20 | 1.87 | 1.34 |
| Sanidin, KAlSi3O8 | 6.57 | 2.90 | 6.56 | 7.22 | 18.49 | 5.03 | 2.66 | 6.63 |
| Anorthite, CaAl2Si2O8 | - | 4.64 | 7.17 | 4.47 | - | 11.51 | 21.01 | 15.20 |
| Sample | 10% HNO3 | |||
|---|---|---|---|---|
| CaO | MgO | K2O | SO4 −2 | |
| Arumetsa(A) + 40%f(A) | 0.83 | 0.18 | 0.03 | 7.47 |
| A + 40%Auvere FA | 5.03 | 1.32 | 11.07 | 0.44 |
| A + 40%DeSOx | 6.04 | 0.80 | 15.67 | 3.75 |
| A + 40%CD | 9.11 | 2.04 | 16.33 | 17.81 |
| Illite(I) + 40%f(I) | 2.26 | 0.15 | 0.07 | 7.52 |
| I + 40%Auvere FA | 8.81 | 7.23 | 11.68 | 0.45 |
| I + 40%DeSOx | 8.66 | 1.22 | 15.11 | 14.00 |
| I + 40%CD | 11.07 | 5.91 | 15.09 | 12.86 |
| 10% NaOH | ||||
| Arumetsa(A) + 40%f(A) | 3.74 | 0.014 | 4.48 | 96.42 |
| A + 40%Auvere FA | 0.35 | 0.012 | 5.64 | 4.32 |
| A + 40%DeSOx | 0.72 | 0.008 | 5.39 | 0.57 |
| A + 40%CD | 0.72 | 0.005 | 8.05 | 0.93 |
| Illite(I) + 40%f(I) | 0.93 | 0.072 | 2.16 | 56.18 |
| I + 40%Auvere FA | 0.55 | 0.029 | 3.51 | 2.93 |
| I + 40%DeSOx | 0.57 | 0.015 | 5.58 | 1.21 |
| I + 40%CD | 0.67 | 0.012 | 8.41 | 1.88 |
| Sample | E/kJ mol−1 | |
|---|---|---|
| Step I | Step II | |
| Variation/Average | Variation/Average | |
| Arumetsa clay(A) + 40%fired(f)(A) | 75–182/128 | - |
| A + 20%f(A) + 20%Auvere FA, H | 164–193/202 | 194–210/205 |
| A + 20%f(A) + 20%DeSOx, H | 119–169/138 | 190–201/197 |
| A + 20%f(A) + 20%CD, H | 14–114/73 | 201–213/207 |
| A + 40%Auvere FA, H | 86–194/135 | 15–390/236 |
| A + 40%DeSOx, H | 78–158/119 | 153–222/164 |
| A + 40%CD, H | 77–148/117 | 211–221/214 |
| A + 20%CD, H + 20% Auvere FA, H | 111–168/130 | 158–217/199 |
| A + 20%CD, H + 20% DeSOx, H | 75–124/93 | 189–203/193 |
| Illitic clay(I) + 40%f(I) | 9–206/113 | - |
| I + 20%f(I) + 20%Auvere FA, H | 144–331/185 | 194–235/212 |
| I + 20%f(I) + 20%CD, H | 18–76/35 | 206–220/213 |
| I + 40% Auvere FA, H | 94–201/170 | 85–220/204 |
| I + 40%CD, H | 11–141/76 | 154–220/208 |
| I + 20%CD, H + 20% Auvere FA, H | 125–188/163 | 186–223/207 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaljuvee, T.; Štubňa, I.; Húlan, T.; Uibu, M.; Einard, M.; Traksmaa, R.; Viljus, M.; Jefimova, J.; Trikkel, A. Thermal Behavior of Ceramic Bodies Based on Estonian Clay from the Arumetsa Deposit with Oil Shale Ash and Clinker Dust Additives. Processes 2022, 10, 46. https://doi.org/10.3390/pr10010046
Kaljuvee T, Štubňa I, Húlan T, Uibu M, Einard M, Traksmaa R, Viljus M, Jefimova J, Trikkel A. Thermal Behavior of Ceramic Bodies Based on Estonian Clay from the Arumetsa Deposit with Oil Shale Ash and Clinker Dust Additives. Processes. 2022; 10(1):46. https://doi.org/10.3390/pr10010046
Chicago/Turabian StyleKaljuvee, Tiit, Igor Štubňa, Tomáš Húlan, Mai Uibu, Marve Einard, Rainer Traksmaa, Mart Viljus, Jekaterina Jefimova, and Andres Trikkel. 2022. "Thermal Behavior of Ceramic Bodies Based on Estonian Clay from the Arumetsa Deposit with Oil Shale Ash and Clinker Dust Additives" Processes 10, no. 1: 46. https://doi.org/10.3390/pr10010046
APA StyleKaljuvee, T., Štubňa, I., Húlan, T., Uibu, M., Einard, M., Traksmaa, R., Viljus, M., Jefimova, J., & Trikkel, A. (2022). Thermal Behavior of Ceramic Bodies Based on Estonian Clay from the Arumetsa Deposit with Oil Shale Ash and Clinker Dust Additives. Processes, 10(1), 46. https://doi.org/10.3390/pr10010046






