Synthesis, Characterization and Gas Adsorption of Unfunctionalized and TEPA-Functionalized MSU-2 †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Unfunctionalized MSU-2
2.3. Synthesis of TEPA-Functionalized MSU-2
2.4. CO2 Adsorption Experiment
2.5. Characterization
3. Results and Discussion
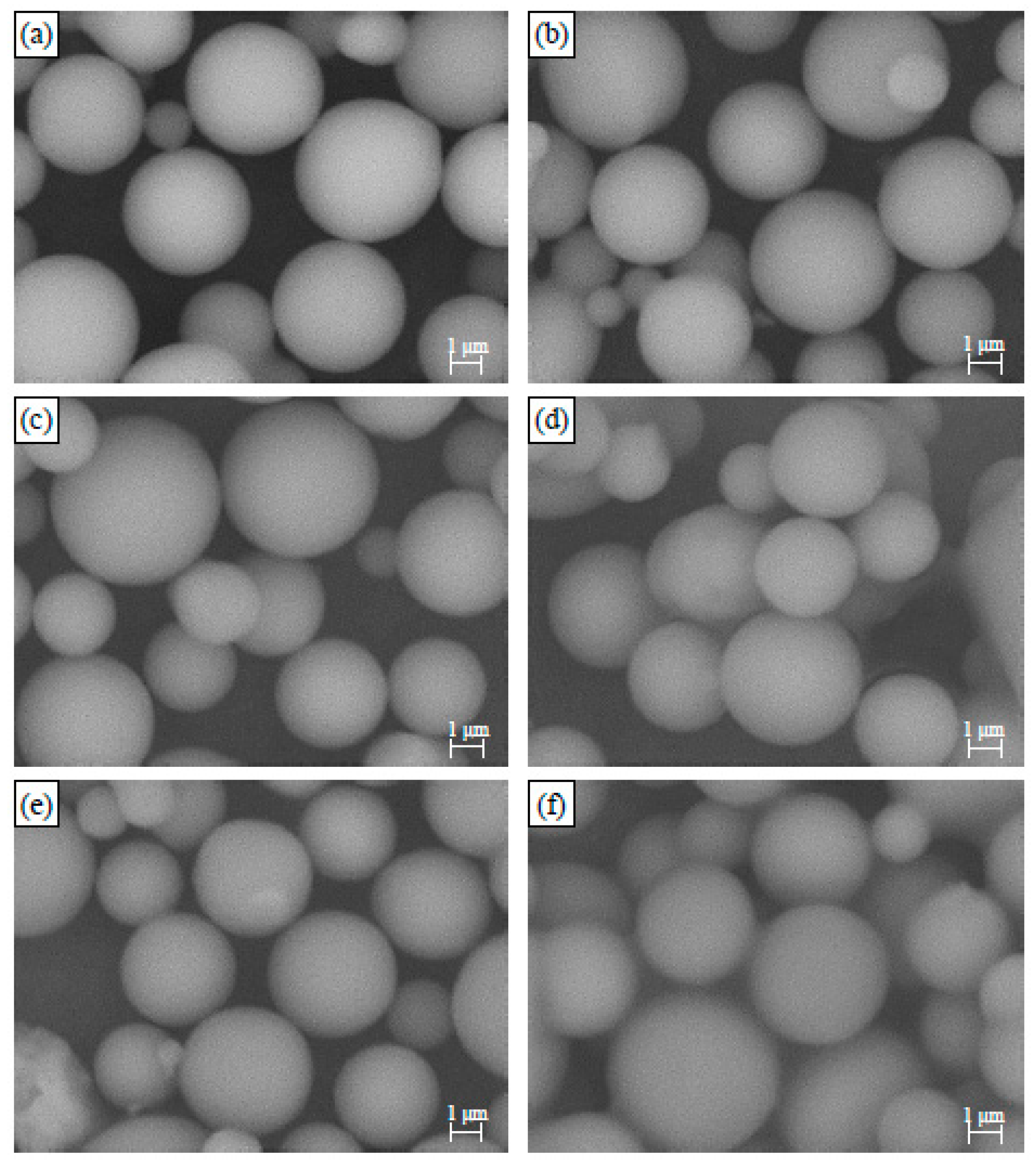
3.1. Morphology Analysis
3.2. Pore Characteristics and Elemental Analysis
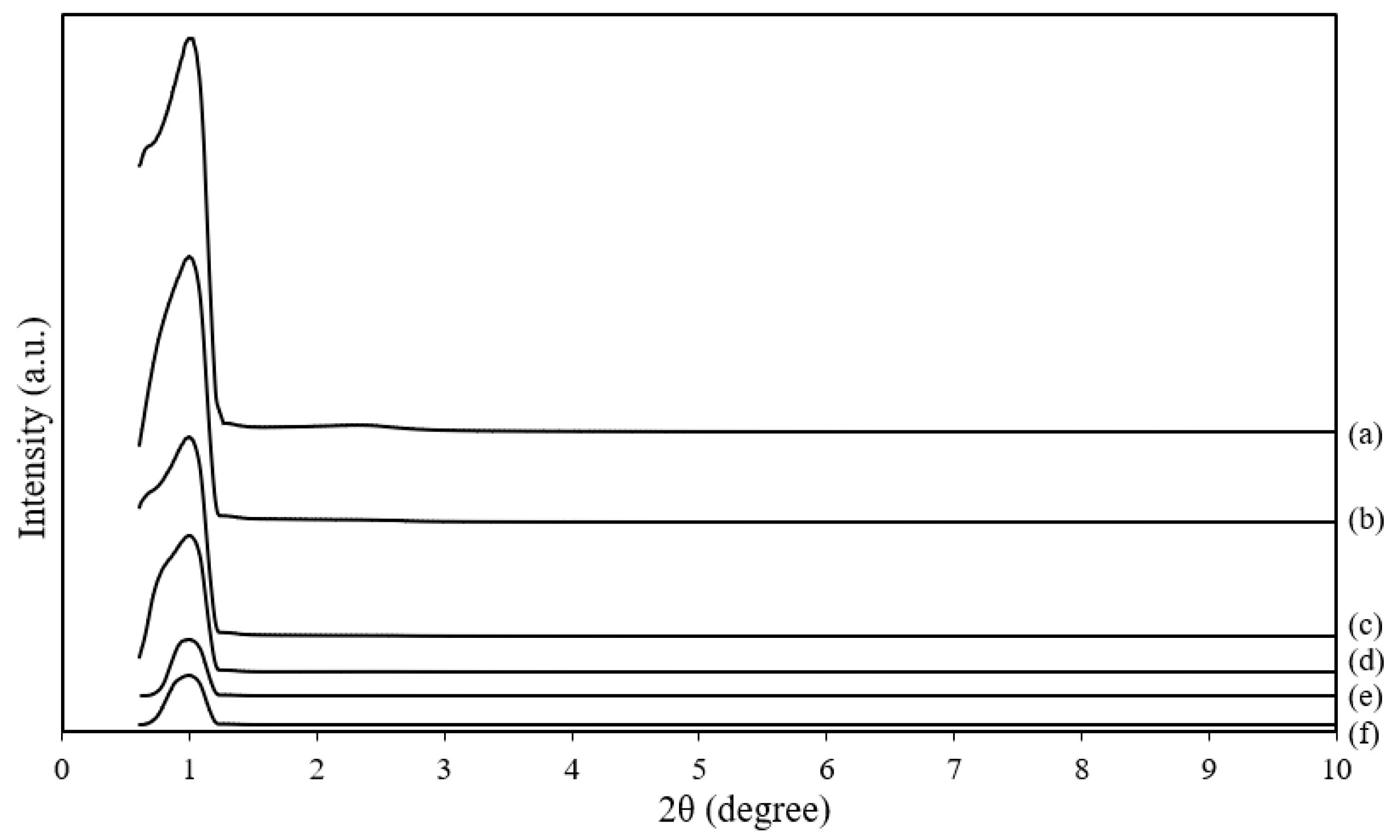
3.3. Crystallinity Analysis
3.4. Functional Groups
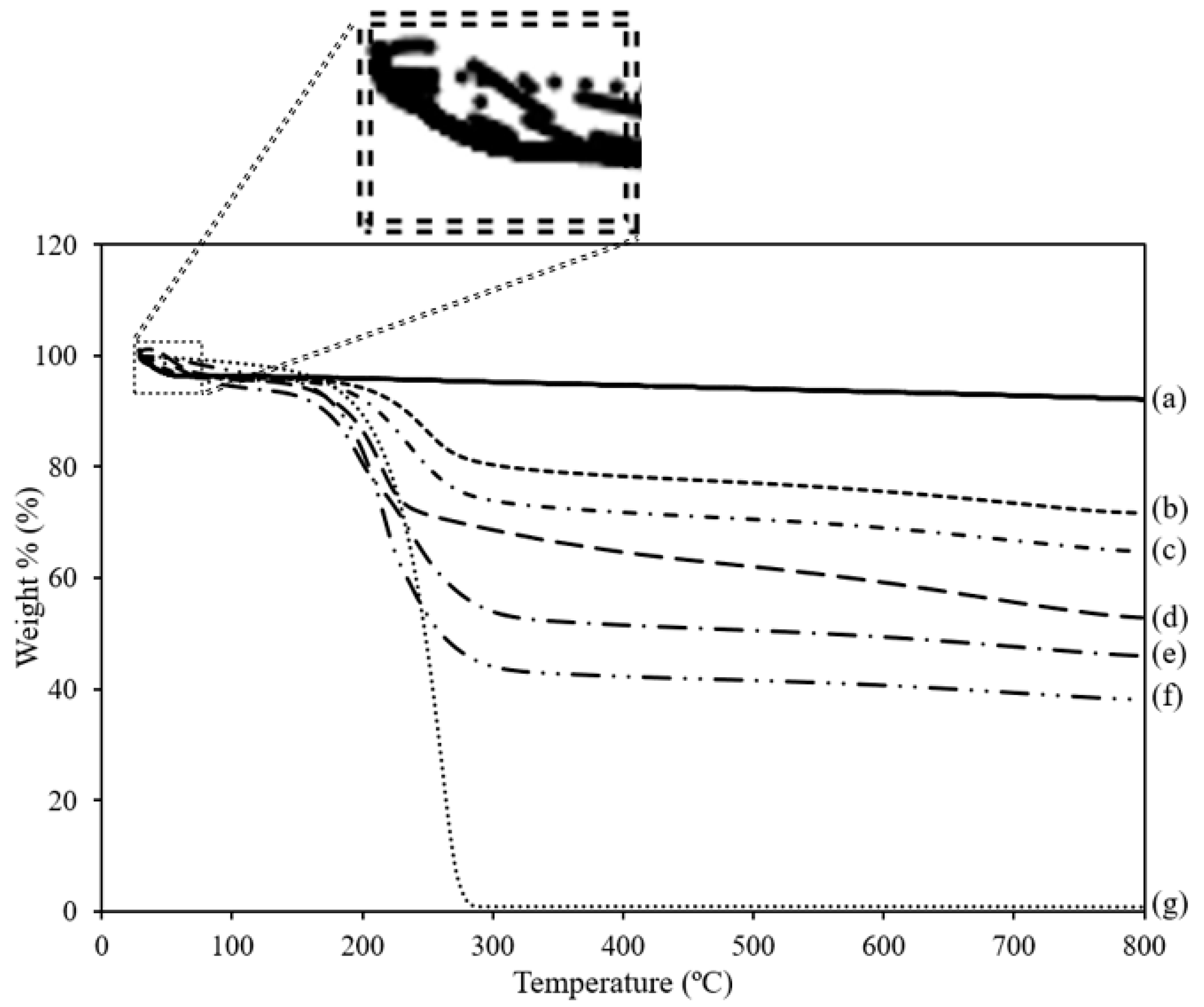
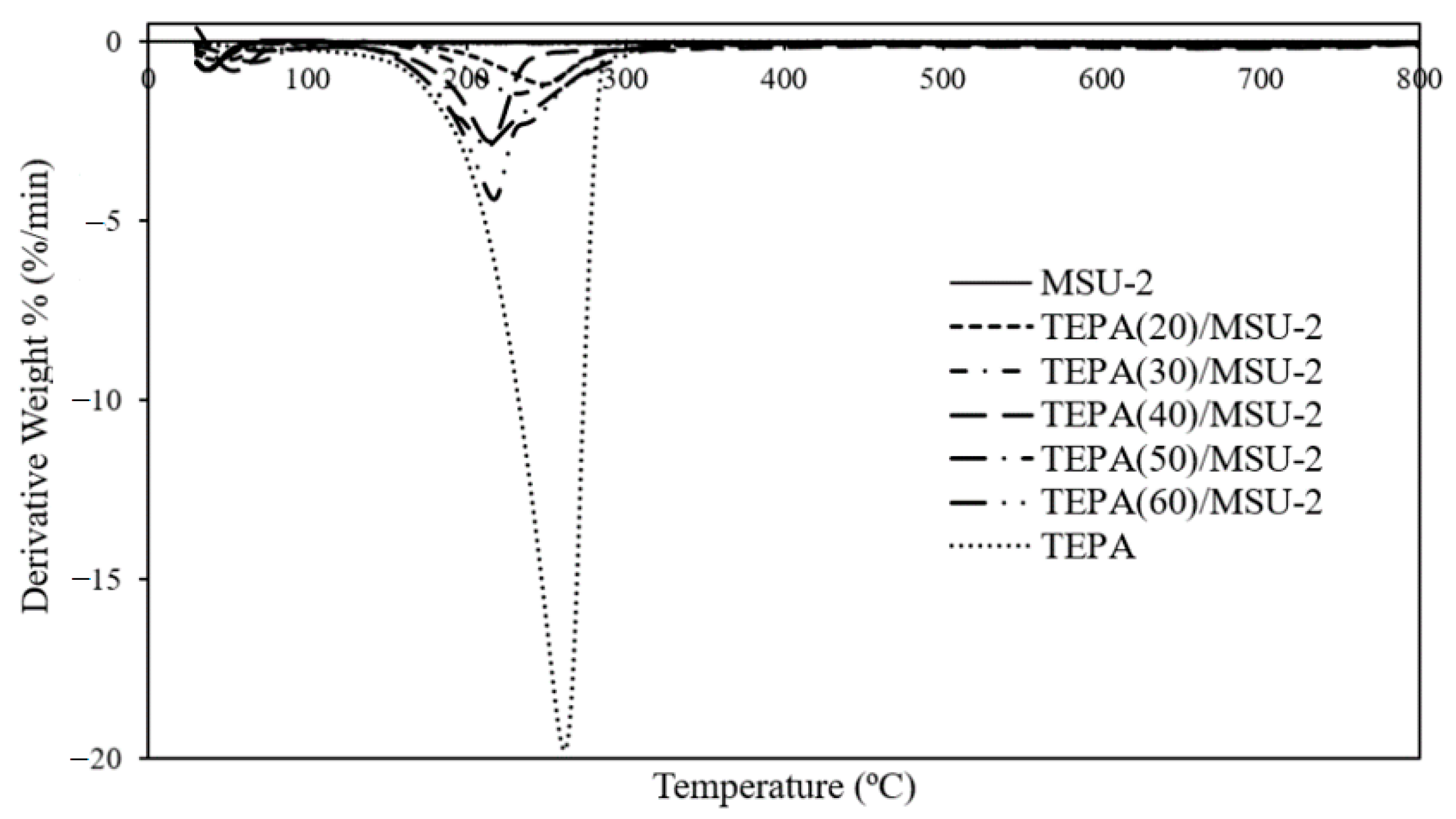
3.5. Thermal Analysis
3.6. Effect of TEPA Loading on CO2 Adsorption Capacity of Adsorbents
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hauchhum, L.; Mahanta, P. Carbon dioxide adsorption on zeolites and activated carbon by pressure swing adsorption in a fixed bed. Int. J. Energy Environ. Eng. 2014, 5, 349–356. [Google Scholar] [CrossRef]
- Global Energy & CO2 Status Report 2019. Available online: https://www.iea.org/reports/global-energy-co2-status-report-2019 (accessed on 14 July 2022).
- Robertson, D. Health effects of increase in concentration of carbon dioxide in the atmosphere. Curr. Sci. 2006, 90, 1607–1609. [Google Scholar]
- Mei, W.; Primeau, F.; McWilliams, J.C.; Pasquero, C. Sea surface height evidence for long-term warming effects of tropical cyclones on the ocean. Proc. Natl. Acad. Sci. USA 2013, 110, 15207–15210. [Google Scholar] [CrossRef] [PubMed]
- Songolzadeh, M.; Soleimani, M.; Ravanchi, M.T.; Songolzadeh, M. Carbon Dioxide Separation from Flue Gases: A Technological, Review Emphasizing Reduction in Greenhouse Gas Emissions. Sci. World J. 2014, 14, 828131. [Google Scholar] [CrossRef] [PubMed]
- Bounaceur, R.; Lape, N.; Roizard, D.; Vallieres, C.; Favre, E. Membrane processes for post-combustion carbon dioxide capture: A parametric study. Energy 2006, 31, 2556–2570. [Google Scholar] [CrossRef]
- Gupta, M.; Coyle, I.; Thambimuthu, K. CO2 Capture Technologies and Opportunities in Canada: “Strawman Document for CO2 Capture and Storage (CC&S) Technology Roadmap”, 1st ed.; Canadian CC& S Technology Roadmap Workshop: Calgary, AB, Canada, 2003. [Google Scholar]
- Lu, C.; Bai, H.; Su, F.; Chen, W.; Hwang, J.F.; Lee, H.-H. Adsorption of Carbon Dioxide from Gas Streams via Mesoporous Spherical-Silica Particles. J. Air Waste Manag. Assoc. 2010, 60, 489–496. [Google Scholar] [CrossRef]
- Meth, S.; Goeppert, A.; Prakash, G.K.S.; Olah, G.A. Silica Nanoparticles as Supports for Regenerable CO2 Sorbents. Energy Fuels 2012, 26, 3082–3090. [Google Scholar] [CrossRef]
- Olajire, A.A. CO2 capture and separation technologies for end-of-pipe applications—A review. Energy 2010, 35, 2610–2628. [Google Scholar] [CrossRef]
- Jiao, J.; Lv, P.; Wang, L.; Dan, S.; Qi, L.; Cui, Y. CO2 capture of amino functionalized three-dimensional worm-hole mesostructured MSU-J silica. J. Porous Mater. 2014, 21, 775–781. [Google Scholar] [CrossRef]
- Kapdi, S.; Vijay, V.; Rajesh, S.; Prasad, R. Biogas scrubbing, compression and storage: Perspective and prospectus in Indian context. Renew. Energy 2005, 30, 1195–1202. [Google Scholar] [CrossRef]
- Adsorption. Available online: https://www.slideshare.net/Kamyaparashar/adsorption-presentation-44669901 (accessed on 14 July 2022).
- Tyagi, V.; Ratna Sagar, P. Essential Chemistry Xii; Ratna Sagar P. Ltd.: Delhi, India, 2009. [Google Scholar]
- Anusha, G. Feasibility Studies on the Removal of Iron and Fluoride from Aqueous Solution by Adsorption Using Agro Based Waste Materials. Ph.D. Thesis, Anna University, Chennai, India, 2015. [Google Scholar]
- Gargiulo, N.; Pepe, F.; Caputo, D. CO2 Adsorption by Functionalized Nanoporous Materials: A Review. J. Nanosci. Nanotechnol. 2014, 14, 1811–1822. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, Z.; Xu, X.; Wang, J. Review on porous nanomaterials for adsorption and photocatalytic conversion of CO2. Chin. J. Catal. 2017, 38, 1956–1969. [Google Scholar] [CrossRef]
- Ma, C.; Bai, J.; Hu, X.; Jiang, Z.; Wang, L. Nitrogen-doped porous carbons from polyacrylonitrile fiber as effective CO2 ad-sorbents. J. Environ. Sci. 2023, 125, 533–543. [Google Scholar] [CrossRef]
- Usseinov, A.B.; Akilbekov, A.T.; Kotomin, E.A.; Popov, A.I.; Seitov, D.D.; Nekrasov, K.A.; Giniyatova, S.G.; Karipbayev, Z.T. The first principles calculations of CO2 adsorption on (101––0100) ZnO surface. AIP Conf. Proc. 2019, 2174, 020181. [Google Scholar]
- Lee, G.; Yoo, D.K.; Ahmed, I.; Lee, H.J.; Jhung, S.H. Metal-organic frameworks composed of nitro groups: Preparation and applications in adsorption and catalysis. Chem. Eng. J. 2023, 451, 138538. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.; Hu, X. Adsorption of CO2 by a novel zeolite doped amine modified ternary aerogels. Environ. Res. 2022, 214, 113855. [Google Scholar] [CrossRef]
- Lashaki, M.J.; Ziaei-Azad, H.; Sayari, A. Unprecedented improvement of the hydrothermal stability of amine-grafted MCM-41 silica for CO2 capture via aluminum incorporation. Chem. Eng. J. 2022, 450, 138393. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, X.; Wu, X.; Liu, M.; Shi, R.; Yu, X. Pentaethylenehexamine loaded SBA-16 for CO2 capture from simulated flue gas. Powder Technol. 2017, 318, 186–192. [Google Scholar] [CrossRef]
- Jiao, J.; Cao, J.; Lv, P.P. Amine-immobilized Three-dimensional Wormhole Mesostructured MSU-J Silica for CO2 Adsorption: Effect of Amine Loading and Temperature on the Adsorption Capacity. Chem. Lett. 2015, 44, 928–930. [Google Scholar] [CrossRef]
- Kishor, R.; Ghoshal, A.K. Amine-Modified Mesoporous Silica for CO2 Adsorption: The Role of Structural Parameters. Ind. Eng. Chem. Res. 2017, 56, 6078–6087. [Google Scholar] [CrossRef]
- Wang, X.; Li, H.; Liu, H.; Hou, X. AS-synthesized mesoporous silica MSU-1 modified with tetraethylenepentamine for CO2 adsorption. Microporous Mesoporous Mater. 2011, 142, 564–569. [Google Scholar] [CrossRef]
- Chen, C.; Kim, J.; Ahn, W.-S. CO2 capture by amine-functionalized nanoporous materials: A review. Korean J. Chem. Eng. 2014, 31, 1919–1934. [Google Scholar] [CrossRef]
- Wang, X.; Schwartz, V.; Clark, J.C.; Ma, X.; Overbury, S.H.; Xu, X.; Song, C. Infrared Study of CO2 Sorption over “Molecular Basket” Sorbent Consisting of Polyethylenimine-Modified Mesoporous Molecular Sieve. J. Phys. Chem. C 2009, 113, 7260–7268. [Google Scholar] [CrossRef]
- Jiao, J.; Cao, J.; Xia, Y.; Zhao, L. Improvement of adsorbent materials for CO2 capture by amine functionalized mesoporous silica with worm-hole framework structure. Chem. Eng. J. 2016, 306, 9–16. [Google Scholar] [CrossRef]
- Beaudet, L.; Hossain, A.K.-Z.; Mercier, L. Direct Synthesis of Hybrid Organic−Inorganic Nanoporous Silica Microspheres. 1. Effect of Temperature and Organosilane Loading on the Nano- and Micro-Structure of Mercaptopropyl-Functionalized MSU Silica. Chem. Mater. 2002, 15, 327–334. [Google Scholar] [CrossRef]
- Boissière, C.; Larbot, A.; van der Lee, A.; Kooyman, P.J.; Prouzet, E. A New Synthesis of Mesoporous MSU-X Silica Controlled by a Two-Step Pathway. Chem. Mater. 2000, 12, 2902–2913. [Google Scholar] [CrossRef]
- Pérez-Quintanilla, D.; Sánchez, A.; del Hierro, I.; Fajardo, M.; Sierra, I. Synthesis and characterization of novel mesoporous silicas of the MSU-X family for environmental applications. J. Nanosci. Nanotechnol. 2009, 9, 4901–4909. [Google Scholar] [CrossRef]
- Biswas, K.; Ray, J.C.; Choi, J.-S.; Ahn, W.-S. Morphology control of MSU-1 silica particles. J. Non-Cryst. Solids 2008, 354, 1–9. [Google Scholar] [CrossRef]
- Biswas, K.; Jang, S.-H.; Ahn, W.-S.; Baik, Y.-S.; Cheong, W.J. Synthesis of MSU-1 silica particles with spherical morphology. Stud. Surf. Sci. Catal. 2007, 165, 587–590. [Google Scholar]
- Boissiere, C.; Kuemmel, M.; Persin, M.; Larbot, A.; Prouzet, E. Spherical MSU-1 mesoporous silica particles tuned for HPLC. Adv. Funct. Materials. 2001, 11, 129–135. [Google Scholar] [CrossRef]
- Lee, G.; Youn, H.-K.; Jin, M.-J.; Cheong, W.-J.; Ahn, W.-S. Synthesis of organic–inorganic hybrid MSU-1 for separation and catalytic applications. Microporous Mesoporous Mater. 2010, 132, 232–238. [Google Scholar] [CrossRef]
- Martines, M.A.; Yeong, E.; Larbot, A.; Prouzet, E. Temperature dependence in the synthesis of hexagonal MSU-3 type mesoporous silica synthesized with Pluronic P123 block copolymer. Microporous Mesoporous Mater. 2004, 74, 213–220. [Google Scholar] [CrossRef]
- Rahmam, S.B. Optimization of Surface Treatment and Functionalization of Multiwalled Carbon Nanotubes (MWCNT) for Carbon Dioxide (CO2) Adsorption. In Chemical Engineering; Universiti Teknologi PETRONAS: Bandar Seri Iskandar, Malaysia, 2014. [Google Scholar]
- Talavera-Pech, W.; Avila-Ortega, A.; Pacheco Catalán, D.; Quintana-Owen, P.; Barron-Zambrano, J. Effect of Functionaliza-tion Synthesis Type of Amino-MCM-41 Mesoporous Silica Nanoparticles on Its RB5 Adsorption Capacity and Kinetics. Silicon 2018, 11, 1547–1555. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area and Porosity. Ber. Bunsenges. Phys. Chem. 1982, 86, 957. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Sing, K. Adsorption by Powders and Porous Solids; Elsevier Ltd.: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Lee, X.Y.; Chew, T.L.; Oh, P.C.; Ho, C.-D.; Ong, Y.T. Synthesis and Characterization of MSU-2 for CO2 Adsorption. IOP Conf. Series Mater. Sci. Eng. 2020, 991, 012076. [Google Scholar] [CrossRef]
- Wang, D.; Wang, X.; Ma, X.; Fillerup, E.; Song, C. Three-dimensional molecular basket sorbents for CO2 capture: Effects of pore structure of supports and loading level of polyethylenimine. Catal. Today 2014, 233, 100–107. [Google Scholar] [CrossRef]
- Watabe, T.; Yogo, K. Efficient CO2 Adsorption on Amine-impregnated Mesoporous Silica Sorbents: Interpretation of Moderate Amine Loading. Chem. Lett. 2014, 43, 790–792. [Google Scholar] [CrossRef]
- Park, I.; Wang, Z.; Pinnavaia, T.J. Assembly of Large-Pore Silica Mesophases with Wormhole Framework Structures from α,ω-Diamine Porogens. Chem. Mater. 2005, 17, 383–386. [Google Scholar] [CrossRef]
- Teng, Z.; Lu, Z.; Li, J.; Zhuang, J.; Yang, W. The effect of 1-propanol on structure and hydrothermal stability of MSU-4 silica spheres. Colloids Surf. A Physicochem. Eng. Asp. 2011, 384, 200–204. [Google Scholar] [CrossRef]
- Bellamy, L.J. Organo-Silicon Compounds. In The Infra-Red Spectra of Complex Molecules; Bellamy, L.J., Ed.; Springer: Dordrecht, The Netherlands, 1975; pp. 374–384. [Google Scholar]
- Smith, A.L. Analysis of Silicones; Wiley: New York, NY, USA, 1974; Volume 41. [Google Scholar]
- Lin, S.-P. Introduction of Molecular Vibrations & IR Spectroscopy. In Modern Optical Spectroscopy; Institute of Biomedical Engineering, National Chung Hsing University: Taiwan, 2016. [Google Scholar]
- Oxtoby, D.W.; Gillis, H.P.; Butler, L.J. Principles of Modern Chemistry; Cengage Learning: Boston, MA, USA, 2015. [Google Scholar]
- Lin, L.-Y.; Bai, H. Continuous generation of mesoporous silica particles via the use of sodium metasilicate precursor and their potential for CO2 capture. Microporous Mesoporous Mater. 2010, 136, 25–32. [Google Scholar] [CrossRef]
- Dao, D.S.; Yamada, H.; Yogo, K. Large-Pore Mesostructured Silica Impregnated with Blended Amines for CO2 Capture. Ind. Eng. Chem. Res. 2013, 52, 13810–13817. [Google Scholar] [CrossRef]
- Dao, D.S.; Yamada, H.; Yogo, K. Response Surface Optimization of Impregnation of Blended Amines into Mesoporous Silica for High-Performance CO2 Capture. Energy Fuels 2015, 29, 985–992. [Google Scholar] [CrossRef]



| Sample | BET Specific Surface Area, SBET (m2/g) | Total Pore Volume, Vp (cm3/g) | N Content (wt%) |
|---|---|---|---|
| MSU-2 | 1223.13 | 0.87 | 0.00 |
| TEPA(20)/MSU-2 | 397.20 | 0.34 | 7.62 |
| TEPA(30)/MSU-2 | 140.34 | 0.16 | 8.97 |
| TEPA(40)/MSU-2 | 27.08 | 0.03 | 11.91 |
| TEPA(50)/MSU-2 | 0.29 | ~0.00 | 15.80 |
| TEPA(60)/MSU-2 | 0.12 | ~0.00 | 19.46 |
| Wavenumber (cm−1) | Title 3 |
|---|---|
| ∼3467 # | O-H stretching vibrations of the hydrogen bonded surface silanol groups and the remaining adsorbed water molecules |
| 2950 * | Asymmetric stretching vibration of the –CH2 groups of TEPA chains |
| 2843 * | Symmetric stretching vibration of the –CH2 groups of TEPA chains |
| 1647 # | Deformation vibrations of physically adsorbed water molecules |
| 1571 * | Bending vibration (δ(N–H)) of –NH– |
| 1477 * | Deformation vibration of –NH2 |
| 1310 * | Stretching vibration of C–N |
| 1077 # | Asymmetrical stretching (νas) of siloxane (-Si-O-Si-) band |
| 800 # | Si-O bond symmetrical stretching (νs) of the silanol groups |
| 461 # | Bending vibration (δ) of siloxane (-Si-O-Si-) band |
| Adsorbents | Weight Loss of First-Step Degradation at 30–80 °C (%) | Weight Loss of Second-Step Degradation at 190–290 °C (%) |
|---|---|---|
| MSU-2 | 3.62 | - |
| TEPA(20)/MSU-2 | 3.98 | 16.53 |
| TEPA(30)/MSU-2 | 4.09 | 22.94 |
| TEPA(40)/MSU-2 | 4.25 | 26.24 |
| TEPA(50)/MSU-2 | 5.34 | 41.10 |
| TEPA(60)/MSU-2 | 3.15 | 52.95 |
| TEPA | - | 99.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, X.Y.; Viriya, V.; Chew, T.L.; Oh, P.C.; Ong, Y.T.; Ho, C.-D.; Jawad, Z.A. Synthesis, Characterization and Gas Adsorption of Unfunctionalized and TEPA-Functionalized MSU-2. Processes 2022, 10, 1943. https://doi.org/10.3390/pr10101943
Lee XY, Viriya V, Chew TL, Oh PC, Ong YT, Ho C-D, Jawad ZA. Synthesis, Characterization and Gas Adsorption of Unfunctionalized and TEPA-Functionalized MSU-2. Processes. 2022; 10(10):1943. https://doi.org/10.3390/pr10101943
Chicago/Turabian StyleLee, Xin Ying, Vinosha Viriya, Thiam Leng Chew, Pei Ching Oh, Yit Thai Ong, Chii-Dong Ho, and Zeinab Abbas Jawad. 2022. "Synthesis, Characterization and Gas Adsorption of Unfunctionalized and TEPA-Functionalized MSU-2" Processes 10, no. 10: 1943. https://doi.org/10.3390/pr10101943





