Synthesis, In Silico, and In Vitro Biological Evaluation of New Furan Hybrid Molecules
Abstract
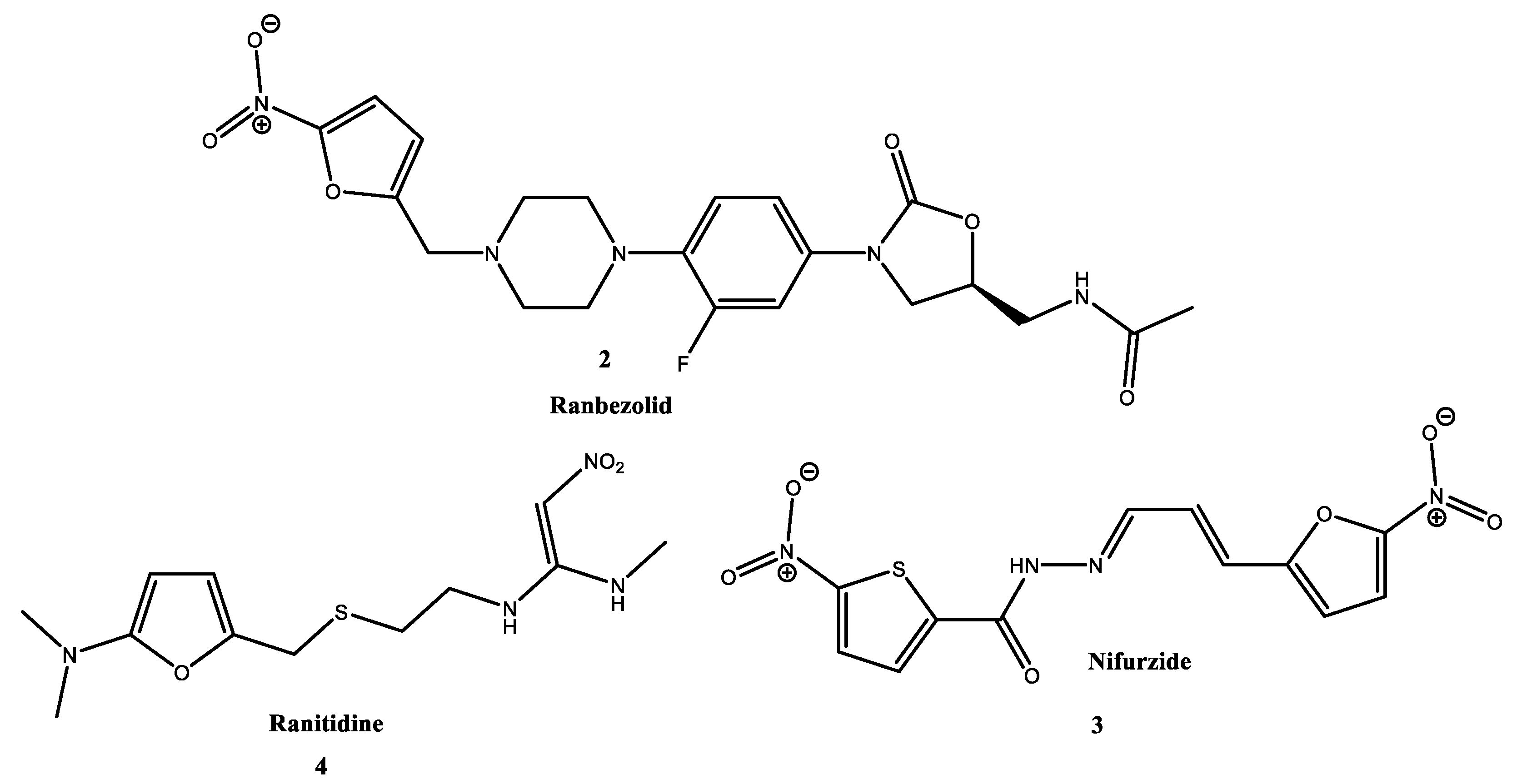
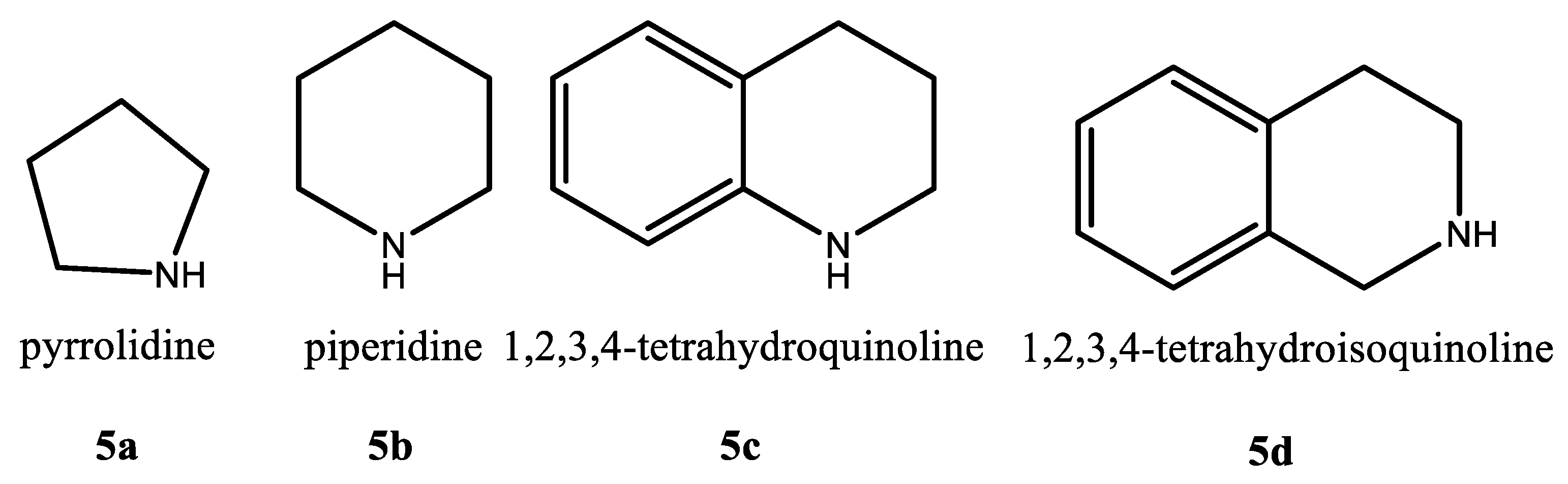
:1. Introduction
2. Materials and Methods
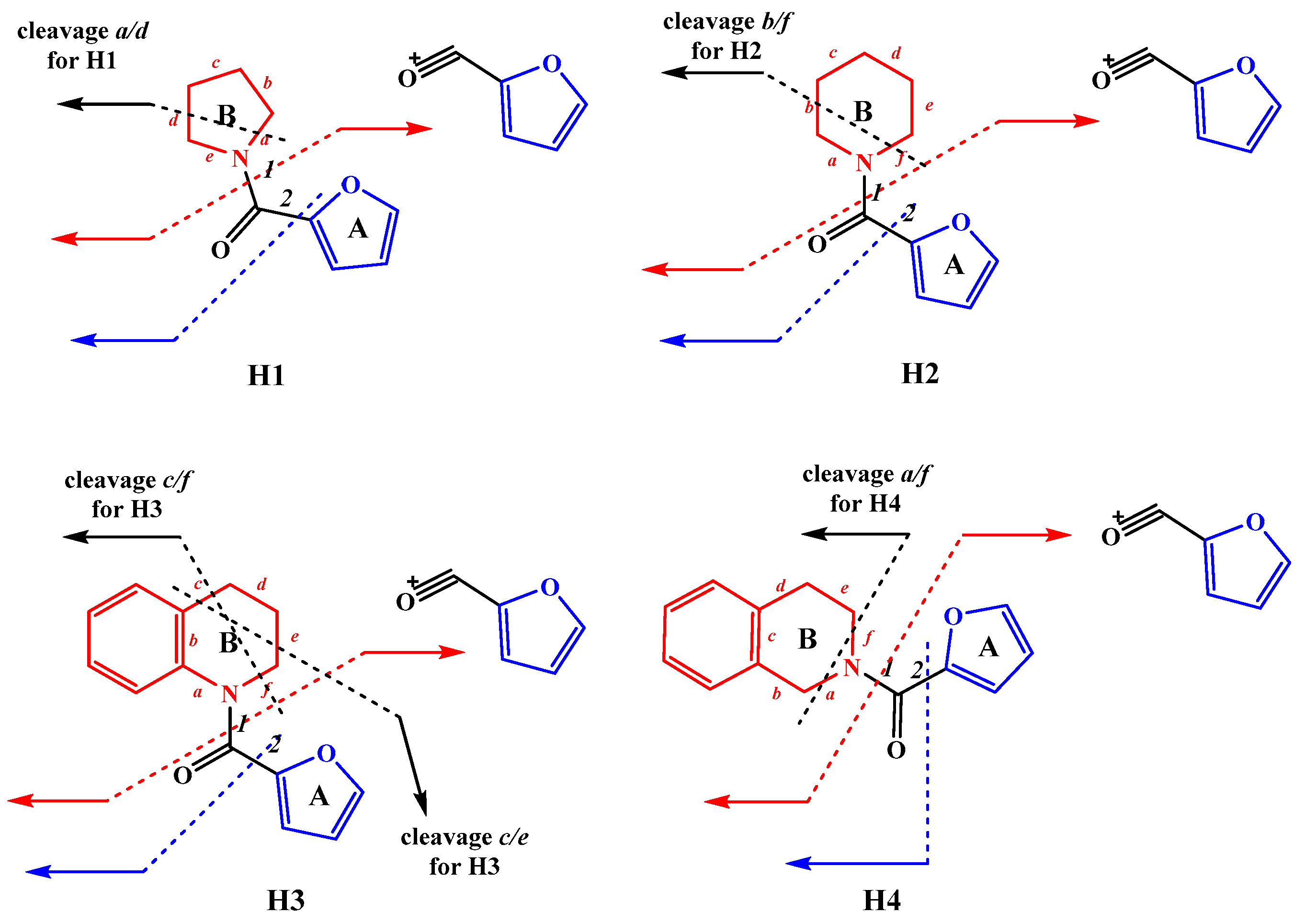
2.1. Synthesis
2.2. Biological Evaluation
2.3. Biological Experiments
2.3.1. Hydrogen Peroxide Scavenging Activity (HPSA)
2.3.2. Metal-Chelating Activity (MChA) on Ferrous Ions
2.3.3. Inhibition of Albumin Denaturation (IAD)
2.3.4. Antitryptic Activity (ATA)
2.4. Physicochemical Characterization
2.4.1. Determination of Lipophilicity as RM Values
2.4.2. Prediction of Anti-Inflammatory and Anti-Arthritic Activity
2.5. Statistical Analysis
3. Results
3.1. Synthesis
3.2. Biological Evaluation
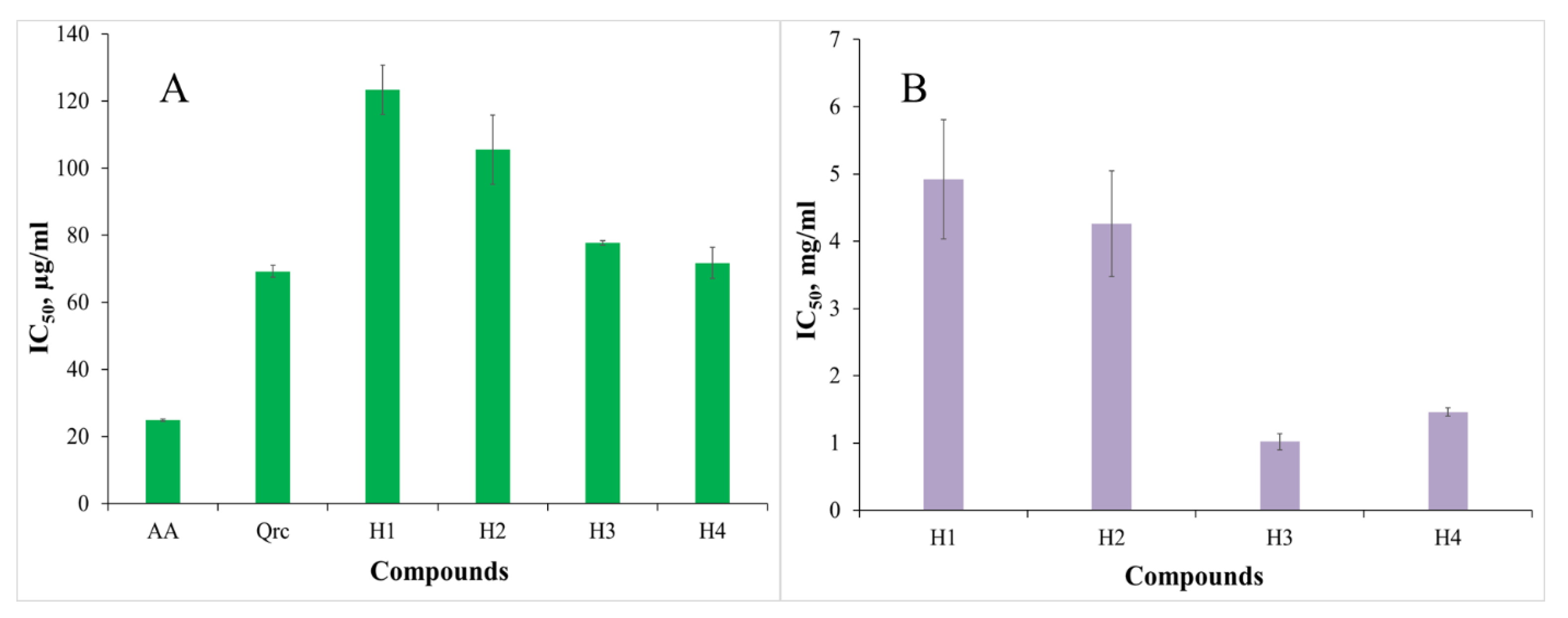
3.2.1. Hydrogen Peroxide Scavenging Activity
3.2.2. Metal-Chelating Activity (MChA) on Ferrous Ions
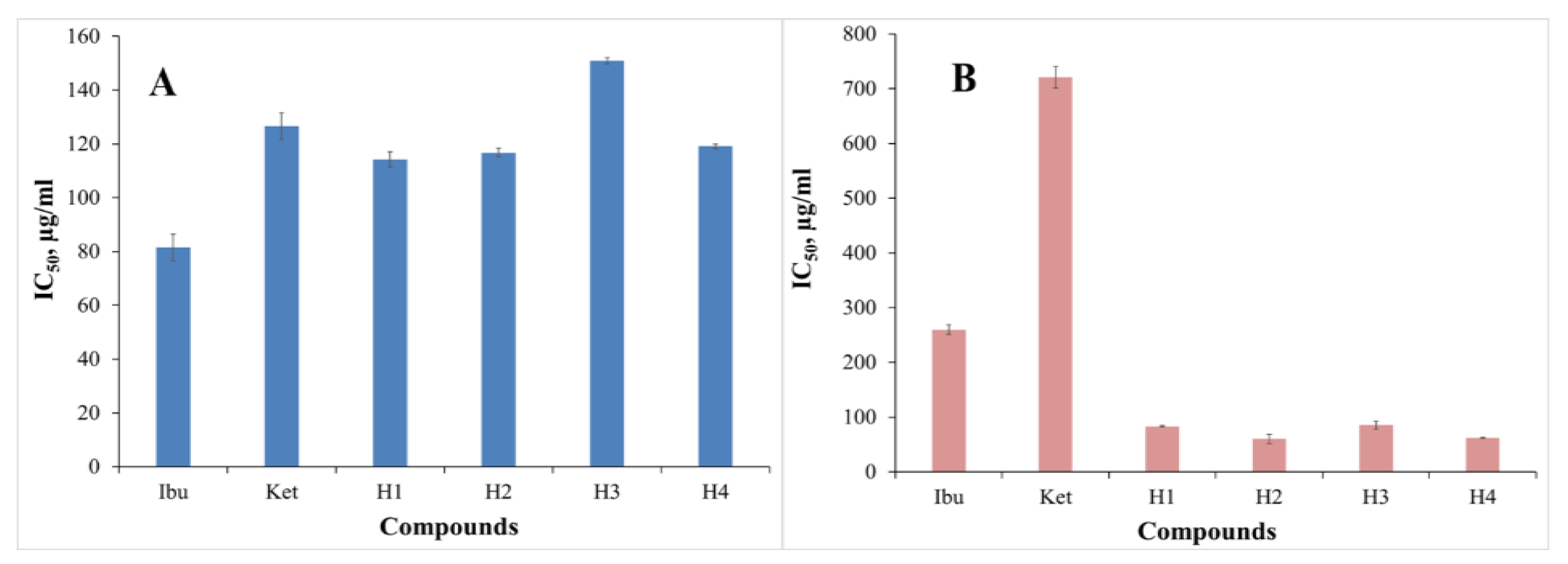
3.2.3. Inhibition of Albumin Denaturation (IAD)
3.2.4. Antitryptic Activity (ATA)
3.2.5. Lipophilicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Demicheva, L. Biological activity of furan derivatives (review). Chem. Heterocycl. Compd. 1993, 29, 243–267. [Google Scholar] [CrossRef]
- Duffy, L.; Smith, A.D. Nitrofurantoin macrocrystals prevent bacteriuria in intermittment self-catheterization. Urology 1982, 20, 47–49. [Google Scholar] [CrossRef]
- Naruganahalli, K.S.; Shirumalla, R.K.; Bansal, V.; Gupta, J.B.; Das, B.; Ray, A. Ranbezolid, a novel oxazolidinone antibacterial: In vivo characterisation of monoamine oxidase inhibitory potential in conscious rats. Eur. J. Pharmacol. 2006, 545, 167–172. [Google Scholar] [CrossRef]
- Delsarte, A.; Faway, M.; Frère, J.M.; Coyette, J.; Calberg-Bacq, C.M.; Heinen, E. Nifurzide, a nitrofuran antiinfectious agent: Interaction with Escherichia coli cells. Antimicro. Agents Chemother. 1981, 19, 477–486. [Google Scholar] [CrossRef] [Green Version]
- White, C.M.; Hernandez, A.V. Ranitidine and Risk of N-Nitrosodimethylamine (NDMA) Formation. JAMA 2021, 326, 225–227. [Google Scholar] [CrossRef]
- Mermer, A.; Keles, T.; Sirin, Y. Recent studies of nitrogen containing heterocyclic compounds as novel antiviral agents: A review. Bioorg. Chem. 2021, 114, 105076. [Google Scholar] [CrossRef]
- Li, Y.-S.; Liu, X.-Y.; Zhao, D.-S.; Liao, Y.-X.; Zhang, L.-H.; Zhang, F.-Z.; Song, G.-P.; Cui, Z.-N. Tetrahydroquinoline and tetrahydroisoquinoline derivatives as potential selective PDE4B inhibitors. Bioorg. Med. Chem. Lett. 2018, 28, 3271–3275. [Google Scholar] [CrossRef]
- Zeni, G.; Lüdtke, D.; Nogueira, C.; Panatieri, R.; Braga, A.; Silveira, C.; Stefani, H.; Rocha, J. New acetylenic furan derivatives: Synthesis and anti-inflammatory activity. Tetrahedron Lett. 2001, 42, 8927–8930. [Google Scholar] [CrossRef]
- Janczewski, Ł.; Zieliński, D.; Kolesińska, B. Synthesis of amides and esters containing furan rings under microwave-assisted conditions. Open Chem. 2021, 19, 265–280. [Google Scholar] [CrossRef]
- Qian, Z.; Li, Q.; Wang, L.; Fu, F.; Liu, X. The chemical effect of furfural amide on the enhanced performance of the diphenolic acid derived bio-polybenzoxazine resin. J. Poly. Sci. 2021, 59, 2057–2068. [Google Scholar] [CrossRef]
- Malladi, S.; Venkata Nadh, R.; Suresh Babu, K.; Suri Babu, P. Synthesis and antibacterial activity studies of 2,4-di substituted furan derivatives. Beni-Suef Univ. J. Basic App. Sci. 2017, 6, 345–353. [Google Scholar] [CrossRef]
- Manolov, S.; Ivanov, I.; Bojilov, D. Synthesis of New 1,2,3,4-Tetrahydroquinoline Hybrid of Ibuprofen and Its Biological Evaluation. Molbank 2022, 1, M1350. [Google Scholar] [CrossRef]
- Sirin, S.; Duyar, H.; Aslım, B.; Seferoğlu, Z. Synthesis and biological activity of pyrrolidine/piperidine substituted 3-amido-9-ethylcarbazole derivatives. J. Mol. Struct. 2021, 1242, 130687. [Google Scholar] [CrossRef]
- Sakat, S.S.; Juvekar, A.R.; Gambhire, M.N. In-vitro antioxidant and anti-inflammatory activity of methanol extract of Oxalis corniculata linn. Int. J. Pharm. Pharm. Sci. 2010, 2, 146–155. [Google Scholar]
- Manolov, S.; Ivanov, I.; Bojilov, D. Microwave-assisted synthesis of 1,2,3,4-tetrahydroisoquinoline sulfonamide derivatives and their biological evaluation. J. Serb. Chem. Soc. 2021, 86, 139–151. [Google Scholar] [CrossRef]
- Oyedapo, O.O.; Famurewa, A.J. Antiprotease and membrane stabilizing activities of extracts of fagara zanthoxyloides, olax subscorpioides and tetrapleura tetraptera. Int. J. Pharmacogn. 1995, 33, 65–69. [Google Scholar] [CrossRef]
- Pontiki, E.; Hadjipavlou-Litina, D. Synthesis and pharmacochemical evaluation of novel aryl-acetic acid inhibitors of lipoxygenase, antioxidants, and anti-inflammatory agents. Bioorg. Med. Chem. 2007, 15, 5819–5827. [Google Scholar] [CrossRef]
- Sadym, A.; Lagunin, A.; Filimonov, D.; Poroikov, V. Prediction of biological activity spectra via the Internet. SAR QSAR Environ. Res. 2003, 14, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Filimonov, D.; Lagunin, A.; Gloriozova, T.; Rudik, A.; Druzhilovskii, D.; Pogodin, P.; Poroikov, V. Prediction of the Biological Activity Spectra of Organic Compounds Using the Pass Online Web Resource. Chem. Heterocycl. Compd. 2014, 50, 444–457. [Google Scholar] [CrossRef]
- Das, S.; Addis, D.; Zhou, S.; Junge, K.; Beller, M. Zinc-catalyzed reduction of amides: Unprecedented selectivity and functional group tolerance. J. Am. Chem. Soc. 2010, 132, 1770–1771. [Google Scholar] [CrossRef] [PubMed]
- Pathak, U.; Bhattacharyya, S.; Pandey, L.; Mathur, S.; Jain, R. An easy access to tertiary amides from aldehydes and N, N-dialkylchlorothiophosphoramidates. RSC Adv. 2014, 4, 3900. [Google Scholar] [CrossRef]
- Khosravi, K.; Naserifar, S. 1,1,2,2-Tetrahydroperoxy-1,2-diphenylethane: An efficient and high oxygen content oxidant in various oxidative reactions. Tetrahedron 2018, 74, 6584–6592. [Google Scholar] [CrossRef]
- Ramkumar, R.; Chandrasekaran, S. Catalyst-free, metal-free, and chemoselective transformation of activated secondary amides. Synthesis 2019, 51, 921–932. [Google Scholar] [CrossRef] [Green Version]
- Chalana, A.; Kumar, R.; Karri, R.; Kumar, K.; Kumar, B.; Roy, G. Interplay of the intermolecular and intramolecular interactions in stabilizing the thione-based copper(I) complexes and their significance in protecting the biomolecules against metal-mediated oxidative damage. Polyhedron 2022, 215, 115647. [Google Scholar] [CrossRef]
- Galano, A.; Macías-Ruvalcaba, A.; Campos, M.; Pedraza-Chaverri, J. Mechanism of the OH radical scavenging activity of nordihydroguaiaretic acid: A combined theoretical and experimental study. J. Phys. Chem. B 2010, 114, 6625–6635. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutterdge, J.M.C. Free Radicals in Biology and Medicine, 5th ed.; Oxford Academic: Oxford, UK, 2015; Available online: https://doi.org/10.1093/acprof:oso/9780198717478.001.0001 (accessed on 5 August 2022).
- Khan, A.U. Singlet molecular oxygen from superoxide anion and sensitized fluorescence of organic molecules. Science 1970, 168, 467–477. [Google Scholar] [CrossRef]
- Kellog, E.W.; Fridovich, I. Superoxide, hydrogen peroxide, and singlet oxygen in lipid peroxidation by a xanthine oxidase system. J. Biol. Chem. 1975, 250, 8812–8817. Available online: https://www.jbc.org/article/S0021-9258(19)40745-X/pdf (accessed on 15 August 2022). [CrossRef]
- Bandgar, B.P.; Adsul, L.K.; Lonikar, S.V.; Chavan, H.V.; Shringare, S.N.; Patil, S.A.; Jalde, S.S.; Koti, B.A.; Dhole, N.A.; Gacche, R.N.; et al. Synthesis of novel carbazole chalcones as radical scavenger, antimicrobial and cancer chemopreventive agents. J. Enzyme Inhib. Med. Chem. 2013, 28, 593–600. [Google Scholar] [CrossRef] [Green Version]
- Vane, J.R.; Botting, R.M. New insights into the mode of action of anti-inflammatory drug. Inflamm. Res. 1995, 44, 1–10. [Google Scholar] [CrossRef]
- Jayashree, V.; Bagyalakshmi, S.; Manjula Devi, K.; Richard Daniel, D. In-vitro anti-inflamatory activity of 4-benzylpiperidine. Asian J. Pharm. Clin. Res. 2016, 9, 108–110. [Google Scholar] [CrossRef]
- Mondal, M.; Lakshmi, P.; Krishna, R.; Sakthivel, N. Molecular interaction between human serum albumin (HSA) and phloroglucinol derivative that shows selective anti-proliferative potential. J. Lumin. 2017, 192, 990–998. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, D.; Hoekman, D.H. Exploring QSAR: Hydrophobic, Electronic, and Steric Constants; American Chemical Society: Washington, DC, USA, 1995; Available online: https://www.amazon.com/Exploring-QSAR-Hydrophobic-Electronic-Profession-al/dp/0841229910 (accessed on 2 August 2022).


| Compounds | IC50± SD, μg/mL | IC50± SD, mg/mL | RM ± SD | Pa | |||
|---|---|---|---|---|---|---|---|
| HPSA | IAD | ATA | MChA | cAnti-I | cAnti-A | ||
| AA | 24.84 ± 0.35 | - | - | - | - | - | - |
| Qrc | 69.25 ± 1.82 | - | - | - | - | - | - |
| Ibu | - | 81.50 ± 4.95 | 259.82 ± 9.14 | - | 1.11 ± 0.010 | 0.903 | 0.573 |
| Ket | - | 126.58 ± 5.00 | 720.57 ± 19.78 | - | 1.54 ± 0.015 | 0.925 | 0.469 |
| H1 | 123.33 ± 7.41 | 114.31 ± 2.88 | 83.25 ± 1.69 | 4.92 ± 0.89 | 1.04 ± 0.023 | 0.224 | - |
| H2 | 105.52 ± 10.33 | 116.76 ± 1.61 | 60.21 ± 8.16 | 4.26 ± 0.79 | 0.94 ± 0.016 | 0.226 | - |
| H3 | 77.75 ± 0.67 | 150.99 ± 1.16 | 85.33 ± 7.26 | 1.02 ± 0.12 | 1.01 ± 0.030 | - | - |
| H4 | 71.72 ± 4.63 | 119.08 ± 0.92 | 62.23 ± 0.83 | 1.46 ± 0.06 | 0.87 ± 0.015 | 0.201 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manolov, S.; Ivanov, I.; Bojilov, D.; Nedialkov, P. Synthesis, In Silico, and In Vitro Biological Evaluation of New Furan Hybrid Molecules. Processes 2022, 10, 1997. https://doi.org/10.3390/pr10101997
Manolov S, Ivanov I, Bojilov D, Nedialkov P. Synthesis, In Silico, and In Vitro Biological Evaluation of New Furan Hybrid Molecules. Processes. 2022; 10(10):1997. https://doi.org/10.3390/pr10101997
Chicago/Turabian StyleManolov, Stanimir, Iliyan Ivanov, Dimitar Bojilov, and Paraskev Nedialkov. 2022. "Synthesis, In Silico, and In Vitro Biological Evaluation of New Furan Hybrid Molecules" Processes 10, no. 10: 1997. https://doi.org/10.3390/pr10101997








