Evaluation of Antiaging Effect of Sheep Placenta Extract Using SAMP8 Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Sheep Placenta Extract Preparation and Dosage
2.3. Experimental Animal Grouping
2.4. Aging Index
2.5. Serum Biochemical Parameters Analysis
2.6. Histological Analysis
2.7. Statistical Analysis
3. Results
3.1. Effects of SPE on Food Intake, Water Intake, Body Weight, and Organ Weight of SAMP8 Mice
3.2. Effect of SPE on Aging Index of Senescence-Accelerated Mice
3.3. Effects of SPE on Serum Biochemical Parameters
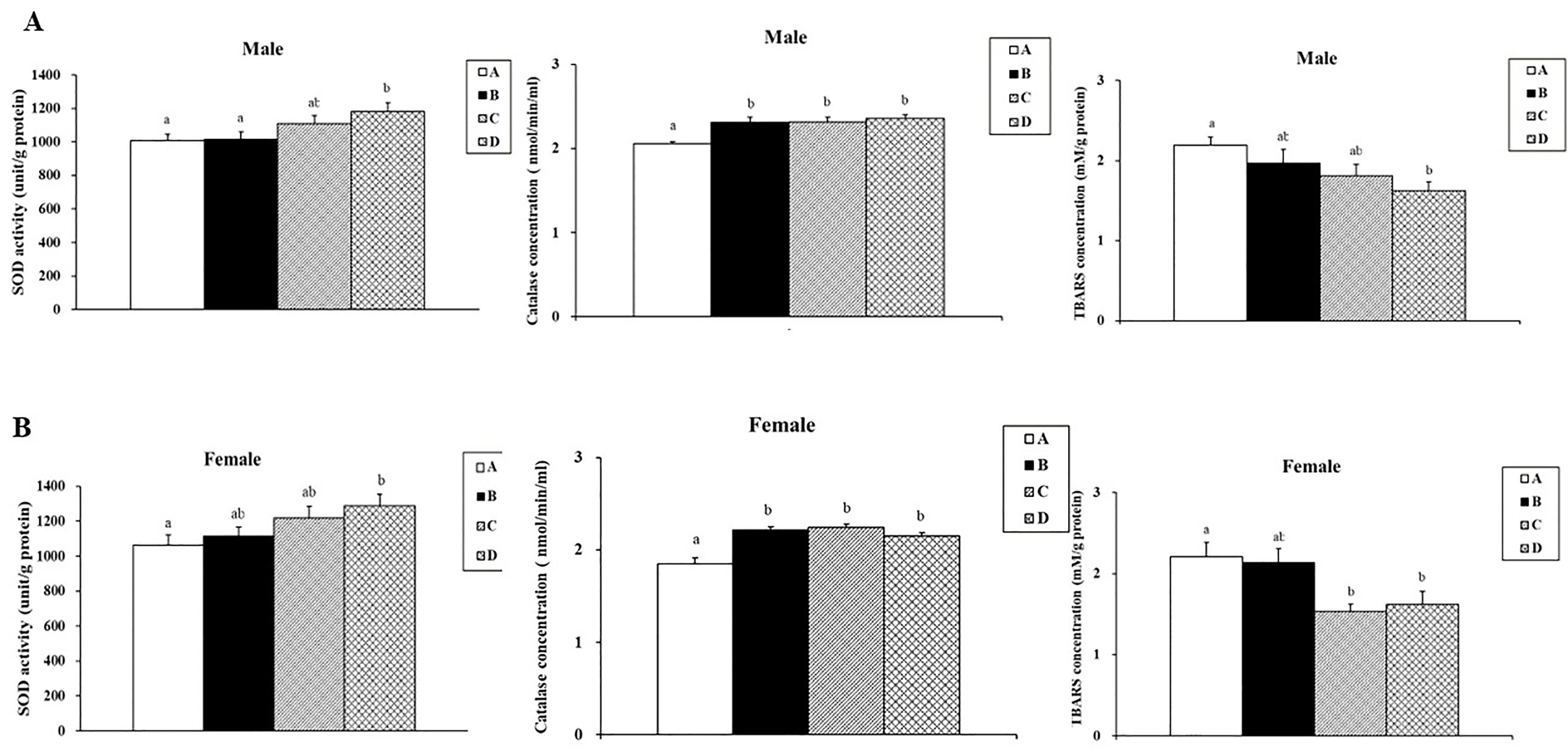
3.4. Effects of SPE on SOD Activities, Catalase Activities, and Lipid Peroxidation in Aging Mice
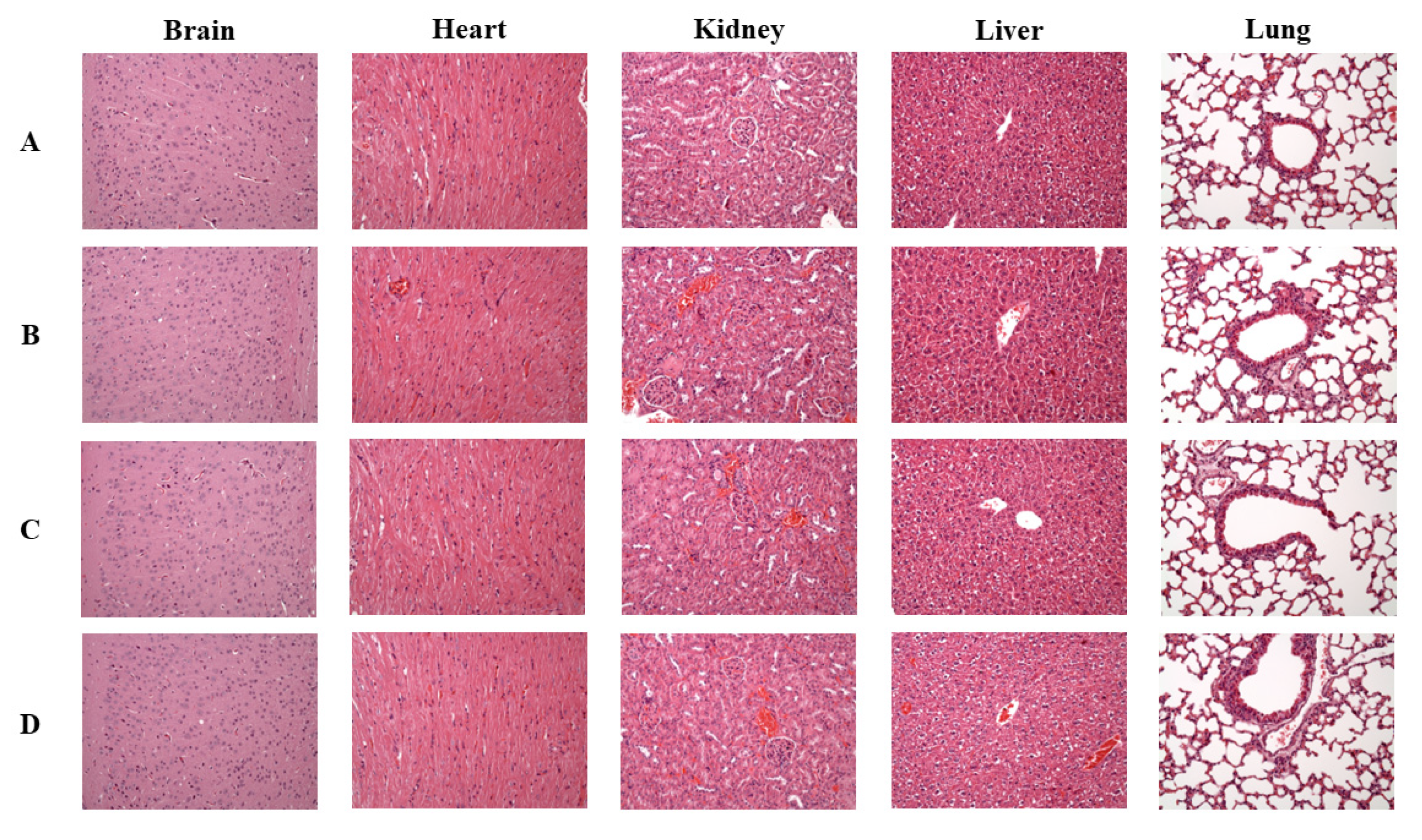
3.5. Effects of SPE on Histology of Vital Organs in Aging Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, C.; Groom, K.M.; Oyston, C.; Chamley, L.W.; Clark, A.R.; James, J.L. The placenta in fetal growth restriction: What is going wrong? Placenta 2020, 96, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Brett, K.E.; Ferraro, Z.M.; Yockell-Lelievre, J.; Gruslin, A.; Adamo, K.B. Maternal–fetal nutrient transport in pregnancy pathologies: The role of the placenta. Int. J. Mol. Sci. 2014, 15, 16153–16185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasool, A.; Alvarado-Flores, F.; O’Tierney-Ginn, P. Placental impact of dietary supplements: More than micronutrients. Clin. Ther. 2021, 43, 226–245. [Google Scholar] [CrossRef] [PubMed]
- Ghoneum, M.; El-Gerbed, M.S. Human placental extract ameliorates methotrexate-induced hepatotoxicity in rats via regulating antioxidative and anti-inflammatory responses. Cancer Chemother. Pharmacol. 2021, 88, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Sarkar, R.; Saha, P.; Maity, A.; Sarkar, T.; Das, D.; Chakraborty, P.D.; Bandyopadhyay, S.; Ghosh, C.K.; Karmakar, S. Effect of human placental extract in the management of biofilm mediated drug resistance–A focus on wound management. Microb. Pathog. 2017, 111, 307–315. [Google Scholar] [CrossRef]
- Kaneko, Y.; Sano, M.; Seno, K.; Oogaki, Y.; Takahashi, H.; Ohkuchi, A.; Yokozawa, M.; Yamauchi, K.; Iwata, H.; Kuwayama, T. Olive leaf extract (OleaVita) suppresses inflammatory cytokine production and NLRP3 inflammasomes in human placenta. Nutrients 2019, 11, 970. [Google Scholar] [CrossRef] [Green Version]
- Mumtaz, S.M.; Goyal, R.K.; Ameen, A.; Alexandrovich, B.I.; Gupta, M. Animal Placental Therapy: An Emerging Tool for Health Care. Curr. Tradit. Med. 2022, 8, 20–30. [Google Scholar] [CrossRef]
- Pogozhykh, O.; Prokopyuk, V.; Figueiredo, C.; Pogozhykh, D. Placenta and placental derivatives in regenerative therapies: Experimental studies, history, and prospects. Stem Cells Int. 2018, 2018, 4837930. [Google Scholar] [CrossRef]
- Pan, S.Y.; Chan, M.K.; Wong, M.B.; Klokol, D.; Chernykh, V. Placental therapy: An insight to their biological and therapeutic properties. Blood 2017, 4, 12. [Google Scholar]
- Ober, W.B. Notes on placentophagy. Bull. N. Y. Acad. Med. 1979, 55, 591. [Google Scholar]
- Hayes, E.H. Consumption of the placenta in the postpartum period. J. Obstet. Gynecol. Neonatal. Nurs. 2016, 45, 78–89. [Google Scholar] [CrossRef]
- Koike, K.; Yamamoto, Y.; Suzuki, N.; Yamazaki, R.; Yoshikawa, C.; Takano, F.; Takuma, K.; Sugiura, K.; Inoue, M. Efficacy of porcine placental extract on climacteric symptoms in peri-and postmenopausal women. Climacteric 2012, 16, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Silini, A.R.; Cargnoni, A.; Magatti, M.; Pianta, S.; Parolini, O. The long path of human placenta, and its derivatives, in regenerative medicine. Front. Bioeng. Biotechnol. 2015, 3, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Hou, Y.; Cheng, Y. Polypeptide preparation of sheep placenta and its free radicals scavenging activity. China Brew. 2014, 33, 89–93. [Google Scholar]
- Wu, Y. Study on the new method of extraction placental peptide and analysis of nutritive composition in sheep placenta. Food Sci. 2005, 26, 295–297. [Google Scholar]
- Zhang, S.; Fang, F.; Gu, Y.; Zhang, Y.; Tao, Y.; Zhang, X. Effects of goat placental preparations on relieving fatigue and anti-oxidation in mice. J. Tradit. Chin. Vet. Med. 2008, 6, 8–10. [Google Scholar]
- Hou, Y.; Zhou, J.; Liu, W.; Cheng, Y.; Wu, L.; Yang, G. Preparation and characterization of antioxidant peptides from fermented goat placenta. Korean J. Food Sci. Anim. Resour. 2014, 34, 769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, P.-F.; Baskaran, R.; Kuo, C.-H.; Day, C.H.; Chen, R.-J.; Ho, T.-J.; Yeh, Y.-L.; Padma, V.V.; Lai, C.-H.; Huang, C.-Y. Bioactive dipeptide from potato protein hydrolysate combined with swimming exercise prevents high fat diet induced hepatocyte apoptosis by activating PI3K/Akt in SAMP8 mouse. Mol. Biol. Rep. 2021, 48, 2629–2637. [Google Scholar] [CrossRef] [PubMed]
- Takeda, T.; Matsushita, T.; Kurozumi, M.; Takemura, K.; Higuchi, K.; Hosokawa, M. Pathobiology of the senescence-accelerated mouse (SAM). Exp. Gerontol. 1997, 32, 117–127. [Google Scholar] [CrossRef]
- Akiguchi, I.; Pallàs, M.; Budka, H.; Akiyama, H.; Ueno, M.; Han, J.; Yagi, H.; Nishikawa, T.; Chiba, Y.; Sugiyama, H. SAMP8 mice as a neuropathological model of accelerated brain aging and dementia: Toshio Takeda’s legacy and future directions. Neuropathology 2017, 37, 293–305. [Google Scholar] [CrossRef] [Green Version]
- Lagartos-Donate, M.; Gonzáles-Fuentes, J.; Marcos-Rabal, P.; Insausti, R.; Arroyo-Jiménez, M. Pathological and non-pathological aging, SAMP8 and SAMR1. What do hippocampal neuronal populations tell us? bioRxiv 2019, bioRxiv:598599. [Google Scholar]
- Chen, Y.-J.; Baskaran, R.; Shibu, M.A.; Lin, W.-T. Anti-Fatigue and Exercise Performance Improvement Effect of Glossogyne tenuifolia Extract in Mice. Nutrients 2022, 14, 1011. [Google Scholar] [CrossRef]
- Liu, J.; Luo, S.; Yang, J.; Ren, F.; Zhao, Y.; Luo, H.; Ge, K.; Zhang, H. The protective effect of sheep placental extract on concanavalin A-induced liver injury in mice. Molecules 2018, 24, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolduc, J.A.; Collins, J.A.; Loeser, R.F. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic. Biol. Med. 2019, 132, 73–82. [Google Scholar] [CrossRef]
- Padma, V.V.; Sowmya, P.; Felix, T.A.; Baskaran, R.; Poornima, P. Protective effect of gallic acid against lindane induced toxicity in experimental rats. Food Chem. Toxicol. 2011, 49, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, R.; Poornima, P.; Huang, C.Y.; Padma, V.V. Neferine prevents NF-κB translocation and protects muscle cells from oxidative stress and apoptosis induced by hypoxia. Biofactors 2016, 42, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Ataie, Z.; Mehrani, H.; Ghasemi, A.; Farrokhfall, K. Cinnamaldehyde has beneficial effects against oxidative stress and nitric oxide metabolites in the brain of aged rats fed with long-term, high-fat diet. J. Funct. Foods 2019, 52, 545–551. [Google Scholar] [CrossRef]
- Song, H.; Zang, C.; Liu, X.; Hou, H.; LI, D. Anti-aging function of placenta freeze-dried powder on mice. Chin. J. Biochem. Pharm. 2014, 65–67. [Google Scholar]
- Xinghuai, Z.; Guiqin, Y.; Guangjin, H. Effect of preparations from deer fetus and placenta on anti-aging of old male rats. J. Shenyang Agric. Univ. 2005, 36, 233–235. [Google Scholar]
- Mitsui, Y.; Bagchi, M.; Marone, P.A.; Moriyama, H.; Bagchi, D. Safety and toxicological evaluation of a novel, fermented, peptide-enriched, hydrolyzed swine placenta extract powder. Toxicol. Mech. Methods 2015, 25, 13–20. [Google Scholar] [CrossRef]
- Park, S.; Phark, S.; Lee, M.; Lim, J.; Sul, D. Anti-oxidative and anti-inflammatory activities of placental extracts in benzo [a] pyrene-exposed rats. Placenta 2010, 31, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Sur, T.K.; Biswas, T.K.; Ali, L.; Mukherjee, B. Anti-inflammatory and anti-platelet aggregation activity of human placental extract. Acta Pharmacol. Sin. 2003, 24, 187–192. [Google Scholar] [PubMed]
- Togashi, S.-I.; Takahashi, N.; Kubo, Y.; Shigihara, A.; Higashiyama, K.; Watanabe, S.; Fukui, T. Purification and identification of antioxidant substances in human-placenta extracts. J. Health Sci. 2000, 46, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Lephart, E.D. Skin aging and oxidative stress: Equol’s anti-aging effects via biochemical and molecular mechanisms. Ageing Res. Rev. 2016, 31, 36–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Chen, J.; Mao, X.; Qi, P.; Zhang, X. Anti-inflammatory and anti-aging evaluation of pigment–protein complex extracted from Chlorella pyrenoidosa. Mar. Drugs 2019, 17, 586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roubenoff, R.; Harris, T.B.; Abad, L.W.; Wilson, P.W.; Dallal, G.E.; Dinarello, C.A. Monocyte cytokine production in an elderly population: Effect of age and inflammation. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 1998, 53, M20–M26. [Google Scholar] [CrossRef]

| Group | Body Weight (g) | Food Intake (g/Day) | Water Intake (mL/Day) | ||
|---|---|---|---|---|---|
| Initial | Final | Gain | |||
| A | 29.81 ± 0.58 | 31.49 ± 2.02 | 1.69 ± 0.43 | 4.61 ± 0.08 | 6.54 ± 0.09 |
| B | 30.24 ± 0.67 | 32.01 ± 0.65 | 1.70 ± 0.56 | 4.73 ± 0.10 | 6.93 ± 0.14 |
| C | 29.52 ± 0.67 | 32.76 ± 0.63 | 2.80 ± 0.52 | 4.74 ± 0.08 | 6.65 ± 0.14 |
| D | 29.68 ± 0.85 | 30.76 ± 0.80 | 1.08 ± 0.31 | 4.87 ± 0.03 | 6.95 ± 0.14 |
| Group | Body Weight (g) | Food Intake (g/Day) | Water Intake (mL/Day) | ||
|---|---|---|---|---|---|
| Initial | Final | Gain | |||
| A | 25.58 ± 0.53 | 26.73 ± 0.54 | 1.16 ± 0.24 | 4.28 ± 0.09 | 5.01 ± 0.10 |
| B | 25.85 ± 0.57 | 27.39 ± 0.43 | 1.59 ± 0.62 | 4.08 ± 0.09 | 5.00 ± 0.06 |
| C | 25.80 ± 0.55 | 27.17 ± 0.56 | 1.23 ± 0.27 | 4.16 ± 0.08 | 5.04 ± 0.04 |
| D | 25.55 ± 0.42 | 27.36 ± 0.50 | 1.82 ± 0.49 | 4.34 ± 0.09 | 5.18 ± 0.06 |
| Group | Relative Organ Weights (g/100 g Body Weight) | |||||
|---|---|---|---|---|---|---|
| Brain | Heart | Liver | Spleen | Lung | Kidney | |
| A | 1.41 ± 0.03 | 0.63 ± 0.02 | 4.46 ± 0.14 | 0.29 ± 0.03 | 0.67 ± 0.02 | 1.60 ± 0.04 |
| B | 1.38 ± 0.04 | 0.61 ± 0.02 | 4.58 ± 0.07 | 0.33 ± 0.04 | 0.66 ± 0.01 | 1.62 ± 0.04 |
| C | 1.30 ± 0.04 | 0.58 ± 0.02 | 4.40 ± 0.14 | 0.27 ± 0.02 | 0.66 ± 0.07 | 1.67 ± 0.11 |
| D | 1.42 ± 0.03 | 0.63 ± 0.01 | 4.81 ± 0.10 | 0.32 ± 0.02 | 0.72 ± 0.04 | 1.75 ± 0.06 |
| Group | Relative Organ Weights (g/100 g Body Weight) | |||||
|---|---|---|---|---|---|---|
| Brain | Heart | Liver | Spleen | Lung | Kidney | |
| A | 1.56 ± 0.06 | 0.65 ± 0.01 | 4.98 ± 0.11 | 0.42 ± 0.02 | 0.82 ± 0.02 | 1.42 ± 0.06 |
| B | 1.58 ± 0.07 | 0.60 ± 0.02 | 4.66 ± 0.14 | 0.42 ± 0.03 | 0.79 ± 0.04 | 1.40 ± 0.03 |
| C | 1.55 ± 0.07 | 0.62 ± 0.02 | 4.65 ± 0.15 | 0.37 ± 0.01 | 0.77 ± 0.02 | 1.42 ± 0.02 |
| D | 1.61 ± 0.08 | 0.59 ± 0.02 | 4.57 ± 0.13 | 0.40 ± 0.02 | 0.86 ± 0.05 | 1.38 ± 0.04 |
| Group | A | B | C | D |
|---|---|---|---|---|
| Behavior | ||||
| Reactivity | 0.10 ± 0.10 | 0.00 ± 0.00 | 0.11 ± 0.11 | 0.00 ± 0.00 |
| Passivity | 0.40 ± 0.16 | 0.11 ± 0.11 | 0.22 ± 0.15 | 0.00 ± 0.00 |
| Skin | ||||
| Glossiness | 1.60 ± 0.16 a | 1.00 ± 0.00 b | 0.78 ± 0.15 b | 0.67 ± 0.17 b |
| Coarseness | 1.30 ± 0.15 | 1.67 ± 0.17 | 1.44 ± 0.18 | 1.44 ± 0.18 |
| Hair loss | 1.50 ± 0.22 | 1.44 ± 0.18 | 1.22 ± 0.15 | 1.44 ± 0.18 |
| Ulcer | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Eyes | ||||
| Periophthalmic lesion | 0.20 ± 0.13 | 0.11 ± 0.11 | 0.00 ± 0.00 | 0.11 ± 0.11 |
| Spine | ||||
| Lordokyphosis | 1.60 ± 0.16 a | 1.00 ± 0.00 b | 1.00 ± 0.00 b | 1.00 ± 0.00 b |
| Total | 6.70 ± 0.26 a | 5.33 ± 0.24 b | 4.78 ± 0.32 b | 4.67 ± 0.17 b |
| Group | A | B | C | D |
|---|---|---|---|---|
| Behavior | ||||
| Reactivity | 0.78 ± 0.22 | 0.78 ± 0.22 | 0.56 ± 0.18 | 0.67 ± 0.17 |
| Passivity | 1.11 ± 0.11 | 0.78 ± 0.15 | 0.89 ± 0.11 | 0.56 ± 0.24 |
| Skin | ||||
| Glossiness | 0.89 ± 0.11 | 0.33 ± 0.17 | 0.56 ± 0.18 | 0.67 ± 0.17 |
| Coarseness | 0.78 ± 0.15 | 0.56 ± 0.18 | 0.89 ± 0.11 | 0.67 ± 0.24 |
| Hair loss | 0.78 ± 0.22 | 0.67 ± 0.24 | 0.67 ± 0.17 | 0.67 ± 0.17 |
| Ulcer | 0.44 ± 0.18 | 0.56 ± 0.18 | 0.11 ± 0.11 | 0.22 ± 0.15 |
| Eyes | ||||
| Periophthalmic lesion | 0.78 ± 0.22 | 0.78 ± 0.15 | 0.44 ± 0.18 | 0.67 ± 0.24 |
| Spine | ||||
| Lordokyphosis | 1.00 ± 0.00 a | 0.78 ± 0.15 ab | 0.56 ± 0.18 b | 0.44 ± 0.18 b |
| Total | 6.56 ± 0.18 a | 5.22±0.32 b | 4.67 ± 0.17 b | 4.56 ± 0.18 b |
| Group | A | B | C | D |
|---|---|---|---|---|
| Glucose (mg/dL) | 112.02 ± 0.99 | 109.56 ± 1.22 | 114.21 ± 0.64 | 109.86 ± 1.95 |
| Total protein (g/dL) | 5.29 ± 0.05 | 5.40 ± 0.06 | 5.38 ± 0.07 | 5.36 ± 0.03 |
| Albumin (g/dL) | 2.95 ± 0.11 | 3.12 ± 0.08 | 3.06 ± 0.06 | 2.88 ± 0.08 |
| Triglyceride (mg/dL) | 108.65 ± 0.56 | 103.41 ± 0.54 | 104.43 ± 1.95 | 106.41 ± 0.11 |
| Total cholesterol (mg/dL) | 116.48 ± 1.81 | 114.91 ± 2.64 | 118.00 ± 2.99 | 115.49 ± 1.83 |
| HDL (mg/dL) | 53.57 ± 1.65 | 54.22 ± 1.72 | 56.35 ± 1.53 | 56.21 ± 1.03 |
| LDL (mg/dL) | 7.20 ± 0.14 | 7.06 ± 0.13 | 7.24 ± 0.09 | 7.29 ± 0.15 |
| AST (U/L) | 89.14 ± 0.75 | 87.06 ± 0.86 | 88.87 ± 0.42 | 88.09 ± 0.38 |
| ALT (U/L) | 59.51 ± 0.91 | 61.05 ± 0.87 | 59.49 ± 0.64 | 59.57 ± 0.42 |
| BUN (mg/dL) | 25.91 ± 1.22 | 26.81 ± 0.39 | 26.99 ± 0.46 | 27.72 ± 0.70 |
| Creatinine (mg/dL) | 0.30 ± 0.02 | 0.32 ± 0.01 | 0.29 ± 0.02 | 0.33 ± 0.02 |
| Sodium (mg/dL) | 6.46 ± 0.10 | 6.77 ± 0.11 | 6.52 ± 0.13 | 6.68 ± 0.08 |
| Potassium (mg/dL) | 7.71 ± 0.13 | 7.94 ± 0.12 | 7.86 ± 0.10 | 7.75 ± 0.15 |
| Uric acid (mg/dL) | 4.41 ± 0.05 | 4.38 ± 0.08 | 4.54 ± 0.07 | 4.57 ± 0.08 |
| Creatine kinase (U/L) | 260.52 ± 2.26 | 259.76 ± 3.23 | 264.30 ± 1.75 | 262.96 ± 1.88 |
| Ketones (mmol/L) | 0.66 ± 0.04 | 0.65 ± 0.02 | 0.68 ± 0.04 | 0.62 ± 0.07 |
| Free fatty acids (mmol/L) | 0.72 ± 0.08 | 0.79 ± 0.02 | 0.76 ± 0.01 | 0.73 ± 0.04 |
| IL-6 (pg/mL) | <1.5 | <1.5 | <1.5 | <1.5 |
| TNF-α (pg/mL) | <0.106 | <0.106 | <0.106 | <0.106 |
| Group | A | B | C | D |
|---|---|---|---|---|
| Glucose (mg/dL) | 116.12 ± 1.43 | 115.66 ± 1.15 | 113.40 ± 1.84 | 114.51 ± 2.01 |
| Total Protein (g/dL) | 5.36 ± 0.02 | 5.46 ± 0.11 | 5.35 ± 0.04 | 5.37 ± 0.02 |
| Albumin (g/dL) | 3.02 ± 0.04 | 2.93 ± 0.07 | 3.09 ± 0.11 | 2.90 ± 0.08 |
| Triglyceride (mg/dL) | 101.27 ± 1.66 | 103.22 ± 0.68 | 103.18 ± 1.00 | 104.65 ± 0.78 |
| Total cholesterol (mg/dL) | 117.99 ± 2.10 | 120.29 ± 2.20 | 116.88 ± 1.96 | 120.43 ± 2.39 |
| HDL (mg/dL) | 55.14 ± 2.03 | 54.16 ± 1.84 | 56.99 ± 1.13 | 57.03 ± 1.70 |
| LDL (mg/dL) | 7.36 ± 0.11 | 7.28 ± 0.12 | 7.22 ± 0.16 | 7.42 ± 0.09 |
| AST (U/L) | 88.94 ± 0.51 | 88.33 ± 0.33 | 87.36 ± 0.55 | 88.09 ± 0.63 |
| ALT (U/L) | 59.33 ± 0.97 | 61.31 ± 1.18 | 60.41 ± 0.50 | 58.87 ± 1.38 |
| BUN (mg/dL) | 27.98 ± 0.46 | 28.18 ± 0.47 | 25.62 ± 1.38 | 28.07 ± 0.73 |
| Creatinine (mg/dL) | 0.31 ± 0.01 | 0.28 ± 0.02 | 0.30 ± 0.01 | 0.33 ± 0.03 |
| Sodium (mg/dL) | 6.59 ± 0.21 | 6.64 ± 0.18 | 6.67 ± 0.11 | 6.75 ± 0.19 |
| Potassium (mg/dL) | 7.89 ± 0.10 | 7.83 ± 0.07 | 7.75 ± 0.09 | 7.79 ± 0.11 |
| Uric acid (mg/dl) | 4.64 ± 0.04 | 4.56 ± 0.07 | 4.73 ± 0.10 | 4.68 ± 0.02 |
| Creatine kinase (U/L) | 259.32 ± 1.10 | 262.86 ± 0.71 | 261.66 ± 0.63 | 260.96 ± 1.10 |
| Ketone (mmol/L) | 0.72 ± 0.07 | 0.69 ± 0.05 | 0.67 ± 0.04 | 0.70 ± 0.06 |
| Free fatty acids (mmol/L) | 0.73 ± 0.03 | 0.70 ± 0.06 | 0.76 ± 0.03 | 0.74 ± 0.02 |
| IL-6 (pg/mL) | <1.5 | <1.5 | <1.5 | <1.5 |
| TNF-α (pg/mL) | <0.106 | <0.106 | <0.106 | <0.106 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, M.-Y.; Yang, C.-P.O.; Li, W.-C.; Yang, Y.-M.; Huang, Y.-J.; Wang, M.-F.; Lin, W.-T. Evaluation of Antiaging Effect of Sheep Placenta Extract Using SAMP8 Mice. Processes 2022, 10, 2242. https://doi.org/10.3390/pr10112242
Chou M-Y, Yang C-PO, Li W-C, Yang Y-M, Huang Y-J, Wang M-F, Lin W-T. Evaluation of Antiaging Effect of Sheep Placenta Extract Using SAMP8 Mice. Processes. 2022; 10(11):2242. https://doi.org/10.3390/pr10112242
Chicago/Turabian StyleChou, Ming-Yu, Chi-Pei Ou Yang, Wen-Ching Li, Yao-Ming Yang, Yu-Ju Huang, Ming-Fu Wang, and Wan-Teng Lin. 2022. "Evaluation of Antiaging Effect of Sheep Placenta Extract Using SAMP8 Mice" Processes 10, no. 11: 2242. https://doi.org/10.3390/pr10112242
APA StyleChou, M.-Y., Yang, C.-P. O., Li, W.-C., Yang, Y.-M., Huang, Y.-J., Wang, M.-F., & Lin, W.-T. (2022). Evaluation of Antiaging Effect of Sheep Placenta Extract Using SAMP8 Mice. Processes, 10(11), 2242. https://doi.org/10.3390/pr10112242







