Effect of Sulfate, Citrate, and Tartrate Anions on the Liquid-Liquid Equilibrium Behavior of Water + Surfactant
Abstract
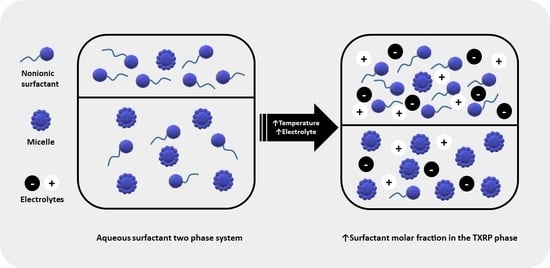
:1. Introduction
2. Materials and Methods
2.1. Experimental Procedure
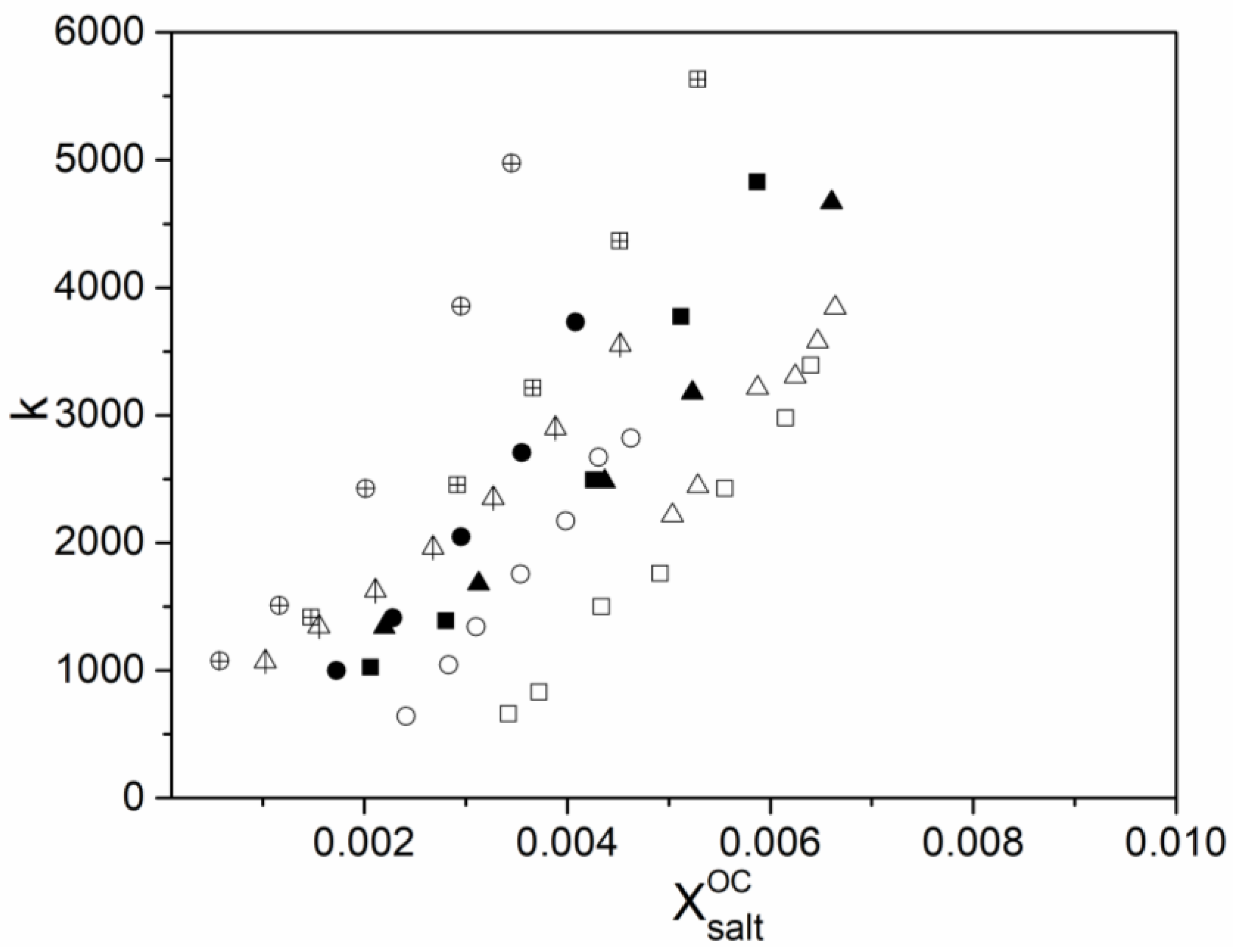
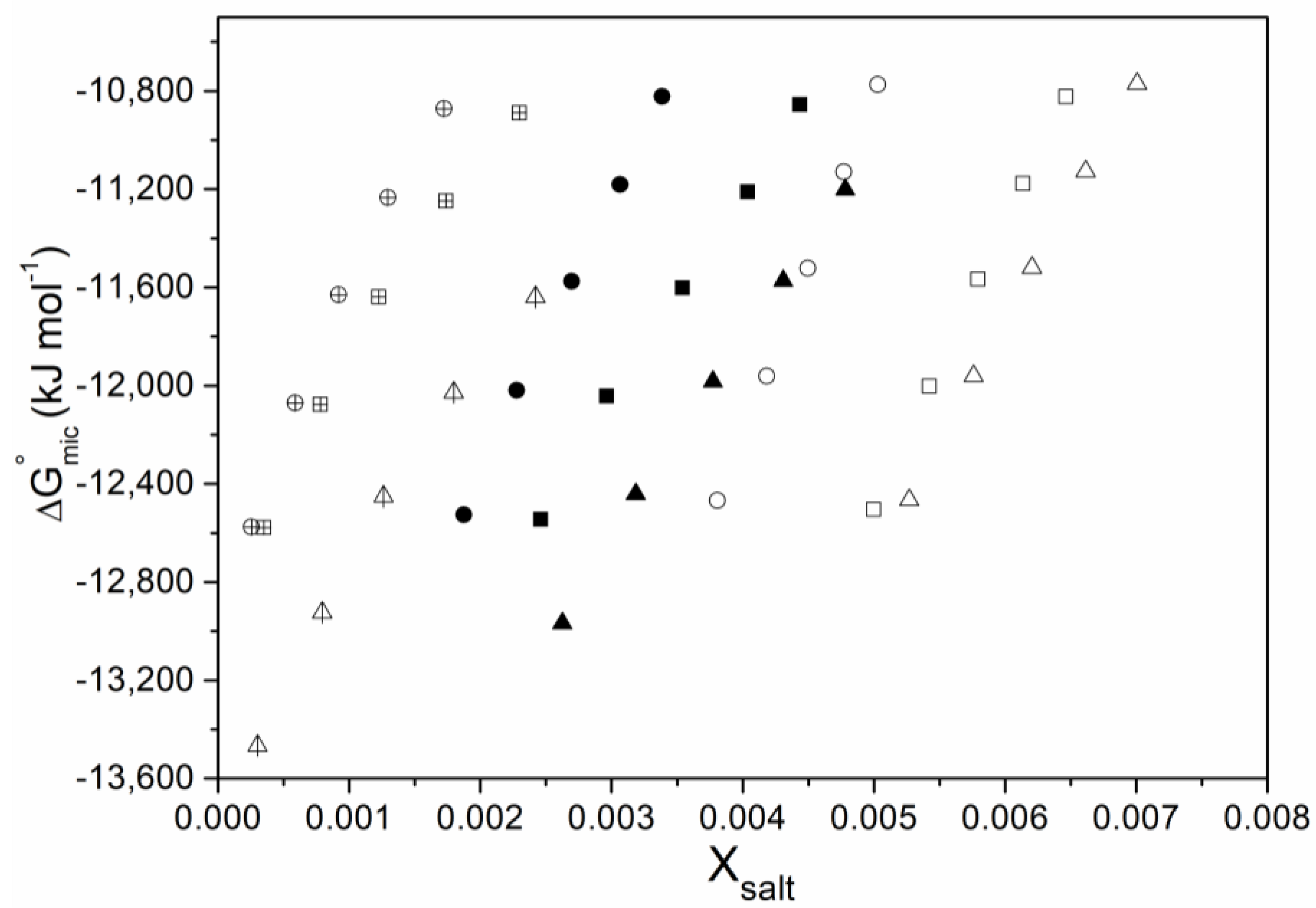
2.2. Determination of Thermodynamic Parameters
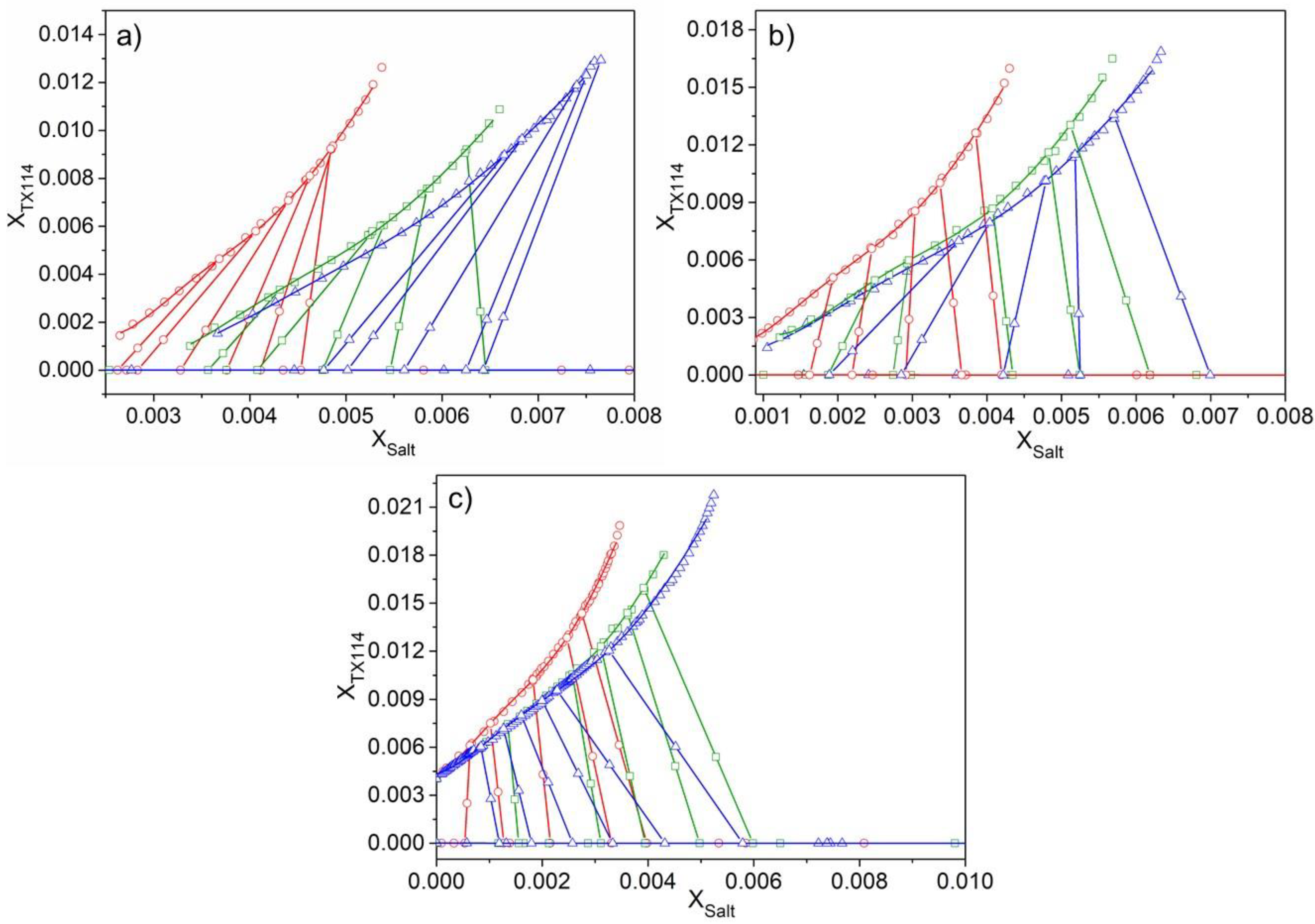
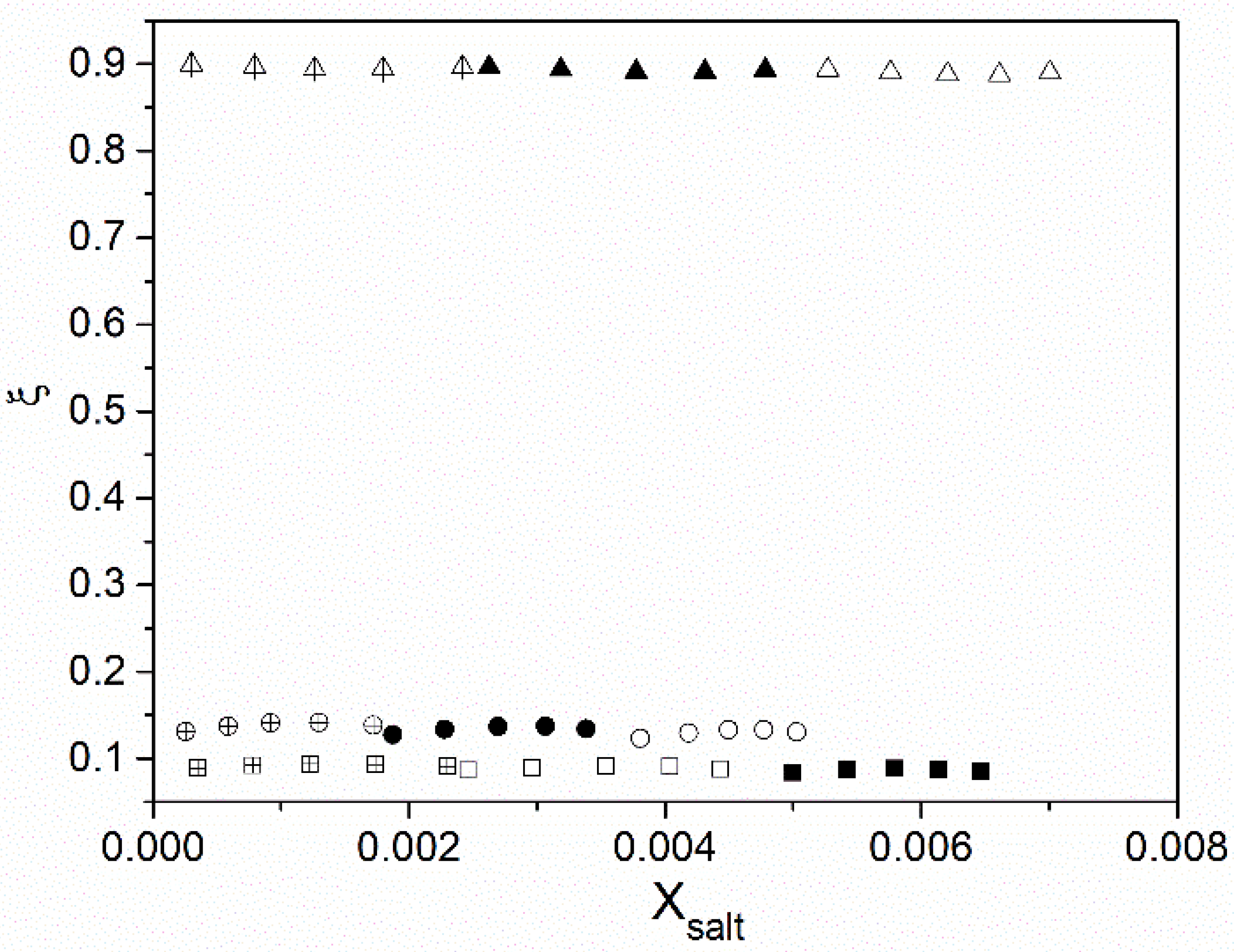
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chakraborty, A.; Sen, K. Phase separation in aqueous systems for realizing virtually significant extractions. RSC Adv. 2014, 4, 64328–64335. [Google Scholar] [CrossRef]
- Kepka, C.; Rhodin, J.; Lemmens, R.; Tjerneld, F.; Gustavsson, P.-E. Extraction of plasmid DNA from Escherichia coli cell lysate in a thermoseparating aqueous two-phase system. J. Chromatogr. A 2004, 1024, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.V.; Nerli, B.B. Molecular features determining different partitioning patterns of papain and bromelain in aqueous two-phase systems. Int. J. Biol. Macromol. 2013, 61, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, W.; Liu, Y.; Zhi, W.; Han, J.; Wang, Y.; Ni, L. Green separation of bromelain in food sample with high retention of enzyme activity using recyclable aqueous two-phase system containing a new synthesized thermo-responsive copolymer and salt. Food Chem. 2019, 282, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Chow, Y.H.; Yap, Y.J.; Tan, C.P.; Anuar, M.S.; Tejo, B.A.; Show, P.L.; Ariff, A.B.; Ng, E.-P.; Ling, T.C. Characterization of bovine serum albumin partitioning behaviors in polymer-salt aqueous two-phase systems. J. Biosci. Bioeng. 2015, 120, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, S.; Rahbar Shahrouzi, J.; Towfighi, F.; Baradar Khoshfetrat, A. Partitioning of cefazolin in aqueous two-phase systems containing poly (ethylene glycol) and sodium salts (citrate, tartrate, and sulphate). Fluid Ph. Equilibria 2019, 488, 54–61. [Google Scholar] [CrossRef]
- Junqueira, C.M.; da Silva Cabral, D.; Penido, J.A.; Mageste, A.B.; Virtuoso, L.S. How does the use of surfactants in polymer-salt based aqueous two-phase systems affect the annatto dye (Bixa orellana L.) partitioning? Fluid Ph. Equilibria 2018, 478, 14–22. [Google Scholar] [CrossRef]
- Teng, H.; Wang, X.; Hou, Y.; Chen, Y.; Yang, C.; Shen, T. Properties and Extraction for [Ni(NH3)6]2+ of ATPS-a Formed by Aqueous Cationic–Anionic Surfactant Mixtures. J. Dispers. Sci. Technol. 2016, 37, 830–835. [Google Scholar] [CrossRef]
- Kastelic, M.; Vlachy, V. Theory for the Liquid–Liquid Phase Separation in Aqueous Antibody Solutions. J. Phys. Chem. B 2018, 122, 5400–5408. [Google Scholar] [CrossRef]
- Hinze, W.L.; Pramauro, E. A Critical Review of Surfactant-Mediated Phase Separations (Cloud-Point Extractions): Theory and Applications. Crit. Rev. Anal. Chem. 1993, 24, 133–177. [Google Scholar] [CrossRef]
- Malpiedi, L.P.; Nerli, B.B.; Abdala, D.S.P.; Pessôa-Filho, P.d.A.; Pessoa, A. Aqueous micellar systems containing Triton X-114 and Pichia pastoris fermentation supernatant: A novel alternative for single chain-antibody fragment purification. Sep. Purif. Technol. 2014, 132, 295–301. [Google Scholar] [CrossRef]
- Teodorowicz, M.; Perdijk, O.; Verhoek, I.; Govers, C.; Savelkoul, H.F.J.; Tang, Y.; Wichers, H.; Broersen, K. Optimized Triton X-114 assisted lipopolysaccharide (LPS) removal method reveals the immunomodulatory effect of food proteins. PLoS ONE 2017, 12, e0173778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Zhu, C.; Fan, D.; Ma, X.; Mi, Y.; Xue, W. A Two-Step Protocol to Remove Endotoxins from Human-Like Collagen. Sep. Sci. Technol. 2015, 50, 993–1001. [Google Scholar] [CrossRef]
- Duarte, A.W.F.; Lopes, A.M.; Molino, J.V.D.; Pessoa, A.; Sette, L.D. Liquid–liquid extraction of lipase produced by psychrotrophic yeast Leucosporidium scottii L117 using aqueous two-phase systems. Sep. Purif. Technol. 2015, 156, 215–225. [Google Scholar] [CrossRef] [Green Version]
- Szymczyk, K.; Taraba, A. Aggregation behavior of Triton X-114 and Tween 80 at various temperatures and concentrations studied by density and viscosity measurements. J. Therm. Anal. Calorim. 2016, 126, 315–326. [Google Scholar] [CrossRef] [Green Version]
- Cordisco, E.; Haidar, C.N.; Goñi, R.; Nerli, B.B.; Malpiedi, L.P. Physicochemical characterization of aqueous micellar systems formed by environmentally friendly salts. Fluid Ph. Equilibria 2015, 393, 111–116. [Google Scholar] [CrossRef]
- Ritter, E.; Racheva, R.; Storm, S.; Müller, S.; Ingram, T.; Smirnova, I. Influence of Inorganic Salts on the Phase Equilibrium of Triton X-114 Aqueous Two-Phase Systems. J. Chem. Eng. Data 2016, 61, 1496–1501. [Google Scholar] [CrossRef]
- Sayem Alam, M.; Mandal, A.B. The clouding phenomena of mixed surfactant (non-ionic Triton X-114+cationic gemini 16-5-16) solutions: Influence of inorganic and organic additives on the cloud point. J. Mol. Liq. 2015, 212, 237–244. [Google Scholar] [CrossRef]
- Mohammad, S.; Held, C.; Altuntepe, E.; Köse, T.; Sadowski, G. Influence of Salts on the Partitioning of 5-Hydroxymethylfurfural in Water/MIBK. J. Phys. Chem. B 2016, 120, 3797–3808. [Google Scholar] [CrossRef]
- Xie, X.; Han, J.; Zhang, Q.; Rao, W.; Wang, Y.; Wu, J.; Ni, L. Cloud point behavior of thermosensitive triblock copolymer L61 in the presence of electrolytes. J. Dispers. Sci. Technol. 2017, 38, 494–497. [Google Scholar] [CrossRef]
- Koga, Y. Chapter VIII-The Effects of Salts on the Molecular Organization of H2O: 1-Propanol (1P)-Probing Methodology. In Solution Thermodynamics and its Application to Aqueous Solutions; Koga, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 205–239. [Google Scholar]
- Sun, P.; Huang, K.; Liu, H. Specific Salt Effect on the Interaction between Rare Earth Ions and Trioctylphosphine Oxide Molecules at the Organic–Aqueous Two-Phase Interface: Experiments and Molecular Dynamics Simulations. Langmuir 2018, 34, 11374–11383. [Google Scholar] [CrossRef] [PubMed]
- Merchuk, J.C.; Andrews, B.A.; Asenjo, J.A. Aqueous two-phase systems for protein separation: Studies on phase inversion. J. Chromatogr. B Biomed. Appl. 1998, 711, 285–293. [Google Scholar] [CrossRef]
- Corkill, J.M.; Goodman, J.F.; Harrold, S.P. Thermodynamics of micellization of non-ionic detergents. Trans. Faraday Soc. 1964, 60, 202–207. [Google Scholar] [CrossRef]
- Zana, R. Critical Micellization Concentration of Surfactants in Aqueous Solution and Free Energy of Micellization. Langmuir 1996, 12, 1208–1211. [Google Scholar] [CrossRef]
- Sadeghi, R.; Jamehbozorg, B. The salting-out effect and phase separation in aqueous solutions of sodium phosphate salts and poly(propylene glycol). Fluid Ph. Equilibria 2009, 280, 68–75. [Google Scholar] [CrossRef]
- Ebrahimi, N.; Sadeghi, R.; Ameen, B.A.H. Thermodynamics of clouding process in 1-butanol + water mixtures in the presence and absence of sugars. J. Mol. Liq. 2019, 278, 164–174. [Google Scholar] [CrossRef]
- Marcus, Y. Thermodynamics of ion hydration and its interpretation in terms of a common model. Pure AppI. Chem. 1987, 59, 1093–1101. [Google Scholar] [CrossRef] [Green Version]
- Marcus, Y. Thermodynamics of solvation of ions. Part 6.—The standard partial molar volumes of aqueous ions at 298.15 K. J. Chem. Soc. Faraday Trans. 1993, 89, 713–718. [Google Scholar] [CrossRef]
- Apelblat, A.; Manzurola, E. Apparent molar volumes of organic acids and salts in water at 298.15 K. Fluid Ph. Equilibria 1990, 60, 157–171. [Google Scholar] [CrossRef]
- Clegg, S.L.; Wexler, A.S. Densities and Apparent Molar Volumes of Atmospherically Important Electrolyte Solutions. 1. The Solutes H2SO4, HNO3, HCl, Na2SO4, NaNO3, NaCl, (NH4)2SO4, NH4NO3, and NH4Cl from 0 to 50 °C, Including Extrapolations to Very Low Temperature and to the Pure Liquid State, and NaHSO4, NaOH, and NH3 at 25 °C. J. Phys. Chem. A 2011, 115, 3393–3460. [Google Scholar] [CrossRef]
- Marcus, Y. Thermodynamics of solvation of ions. Part 5.—Gibbs free energy of hydration at 298.15 K. J. Chem. Soc. Faraday Trans. 1991, 87, 2995–2999. [Google Scholar] [CrossRef]
- Carale, T.R.; Pham, Q.T.; Blankschtein, D. Salt effects on intramicellar interactions and micellization of nonionic surfactants in aqueous solutions. Langmuir 1994, 10, 109–121. [Google Scholar] [CrossRef]
- Aravopoulou, D.; Kyriakos, K.; Miasnikova, A.; Laschewsky, A.; Papadakis, C.M.; Kyritsis, A. Comparative Investigation of the Thermoresponsive Behavior of Two Diblock Copolymers Comprising PNIPAM and PMDEGA Blocks. J. Phys. Chem. B 2018, 122, 2655–2668. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Han, J.; Wang, Y.; Wang, L.; Li, C.; Mao, Y.; Ni, L.; Zhang, W. The Cloud Point Behaviors and the Liquid–Liquid Equilibrium of L31—Inorganic Sodium Salt Aqueous Two-Phase Systems. J. Dispers. Sci. Technol. 2019, 40, 777–783. [Google Scholar] [CrossRef]
- Ananthapadmanabhan, K.P.; Goddard, E.D. Aqueous biphase formation in polyethylene oxide-inorganic salt systems. Langmuir 1987, 3, 25–31. [Google Scholar] [CrossRef]
- Stubicar, N.; Petres, J.J. Micelle formation by tritons in aqueous solutions. Croat. Chem. Acta 1981, 255–266. [Google Scholar]
- Patil, S.; Rakshit, A.; Sharma, K.; Rohit, K.; Rana, A. Physicochemical Studies of Nonionic Surfactants, C12E12 and C12E15: Effect of pH and NaCl. J. Surf. Sci. Technol. 2004, 20, 87. [Google Scholar]
- Rosen, M.J. Surfactant and Interfacial Phenomena, 2nd ed.; John Willey & Sons Inc.: New York, NY, USA, 1989. [Google Scholar]
- Burakowski, A.; Gliński, J.; Riederer, M. Hydration of polyethylene glycol monododecyl ethers (C12Ei, for i = 6 and 10) in their diluted aqueous solutions. J. Mol. Liq. 2019, 276, 179–183. [Google Scholar] [CrossRef]
- Nucci, N.V.; Vanderkooi, J.M. Effects of salts of the Hofmeister series on the hydrogen bond network of water. J. Mol. Liq. 2008, 143, 160–170. [Google Scholar] [CrossRef] [Green Version]
- Rogers, R.D.; Bauer, C.B. Partitioning behavior of group 1 and 2 cations in poly(ethylene glycol)-based aqueous biphasic systems. J. Chromatogr. B Biomed. Appl. 1996, 680, 237–241. [Google Scholar] [CrossRef]
- Zafarani-Moattar, M.T.; Hamzehzadeh, S. Effect of pH on the phase separation in the ternary aqueous system containing the hydrophilic ionic liquid 1-butyl-3-methylimidazolium bromide and the kosmotropic salt potassium citrate at T = 298.15K. Fluid Ph. Equilibria 2011, 304, 110–120. [Google Scholar] [CrossRef]
- Hooshyar, H.; Sadeghi, R. Influence of sodium salts on the micellization and interfacial behavior of cationic surfactant dodecyltrimethylammonium bromide in aqueous solution. J. Chem. Eng. Data 2015, 60, 983–992. [Google Scholar] [CrossRef]
- Sadeghi, R.; Jahani, F. Salting-In and Salting-Out of Water-Soluble Polymers in Aqueous Salt Solutions. J. Phys. Chem. B 2012, 116, 5234–5241. [Google Scholar] [CrossRef] [PubMed]
| Anion | ||||
|---|---|---|---|---|
| −1110.54 | −967.65 | 91.07 | −233.96 | |
| −2645.28 | −1781.49 | 126.51 | −990.30 | |
| −1007.53 | −846.60 | 115.35 | −276.27 |
| Anion | |||
|---|---|---|---|
| 56.1 | −15.8 | −65.3 | |
| 95.4 | −15.9 | −123.1 | |
| 76.7 | −9.2 | −58.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jimenez, O.A.Q.; Costa, J.M.; de Souza, B.R.; Medeiros, A.C.; Monteiro-Junior, E.G.; Basso, R.C. Effect of Sulfate, Citrate, and Tartrate Anions on the Liquid-Liquid Equilibrium Behavior of Water + Surfactant. Processes 2022, 10, 2023. https://doi.org/10.3390/pr10102023
Jimenez OAQ, Costa JM, de Souza BR, Medeiros AC, Monteiro-Junior EG, Basso RC. Effect of Sulfate, Citrate, and Tartrate Anions on the Liquid-Liquid Equilibrium Behavior of Water + Surfactant. Processes. 2022; 10(10):2023. https://doi.org/10.3390/pr10102023
Chicago/Turabian StyleJimenez, Otto A. Q., Josiel M. Costa, Bruno R. de Souza, Abimael C. Medeiros, Edson G. Monteiro-Junior, and Rodrigo C. Basso. 2022. "Effect of Sulfate, Citrate, and Tartrate Anions on the Liquid-Liquid Equilibrium Behavior of Water + Surfactant" Processes 10, no. 10: 2023. https://doi.org/10.3390/pr10102023