Changing Ready-to-Drink Coffee Aroma Using Dielectric Barrier Discharge Plasma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Ready-to-Drink Coffee Preparation
2.3. Plasma Treatment
2.4. GC-MS Analysis
2.5. Aroma Profile
2.6. Statistical Analysis
3. Results and Discussion
3.1. Volatile Profile
3.2. Aroma Profile
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mordor_Intelligence. Ready-to-Drink (RTD) Coffee Market—Growth, Trends, COVID-19 Impact, and Forecasts (2022–2027); Mordor: Hyderabad, India, 2022. [Google Scholar]
- Stiefel, C.; Lindemann, B.; Morlock, G.E. Non-target bioactive compound profiles of coffee roasts and preparations. Food Chem. 2022, 391, 133263. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, M.; Murakami, K.; Ikeda, M.; Iwatsuki, K.; Kokubo, S.; Wada, A.; Tokuno, K.; Onishi, M.; Iwabuchi, H.; Tanaka, K. Characterization of flavor compounds released during grinding of roasted robusta coffee beans. Food Sci. Technol. Res. 2005, 11, 298–307. [Google Scholar] [CrossRef] [Green Version]
- Kumazawa, K.; Masuda, H. Investigation of the change in the flavor of a coffee drink during heat processing. J. Agric. Food Chem. 2003, 51, 2674–2678. [Google Scholar] [CrossRef] [PubMed]
- Buffo, R.A.; Cardelli-Freire, C. Coffee flavour: An overview. Flavour Fragr. J. 2004, 19, 99–104. [Google Scholar] [CrossRef]
- Mahingsapun, R.; Tantayotai, P.; Panyachanakul, T.; Samosorn, S.; Dolsophon, K.; Jiamjariyatam, R.; Lorliam, W.; Srisuk, N.; Krajangsang, S. Enhancement of Arabica coffee quality with selected potential microbial starter culture under controlled fermentation in wet process. Food Biosci. 2022, 48, 101819. [Google Scholar] [CrossRef]
- Krajangsang, S.; Seephin, P.; Tantayotai, P.; Mahingsapun, R.; Meeampun, Y.; Panyachanakul, T.; Samosorn, S.; Dolsophon, K.; Jiamjariyatam, R.; Lorliam, W.; et al. New approach for screening of microorganisms from Arabica coffee processing for their ability to improve Arabica coffee flavor. 3 Biotech 2022, 12, 143. [Google Scholar] [CrossRef]
- Gancarz, M.; Dobrzański, B.; Malaga-Toboła, U.; Tabor, S.; Combrzyński, M.; Ćwikła, D.; Strobel, W.R.; Oniszczuk, A.; Karami, H.; Darvishi, Y.; et al. Impact of Coffee Bean Roasting on the Content of Pyridines Determined by Analysis of Volatile Organic Compounds. Molecules 2022, 27, 1559. [Google Scholar] [CrossRef]
- Campelo, P.H.; Alves Filho, E.G.; Silva, L.M.A.; de Brito, E.S.; Rodrigues, S.; Fernandes, F.A.N. Modulation of aroma and flavor using dielectric barrier discharge plasma technology in a juice rich in terpenes and sesquiterpenes. LWT 2020, 130, 109644. [Google Scholar] [CrossRef]
- Rodrigues, S.; Fernandes, F.A.N. Glow Discharge Plasma Processing for the Improvement of Pasteurized Orange Juice’s Aroma and Off-Flavor. Processes 2022, 10, 1812. [Google Scholar] [CrossRef]
- Fernandes, F.A.N.; Rodrigues, S. Cold Plasma Processing on Fruits and Fruit Juices: A Review on the Effects of Plasma on Nutritional Quality. Processes 2021, 9, 2098. [Google Scholar] [CrossRef]
- Campelo, P.H.; Alves Filho, E.G.; Silva, L.M.A.; de Brito, E.S.; Rodrigues, S.; Fernandes, F.A.N. Modulation of aroma and flavor using glow discharge plasma technology. Innov. Food Sci. Emerg. Technol. 2020, 62, 102363. [Google Scholar] [CrossRef]
- Rodríguez, Ó.; Gomes, W.F.; Rodrigues, S.; Fernandes, F.A.N. Effect of indirect cold plasma treatment on cashew apple juice (Anacardium occidentale L.). LWT—Food Sci. Technol. 2017, 84, 457–463. [Google Scholar] [CrossRef]
- Batista, J.D.F.; Dantas, A.M.; Santos Fonseca, J.V.; Madruga, M.S.; Fernandes, F.A.N.; Rodrigues, S.; da Silva Campelo Borges, G. Effects of cold plasma on avocado pulp (Persea americana Mill.): Chemical characteristics and bioactive compounds. J. Food Process. Preserv. 2021, 45, 15179. [Google Scholar] [CrossRef]
- Córdoba, N.; Moreno, F.L.; Osorio, C.; Velásquez, S.; Ruiz, Y. Chemical and sensory evaluation of cold brew coffees using different roasting profiles and brewing methods. Food Res. Int. 2021, 141, 110141. [Google Scholar] [CrossRef] [PubMed]
- Grosch, W. Evaluation of the Key Odorants of Foods by Dilution Experiments, Aroma Models and Omission. Chem. Senses 2001, 26, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Usami, A.; Ono, T.; Marumoto, S.; Miyazawa, M. Comparison of volatile compounds with characteristic odor in flowers and leaves of Nojigiku (Chrysanthemum japonense). J. Oleo Sci. 2013, 62, 631–636. [Google Scholar] [CrossRef] [Green Version]
- Padrayuttawat, A.; Yoshizawa, T.; Tamura, H.; Tokunaga, T. Optical isomers and odor thresholds of volatile constituents in Citrus sudachi. Food Sci. Technol. Int. 1997, 3, 402–408. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Z.; Chen, J.; Niu, Y.; Chen, F. Characterization of the key odorants of fennel essential oils of different regions using GC–MS and GC–O combined with partial least squares regression. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1063, 226–234. [Google Scholar] [CrossRef]
- Fazzalari, F.A. Compilation of Odor and Taste Threshold Values Data; American Society for Testing and Materials: Philadelphia, PA, USA, 1978. [Google Scholar]
- Buttery, R.G.; Ling, L.C.; Light, D.M. Tomato Leaf Volatile Aroma Components. J. Agric. Food Chem. 1987, 35, 1039–1042. [Google Scholar] [CrossRef]
- Qian, M.C.; Wang, Y. Seasonal variation of volatile composition and odor activity value of “Marion” (Rubus spp. hyb) and “Thornless Evergreen” (R. laciniatus L.) blackberries. J. Food Sci. 2005, 70, 13–20. [Google Scholar] [CrossRef]
- Gao, X.; Feng, T.; Sheng, M.; Wang, B.; Wang, Z.; Shan, P.; Zhang, Y.; Ma, H. Characterization of the aroma-active compounds in black soybean sauce, a distinctive soy sauce. Food Chem. 2021, 364, 130334. [Google Scholar] [CrossRef]
- Vázquez-Araújo, L.; Enguix, L.; Verdú, A.; García-García, E.; Carbonell-Barrachina, A.A. Investigation of aromatic compounds in toasted almonds used for the manufacture of turrón. Eur. Food Res. Technol. 2008, 227, 243–254. [Google Scholar] [CrossRef]
- Zhai, X.; Yang, M.; Zhang, J.; Zhang, L.; Tian, Y.; Li, C.; Bao, L.; Ma, C.; Abd El-Aty, A.M. Feasibility of Ultrasound-Assisted Extraction for Accelerated Cold Brew Coffee Processing: Characterization and Comparison With Conventional Brewing Methods. Front. Nutr. 2022, 9, 849811. [Google Scholar] [CrossRef]
- Hu, C.J.; Li, D.; Ma, Y.X.; Zhang, W.; Lin, C.; Zheng, X.Q.; Liang, Y.R.; Lu, J.L. Formation mechanism of the oolong tea characteristic aroma during bruising and withering treatment. Food Chem. 2018, 269, 202–211. [Google Scholar] [CrossRef]
- Amirabadi, S.; Milani, J.M.; Sohbatzadeh, F. Application of dielectric barrier discharge plasma to hydrophobically modification of gum arabic with enhanced surface properties. Food Hydrocoll. 2020, 104, 105724. [Google Scholar] [CrossRef]
- Deng, J.; He, L.; Zhao, B.; Chen, Q. Effects of air relative humidity on spectral characteristics of dielectric barrier discharge plasma assisted combustion reactor. Vacuum 2020, 175, 109189. [Google Scholar] [CrossRef]
- Ramanujam, M.; Mix, R.; Wagner, M.; Friedrich, J.F. Effect of Br gassing after Ar plasma treatment of polyolefins. J. Adhes. Sci. Technol. 2013, 27, 1828–1839. [Google Scholar] [CrossRef]
- Castro, D.R.G.; Mar, J.M.; Silva, L.S.; Silva, K.A.; Sanches, E.A.; Bezerra, J.A.; Rodrigues, S.; Fernandes, F.A.N.; Campelo, P.H. Dielectric barrier atmospheric cold plasma applied on camu-camu juice processing: Effect of the excitation frequency. Food Res. Int. 2020, 131, 109044. [Google Scholar] [CrossRef] [PubMed]

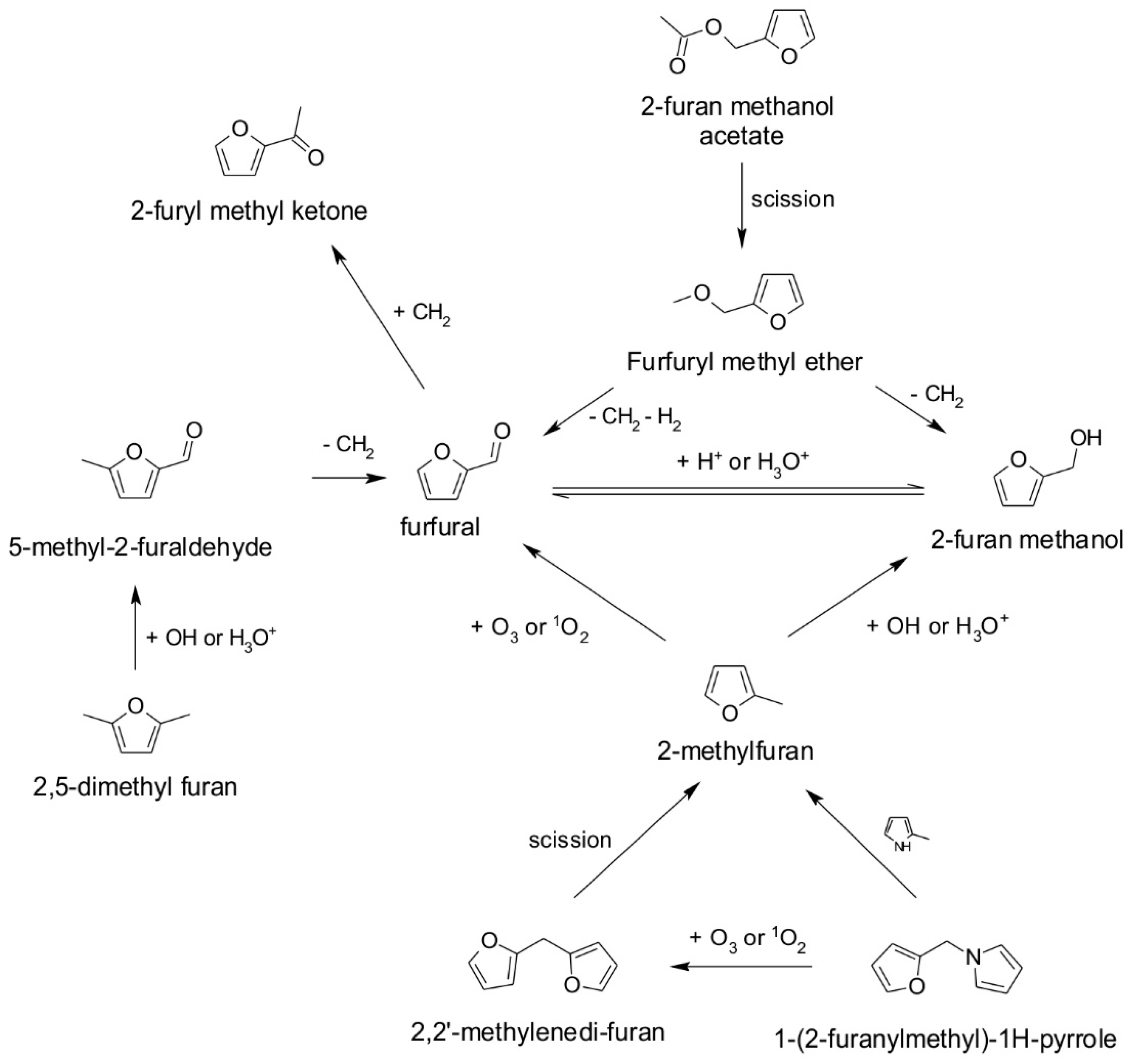
| Retention Time (min) | Compound | Kovats Index | m/z | Odor Threshold in Water (mg/L) | Odor Description |
|---|---|---|---|---|---|
| Aldehydes and Alcohols | |||||
| 2.12 | 2-methylbutanal and 3-methylbutanal | 763 | 44, 58, 71 | 0.002 | Cocoa |
| 3.02 | 1,5-hexadien-3-ol | 781 | 57, 29, 39 | 0.5 | -- |
| 4.60 | hexanal | 813 | 44, 56, 72 | 0.0045 | Green |
| Furans | |||||
| 1.64 | 2-methylfuran | 753 | 82, 53, 39 | -- | -- |
| 2.44 | 2,5-dimethyl furan | 769 | 96, 43, 53 | 0.1 | Meaty |
| 5.40 | furfuryl methyl ether | 829 | 81, 112, 53 | -- | -- |
| 5.79 | furfural | 837 | 96, 39, 67 | 0.77 | Bready |
| 6.67 | 2-furan methanol | 855 | 98, 41, 81 | 2.0 | Bready |
| 9.00 | 2-furyl methyl ketone | 902 | 95, 110, 39 | 10.0 | Balsamic |
| 11.62 | 5-methyl-2-furaldehyde | 955 | 110, 53, 81 | 1.11 | Caramellic |
| 12.78 | 2-pentyl furan | 979 | 81, 138, 53 | 0.006 | Fruity |
| 13.32 | 2-furan methanol acetate | 990 | 81, 98, 140 | 0.1 | Fruity |
| 18.03 | 2,2′-methylenedi furan | 1085 | 91, 148, 39 | -- | -- |
| 18.54 | 2-furan methanol propionate | 1095 | 81, 98, 154 | -- | -- |
| Pyridines, Pyrazines, and Tetrazoles | |||||
| 3.27 | pyridine | 786 | 79, 52, 39 | 2.0 | Fishy |
| 4.92 | 5-hydrazino-1H-1,2,3,4-tetrazole | 820 | 72, 100, 207 | -- | -- |
| 5.52 | 4-methyl pyrimidine | 832 | 94, 40, 53 | -- | -- |
| 9.17 | 2,6-dimethylpyrazine | 906 | 108, 42, 67 | 10.0 | Chocolate |
| 9.27 | 2-ethylpyrazine and 2,3-dimethylpyrazine | 908 | 107, 80, 53 | 2.5 | Nutty |
| 11.94 | 3-ethyl-2,5-dimethylpyrazine | 962 | 135, 42, 108 | 0.001 | Nutty |
| 17.87 | 2-ethyl-3,5-dimethyl pyrazine | 1082 | 135, 42, 108 | 0.001 | Nutty |
| Pyrroles | |||||
| 3.14 | 2-methyl-1H-pyrrole | 784 | 80, 53, 28 | 0.017 | -- |
| 13.90 | 1-methyl-1H-pyrrole-2-carbaldehyde | 1001 | 109, 53, 80 | 19.6 | -- |
| 23.20 | 1-(2-furanylmethyl)-1H-pyrrole | 1190 | 81, 147, 53 | 0.1 | Vegetable |
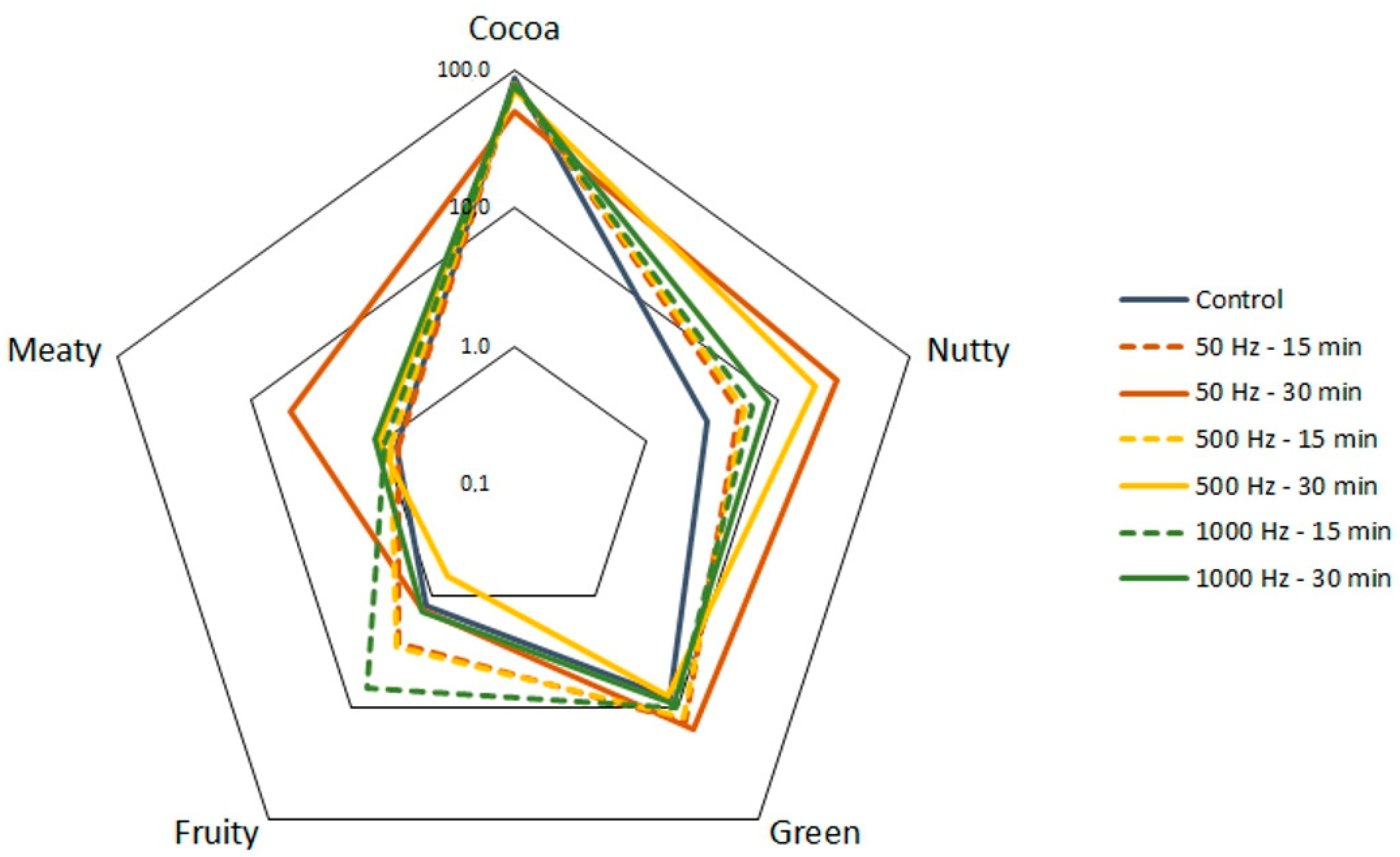
| Compound | Control | 50 Hz 15 min | 50 Hz 30 min | 500 Hz 15 min | 500 Hz 30 min | 1000 Hz 15 min | 100 Hz 30 min |
|---|---|---|---|---|---|---|---|
| 2-methylfuran | 13.4 ± 0.6 b | 8.7 ± 0.4 d | 11.3 ± 0.5 c | 8.0 ± 0.4 d | 13.5 ± 0.9 b | 17.4 ± 1.2 a | 11.5 ± 1.5 bc |
| 2-methylbutanal and 3-methylbutanal | 21.2 ± 1.5 a | 10.2 ± 1.0 c | 2.7 ± 0.3 e | 11.7 ± 0.8 c | 8.3 ± 0.5 d | 11.1 ± 0.9 c | 15.3 ± 1.6 b |
| 2,5-dimethyl furan | 9.4 ± 0.5 b | 4.9 ± 0.5 d | 13.3 ± 0.8 a | 6.5 ± 0.4 c | 6.2 ± 0.4 c | 8.0 ± 0.8 bc | 7.6 ± 0.8 bc |
| 1,5-hexadien-3-ol | 0.6 ± 0.1 b | 1.5 ± 0.3 a | 0.2 ± 0.1 c | 0.7 ± 0.1 b | 0.0 ± 0.0 d | 0.0 ± 0.0 d | 0.5 ± 0.1 b |
| 2-methyl-1H-pyrrole | 2.3 ± 0.4 a | 1.8 ± 0.4 ab | 0.7 ± 0.1 c | 2.3 ± 0.3 a | 1.2 ± 0.2 b | 1.0 ± 0.2 bc | 2.3 ± 0.3 a |
| pyridine | 12.9 ± 0.8 a | 11.4 ± 1.0 ab | 5.2 ± 0.4 d | 10.7 ± 0.8 b | 12.2 ± 0.8 ab | 8.8 ± 0.7 c | 10.6 ± 0.9 b |
| not identified | 0.0 ± 0.0 e | 6.3 ± 0.5 b | 7.0 ± 0.3 b | 4.8 ± 0.3 c | 8.5 ± 0.4 a | 9.4 ± 0.6 a | 3.9 ± 0.3 d |
| hexanal | 4.4 ± 0.2 a | 3.8 ± 0.3 bc | 1.9 ± 0.3 e | 4.1 ± 0.3 ab | 2.1 ± 0.3 e | 2.9 ± 0.3 d | 3.2 ± 0.2 cd |
| 5-hydrazino-tetrazole | 1.0 ± 0.2 d | 3.4 ± 0.2 a | 1.5 ± 0.3 cd | 2.3 ± 0.2 b | 2.1 ± 0.4 bc | 2.0 ± 0.2 bc | 1.6 ± 0.2 c |
| furfuryl methyl ether | 0.8 ± 0.1 b | 0.6 ± 0.2 b | 0.0 ± 0.0 c | 0.8 ± 0.1 b | 2.6 ± 0.4 a | 0.5 ± 0.2 b | 1.0 ± 0.3 b |
| 4-methyl pyrimidine | 2.4 ± 0.5 e | 4.5 ± 0.3 b | 3.4 ± 0.3 d | 4.6 ± 0.4 ab | 5.4 ± 0.3 a | 4.6 ± 0.6 ab | 4.2 ± 0.4 b |
| furfural | 4.5 ± 0.6 c | 7.7 ± 0.6 a | 4.2 ± 0.3 c | 7.4 ± 0.4 a | 6.7 ± 0.4 ab | 5.1 ± 0.4 bc | 7.1 ± 0.2 a |
| 2-furan methanol | 4.1 ± 0.6 e | 8.3 ± 0.4 c | 33.0 ± 2.0 a | 8.0 ± 0.5 c | 13.2 ± 1.1 b | 12.8 ± 1.0 b | 6.7 ± 0.5 d |
| 2-furyl methyl ketone | 1.1 ± 0.1 b | 1.3 ± 0.3 ab | 1.9 ± 0.3 a | 1.4 ± 0.3 ab | 1.2 ± 0.3 b | 1.0 ± 0.3 b | 1.2 ± 0.4 b |
| 2,6-dimethylpyrazine | 0.5 ± 0.2 a | 0.9 ± 0.2 a | 0.5 ± 0.2 a | 0.8 ± 0.3 a | 0.9 ± 0.2 a | 0.8 ± 0.3 a | 0.7 ± 0.3 a |
| 2-ethylpyrazine and 2,3-dimethylpyrazine | 2.3 ± 0.5 b | 2.1 ± 0.3 b | 2.3 ± 0.4 b | 2.3 ± 0.4 b | 3.4 ± 0.3 a | 2.8 ± 0.4 ab | 2.1 ± 0.4 b |
| 5-methyl-2-furaldehyde | 2.7 ± 0.4 bc | 4.0 ± 0.3 a | 2.1 ± 0.3 c | 4.1 ± 0.5 a | 3.4 ± 0.2 ab | 2.9 ± 0.5 b | 3.4 ± 0.5 ab |
| 3-ethyl-2,5-dimethylpyrazine | 0.3 ± 0.1 c | 0.3 ± 0.1 c | 0.7 ± 0.1 ab | 0.3 ± 0.1 bc | 1.0 ± 0.2 a | 0.5 ± 0.1 b | 1.0 ± 0.2 a |
| 2-pentyl furan | 0.2 ± 0.1 b | 0.4 ± 0.1 ab | 0.0 ± 0.0 c | 0.6 ± 0.1 a | 0.0 ± 0.0 c | 0.4 ± 0.2 ab | 0.0 ± 0.0 c |
| 2-furan methanol acetate | 11.2 ± 0.5 a | 10.4 ± 0.3 ab | 3.6 ± 0.3 cd | 11.5 ± 0.5 a | 3.9 ± 0.3 c | 2.8 ± 0.5 d | 9.5 ± 0.4 b |
| 1-methylpyrrole-2-carbaldehyde | 0.9 ± 0.2 cd | 1.8 ± 0.3 a | 0.6 ± 0.1 d | 1.6 ± 0.1 ab | 0.9 ± 0.2 cd | 0.7 ± 0.2 d | 1.2 ± 0.1 bc |
| 2-ethyl-3,5-dimethyl pyrazine | 0.4 ± 0.1 ab | 0.5 ± 0.1 a | 0.3 ± 0.1 ab | 0.5 ± 0.1 a | 0.4 ± 0.1 ab | 0.4 ± 0.1 ab | 0.2 ± 0.1 b |
| 2,2′-methylenedi furan | 0.4 ± 0.1 a | 0.4 ± 0.1 a | 0.3 ± 0.1 a | 0.3 ± 0.1 a | 0.4 ± 0.1 a | 0.4 ± 0.1 a | 0.2 ± 0.1 a |
| 2-furan methanol propionate | 0.3 ± 0.1 ab | 0.4 ± 0.1 a | 0.1 ± 0.1 b | 0.3 ± 0.1 ab | 0.0 ± 0.0 b | 0.4 ± 0.1 a | 0.2 ± 0.1 ab |
| 2-nonen-1-ol | 0.0 ± 0.0 c | 0.5 ± 0.1 ab | 0.7 ± 0.2 a | 0.5 ± 0.1 ab | 0.2 ± 0.1 b | 0.3 ± 0.1 b | 0.0 ± 0.0 c |
| 1-(2-furanylmethyl)pyrrole | 0.4 ± 0.1 a | 0.3 ± 0.1 ab | 0.1 ± 0.0 b | 0.3 ± 0.1 ab | 0.0 ± 0.0 c | 0.1 ± 0.0 b | 0.2 ± 0.0 ab |
| 2-decen-1-ol | 0.0 ± 0.0 c | 0.4 ± 0.1 a | 0.3 ± 0.1 ab | 0.2 ± 0.1 ab | 0.0 ± 0.0 c | 0.1 ± 0.1 b | 0.0 ± 0.0 c |
| not identified | 0.1 ± 0.0 b | 0.1 ± 0.0 b | 0.0 ± 0.0 b | 0.1 ± 0.0 b | 0.2 ± 0.1 ab | 0.3 ± 0.1 a | 0.1 ± 0.1 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, S.; Fernandes, F.A.N. Changing Ready-to-Drink Coffee Aroma Using Dielectric Barrier Discharge Plasma. Processes 2022, 10, 2056. https://doi.org/10.3390/pr10102056
Rodrigues S, Fernandes FAN. Changing Ready-to-Drink Coffee Aroma Using Dielectric Barrier Discharge Plasma. Processes. 2022; 10(10):2056. https://doi.org/10.3390/pr10102056
Chicago/Turabian StyleRodrigues, Sueli, and Fabiano A. N. Fernandes. 2022. "Changing Ready-to-Drink Coffee Aroma Using Dielectric Barrier Discharge Plasma" Processes 10, no. 10: 2056. https://doi.org/10.3390/pr10102056





