Abstract
Although water contamination with drug residues is a threat to public health, there are currently barely any effective methods of purifying water from pharmaceutical substances. In this study, continuous-flow sonoplasma treatment was used for the complete degradation of tetracycline and ciprofloxacin in polluted municipal water. The addition of CeO2 nanoparticles as catalysts significantly increased the degradation rate of the antibiotics, and a degradation degree of 70% was achieved. The presence of reactive oxygen species in the CeO2-nanoparticle-containing sonoplasma-treated system was experimentally proven for the first time using the chemiluminescence technique.
1. Introduction
Increasing economic growth leads to an increase in drug residue contamination in groundwater. Antibiotics, hormones, anabolics and tranquilizers are common synthetic contaminants detected in the wastewater of pharmaceutical factories, urban wastewater treatment plants, hospitals and landfills, as well as livestock and aquaculture industries [1,2,3,4]. Drug residues that accumulate in ground and surface waters are consumed by various “intact” organisms (fish, animals and humans), which negatively affects the environment and public health [1,2,4,5].
The growing volume of human-produced wastewater and more stringent environmental and water use regulations require the development of new approaches that can improve the existing treatment methods [6,7,8,9]. The current research on water treatment is focused on a group of methods named “advanced oxidation processes” (AOP). These methods include the generation of OH• radicals by various techniques, such as photocatalysis, use of ultrasound, microwaves, ozonation and others, as well as their combinations [6,9].
Wastewater ozonation has been widely used over the last century for disinfection, the removal of organic contaminants and for the treatment of taste and odor issues [10,11]. The redox potential of ozone is higher than that of oxygen and chlorine, and it has a higher oxidative effect [12,13]. Ozonation treatment mechanisms include both direct oxidation by ozone molecules and the reactions of ROS (atomic oxygen, hydroxyl radicals and hydroperoxide radicals) that are formed in ozone decomposition processes [11,14]. Different techniques can be combined with ozonation to promote radical formation.
Ozonation with simultaneous UV photolysis has been applied for the removal of several organic species, including hormones, alkenes, phenols and aromatic compounds. In all cases, the complete mineralization of contaminants was achieved [11,14,15]. Nevertheless, water must be sufficiently transparent for the effective use of UV/ozonation technology, and the process is very difficult to use with heavily contaminated wastewaters.
However, the ozonation method is not free from disadvantages [6,9,11,14]. Ozone is a toxic gas, and the strict observance of safety rules is required in treatment plants. Moreover, the incorrect selection of ozone concentration or effluent treatment method often results in toxic byproducts. Water that is saturated with an air–ozone mixture has high oxidation activity and easily corrodes the materials in treatment equipment and reactors. Water ozonation results in a high content of assimilable organic carbon, which is easily absorbed by microorganisms, thus leading to the bacterial contamination of treated water.
Ultrasound treatment is an AOP, which involves the production of hydroxyl radicals via the pyrolysis of water in cavitation bubbles. Although the decomposition of organic contaminants by ultrasound alone has demonstrated poor cost effectiveness, the combination of ultrasound and other ROS formation methods is quite a promising approach [16].
Ultrasonic treatment can be combined with ozonation. The ultrasonic treatment of a liquid medium causes cavitation and micro-turbulence, which promote the degradation of ozone and the formation of hydroxyl radicals [17,18,19,20,21]. Under ultrasound, ozone consumption can be reduced by 60–70%. Such an approach has shown good efficiency in the purification of water from aromatic compounds, textile dyes and in wastewater treatment in distilleries. Combinations of ultrasound with photolysis and photocatalysis have also demonstrated good results in experiments with phenols, organic dyes and antibiotics [16,22,23,24].
Plasma-based water purification methods provide the effective oxidation of organic contaminants by radicals (similarly to other AOP) and by other ROS formed at discharge in air (i.e., ozone, singlet oxygen, the nitrate radical and super oxide) [25]. Currently, many universities are conducting a cycle of research works on the use of plasma processes for the purification of drug-contaminated waters [25,26,27,28,29]. When plasma is generated in air or in water vapor, the efficiency of plasma treatment depends on the kinetics of radical transfer from the gas phase to the water solution [25].
A unique combination of ultrasonic and plasma treatment (sonoplasma) has recently been applied for flow-mode water treatment [30]. Simultaneous hydrodynamic cavitation and plasma discharge treatment causes shockwaves via the action of collapsing bubbles, ultraviolet radiation, hydroxyl radicals and plasma-induced ROS. The new method has proven to be highly effective in the removal of the organic dye E132 and E. coli disinfection [30]. The high rate of flow-mode sonoplasma treatment makes this method potentially scalable for industrial applications. However, this very new AOP process has been barely reported in the literature [30], and comprehensive research is needed to develop approaches for the practical use of sonoplasma.
As mentioned earlier, the introduction of catalysts to wastewater can significantly amplify the yields of hydroxyl radicals in several AOP. Homogeneous catalysts (metal ions) and heterogeneous catalysts (insoluble metal oxides, etc.) are used to promote the formation of reactive oxygen species during ozonation [15,31,32]. Semiconducting nanoparticles can also act as sonocatalysts, generating electron–hole pairs under ultrasound, followed by the formation of hydroxyl radicals in water solution [33,34,35]. Nevertheless, the catalyst itself remains in the water after treatment and becomes a contaminator, which can affect living organisms [6,11,24]. From this point of view, water-insoluble catalysts are preferable to metal ions, and additional stages should be added to water treatment processes to remove catalysts after AOP.
The objective of this work was to analyze the prospects of using the sonoplasma process for cleaning antibiotics-contaminated wastewater. The efficiency of sonoplasma treatment and the effect of a water-insoluble CeO2 nanocatalyst added to the discharge zone were investigated using common wastewater contaminants—the antibiotics tetracycline and ciprofloxacin [36]. Antibiotics degradation efficiency and ROS production were investigated by means of UV-vis spectroscopy and chemiluminescence measurements. The water treatment plant used in this work is potentially up-scalable to the industrial level and allows antibiotics to be effectively removed in a flow-mode regime. The perspectives of upgrading the plant with a membrane filtration module are also discussed.
2. Materials and Methods
2.1. Reagents
The following compounds were used to prepare water solutions: tetracycline (Biosintez, Russia, Penza; medical grade), ciprofloxacin (Ozon Pharm, Russia, Zhigulevsk; medical grade). Tetracycline and ciprofloxacin pills were dissolved, and the solutions were filtered to remove the insoluble auxiliary components, such as cellulose, and then diluted to obtain the desired concentrations of 40 mg/L and 50 mg/L, respectively. The solutions were prepared using municipal water. A detailed analysis of water composition was carried out. The analytical results are included in the supplementary materials, and the effects of municipal water admixtures are described in the Results and Discussion section.
2.2. CeO2 Preparation
CeO2 was synthetized using a previously reported method [37]. To obtain the cerium dioxide colloidal solution, ceric ammonium nitrate (Sigma-Aldrich) was dissolved in distilled water to give a solution with a concentration of 0.1 M. An amount of 40 mL of this solution was then placed in a 100 mL Teflon autoclave and treated at 230 °C for 24 h. Under hydrothermal treatment, ceric ammonium nitrate hydrolyzes, and CeO2 nanoparticles precipitate according to the following equation (1):
(NH4)2Ce(NO3)6 + 2H2O ↔ 2NH4NO3 + 4HNO3 + ↓ CeO2
After treatment, the autoclaves were cooled in air. In each case, the solid phase was separated by centrifugation and re-dispersed in distilled water, forming electrostatically stabilized CeO2 colloid solutions.
2.3. Methods of Analysis
The antibiotic concentrations were measured in the wavelength range 200–500 nm with 0.1 nm resolution using a SF-2000 (OKB Spectrum, Russia, Saint-Petersburg) spectrometer. The absorption peaks at 320 and 375 nm were used to measure tetracycline and ciprofloxacin concentrations in municipal water, respectively [22,38]. The intensities of these peaks depend linearly on the concentration of antibiotics in the range of 5–100 mg/l.
The phase composition of the cerium precipitates was determined using X-ray phase analysis on a Bruker D8 Advance diffractometer (Cu K-α radiation, Ni filter and LYNXEYE detector) (Blue Scientific, United Kingdom, Cambridge). The diffraction data were collected in the 2θ angle range from 15 to 100° in 0.02° increments with accumulation time of 0.3 s/step.
Dynamic light scattering spectroscopy data were obtained using a Photocor Complex multi-angle particle size analyzer (Photocor Ltd., Russia, Moscow) at a temperature of 20 °C and an accumulation time of 60 s.
Chemiluminescence analysis was performed at room temperature on a 12-channel Lum-1200 chemiluminometer (llc “DISoft”, Russia, Moscow). A highly sensitive luminol analog, L-012 (8-amino-5-chloro-7-phenyl-pyrido[3,4-d]pyridazine-1,4(2H,3H)dione), was used as a chemiluminescent probe. L-012 is a chemiluminescent probe, which is sensitive to the formation of free radicals, such as hydroxyl radicals, hydrogen peroxide, reactive chlorine species, etc. [39,40,41]. Briefly, an aqueous solution of L-012 (5 µM) was added to a plastic cuvette containing phosphate-buffered solution (100 mM, pH 7.4), and luminescence was recorded for 30–60 s. An aliquot (100 µL) of the analyzed sample was then added to the system without interrupting signal recording. Luminescence was registered for at least 10 min.
2.4. Sonoplasma Experimental Setup
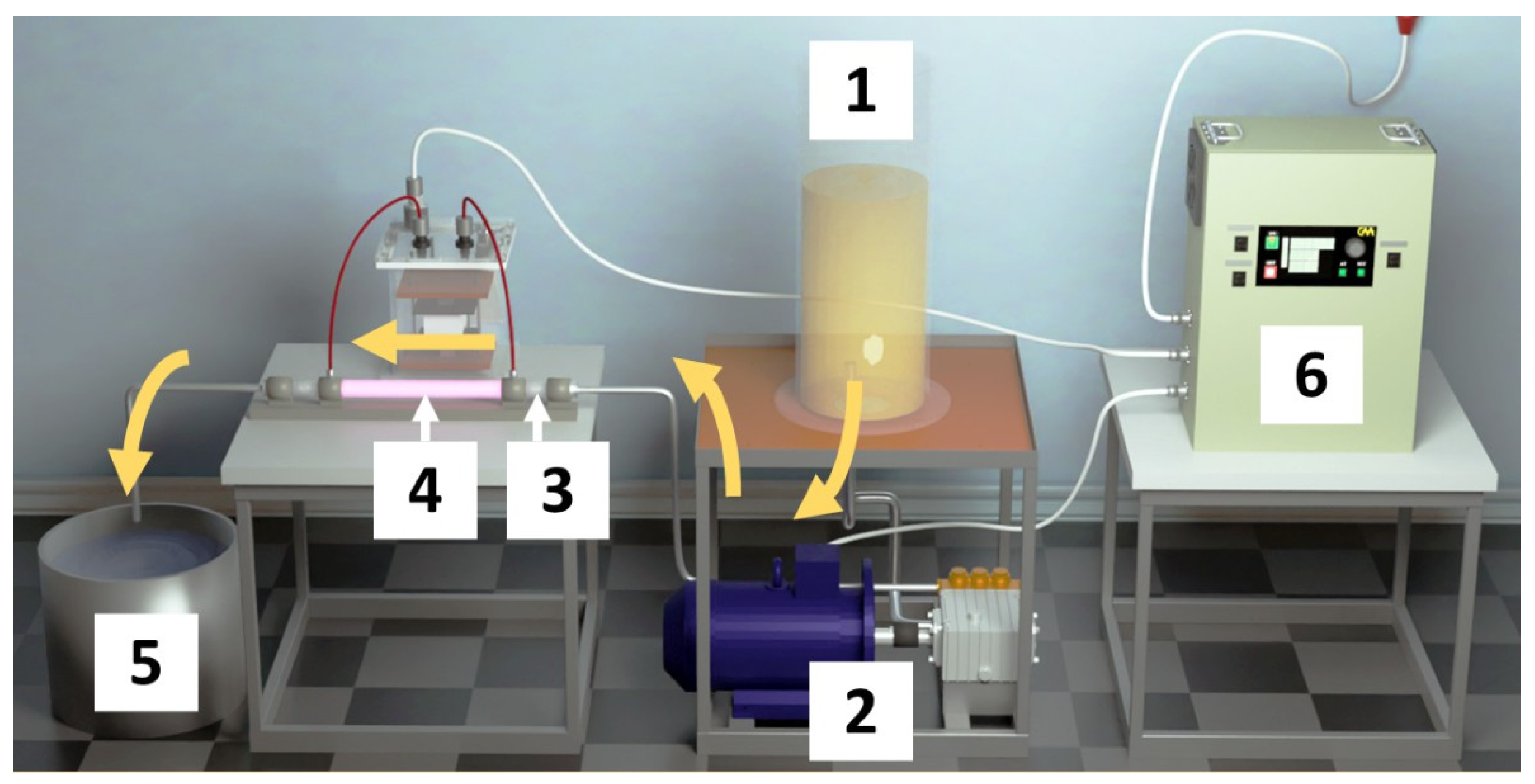
Figure 1 shows a laboratory setup for sonoplasma wastewater treatment with a capacity of 1 m3/h. This setup allows water to continuously flow through the cavitation region where sonoplasma processing takes place (mark (4) in Figure 1). The hydrodynamic emitter (mark (3) in Figure 1) effectively introduces acoustic energy into the liquid flow. An alternating voltage with a frequency of 43 kHz and an amplitude of up to 8.6 kV was used to excite discharge. In this mode, the plasma discharge was observed to burn stably, and the current amplitude on the secondary winding of the transformer was 0.6 A. The hydrodynamic radiator generates vibrations in a wide frequency range, from 0.3 to 60 kHz, at a maximum intensity of 1.5–3.5 w/cm2.
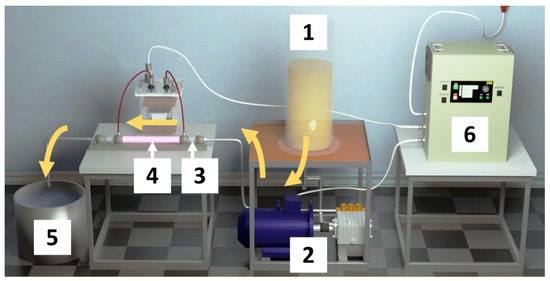
Figure 1.
Laboratory flow reactor (1 m3/h) for sonoplasma wastewater treatment: (1) contaminated water tank (CeO2 catalyst is added to the water); (2) high-pressure pump; (3) hydrodynamic emitter; (4) discharge camera; (5) treated water tank; (6) power supply.
The laboratory setup is potentially scalable and could combine hydrodynamic cavitation and plasma in industrial plants for water treatment in the flow-mode regime.
2.5. Sonoplasma Treatment Procedure
Solutions of antibiotics in municipal water with concentrations of 40 mg/l for tetracycline and 50 mg/l for ciprofloxacin were prepared as described above. Similar solutions with the addition of a CeO2 suspension, with a particle size of 100 nm and concentration of 5 mg/l, were prepared. The obtained solutions were passed through the discharge chamber of the sonoplasma experimental setup 1, 2 or 3 times.
The chemical analysis of municipal water was performed by the BWT BARRIER RUS laboratory, Moscow, Russia, which was approved in accordance with the standards of the International Organization for Standardization.
3. Results
3.1. Degradation of Tetracycline and Ciprofloxacin in a Sonoplasma Reactor
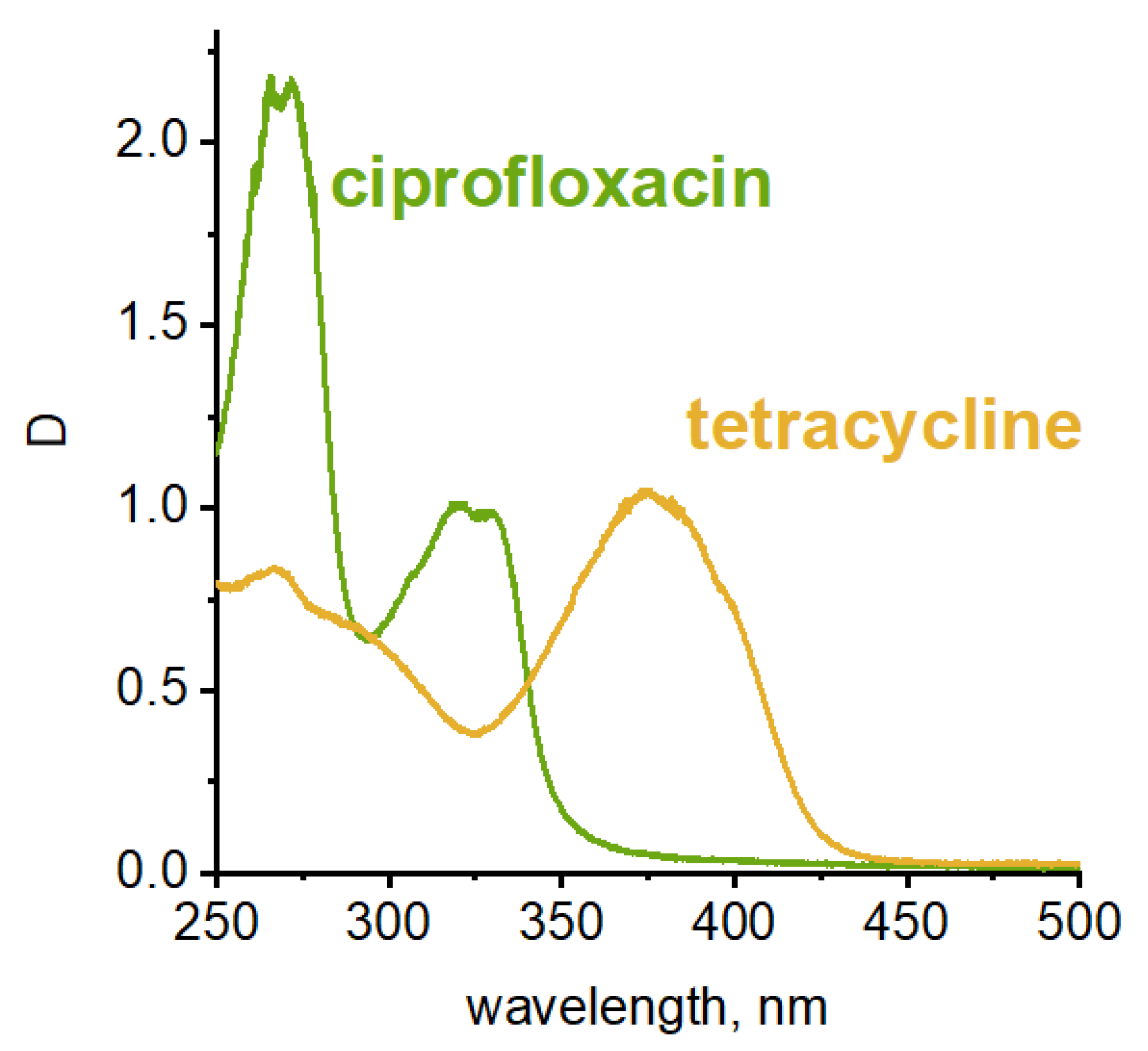
Antibiotic solutions were treated with sonoplasma in a flow reactor. The treatment cycle (5 msec) was repeated several times, and water samples were taken after each cycle. The concentrations of tetracycline and ciprofloxacin in water after treatment were measured using absorption bands in UV-visible spectra (320 and 375 nm, respectively; see Figure 2) [22,38].
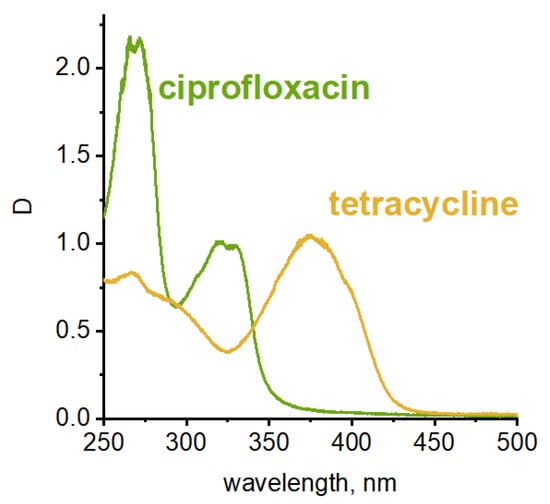
Figure 2.
UV-vis spectra of tetracycline and ciprofloxacin.
It is necessary to note that antibiotics of medical grade were used for the experiment. In the case of tetracycline, the tablet shell contains the dye E122 azorubine. However, its absorption maximum is at 510 nm, meaning that it does not interfere with the tetracycline absorption band. The concentration of azorubine is also very low compared to the antibiotic, and its absorption bands are barely detectable [42,43].
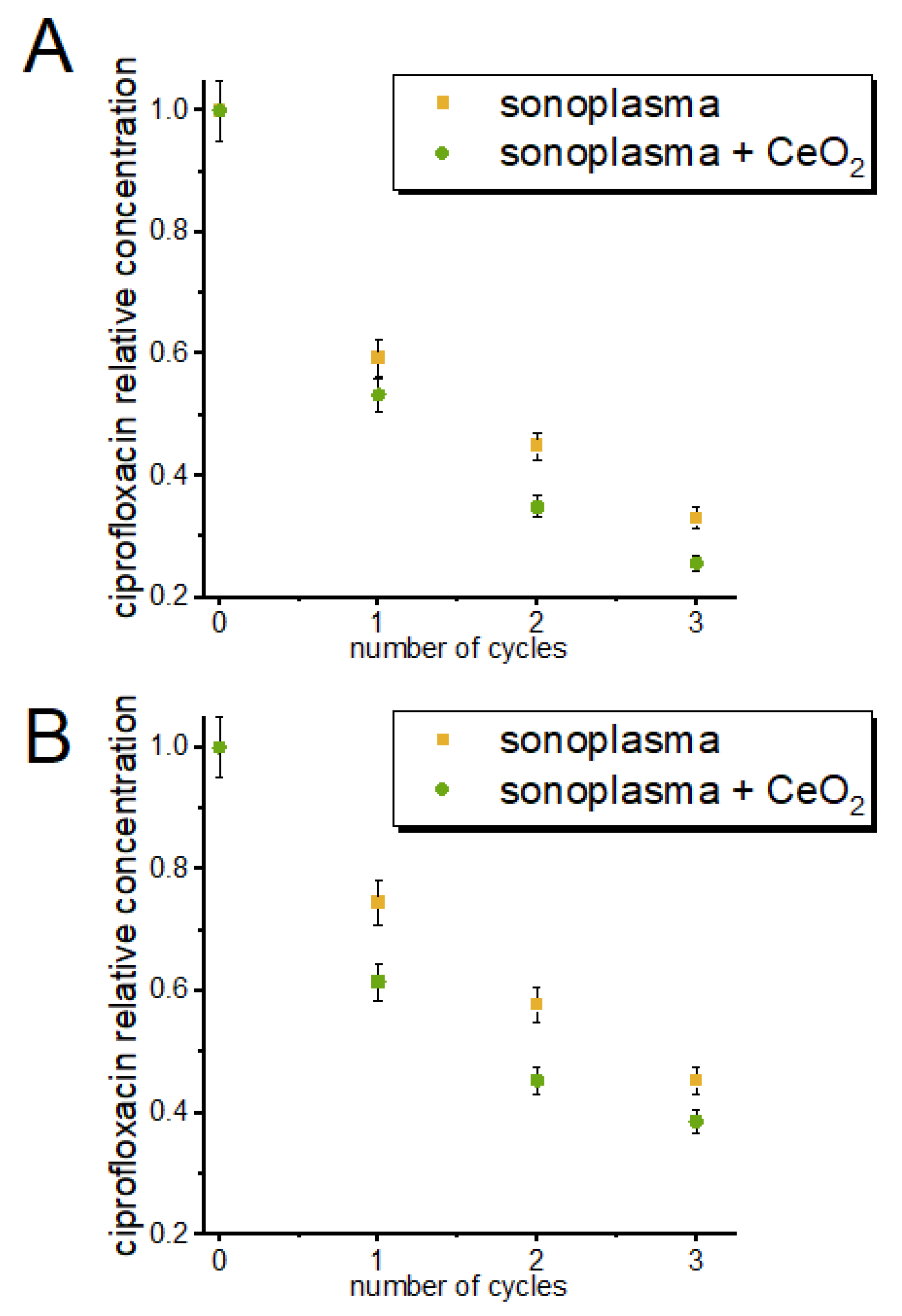
The degree of antibiotic degradation depended on the number of treatment cycles (passes through the discharge zone), as shown in Figure 3. The concentration of tetracycline was reduced by 41% after the first cycle, whereas the concentration of ciprofloxacin was reduced by 25%. After three treatment cycles, decomposition yields of 67% and 55% were achieved for tetracycline and ciprofloxacin, respectively.
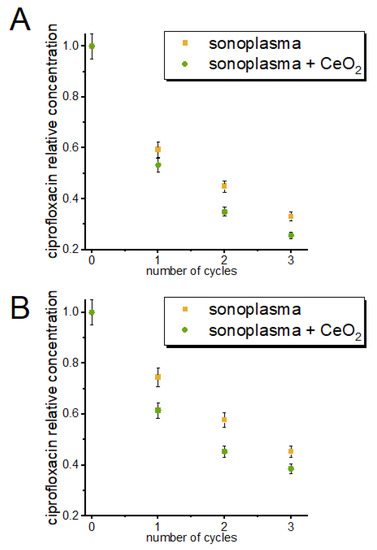
Figure 3.
Change in the concentrations of tetracycline (A) and ciprofloxacin (B) in solution with the number of sonoplasma treatment cycles.
3.2. Mechanism of Action
The mechanisms of ROS formation in aqueous media under ultrasound cavitation have been extensively investigated over several decades [44,45,46]. The collapse of cavitation bubbles at high rates causes local energy excesses and plasma formation (ionization of water molecules under high temperatures and pressures). The sonolysis of aqueous solutions results in the dissociation of water molecules into and radicals (2). Further reactions of radicals lead to the formation of hydrogen peroxide () and hydroperoxide radicals () (3 and 4). Sonoluminescence at low acoustic intensities is generally related to the emission of excited radicals, which are formed in recombination reactions (5–7).
The sonoplasma technique used in this work combines ultrasonic cavitation and low-temperature plasma. Plasma was induced using alternating voltage at a frequency of 43 kHz directly in water media using cavitating bubbles as gas discharge microchambers. This method allows the yield of reactive intermediates in cavitation bubbles to be amplified and thereafter promotes the formation of ROS in the water volume at relatively low acoustic intensities (1.5–3.5 W/cm2) and frequencies (under 60 kHz). The spectrum of the sonoplasma produced in the equipment used was measured in a previous work [30]. Emission at 280–330 nm with a peak at 310 nm was attributed to the excited radicals [44,45], and no continuum of blackbody irradiation was detected, likely because of the very low acoustic intensities.
In ultrasound, degradation can occur via pyrolysis, as well as via the interactions between the pollutants and the hydroxyl radical oxidation. The contribution of the pyrolysis process to the overall degradation depends on the proximity of the pollutants to the cavitation bubbles, with this proximity being determined by the hydrophobic character of compounds [47]. Neither of the antibiotics studied showed any significant hydrophobicity, meaning that this mechanism can be discarded.
The decreases in tetracycline and ciprofloxacin concentrations are principally caused by oxidation with the ROS formed under the sonoplasma treatment. The mechanisms of the photocatalytic and sonocatalytic decomposition of tetracycline and ciprofloxacin have been studied in detail [24,33,34,48]. If we consider that the composition of the ROS produced by different AOP in aqueous solutions (, and ) depends more on the pH than on the method of water molecule decomposition (photocatalysis, sonolysis, sonocatalysis and plasmolysis), we can assume that the reaction pathways of tetracycline and ciprofloxacin destruction are similar to those revealed in other works at the same pH [9,24,33,34,48].
Ciprofloxacin degradation in an ultrasound process can take place via the cleavage of C–N and C–C bonds [49]. The produced aromatic compounds transform into aliphatic by-products via the cleavage of the aromatic ring by further OH- radical attacks [34]. These by-products could then potentially be converted into H2O and CO2 via further mineralization.
A similar mechanism has been proposed for the ultrasound treatment of tetracycline [50,51]. The generated radicals attack the parent molecule via either ring opening or bond cleavage reactions to form intermediate species that are eventually broken down into simple inorganic molecules. Several parallel degradation routes were proposed after a study of the LC-MS data. The tetracycline molecule may undergo deprotonation, dehydroxylation, deethylation, addition and carboxylation reactions, and benzene ring cleavage. However, all of the intermediate products finally mineralize into smaller inorganic molecules, such as NH4+, NO3-, H2O, CO2, etc.
The crucial difference in water treatment with sonoplasma is the higher rate of antibiotic degradation compared to other AOP. This is evidently accomplished by the high rates of ROS production and the highly homogeneous distribution of ROS in the water volume. The typical halftimes of tetracycline and ciprofloxacin removal from water solutions via adsorption [52], ozonolysis [38], photolysis [22,23], photocatalysis [24] and sonocatalysis [33,34,35] range from tens of minutes to several hours, whereas sonoplasma treatment provides a degradation of 40–75% antibiotics after one or two cycles (5 ms/cycle) of exposure in the flow reactor. The degradation pathways of ciprofloxacin and tetracycline under sonic and sonocatalytic treatment have been studied elsewhere with the use of MS analysis of the treated solution [33,34,49,50,51].
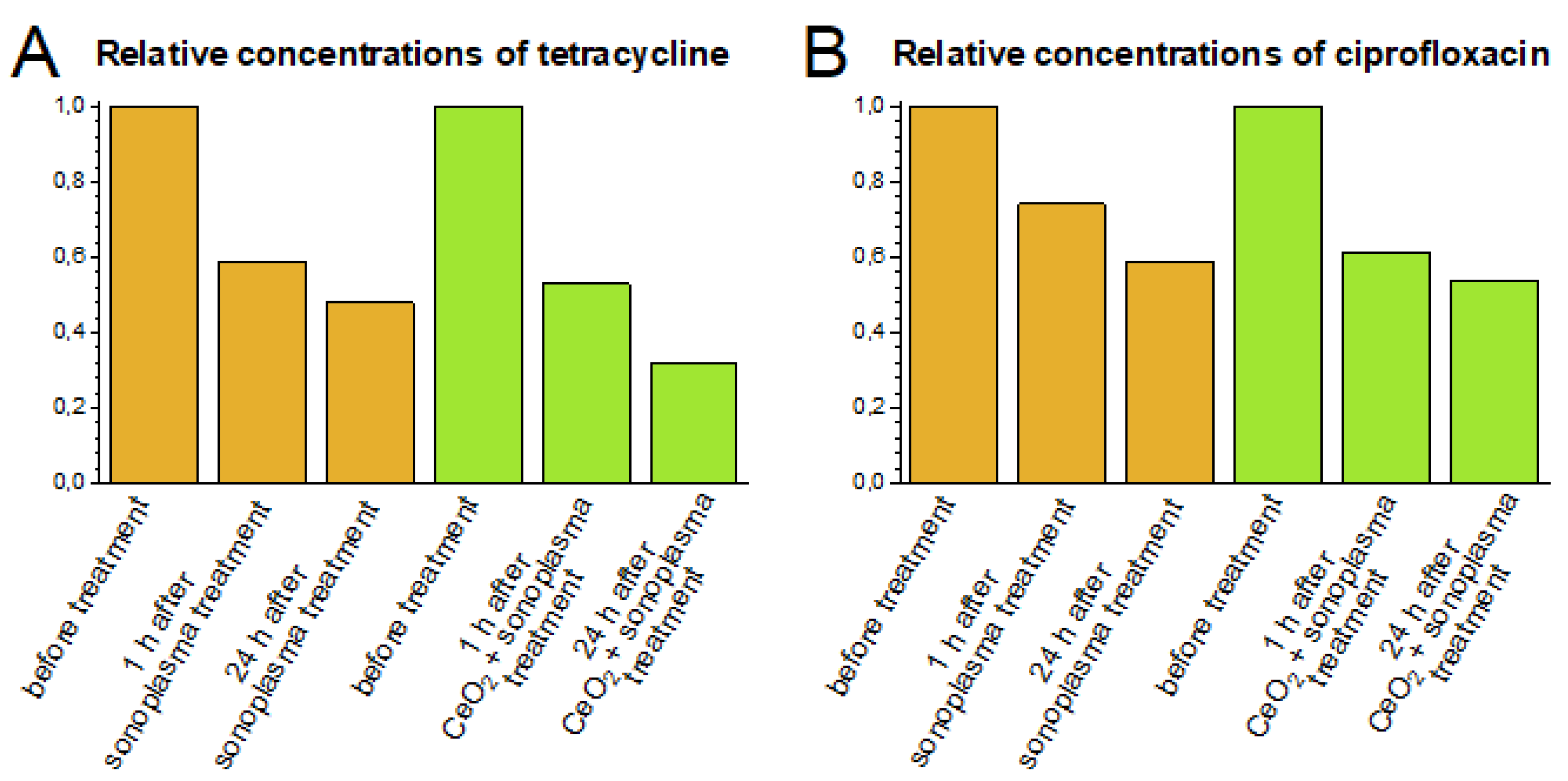
The degradation of antibiotics continues for several days after sonoplasma treatment. To study this prolonged degradation effect, plasma-treated samples were kept in a dark place at an ambient temperature of 22 °C. The concentrations of tetracycline and ciprofloxacin 24 h after one cycle of sonoplasma treatment were 48% and 59% of the initial values, respectively (Figure 4). Similar prolonged degradation after initial sonoplasma treatment has been described previously [30].
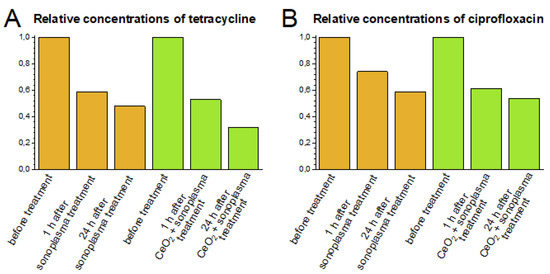
Figure 4.
Relative concentrations of tetracycline (A) and ciprofloxacin (B) in solutions before treatment and 1 and 24 hours after the sonoplasma treatment, with and without CeO2. Antibiotics (tetracycline and ciprofloxacin) and the CeO2 solution were added in a 10/1 molar ratio.
The concentrations of antibiotics measured 1–24 h after sonoplasma treatment represent the total effect of degradation due to reactions with short-lifetime ROS (i.e., radicals formed under sonoplasma action) followed by oxidation by stable ROS (i.e., hydrogen peroxide formed under sonoplasma action, which remains in the water for several hours). A rapid decrease in the antibiotic concentration occurs after the first hour of sonoplasma treatment, and slower decomposition is observed over the next twenty-four hours (Figure 4). It would appear that reactions with unstable ROS have the most significant impact; however, the experimental conditions do not allow this effect to be distinguished directly.
3.3. Effect of Co-Existing Admixtures in the Municipal Water Matrix
In real applications, the sonocatalytic process is affected by the presence of co-existing water matrix chemicals. The presence of admixtures causes a decrease in pollutant removal efficiency for the following reasons: (i) the adsorption of anions, leading to the filling of the catalyst surface pores and reductions in both the adsorption capacity and surface catalytic activity of CeO2; (ii) the quenching of free oxidizing radicals and, subsequently, their transformation to less oxidative species. The composition of the municipal water used for the experiments in the present work was investigated using methods recommended by the national environmental protection regulations and the state standards of the Russian Federation for statistical methods of water quality control. Water hardness was 3.6±0.5 mg-equ/l (titrimetric method), and the pH was 7.9±0.2 (potentiometric method). Detailed results of water composition measurements are given in the Supplementary Materials (Table S1).
The concentrations of the most abundant cations were as follows: calcium—53±6 mg/l (titrimetric method); magnesium—11.6±2.1 mg/l (titrimetric method); iron—0.52±0.10 mg/l (atomic absorption spectrometry). It has previously been shown that hardness ions might decrease the efficiency of sonocatalytic pollutant decomposition [33]. The presence of iron ions, on the other hand, makes a Fenton-like process possible. The mechanism of Fenton-like reactions has been described in great detail elsewhere [53]. An increase in the degradation rate in the Fenton oxidation process has been reported to occur when light and ultrasonic irradiation are applied, either separately or simultaneously [54,55]. This is an extension of the Fenton process, which takes advantage of UV-Vis light irradiation at wavelength values above 300 nm. In these conditions, the photolysis of Fe3+ complexes allows Fe2+ regeneration [56].
The anions in the solution act as radical scavengers. The presence of carbonates and nitrates in the treated water can result in a significant reduction in the efficiency of pollutant abatement, as has been shown in several studies [56,57,58,59,60]. For example, in the presence of nitrate ions, the removal efficiency (%) of tetracycline in a sonocatalytic process over 45 min decreased from 87.6 to 66.2% [33]. The anions interact with radicals, which possess the highest oxidation potential, to form less reactive radicals, such as , , , , and others. This is usually viewed as a negative effect, but in this way, a prolonged action effect is achieved. The resulting radicals are longer lived than radicals, and we were thus able to detect the presence of ROS and, subsequently, further pollutant degradation up to as late as 24 hours after treatment was observed.
3.4. Effect of CeO2 Nanoparticles
The addition of solid catalysts to ultrasound systems improves the pollutant degradation efficiency via the following two pathways: 1) the presence of solid particles can provide a priority position for cavitation bubble nucleation, which is conducive to the formation of cavitation bubbles and oxidation radicals (such as , and caused by the “hot spot” effect) [61]; and 2) the semiconductor sonocatalyst can be excited by the “sonoluminescence” effect caused by the ultrasound to generate electron–hole pairs, which further leads to the generation of oxidation radicals [33,34,35,62,63,64]. Sonication and catalytic degradation are known to provide a synergetic effect, as sonication increases the effectiveness of the catalytic process. The mass transfer of the organic pollutant to the catalyst surface is increased because of the deaggregation induced by ultrasonic irradiation. Catalyst particle aggregation in aqueous solution is prevented by the physical effects of acoustic cavitation, which lead to an increase in the active surface area. The ultrasonic waves continuously clean the catalyst surface to avoid the accumulation of pollutants and the intermediates produced during degradation [63]. Combining ultrasound with photolysis and photocatalysis has demonstrated good results in experiments with phenols, organic dyes and antibiotics [16,22,23,24].
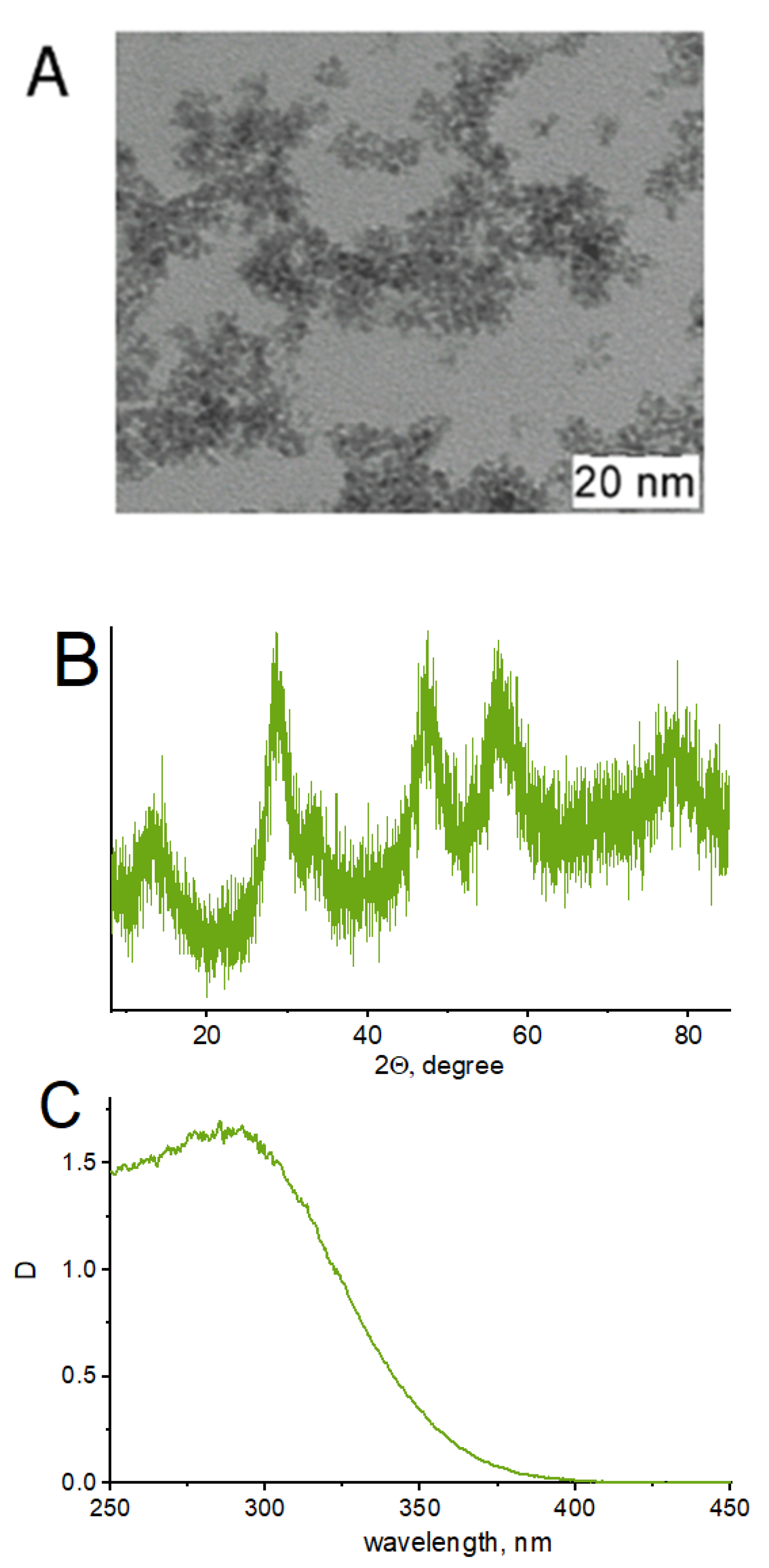
Although ZnO and titanium dioxide have been principally used as catalysts in previously cited works, these particles have recently been proven to be toxic [65]. CeO2, on the other hand, is non-toxic, abundant and inexpensive. The sonocatalytic and photocatalytic activities of nanosized CeO2 have been successfully evaluated for the degradation of refractory compounds in aqueous solutions [66,67]. Moreover, cerium compounds have already been successfully used in the decomposition of tetracycline [32]. In this research work, CeO2 (ceria) was chosen as the sonocatalyst for target pollutant degradation due to its Ce3+/Ce4+ redox transition property and good interfacial charge transfer. The characteristics of the CeO2 nanocatalyst colloidal solution are presented in Figure 5. According to XRD studies, CeO2 has a fluorite-type structure (PDF 34-394) with a coherent scattering region at around 3 nm. Aggregation in water solution results in the formation of particles with hydrodynamic radii of around 8, 40 and 120 nm. When CeO2 is added to the reaction system, it acts as a photocatalyst of ROS production under UV irradiation.
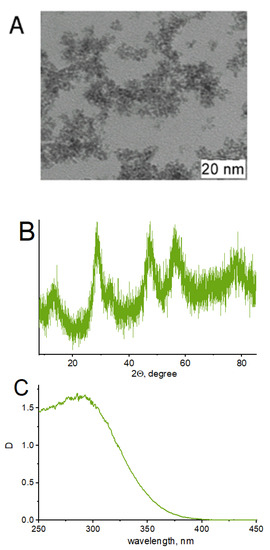
Figure 5.
TEM image (A); XRD data (B); and UV-vis spectrum (C) of CeO2 nanoparticles.
The CeO2 colloidal solution demonstrates strong absorption in the UV region with a peak at around 290 nm (Figure 5C), which partly overlaps with tetracycline and ciprofloxacin spectra. However, the antibiotics’ spectra did not change after the addition of CeO2 because the nanocatalyst concentrations in the experiment were an order of magnitude lower than the concentrations of the antibiotics.
To test the possibility of increasing the efficiency of the purification process with the introduction of a nanocatalyst, we conducted experiments in which a CeO2 colloidal solution was added to the antibiotic solution before treatment. Our experiments showed that, with the addition of CeO2, the efficiency of the tetracycline and ciprofloxacin degradation processes increased by 6% and 14% for tetracycline and ciprofloxacin, respectively, for the first treatment cycle (Figure 3).
The addition of the CeO2 colloidal solution promoted the effect of prolonged degradation after treatment; 24 h after one treatment cycle, the concentrations decreased to 30% or 55% of the initial values of tetracycline or ciprofloxacin, respectively (Figure 4). This indirectly confirms that the presence of ceria nanoparticles stimulates the formation of stable ROS.
3.5. Chemiluminescence Studies
In order to investigate the effect of stable ROS on the contaminants in treated water, L-012-activated chemiluminescence measurements were carried out [68,69,70,71,72].
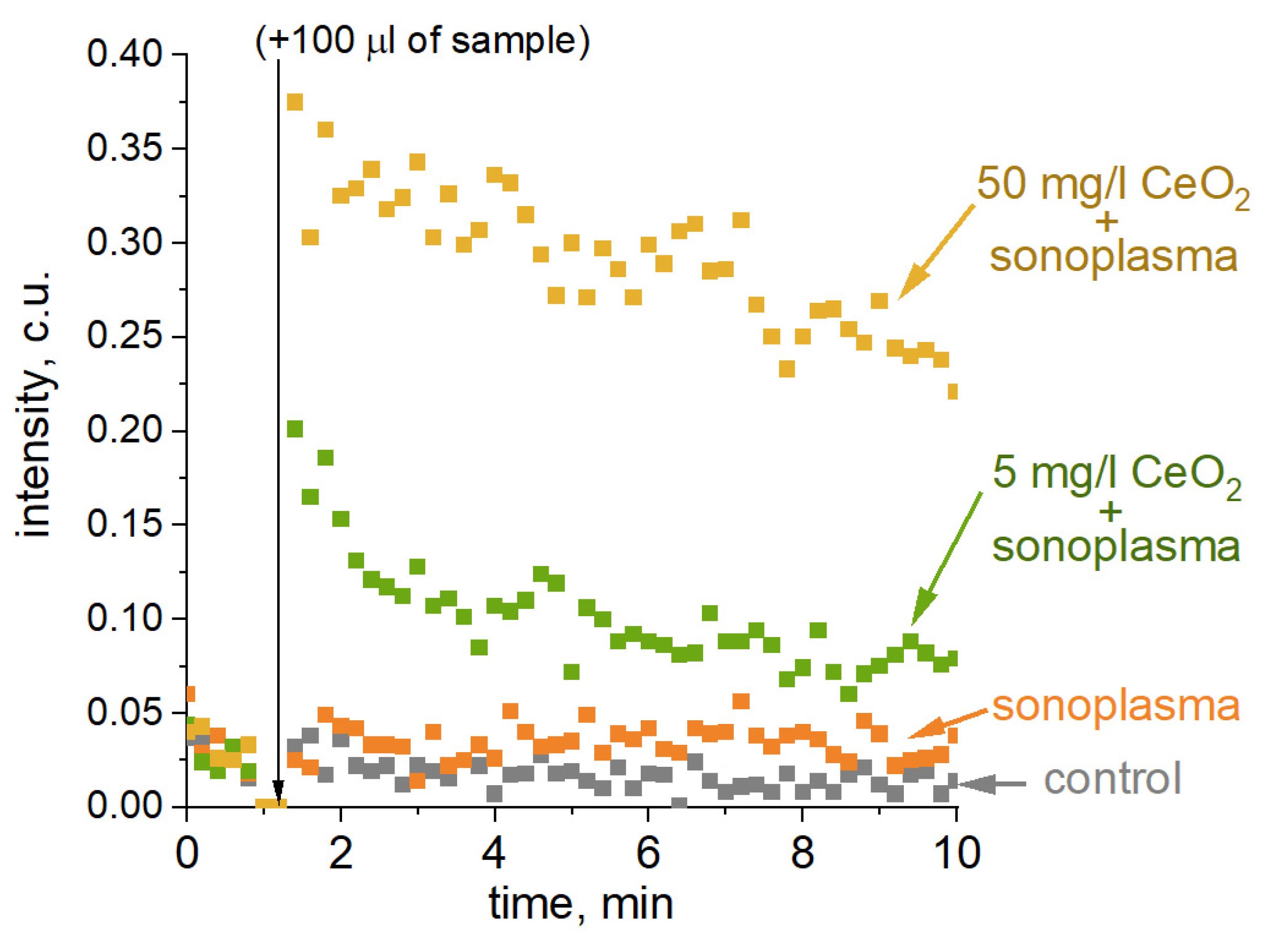
The time dependence of chemiluminescence for untreated and plasma-treated water, containing 5 mg/L and 50 mg/L of CeO2, is shown in Figure 6. The treatment of water without the addition of CeO2 resulted in a slight increase in chemiluminescence intensity. The experiments with CeO2 demonstrate a significant increase in luminescence intensity with an increase in nanocatalyst concentration. It should be noted that 24 h after treatment, the chemiluminescence intensity in water treated with the addition of a 50 mg/L CeO2 colloidal solution was slightly higher than that measured in water without the catalyst. Based on the results of the chemiluminescence experiment, CeO2 promotes the formation of stable ROS, which provide long-term sonoplasma treatment effects.
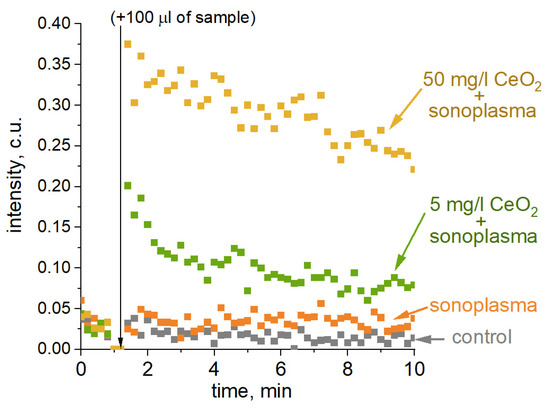
Figure 6.
L-012 chemiluminescence for the samples (from bottom to top): control (phosphate buffer); municipal water; municipal water with 5 mg/L CeO2; municipal water with 50 mg/L CeO2. Chemiluminescence measurements were started 1 hour after one cycle of sonoplasma treatment.
It should be noted that chemiluminescence measurements represent the cumulative effect of different radical sources on L-012. This estimation of the cumulative effect is important when wastewater that contains dissolved chlorides, nitrates, nitrogen, etc., is treated with sonoplasma. Although the composition of stable ROS in the experiment is dominated by hydrogen peroxide, other chlorine and nitrogen derivatives that are produced under sonoplasma can also affect the organic contaminants.
3.6. Possible Modifications to Technological Setup
An analysis of the obtained experimental data suggests that the sonoplasma method and membrane technologies can be effectively combined for wastewater treatment. Reverse osmosis systems are a prospective solution for removing drugs from effluents [73,74]. In reverse osmosis, the contaminated water is pushed through a semi-permeable membrane from a more concentrated solution to a less concentrated one, in the opposite direction to osmosis [75]. The membrane transmits pure water but does not transmit the dissolved substances. Water is pushed through the membrane under high pressure, which is maintained by a pump (usually a multi-stage centrifugal or rotor pump). Two streams are generally obtained as a result of the filtration process: the filtrate and the retentate [76]. A reverse osmosis unit is capable of removing particles as small as 0.001–0.0001 µm from water. Reverse osmosis systems have many advantages:
- high water quality with low energy consumption,
- unlimited productivity at a comparatively small size,
- low OPEX.
Unfortunately, this is not currently a comprehensive method, meaning that it does not meet all the requirements specified in the EU Directive [71]. The filtrate is usually water treated to a degree that makes it suitable for the purpose of reuse in a technological process, or, in the case of so-called clean water, for discharge into surface waters or the soil. The retentate is water that contains all of the substances, including harmful/toxic substances, which have been removed from the water in the filtration process. By volume, the retentate is considerably smaller than the filtrate. However, the contamination load (i.e., the content of harmful substances) is the same as, or very similar to, that of the raw wastewater load. This means that the problem still remains, only in a highly concentrated form; something needs to be done with this concentrated stream.
Sonoplasma can be effectively used in this technological process. Firstly, it is only necessary to process the retentate in this case, meaning that the volume of the treated liquid will be noticeably reduced. Secondly, the processed retentate, with its reduced antibiotics content and enrichment in active radicals, can be mixed with a contaminated stream at the entrance of the membrane complex. In this case, the active radicals will begin to affect the pollutants even at the preliminary stage in the pipeline in front of the membranes, and the process of their mineralization will begin. A similar technological scheme is capable of significantly increasing the efficiency of the membrane unit.
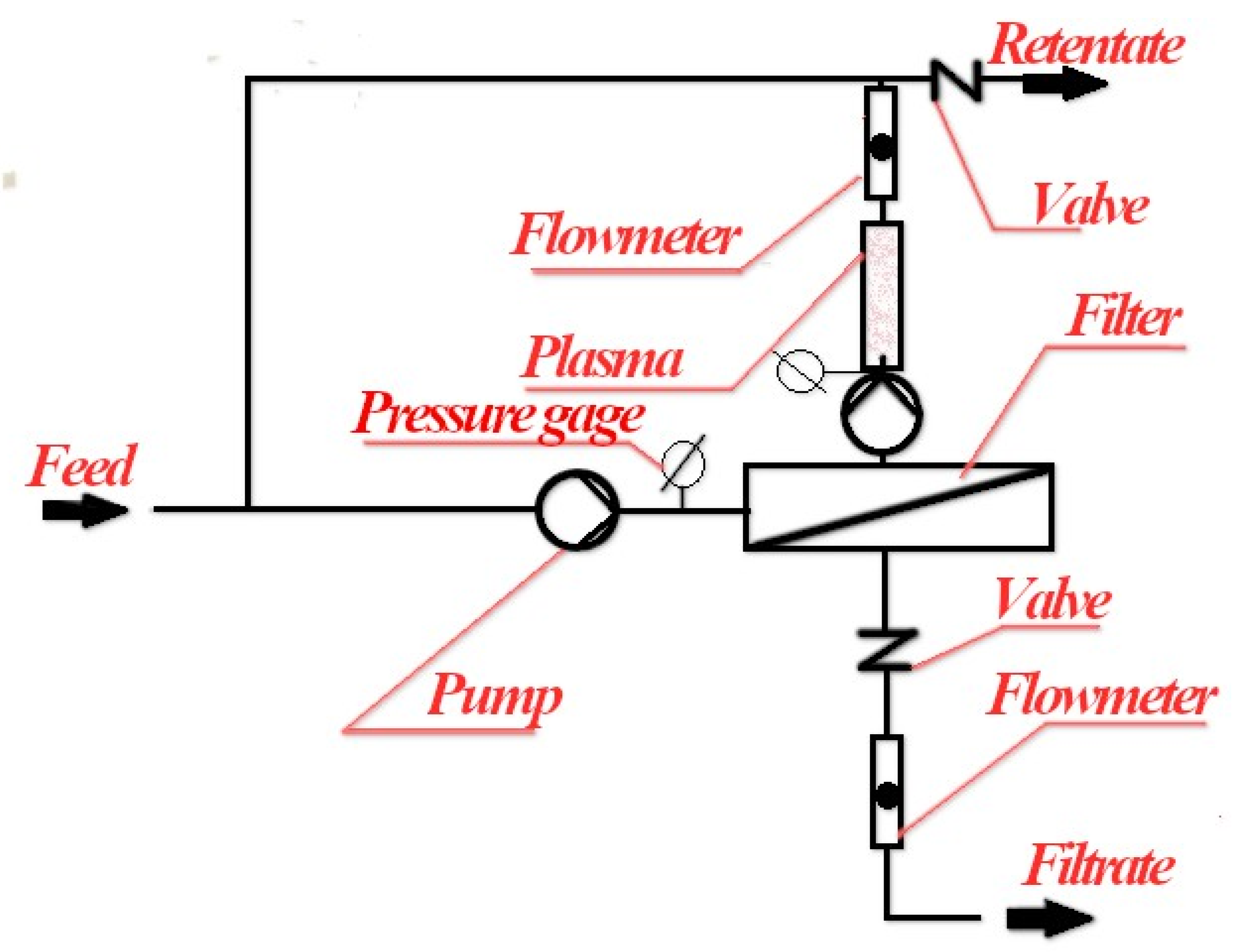
The proposed scheme for a membrane filtration unit in combination with “sonoplasmic” discharge is shown in Figure 7.
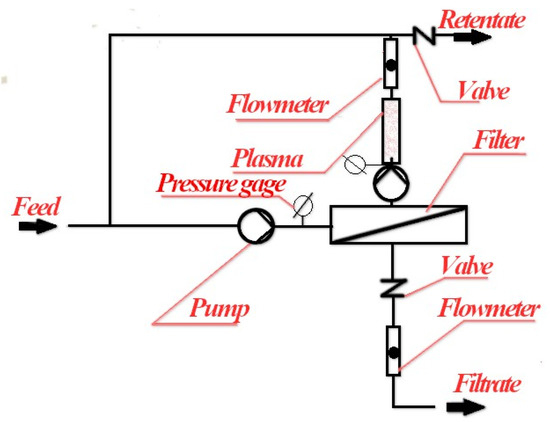
Figure 7.
A possible scheme for using “sonoplasmic” discharge in combination with reverse osmosis.
In this case, it is necessary to be able to send the processed retentate back to the entrance of the membrane reactor. The advantage of such a technological scheme is the possibility of achieving pollutant concentrations that allow discharge into the environment, although not necessarily in a single cycle of sonoplasma treatment. In this case, the discharge of the purified retentate can only be carried out periodically. In addition, the above scheme makes it possible to use the catalysts repeatedly as they will not penetrate the membrane. This possibility can also significantly increase the efficiency of the technological process under consideration. Of course, it will probably be necessary to select specific catalysts that are capable of long-term activity in a plasma discharge for each type of contamination.
4. Conclusions
We showed herein that a flow-mode water treatment under simultaneous hydrodynamic cavitation and plasma makes it possible to effectively decompose tetracycline and ciprofloxacin in very short times. The formation of reactive oxygen species during sonoplasma treatment was shown experimentally for the first time. It is important that the active radicals formed in the water continue to affect the antibiotics even after the end of the plasma treatment. The efficiency of plasma treatment can be enhanced by a CeO2 nanocatalyst. Our experiments showed that the concentration of long-lifetime ROS significantly increases with the addition of CeO2. This increases the efficiency of tetracycline and ciprofloxacin decomposition. An improved experimental setup in which a retentate that is formed in a reverse osmosis process undergoes subsequent sonoplasma treatment was also proposed.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr10102063/s1, Table S1. Composition of municipal water used for the experiments (the methods used to analyze water comply with the national environmental protection regulations and state standards of the Russian Federation for statistical methods of water quality control).
Author Contributions
Conceptualization, V.A. and A.B.; methodology, R.N., A.A. and M.S.; validation, G.C and V.B.; formal analysis, M.S., D.K. and V.B.; investigation, D.K., S.K., V.V. and I.F.; writing—original draft preparation, D.K and V.A.; writing—review and editing, S.K., G.C. and A.A.; visualization, I.F. and S.K.; supervision, A.B. and V.A.; project administration, A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Science and Higher Education of Russia (grant agreement № 075-15-2020-782).
Data Availability Statement
Not applicable.
Acknowledgments
This work was supported by the Ministry of Science and Higher Education of Russia (grant agreement № 075-15-2020-782).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Huang, A.; Yan, M.; Lin, J.; Xu, L.; Gong, H.; Gong, H. A Review of Processes for Removing Antibiotics from Breeding Wastewater. Int. J. Environ. Res. Public Health 2021, 18, 4909. [Google Scholar] [CrossRef]
- Al-Khazrajy, O.S.A.; Bergström, E.; Boxall, A.B.A. Factors Affecting the Dissipation of Pharmaceuticals in Freshwater Sediments. Environ. Toxicol. Chem. 2018, 37, 829–838. [Google Scholar] [CrossRef]
- Rana, M.S.; Lee, S.Y.; Kang, H.J.; Hur, S.J. Reducing Veterinary Drug Residues in Animal Products: A Review. Food Sci. Anim. Resour. 2019, 39, 687–703. [Google Scholar] [CrossRef]
- Mund, M.D.; Khan, U.H.; Tahir, U.; Mustafa, B.-E.; Fayyaz, A. Antimicrobial Drug Residues in Poultry Products and Implications on Public Health: A Review. Int. J. Food Prop. 2017, 20, 1433–1446. [Google Scholar] [CrossRef]
- Boxall, A.B.A.; Sinclair, C.J.; Fenner, K.; Kolpin, D.; Maund, S.J. Peer Reviewed: When Synthetic Chemicals Degrade in the Environment. Environ. Sci. Technol. 2004, 38, 368A–375A. [Google Scholar] [CrossRef]
- Dewil, R.; Mantzavinos, D.; Poulios, I.; Rodrigo, M.A. New Perspectives for Advanced Oxidation Processes. J. Environ. Manag. 2017, 195, 93–99. [Google Scholar] [CrossRef]
- Bowen, W.R. Water Engineering for the Promotion of Peace. Desalin. Water Treat. 2009, 1, 1–6. [Google Scholar] [CrossRef][Green Version]
- Bartl, A. Moving from Recycling to Waste Prevention: A Review of Barriers and Enables. Waste Manag. Res. J. Int. Solid Wastes Public Clean. Assoc. ISWA 2014, 32, 3–18. [Google Scholar] [CrossRef]
- Ahmad, F.; Zhu, D.; Sun, J. Environmental Fate of Tetracycline Antibiotics: Degradation Pathway Mechanisms, Challenges, and Perspectives. Environ. Sci. Eur. 2021, 33, 64. [Google Scholar] [CrossRef]
- Glaze, W.H.; Kang, J.-W.; Chapin, D.H. The Chemistry of Water Treatment Processes Involving Ozone, Hydrogen Peroxide and Ultraviolet Radiation. Ozone Sci. Eng. 1987, 9, 335–352. [Google Scholar] [CrossRef]
- Lim, S.; Shi, J.L.; von Gunten, U.; McCurry, D.L. Ozonation of Organic Compounds in Water and Wastewater: A Critical Review. Water Res. 2022, 213, 118053. [Google Scholar] [CrossRef]
- Suárez, S.; Carballa, M.; Omil, F.; Lema, J.M. How Are Pharmaceutical and Personal Care Products (PPCPs) Removed from Urban Wastewaters? Rev. Environ. Sci. Bio. Technol. 2008, 7, 125–138. [Google Scholar] [CrossRef]
- Fent, K.; Weston, A.A.; Caminada, D. Ecotoxicology of Human Pharmaceuticals. Aquat. Toxicol. 2006, 76, 122–159. [Google Scholar] [CrossRef]
- Manasfi, T. Chapter Four—Ozonation in Drinking Water Treatment: An Overview of General and Practical Aspects, Mechanisms, Kinetics, and Byproduct Formation. In Analysis and Formation of Disinfection Byproducts in Drinking Water; Manasfi, T., Boudenne, J.-L., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 92, pp. 85–116. ISBN 0166-526X. [Google Scholar]
- Carey, J.H. An Introduction to Advanced Oxidation Processes (AOP) for Destruction of Organics in Wastewater. Water Qual. Res. J. 1992, 27, 1–22. [Google Scholar] [CrossRef]
- Mahamuni, N.N.; Adewuyi, Y.G. Advanced Oxidation Processes (AOPs) Involving Ultrasound for Waste Water Treatment: A Review with Emphasis on Cost Estimation. Ultrason. Sonochem. 2010, 17, 990–1003. [Google Scholar] [CrossRef]
- Hart, E.J.; Henglein, A. Sonolysis of Ozone in Aqueous Solution. J. Phys. Chem. 1986, 90, 3061–3062. [Google Scholar] [CrossRef]
- Kidak, R.; Ince, N.H. Catalysis of Advanced Oxidation Reactions by Ultrasound: A Case Study with Phenol. J. Hazard. Mater. 2007, 146, 630–635. [Google Scholar] [CrossRef]
- Yang, L.P.; Hu, W.Y.; Huang, H.M.; Yan, B. Degradation of High Concentration Phenol by Ozonation in Combination with Ultrasonic Irradiation. Desalin. Water Treat. 2010, 21, 87–95. [Google Scholar] [CrossRef]
- Tezcanli-Güyer, G.; Ince, N.H. Individual and Combined Effects of Ultrasound, Ozone and UV Irradiation: A Case Study with Textile Dyes. Ultrasonics 2004, 42, 603–609. [Google Scholar] [CrossRef]
- Wu, Z.; Abramova, A.; Nikonov, R.; Cravotto, G. Sonozonation (Sonication/Ozonation) for the Degradation of Organic Contaminants—A Review. Ultrason. Sonochem. 2020, 68, 105195. [Google Scholar] [CrossRef]
- Werner, J.J.; Arnold, W.A.; McNeill, K. Water Hardness as a Photochemical Parameter: Tetracycline Photolysis as a Function of Calcium Concentration, Magnesium Concentration, and PH. Environ. Sci. Technol. 2006, 40, 7236–7241. [Google Scholar] [CrossRef]
- Wei, X.; Chen, J.; Xie, Q.; Zhang, S.; Ge, L.; Qiao, X. Distinct Photolytic Mechanisms and Products for Different Dissociation Species of Ciprofloxacin. Environ. Sci. Technol. 2013, 47, 4284–4290. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Nguyen, H.T.; Pham, T.-D.; Tran, T.D.; Chu, H.T.; Dang, H.T.; Nguyen, V.-H.; Nguyen, K.M.; Pham, T.T.; Van der Bruggen, B. UV–Visible Light Driven Photocatalytic Degradation of Ciprofloxacin by N,S Co-Doped TiO2: The Effect of Operational Parameters. Top. Catal. 2020, 63, 985–995. [Google Scholar] [CrossRef]
- Foster, J.E. Plasma-Based Water Purification: Challenges and Prospects for the Future. Phys. Plasmas 2017, 24, 55501. [Google Scholar] [CrossRef]
- Šunka, P. Pulse Electrical Discharges in Water and Their Applications. Phys. Plasmas 2001, 8, 2587–2594. [Google Scholar] [CrossRef]
- Yang, Y.; Fridman, A.; Cho, Y.I. Plasma Discharge in Water. In Advances in Heat Transfer; Cho, Y.I., Greene, G.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; Volume 42, pp. 179–292. ISBN 0065-2717. [Google Scholar]
- Malik, M.A.; Ghaffar, A.; Malik, S.A. Water Purification by Electrical Discharges. Plasma Sources Sci. Technol. 2001, 10, 82–91. [Google Scholar] [CrossRef]
- Malik, M.A. Water Purification by Plasmas: Which Reactors Are Most Energy Efficient? Plasma Chem. Plasma Process. 2010, 30, 21–31. [Google Scholar] [CrossRef]
- Abramov, V.O.; Abramova, A.V.; Cravotto, G.; Nikonov, R.V.; Fedulov, I.S.; Ivanov, V.K. Flow-Mode Water Treatment under Simultaneous Hydrodynamic Cavitation and Plasma. Ultrason. Sonochem. 2021, 70, 105323. [Google Scholar] [CrossRef]
- Nawrocki, J.; Kasprzyk-Hordern, B. The Efficiency and Mechanisms of Catalytic Ozonation. Appl. Catal. B Environ. 2010, 99, 27–42. [Google Scholar] [CrossRef]
- Fu, J.; Liu, N.; Mei, L.; Liao, L.; Deyneko, D.; Wang, J.; Bai, Y.; Lv, G. Synthesis of Ce-Doped Mn3Gd7−xCex(SiO4)6O1.5 for the Enhanced Catalytic Ozonation of Tetracycline. Sci. Rep. 2019, 9, 18734. [Google Scholar] [CrossRef]
- Soltani, R.D.C.; Mashayekhi, M.; Naderi, M.; Boczkaj, G.; Jorfi, S.; Safari, M. Sonocatalytic Degradation of Tetracycline Antibiotic Using Zinc Oxide Nanostructures Loaded on Nano-Cellulose from Waste Straw as Nanosonocatalyst. Ultrason. Sonochem. 2019, 55, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Hassani, A.; Khataee, A.; Karaca, S.; Karaca, C.; Gholami, P. Sonocatalytic Degradation of Ciprofloxacin Using Synthesized TiO2 Nanoparticles on Montmorillonite. Ultrason. Sonochem. 2017, 35, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Semeraro, P.; Bettini, S.; Sawalha, S.; Pal, S.; Licciulli, A.; Marzo, F.; Lovergine, N.; Valli, L.; Giancane, G. Photocatalytic Degradation of Tetracycline by ZnO/γ-Fe2O3 Paramagnetic Nanocomposite Material. Nanomaterials 2020, 10, 1458. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Antibiotics in the Aquatic Environment—A Review—Part II. Chemosphere 2009, 75, 435–441. [Google Scholar] [CrossRef]
- Shcherbakov, A.B.; Teplonogova, M.A.; Ivanova, O.S.; Shekunova, T.O.; Ivonin, I.V.; Baranchikov, A.Y.; Ivanov, V.K. Facile Method for Fabrication of Surfactant-Free Concentrated CeO2 Sols. Mater. Res. Express 2017, 4, 55008. [Google Scholar] [CrossRef]
- DeWitte, B.; Dewulf, J.; Demeestere, K.; Van De Vyvere, V.; De Wispelaere, P.; Van Langenhove, H. Ozonation of Ciprofloxacin in Water: HRMS Identification of Reaction Products and Pathways. Environ. Sci. Technol. 2008, 42, 4889–4895. [Google Scholar] [CrossRef]
- Daiber, A.; Oelze, M.; August, M.; Wendt, M.; Sydow, K.; Wieboldt, H.; Kleschyov, A.L.; Munzel, T. Detection of Superoxide and Peroxynitrite in Model Systems and Mitochondria by the Luminol Analogue L-012. Free Radic. Res. 2004, 38, 259–269. [Google Scholar] [CrossRef]
- Zielonka, J.; Lambeth, J.D.; Kalyanaraman, B. On the Use of L-012, a Luminol-Based Chemiluminescent Probe, for Detecting Superoxide and Identifying Inhibitors of NADPH Oxidase: A Reevaluation. Free Radic. Biol. Med. 2013, 65, 1310–1314. [Google Scholar] [CrossRef]
- Smith, M.J.; Fowler, M.; Naftalin, R.J.; Siow, R.C.M. UVA Irradiation Increases Ferrous Iron Release from Human Skin Fibroblast and Endothelial Cell Ferritin: Consequences for Cell Senescence and Aging. Free Radic. Biol. Med. 2020, 155, 49–57. [Google Scholar] [CrossRef]
- Snehalatha, M.; Ravikumar, C.; Hubert Joe, I.; Sekar, N.; Jayakumar, V.S. Spectroscopic Analysis and DFT Calculations of a Food Additive Carmoisine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 72, 654–662. [Google Scholar] [CrossRef]
- Leulescu, M.; Rotaru, A.; Moanţă, A.; Iacobescu, G.; Pălărie, I.; Cioateră, N.; Popescu, M.; Criveanu, M.C.; Morîntale, E.; Bojan, M.; et al. Azorubine: Physical, Thermal and Bioactive Properties of the Widely Employed Food, Pharmaceutical and Cosmetic Red Azo Dye Material. J. Therm. Anal. Calorim. 2021, 143, 3945–3967. [Google Scholar] [CrossRef]
- Didenko, Y.T.; Pugach, S.P. Spectra of Water Sonoluminescence. J. Phys. Chem. 1994, 98, 9742–9749. [Google Scholar] [CrossRef]
- Yasui, K. Multibubble Sonoluminescence from a Theoretical Perspective. Molecules 2021, 26, 4624. [Google Scholar] [CrossRef]
- Baranchikov, A.Y.; Ivanov, V.K.; Tretyakov, Y.D. Sonochemical Synthesis of Inorganic Materials. Russ. Chem. Rev. 2007, 76, 133–151. [Google Scholar] [CrossRef]
- Serna-Galvis, E.A.; Botero-Coy, A.M.; Martínez-Pachón, D.; Moncayo-Lasso, A.; Ibáñez, M.; Hernández, F.; Torres-Palma, R.A. Degradation of Seventeen Contaminants of Emerging Concern in Municipal Wastewater Effluents by Sonochemical Advanced Oxidation Processes. Water Res. 2019, 154, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Shurbaji, S.; Huong, P.T.; Altahtamouni, T.M. Review on the Visible Light Photocatalysis for the Decomposition of Ciprofloxacin, Norfloxacin, Tetracyclines, and Sulfonamides Antibiotics in Wastewater. Catalysts 2021, 11, 437. [Google Scholar] [CrossRef]
- Karim, A.V.; Shriwastav, A. Degradation of Ciprofloxacin Using Photo, Sono, and Sonophotocatalytic Oxidation with Visible Light and Low-Frequency Ultrasound: Degradation Kinetics and Pathways. Chem. Eng. J. 2020, 392, 124853. [Google Scholar] [CrossRef]
- Swamy, N.K.; Mohana, K.N.S.; Yashas, S.R. GNR@CeO2 Heterojunction as a Novel Sonophotocatalyst: Degradation of Tetracycline Hydrochloride, Kinetic Modeling and Synergistic Effects. Colloids Surf. A Physicochem. Eng. Asp. 2022, 639, 128324. [Google Scholar] [CrossRef]
- Xu, L.; Wu, X.-Q.; Wang, F.-J.; Liu, N.-P.; Wang, S.-H.; An, H.-L.; Ju, W.-T.; Lu, W.; Liu, B.; Wang, X.-F.; et al. Sonocatalytic Degradation of Tetracycline by Biobr/Fewo4 Nanomaterials in Collaboration with Potassium Persulfate. SSRN Electron. J. 2022. [Google Scholar] [CrossRef]
- Soori, M.M.; Ghahramani, E.; Kazemian, H.; Al-Musawi, T.J.; Zarrabi, M. Intercalation of Tetracycline in Nano Sheet Layered Double Hydroxide: An Insight into UV/VIS Spectra Analysis. J. Taiwan Inst. Chem. Eng. 2016, 63, 271–285. [Google Scholar] [CrossRef]
- Matavos-Aramyan, S.; Moussavi, M. Advances in Fenton and Fenton Based Oxidation Processes for Industrial Effluent Contaminants Control-A Review. Int. J. Environ. Sci. Nat. Resour. 2017, 2, 555594. [Google Scholar] [CrossRef]
- Saleh, R.; Taufik, A. Degradation of Methylene Blue and Congo-Red Dyes Using Fenton, Photo-Fenton, Sono-Fenton, and Sonophoto-Fenton Methods in the Presence of Iron(II, III) Oxide/Zinc Oxide/Graphene (Fe3O4/ZnO/Graphene) Composites. Sep. Purif. Technol. 2019, 210, 563–573. [Google Scholar] [CrossRef]
- Dükkancı, M. Sono-Photo-Fenton Oxidation of Bisphenol-A over a LaFeO3 Perovskite Catalyst. Ultrason. Sonochem. 2018, 40, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Andreozzi, R.; Caprio, V.; Insola, A.; Marotta, R. Advanced Oxidation Processes (AOP) for Water Purification and Recovery. Catal. Today 1999, 53, 51–59. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Shah, Y.T. Mechanisms for advanced photooxidation of aqueous organic waste compounds. Rev. Chem. Eng. 1998, 14, 1–46. [Google Scholar] [CrossRef]
- González Labrada, K.; Alcorta Cuello, D.R.; Saborit Sánchez, I.; García Batle, M.; Manero, M.-H.; Barthe, L.; Jáuregui-Haza, U.J. Optimization of Ciprofloxacin Degradation in Wastewater by Homogeneous Sono-Fenton Process at High Frequency. J. Environ. Sci. Health Part A 2018, 53, 1139–1148. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-Assisted Photocatalytic Degradation of Azo Dyes in Aqueous Solution: Kinetic and Mechanistic Investigations: A Review. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Sivasankar, T.; Moholkar, V.S. Mechanistic Approach to Intensification of Sonochemical Degradation of Phenol. Chem. Eng. J. 2009, 149, 57–69. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Skorb, E.; Belova, V.; Möhwald, H. Ultrasonic Cavitation at Solid Surfaces. Adv. Mater. 2011, 23, 1922–1934. [Google Scholar] [CrossRef]
- Suslick, K.S.; Didenko, Y.; Fang, M.M.; Hyeon, T.; Kolbeck, K.J.; McNamara, W.B.; Mdleleni, M.M.; Wong, M. Acoustic Cavitation and Its Chemical Consequences. Philos. Trans. R. Soc. London. Ser. A Math. Phys. Eng. Sci. 1999, 357, 335–353. [Google Scholar] [CrossRef]
- Putterman, S.J.; Weninger, K.R. Sonoluminescence: How Bubbles Turn Sound into Light. Annu. Rev. Fluid Mech. 2000, 32, 445–476. [Google Scholar] [CrossRef]
- Geng, N.; Chen, W.; Xu, H.; Ding, M.; Xie, Z.; Wang, A. Removal of Tetracycline Hydrochloride by Z-Scheme Heterojunction Sono-Catalyst Acting on Ultrasound/H2O2 System. Process Saf. Environ. Prot. 2022, 165, 93–101. [Google Scholar] [CrossRef]
- Smijs, T.G.; Pavel, S. Titanium Dioxide and Zinc Oxide Nanoparticles in Sunscreens: Focus on Their Safety and Effectiveness. Nanotechnol. Sci. Appl. 2011, 4, 95–112. [Google Scholar] [CrossRef]
- Ji, P.; Wang, L.; Chen, F.; Zhang, J. Ce3+-Centric Organic Pollutant Elimination by CeO2 in the Presence of H2O2. ChemCatChem 2010, 2, 1552–1554. [Google Scholar] [CrossRef]
- Tizhoosh, N.Y.; Khataee, A.; Hassandoost, R.; Darvishi Cheshmeh Soltani, R.; Doustkhah, E. Ultrasound-Engineered Synthesis of WS2@CeO2 Heterostructure for Sonocatalytic Degradation of Tylosin. Ultrason. Sonochem. 2020, 67, 105114. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Song, G.; Lin, J.M. Reactive oxygen species and their chemiluminescence-detection methods. Trends Anal. Chem. 2006, 25, 985–995. [Google Scholar] [CrossRef]
- Yu, W.; Zhao, L. Chemiluminescence detection of reactive oxygen species generation and potential environmental applications. Trends Analyt. Chem. 2021, 136, 116197. [Google Scholar] [CrossRef]
- Agarwal, A.; Allamaneni, S.S.; Said, T.M. Chemiluminescence technique for measuring reactive oxygen species. Reprod. BioMed. Online 2004, 9, 466–468. [Google Scholar] [CrossRef]
- Su, Y.; Song, H.; Lv, Y. Recent advances in chemiluminescence for reactive oxygen species sensing and imaging analysis. Microchem. J. 2019, 146, 83–97. [Google Scholar] [CrossRef]
- Kobayashi, H.; Gil-Guzman, E.; Mahran, A.M.; Sharma, R.K.; Nelson, D.R.; Agarwal, A. Quality control of reactive oxygen species measurement by luminol-dependent chemiluminescence assay. J. Androl. 2001, 22, 568–574. [Google Scholar] [CrossRef]
- Boleda, M.R.; Majamaa, K.; Aerts, P.; Gómez, V.; Galceran, M.T.; Ventura, F. Removal of drugs of abuse from municipal wastewater using reverse osmosis membranes. Desalination Water Treat. 2010, 21, 122–130. [Google Scholar] [CrossRef]
- Dolar, D.; Pelko, S.; Košutić, K.; Horvat, A.J. Removal of anthelmintic drugs and their photodegradation products from water with RO/NF membranes. Process Saf. Environ. Prot. 2012, 90, 147–152. [Google Scholar] [CrossRef]
- Belkacem, M.; Bekhti, S.; Bensadok, K. Groundwater Treatment by Reverse Osmosis. Desalination 2007, 206, 100–106. [Google Scholar] [CrossRef]
- Almansoori, A.; Saif, Y. Structural Optimization of Osmosis Processes for Water and Power Production in Desalination Applications. Desalination 2014, 344, 12–27. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).