Coal-Scenedesmus Microalgae Co-Firing in a Fixed Bed Combustion Reactor: A Study on CO2, SO2 and NOx Emissions and Ash
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemical Analyses
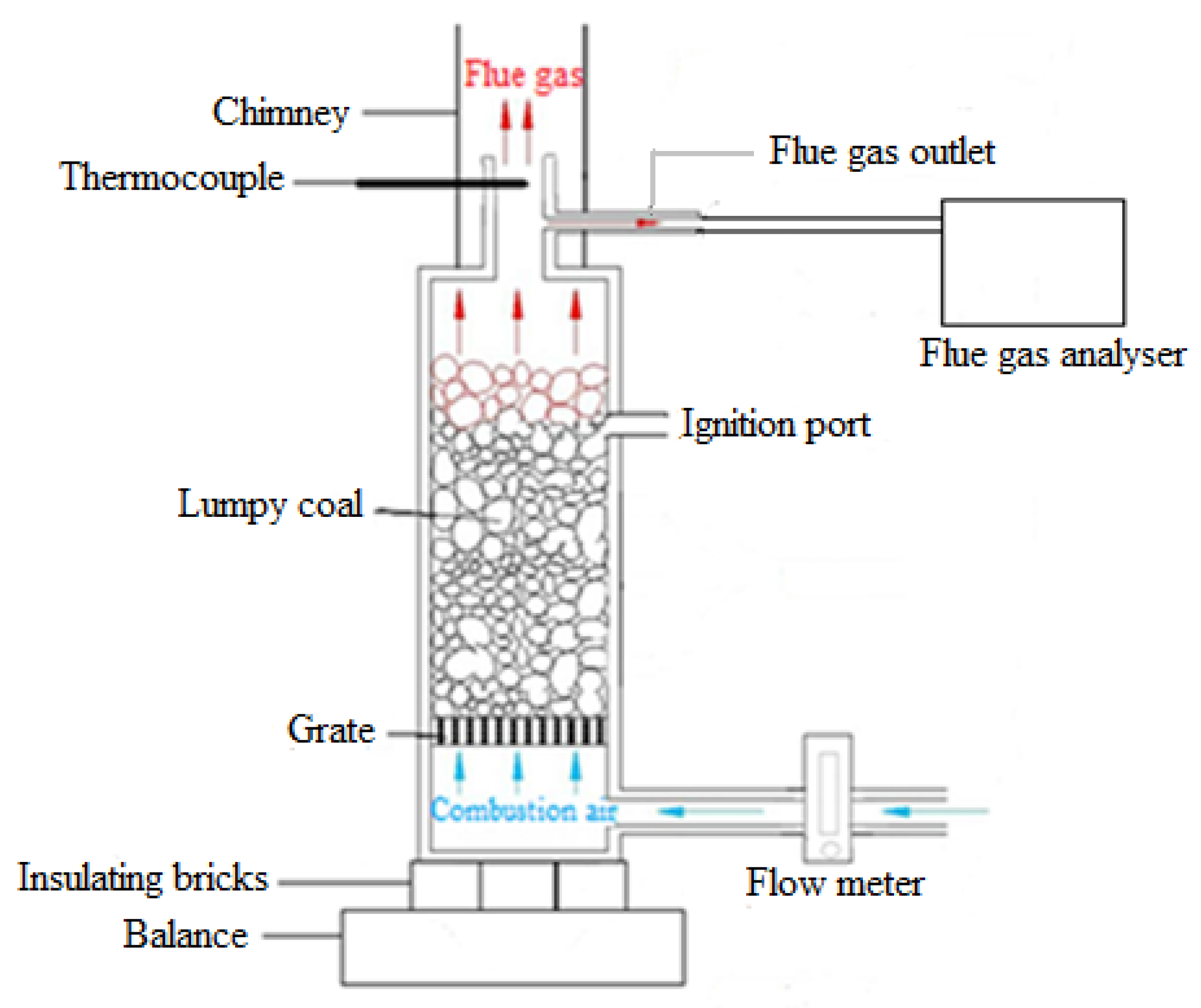
2.3. Combustion System Description
2.4. Combustion Method
2.5. The O2 Referencing
2.6. Ash Elemental Analysis and Imaging
3. Results and Discussion
3.1. Effect of Co-Firing Coal and Scenedesmus Microalgae on Mass Loss
3.2. Effect of Coal–Scenedesmus Microalgae Co-Firing on Combustion Temperature with Time
3.3. Effect of Coal–Scenedesmus Microalgae Co-Firing and Air Flow Rate on Peak Temperature
3.4. Effect of Coal–Scenedesmus Microalgae Co-Firing and Air Flow Rate on CO2 Emissions
3.5. Effect of Coal–Scenedesmus Microalgae Co-Firing and Air Flow Rate on SO2 Emissions
3.6. Effect of Coal–Scenedesmus Microalgae Co-Firing and Air Flow Rate on NOx Emissions
3.7. Combustion Efficiency
3.8. Ash Analysis with XRF
3.9. Scanning Electron Microscopy (SEM)
3.10. Transmission Electron Microscope (TEM)
4. Conclusions
5. Patents
Author Contributions
Funding
Conflicts of Interest
References
- B.P. Statistical Review of World Energy 2021. Available online: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2021-full-report.pdf (accessed on 28 May 2022).
- International Energy Agency. Coal-2021. Available online: https://iea.blob.core.windows.net/assets/f1d724d4-a753-4336-9f6e-64679fa23bbf/Coal2021.pdf (accessed on 28 May 2022).
- Eskom Fact Sheet 2021. Available online: https://www.eskom.co.za/wp-content/uploads/2021/08/CO-0007-Coal-in-SA-Rev-16.pdf (accessed on 10 June 2022).
- Gullison, R.E.; Frumhoff, P.C.; Canadell, J.G.; Field, C.B.; Daniel, C.; Hayhoe, K.; Avissar, R.; Curran, L.M.; Friedlingstein, P.; Jones, C.D.; et al. Tropical forests and climate policy. Science 2007, 316, 985–986. [Google Scholar] [CrossRef] [PubMed]
- Finkelman, R.B.; Wolfe, A.; Hendryx, M.S. The future environmental and health impacts of coal. Energy Geosci. 2021, 2, 99–112. [Google Scholar] [CrossRef]
- Perera, F. Pollution from fossil-fuel combustion is the leading environmental threat to global pediatric health and equity: Solutions exist. Int. J. Environ. Res. Public Health 2018, 15, 16. [Google Scholar] [CrossRef] [PubMed]
- Reducing Greenhouse Gas Emissions: The Carbon Tax Option, Discussion Article for Public Comment; National Treasury: Pretoria, South Africa, 2010.
- Sun, P.; Hui, S.; Gao, Z.; Zhou, Q.; Tan, H.; Zhao, Q.; Xu, T. Experimental investigation on the combustion and heat transfer characteristics of wide size biomass co-firing in 0.2 MW circulating fluidized bed. Appl. Therm. Eng. 2013, 52, 284–292. [Google Scholar] [CrossRef]
- Gao, Y.; Tahmasebi, A.; Dou, J.; Yu, J. Combustion characteristics and air pollutant formation during oxy-fuel co-combustion of microalgae and lignite. Bioresour. Technol. 2016, 207, 276–284. [Google Scholar] [CrossRef]
- Guo, F.; Zhong, Z. Co-combustion of anthracite coal and wood pellets: Thermodynamic analysis, combustion efficiency, pollutant emissions and ash slagging. Environ. Pollut. 2018, 239, 21–29. [Google Scholar] [CrossRef]
- Kanwal, F.; Ahmed, A.; Jamil, F.; Rafiq, S.; Uzair Ayub, H.M.; Ghauri, M.; Khurram, M.S.; Munir, S.; Inayat, A.; Bakar, M.S.A.; et al. Co-combustion of blends of coal and underutilised biomass residues for environmental friendly electrical energy production. Sustainability 2021, 13, 4881. [Google Scholar] [CrossRef]
- Liao, X.; Zhang, S.; Wang, X.; Shao, J.; Zhang, X.; Wang, X.; Yang, H.; Chen, H. Co-combustion of wheat straw and camphor wood with coal slime: Thermal behaviour, kinetics, and gaseous pollutant emission characteristics. Energy 2021, 234, 121292. [Google Scholar] [CrossRef]
- Ashraf, A.; Sattar, H.; Munir, S. A comparative performance evaluation of co-combustion of coal and biomass in drop tube furnace. J. Energy Inst. 2022, 100, 55–65. [Google Scholar] [CrossRef]
- Annamalai, K.; Sweeten, J.; Freeman, M.; Mathur, M.; O’Dowd, W.; Walbert, G.; Jones, S. Co-firing of coal and cattle feedlot biomass (FB) Fuels, Part III: Fouling results from a 500,000 BTU/h pilot plant scale boiler burner. Fuel 2003, 82, 1195–1200. [Google Scholar] [CrossRef]
- Pokothoane, P.S. Analysis of Co-Firing Biomass with South African Coal in Pulverised Coal Boilers. Ph.D. Thesis, University of the Witwatersrand, Johannesburg, South Africa, 2010. [Google Scholar]
- Munir, S.; Nimmo, W.; Gibbs, B.M. The effect of air staged, co-combustion of pulverised coal and biomass blends on NOxemissions and combustion efficiency. Fuel 2011, 90, 126–135. [Google Scholar] [CrossRef]
- Demirbas, A.; Demirbas, M.F. Algae Energy: Algae as a New Source of Biodiesel, 1st ed.; Springer: London, UK; Dordrecht, The Netherlands; Heidelberg, Germany; New York, NY, USA, 2010; p. 199. [Google Scholar]
- Kucukvar, M.; Tatari, O. A comprehensive life cycle analysis of cofiring algae in a coal power plant as a solution for achieving sustainable energy. Energy 2011, 36, 6352–6357. [Google Scholar] [CrossRef]
- Zeelie, B. Processing carbonaceous materials. No.: US 2016/0289567. A1 Patent Application Publication, 6 October 2016. [Google Scholar]
- Baloyi, H.; Dugmore, G. Influences of microalgae biomass on the thermal behaviour of waste coal fines. J. Energy South. Africa 2019, 30, 1–7. [Google Scholar] [CrossRef]
- Magida, N.E.; Bolo, L.L.; Hlangothi, S.P.; Dugmore, G.; Ogunlaja, A.S. Co-combustion Characteristics of coal-Scenedesmus Microalgae Blends and Their Resulting Ash. Combust. Sci. Technol. 2021, 193, 419–436. [Google Scholar] [CrossRef]
- Yoo, C.; Jun, S.Y.; Lee, J.Y.; Ahn, C.Y.; Oh, H.M. Selection of microalgae for lipid production under high levels carbon dioxide. Bioresour. Technol. 2010, 101, S71–S74. [Google Scholar] [CrossRef]
- Tripathi, R.; Singh, J.; Thakur, I.S. Characterization of microalga Scenedesmus sp. ISTGA1 for potential CO2 sequestration and biodiesel production. Renew. Energy 2015, 74, 774–781. [Google Scholar] [CrossRef]
- López-Pacheco, I.Y.; Castillo-Vacas, E.I.; Castañeda-Hernández, L.; Gradiz-Menjivar, A.; Rodas-Zuluaga, L.I.; Castillo-Zacarías, C.; Sosa-Hernández, J.E.; Barceló, D.; Iqbal, H.M.N.; Parra-Saldívar, R. CO2 biocapture by Scenedesmus sp. grown in industrial wastewater. Sci. Total Environ. 2021, 790, 148222. [Google Scholar] [CrossRef]
- Agrawal, A.; Chakraborty, S. A kinetic study of pyrolysis and combustion of microalgae Chlorella vulgaris using thermo-gravimetric analysis. Bioresour. Technol. 2013, 128, 72–80. [Google Scholar] [CrossRef]
- Tahmasebi, A.; Kassim, M.A.; Yu, J.; Bhattacharya, S. Thermogravimetric study of the combustion of Tetraselmis suecica microalgae and its blend with a Victorian brown coal in O2/N2and O2/CO2atmospheres. Bioresour. Technol. 2013, 150, 15–27. [Google Scholar] [CrossRef]
- Sanchez-Silva, L.; López-González, D.; Garcia-Minguillan, A.M.; Valverde, J.L. Pyrolysis, combustion and gasification characteristics of Nannochloropsis gaditana microalgae. Bioresour. Technol. 2013, 130, 321–331. [Google Scholar] [CrossRef]
- Fei, L.; Zhao, B.; Liu, J.; Su, Y. Emission characteristics and formation mechanisms of PM2.5 from co-firing of algal biomass and coal. J. Energy Inst. 2021, 98, 354–362. [Google Scholar] [CrossRef]
- Chen, C.; Ma, X.; Liu, K. Thermogravimetric analysis of microalgae combustion under different oxygen supply concentrations. Appl. Energy 2011, 88, 3189–3196. [Google Scholar] [CrossRef]
- Gil, M.V.; Casal, D.; Pevida, C.; Pis, J.J.; Rubiera, F. Thermal behaviour and kinetics of coal/biomass blends during co-combustion. Bioresour. Technol. 2010, 101, 5601–5608. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, F.; Yang, Q.; Liang, R. Thermogravimetric studies of the behavior of wheat straw with added coal during combustion. Biomass Bioenergy 2009, 33, 50–56. [Google Scholar] [CrossRef]
- Gil, M.V.; Riaza, J.; Álvarez, L.; Pevida, C.; Pis, J.J.; Rubiera, F. Kinetic models for the oxy-fuel combustion of coal and coal/biomass blend chars obtained in N2and CO2 atmospheres. Energy 2012, 48, 510–518. [Google Scholar] [CrossRef]
- Muthuraman, M.; Namioka, T.; Yoshikawa, K. A comparative study on co-combustion performance of municipal solid waste and Indonesian coal with high ash Indian coal: A thermogravimetric analysis. Fuel Process. Technol. 2010, 91, 550–558. [Google Scholar] [CrossRef]
- Åmand, L.E.; Leckner, B. Influence of fuel on the emission of nitrogen oxides (NO and N2O) from an 8-MW fluidized bed boiler. Combust. Flame 1991, 84, 181–196. [Google Scholar] [CrossRef]
- Collings, M.E.; Mann, M.D.; Young, B.C. Effect of Coal Rank and Circulating Fluidized-Bed Operating Parameters on Nitrous Oxide Emissions. Energy Fuels 1993, 7, 554–558. [Google Scholar] [CrossRef]
- Bai, J.; Yu, C.; Li, L.; Wu, P.; Luo, Z.; Ni, M. Experimental Study on the NO and N2O Formation Characteristics during Biomass Combustion. Energy Fuels 2013, 27, 515–522. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, S.; Liu, H.; Yang, J.; Liu, X.; Xu, G. NOx emission characteristics of fluidized bed combustion in atmospheres rich in oxygen and water vapor for high-nitrogen fuel. Fuel 2015, 139, 346–355. [Google Scholar] [CrossRef]
- Pu, G.; Zan, H.; Du, J.; Zhang, X. Study on NO Emission in the Oxy-Fuel Combustion of Co-Firing Coal and Biomass in a Bubbling Fluidized Bed Combustor. BioResources 2016, 12, 1890–1902. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, G.; Wang, X.; Qi, C. Co-combustion of bituminous coal and biomass fuel blends: Thermochemical characterization, potential utilization and environmental advantage. Bioresour. Technol. 2016, 218, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Gani, A.; Morishita, K.; Nishikawa, K.; Naruse, I. Characteristics of co-combustion of low-rank coal with biomass. Energy Fuels 2005, 19, 1652–1659. [Google Scholar] [CrossRef]
- Kadam, K.L. Environmental implications of power generation via coal-microalgae cofiring. Energy 2002, 27, 905–922. [Google Scholar] [CrossRef]
- van Loo, S.; Koppejan, J. The Handbook of Biomass Combustion and Cofiring, 1st ed.; Routledge: London, UK, 2008; p. 464. [Google Scholar]
- Pilusa, T.J.; Huberts, R.; Muzenda, E. Emissions analysis from combustion of eco-fuel briquettes for domestic applications. J. Energy South. Africa 2013, 24, 30–36. [Google Scholar] [CrossRef]
- Fernando, R. Cofiring High Ratios of Biomass with Coal; IEA Clean Coal Centre: London, UK, 2012; p. 70. [Google Scholar]
- Li, J.; Yang, W.; Blasiak, W.; Ponzio, A. Volumetric combustion of biomass for CO2 and NOx reduction in coal-fired boilers. Fuel 2012, 102, 624–633. [Google Scholar] [CrossRef]
- Saikaew, T.; Supudommak, P.; Mekasut, L.; Piumsomboon, P.; Kuchonthara, P. Emission of NOx and N2O from co-combustion of coal and biomasses in CFB combustor. Int. J. Greenh. Gas Control 2012, 10, 26–32. [Google Scholar] [CrossRef]
- Daood, S.S.; Javed, M.T.; Gibbs, B.M.; Nimmo, W. NOx control in coal combustion by combining biomass co-firing, oxygen enrichment and SNCR. Fuel 2013, 105, 283–292. [Google Scholar] [CrossRef]
- Williams, A.; Pourkashanian, M.; Jones, J.M. Combustion of pulverised coal and biomass. Prog. Energy Combust. Sci. 2001, 27, 587–610. [Google Scholar] [CrossRef]
- Ross, A.B.; Jones, J.M.; Chaiklangmuang, S.; Pourkashanian, M.; Williams, A.; Kubica, K.; Andersson, J.T.; Kerst, M.; Danihelka, P.; Bartle, K.D. Measurement and prediction of the emission of pollutants from the combustion of coal and biomass in a fixed bed furnace. Fuel 2002, 81, 571–582. [Google Scholar] [CrossRef]
- Hayhurst, A.N.; Lawrence, A.D. The amounts of NOx and N2O formed in a fluidized bed combustor during the burning of coal volatiles and also of char. Combust. Flame 1996, 105, 341–357. [Google Scholar] [CrossRef]
- Duan, L.; Duan, Y.; Zhao, C.; Anthony, E.J. NO emission during co-firing coal and biomass in an oxy-fuel circulating fluidized bed combustor. Fuel 2015, 150, 8–13. [Google Scholar] [CrossRef]
- McIlveen-Wright, D.R.; Huang, Y.; Rezvani, S.; Mondol, J.D.; Redpath, D.; Anderson, M.; Hewitt, N.J.; Williams, B.C. A Techno-economic assessment of the reduction of carbon dioxide emissions through the use of biomass co-combustion. Fuel 2011, 90, 11–18. [Google Scholar] [CrossRef]
- Sahu, S.G.; Chakraborty, N.; Sarkar, P. Coal-biomass co-combustion: An overview. Renew. Sustain. Energy Rev. 2014, 39, 575–586. [Google Scholar] [CrossRef]
- Díez, L.I.; Lupiáñez, C.; Guedea, I.; Bolea, I.; Romeo, L.M. Anthracite oxy-combustion characteristics in a 90 kWth fluidized bed reactor. Fuel Process. Technol. 2015, 139, 196–203. [Google Scholar] [CrossRef]
- Lunden, M.M.; Yang, N.Y.C.; Headley, T.J.; Shaddix, C.R. Mineral-char interactions during char combustion of a high-volatile coal. Symp. Combust. 1998, 27, 1695–1702. [Google Scholar] [CrossRef]
- van Alphen, C. Factors Influencing Fly Ash Formation and Slag Deposit. Ph.D. Thesis, University of the Witwatersrand, Johannesburg, South Africa, 2005. [Google Scholar]
- Manquais, K.L.; Snape, C.; Mcrobbie, I.; Barker, J.; Pellegrini, V. Comparison of the Combustion Reactivity of TGA and Drop Tube Furnace Chars from a Bituminous Coal. Energy Fuels 2009, 23, 4269–4277. [Google Scholar] [CrossRef]











| Coal | Coalgae® 5% | Coalgae® 10% | Coalgae® 15% | Coalgae® 20% | |
|---|---|---|---|---|---|
| Proximate analysis (wt. %) | |||||
| Moisture | 4.61 | 4.78 | 4.99 | 5.05 | 5.16 |
| Volatiles | 24.5 | 26.9 | 29.1 | 31.7 | 34.9 |
| Ash | 15.0 | 14.9 | 14.7 | 13.9 | 13.0 |
| Fixed carbon | 55.9 | 53.4 | 51.3 | 49.4 | 47.0 |
| Ultimate analysis (wt. %) | |||||
| Carbon | 65.4 | 63.4 | 61.8 | 61.4 | 61.1 |
| Hydrogen | 5.04 | 5.06 | 5.10 | 5.25 | 5.61 |
| Nitrogen | 1.97 | 2.74 | 2.76 | 2.78 | 3.22 |
| Sulphur | 0.49 | 0.47 | 0.40 | 0.39 | 0.37 |
| * Oxygen | 12.1 | 13.4 | 15.2 | 16.3 | 16.7 |
| HHV(MJ/kg) | 25.9 | 25.7 | 25.4 | 25.3 | 25.1 |
| Fuels | Carbon in Fuel (%, db) | Carbon in Ash (%, db) | Combustion Efficiency (%) |
|---|---|---|---|
| Coal | 68.54 | 9.32 | 86.40 |
| Coalgae® 5% | 66.57 | 1.96 | 97.06 |
| Coalgae® 10% | 65.07 | 0.99 | 98.48 |
| Coalgae® 15% | 64.65 | 0.67 | 98.96 |
| Coalgae® 20% | 64.40 | 0.71 | 98.90 |
| Compounds (%) | Coal | Coalgae® 5% | Coalgae® 10% | Coalgae® 15% | Coalgae® 20% |
|---|---|---|---|---|---|
| Al2O3 | 27.726 | 25.719 | 24.13 | 19.475 | 18.191 |
| SiO | 45.989 | 38.601 | 33.748 | 31.063 | 27.828 |
| P2O4 | 1.992 | 4.113 | 4.744 | 5.813 | 7.912 |
| SO3 | _ | 0.172 | 0.179 | 0.207 | 0.216 |
| K2O | 1.051 | 1.219 | 2.151 | 3.029 | 3.134 |
| CaO | 5.624 | 7.987 | 9.818 | 11.451 | 12.714 |
| TiO | 5.861 | 5.741 | 5.575 | 5.526 | 5.151 |
| Fe2O3 | 8.358 | 9.282 | 10.192 | 11.306 | 12.631 |
| MgO | _ | 0.395 | 0.440 | 0.459 | 0.569 |
| * B/A ratio | 0.189 | 0.270 | 0.356 | 0.468 | 0.568 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magida, N.E.; Dugmore, G.; Ogunlaja, A.S. Coal-Scenedesmus Microalgae Co-Firing in a Fixed Bed Combustion Reactor: A Study on CO2, SO2 and NOx Emissions and Ash. Processes 2022, 10, 2183. https://doi.org/10.3390/pr10112183
Magida NE, Dugmore G, Ogunlaja AS. Coal-Scenedesmus Microalgae Co-Firing in a Fixed Bed Combustion Reactor: A Study on CO2, SO2 and NOx Emissions and Ash. Processes. 2022; 10(11):2183. https://doi.org/10.3390/pr10112183
Chicago/Turabian StyleMagida, Nokuthula Ethel, Gary Dugmore, and Adeniyi Sunday Ogunlaja. 2022. "Coal-Scenedesmus Microalgae Co-Firing in a Fixed Bed Combustion Reactor: A Study on CO2, SO2 and NOx Emissions and Ash" Processes 10, no. 11: 2183. https://doi.org/10.3390/pr10112183
APA StyleMagida, N. E., Dugmore, G., & Ogunlaja, A. S. (2022). Coal-Scenedesmus Microalgae Co-Firing in a Fixed Bed Combustion Reactor: A Study on CO2, SO2 and NOx Emissions and Ash. Processes, 10(11), 2183. https://doi.org/10.3390/pr10112183








