Flexible Ring Sensor Array and Machine Learning Model for the Early Blood Leakage Detection during Dialysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Flexible Ring-Light Sensor Array
- Risk level 1: 0 sensors detected (normal)
- Risk level 2: ≦3 sensors detected (Nr = 91 combinations)
- Risk level 3: ≦6 sensors detected (Nr = 153 combinations)
- Risk level 4: ≧7 sensors detected (Nr = 9 combinations)
2.2. Bidirectional Hetero-Associative Memory Machine Learning Model
- Learning stage:
- Bidirectional association stage:
3. Results and Discussions
3.1. Experiment Setup
3.2. Preliminary Detection Tests
3.3. Performance Comparisons
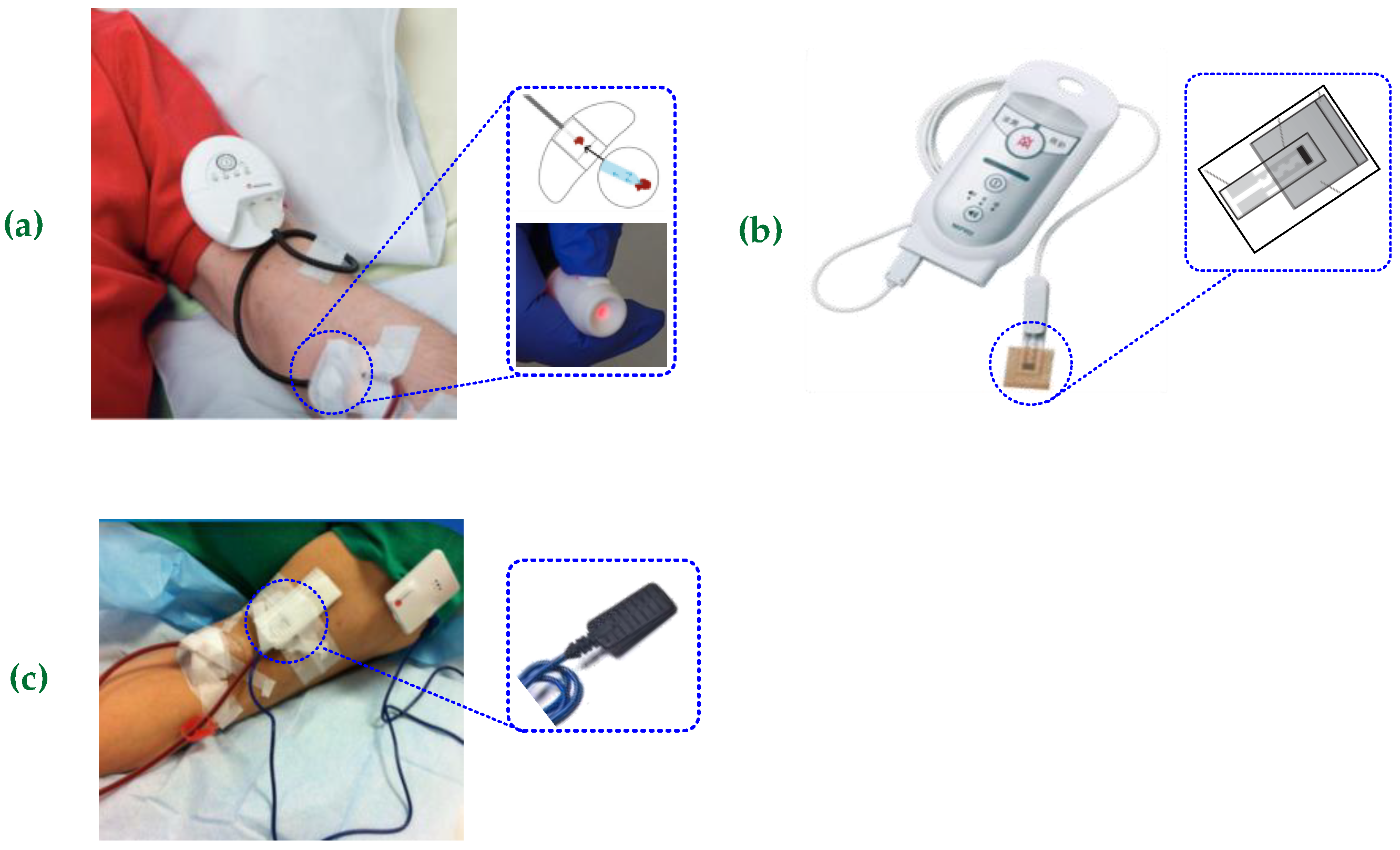
3.4. Proposed System Versus Similar Sensing Devices in the Literature
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| USRDS | US Renal Data System |
| HD | Hemodialysis |
| PD | Peritoneal dialysis |
| AVSs | Arteriovenous shunts |
| AVF | Arteriovenous fistula |
| AVG | Arteriovenous graft |
| PCA | Principal Components Analysis |
| FPCB | Flexible printed circuit boards |
| KΩ | kilo-ohms |
| MΩ | megaohms |
| BHAM | Bidirectional hetero-associative memory |
| CdS | Cadmium sulfide |
| CdSe | Cadmium selenide |
| MSPs | Mapping serial ports |
| ES | Embedded system |
| MCU | Microcontroller |
References
- 2020 Annual Data Report. Available online: https://adr.usrds.org/2020/ (accessed on 20 October 2022).
- Hemodialysis. Available online: https://www.lucenxia.com.my/types-of-peritoneal-dialysis (accessed on 20 October 2022).
- All About Dialysis. Available online: https://www.idcantalya.com/en/dialysis/ (accessed on 20 October 2022).
- Leo, K.M. Haemodialysis Access. Available online: https://www.vascular.com.hk/page37.php (accessed on 20 October 2022).
- Allon, M.; Robbin, M.L. Increasing arteriovenous fistulas in hemodialysis patients: Problems and solutions. Kidney Int. 2002, 62, 1109–1124. [Google Scholar] [CrossRef] [PubMed]
- Murdeshwar, H.N.; Anjum, F. Hemodialysis. 2020. Available online: https://europepmc.org/article/NBK/nbk563296 (accessed on 20 October 2022).
- Azar, A.T.; Canaud, B. Hemodialysis system. In Modelling and Control of Dialysis Systems; Springer: Berlin/Heidelberg, Germany, 2013; pp. 99–166. [Google Scholar]
- Engvall, D.; Stroemsten, P. Detection of blood leakage by detecting a volatile agent. U.S. Patent No. 9,492,091, 15 November 2016. [Google Scholar]
- Nomura, K.I.; Horii, Y.; Kanazawa, S.; Kusaka, Y.; Ushijima, H. Fabrication of a textile-based wearable blood leakage sensor using screen-offset printing. Sensors 2018, 18, 240. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, T.; Kawakubo, Y.; Hara, S.; Hitosugi, T.; Yokoyama, T.; Miki, Y. Blood Leakage Determination Using the Chromaticity of a Color Sensor. Adv. Biomed. Eng. 2019, 8, 177–184. [Google Scholar] [CrossRef]
- Tan, C.-H.; Khaw, J.-Y.; Wong, Y.-C.; Chionh, C.-Y.; Foong, S. BWatch: A Non-invasive Blood Leakage Detection Device using Robust Reflectance-based Sensing. In Proceedings of the 2019 IEEE International Conference on Cybernetics and Intelligent Systems and IEEE Conference on Robotics, Automation and Mechatronics, Bangkok, Thailand, 18–20 November 2019; pp. 350–355. [Google Scholar]
- Redsensemedical Venous Needle Dislodgement. Available online: https://redsensemedical.com/ (accessed on 20 October 2022).
- Hemodialerttm. 2005. Available online: https://www.hemodialert.com/hemodialert-hemodialysis-alarm.php (accessed on 16 October 2022).
- NIPRO. 2014. Available online: https://www.nipro.co.jp/ (accessed on 20 October 2022).
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Chen, W.-L.; Li, C.-M.; Wu, M.-J.; Huang, P.-T.; Chen, Y.-S. Assistive technology using integrated flexible sensor and virtual alarm unit for blood leakage detection during dialysis therapy. Healthc. Technol. Lett. 2016, 3, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.-T.; Jong, T.-L.; Li, C.-M.; Chen, W.-L.; Lin, C.-H. Integrating flexible sensor and virtual self-organizing DC grid model with cloud computing for blood leakage detection during hemodialysis. IEEE Trans. Biomed. Circuits Syst. 2017, 11, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Berberian, N.; Aamir, Z.; Hélie, S.; Chartier, S. Encoding sparse features in a bidirectional associative memory. In Proceedings of the 2016 International Joint Conference on Neural Networks (IJCNN), Vancouver, BC, Canada, 24–29 July 2016; pp. 5119–5126. [Google Scholar]
- Lin, C.-H.; Chen, W.-L.; Kan, C.-D. Inflow and outflow stenoses screening on biophysical experimental arteriovenous graft using big spectral data and bidirectional associative memory machine learning model. IET Cyber-Phys. Syst. Theory Appl. 2019, 4, 139–147. [Google Scholar] [CrossRef]
- Chartier, S.; Giguère, G.; Langlois, D. A new bidirectional heteroassociative memory encompassing correlational, competitive and topological properties. Neural Netw. 2009, 22, 568–578. [Google Scholar] [CrossRef] [PubMed]
- IEC 60601-1-11:2015; Medical Electrical Equipment-Part 1–11: General Requirements for Basic Safety and Essential Performance-Collateral Standard: Requirements for Medical Electrical Equipment and Medical Electrical Systems Used in the Home Healthcare Environment. International Electrotechnical Commission: London, UK, 2015.
- IEEE Std 802.11™; Institute of Electrical and Electronics Engineers et. al. IEEE Computer Society: Part 11: Wireless LAN Medium Access Control (MAC) and Physical Layer (PHY) Specifications. The Institute of Electrical and Electronics Engineers: Piscataway, NJ, USA, 2012; pp. 1–2793.
- Vallozzi, L.; Van Torre, P.; Hertleer, C.; Rogier, H.; Moeneclaey, M.; Verhaevert, J. Wireless communication for firefighters using dual-polarized textile antennas integrated in their garment. IEEE Trans. Antennas Propag. 2010, 58, 1357–1368. [Google Scholar] [CrossRef]
- Ada, L. Cds Cells, Photoresistors, & Light Dependent Resistors (ldr). 2012. Available online: https://learn.adafruit.com/photocells/measuring-light (accessed on 20 October 2022).
- Doering, E. NI myRIO Project Essentials Guide; National Technology and Science Press: Austin, TX, USA, 2014; pp. 165–168. [Google Scholar]
- Lee, H.-C.; Lin, J.-S. An Open-Source Wearable Sensor System for Detecting Extravasation of Intravenous Infusion. IEEE Trans. Instrum. Meas. 2020, 70, 1–11. [Google Scholar] [CrossRef]
- Chuang, H.-C.; Shih, C.-Y.; Chou, C.-H.; Huang, J.-T.; Wu, C.-J. The development of a blood leakage monitoring system for the applications in hemodialysis therapy. IEEE Sens. J. 2015, 15, 1515–1522. [Google Scholar] [CrossRef]
- Zeng, J.-H.; Tsai, Y.-S. A Wireless Hemodialysis Blood Oozing Detection and Alarm System. In International Conference on the Development of Biomedical Engineering in Vietnam; Springer: Singapore, 2018; pp. 441–444. [Google Scholar]
- IEC 60601-1 International Standard, Medical Electrical Equipment–Part 1: General Requirements for Basic Safety and Essential Performance. Available online: https://www.iso.org/standard/41986.html (accessed on 20 October 2022).
- IEC PAS 63023:2016. Medical Electrical System Input Interface for Haemodialysis Equipment for Use of External Alarming Device. Available online: https://webstore.iec.ch/publication/24041 (accessed on 20 October 2022).






| Event | Level 1: Normal Voltage (V) and S | Level 2 Voltage (V) and S | Level 3 Voltage (V) and S | Level 4 Voltage (V) and S | |
|---|---|---|---|---|---|
| Sensor Point | |||||
| 1 | 4.15–4.30, 0 | 3.1–3.48, 1 | 3.1–3.48, 1 | 3.1–3.48, 1 | |
| 2 | 4.15–4.30, 0 | 3.1–3.48, 1 | 3.1–3.48, 1 | 3.1–3.48, 1 | |
| 3 | 4.15–4.30, 0 | 4.15–4.30, 0 | 3.1–3.48, 1 | 3.1–3.48, 1 | |
| 4 | 4.15–4.30, 0 | 4.15–4.30, 0 | 3.1–3.48, 1 | 3.1–3.48, 1 | |
| 5 | 4.15–4.30, 0 | 4.15–4.30, 0 | 4.15–4.30, 0 | 3.1–3.48, 1 | |
| 6 | 4.15–4.30, 0 | 4.15–4.30, 0 | 4.15–4.30, 0 | 3.1–3.48, 1 | |
| 7 | 4.15–4.30, 0 | 4.15–4.30, 0 | 4.15–4.30, 0 | 3.1–3.48, 1 | |
| 8 | 4.15–4.30, 0 | 4.15–4.30, 0 | 4.15–4.30, 0 | 4.15–4.30, 0 | |
| Methods | Bidirectional Hetero-Associative Memory Network (BHAM) | Virtual Self-Organizing DC Grid Model [17] | |
|---|---|---|---|
| Task | |||
| Network Architecture | (8-4-9) | - | |
| Memory Storage | C Matrix (4 × 8): 128 bytes W Matrix (4 × 9): 144 bytes A Matrix (4 × 9): 144 bytes | n2 for matrix Y and 3 × n for vectors, I, V(p), V(p + 1) 520 bytes | |
| Training Data | Connecting Matrices C, W, and A 256 input–output pairs of training patterns | - | |
| Process Unit | Gaussian Function Hard Limit Function | Multiplication, Division, and Subtraction | |
| Algorithm | Bidirectional Associative Memory | Current Injection Method and Jacobi and Gauss–Seidel Method (<25 iterative computations) | |
| Learning Stage | Matrix Operation | - | |
| Recalling Stage | Iteration computation ≤ 2 | - | |
| Execution Time | Average Time: <0.15 ms | Average Time: <0.30 ms | |
| Accuracy | 100% | 100% | |
| Design Items | Sensor | Sensing Direction | Algorithm | Sensitivity | Detector Time | Connect to Nurse Station | Battery Support | The Alarm Unit Set up on the Arm | |
|---|---|---|---|---|---|---|---|---|---|
| Literature | |||||||||
| [8] | Gas detector | One sensor | - | - | <30 s | WiFi Wireless | Yes | Yes | |
| [9] | Wetness sensing | Multi sensors | - | 15 μL | - | - | - | - | |
| [10] | LED color sensors | One sensor | Light intensity | - | - | - | Yes | No | |
| [11] | LED light sensor | One sensor | Light intensity | - | - | No | Yes | Yes | |
| [26] | LED light sensor | One sensor | - | - | - | WiFi Wireless | Yes | Yes | |
| [27] | Photo interrupter | One sensor | Analog | 0.01 mL | 1.6 s | Bluetooth 4.0 | Yes | Yes | |
| [28] | Photodiode | Two sensors | Digital | - | Every 5 s | WiFi Wireless | Yes | No | |
| This Work | Light sensors | Flexible ring array | Digital | 2 mL | <0.15 ms | WiFi Wireless | No | No | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, P.-T.; Lin, C.-H.; Li, C.-M. Flexible Ring Sensor Array and Machine Learning Model for the Early Blood Leakage Detection during Dialysis. Processes 2022, 10, 2197. https://doi.org/10.3390/pr10112197
Huang P-T, Lin C-H, Li C-M. Flexible Ring Sensor Array and Machine Learning Model for the Early Blood Leakage Detection during Dialysis. Processes. 2022; 10(11):2197. https://doi.org/10.3390/pr10112197
Chicago/Turabian StyleHuang, Ping-Tzan, Chia-Hung Lin, and Chien-Ming Li. 2022. "Flexible Ring Sensor Array and Machine Learning Model for the Early Blood Leakage Detection during Dialysis" Processes 10, no. 11: 2197. https://doi.org/10.3390/pr10112197
APA StyleHuang, P.-T., Lin, C.-H., & Li, C.-M. (2022). Flexible Ring Sensor Array and Machine Learning Model for the Early Blood Leakage Detection during Dialysis. Processes, 10(11), 2197. https://doi.org/10.3390/pr10112197








