Evaluating the Performance of a Safe Insulin Supply Chain Using the AHP-TOPSIS Approach
Abstract
:1. Introduction
Background
- Identifying the significance and issues related to insulin in the PSC;
- Identifying the criteria that will be used to measure the performance of the insulin supply chain to maximize its safety; and
- Assessing the priorities and importance of each criterion to maximize safety.
2. Literature Review
2.1. Significance of Insulin as a Medication
2.2. Common Issues of Insulin in the PSC
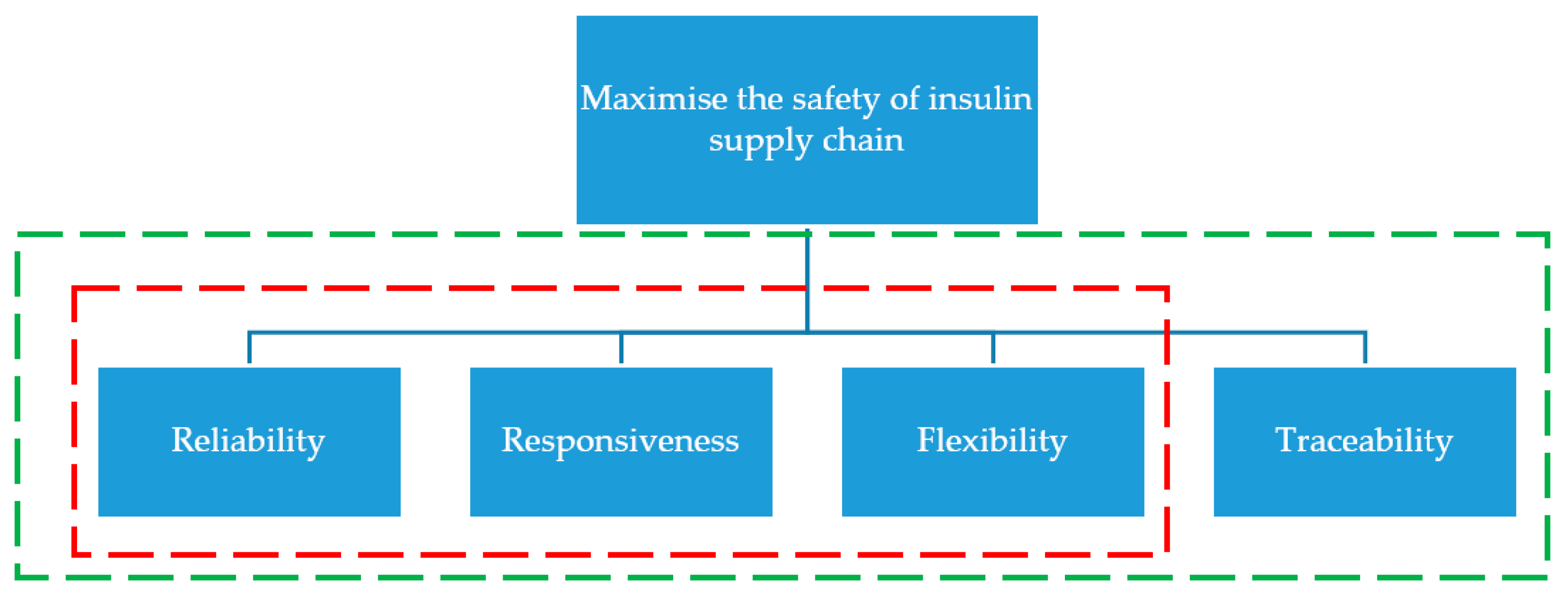
2.3. SCOR for Performance Measures
2.3.1. Reliability
2.3.2. Responsiveness
2.3.3. Flexibility
2.3.4. Traceability Technologies
3. Methodology
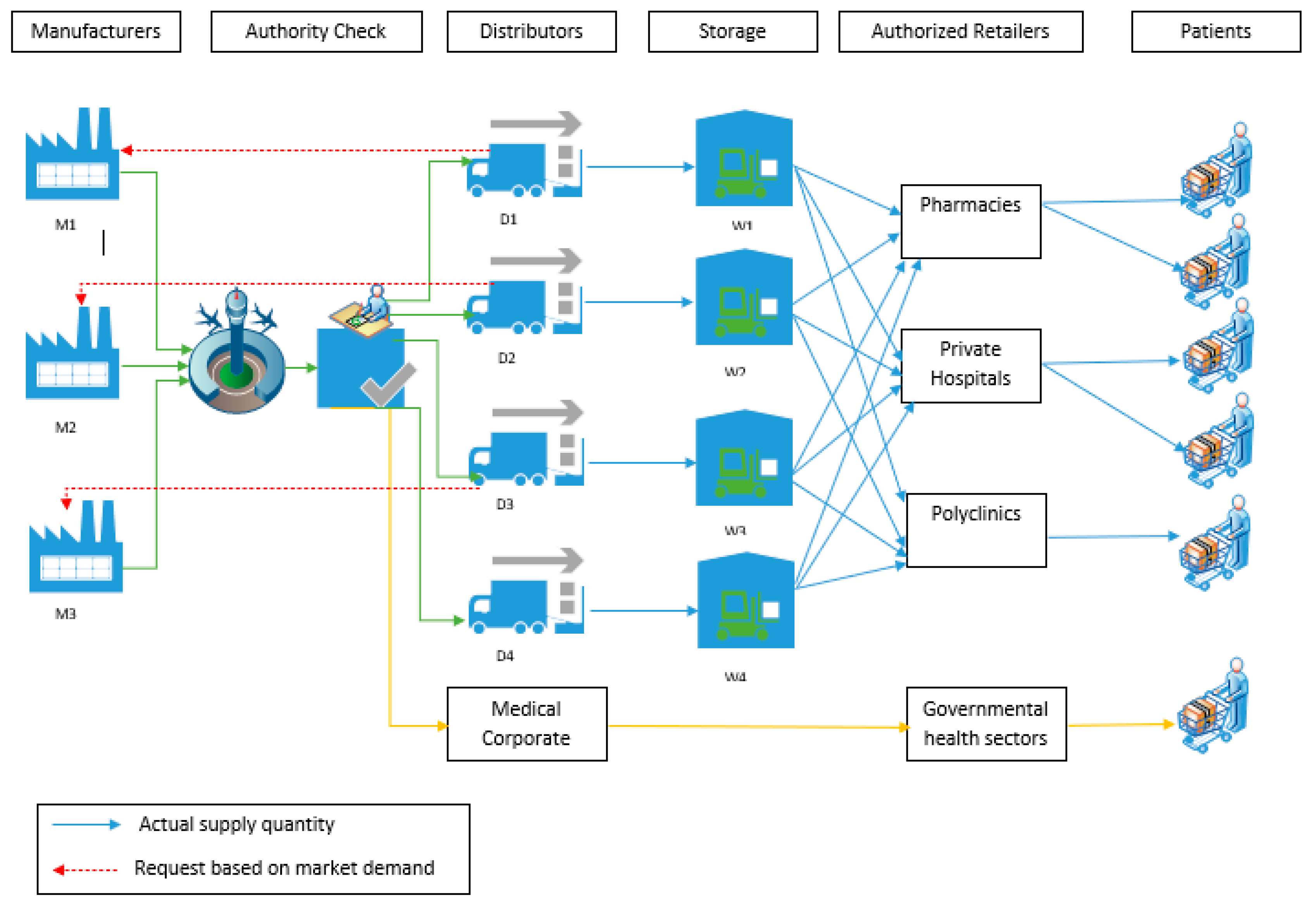
3.1. Model Outline
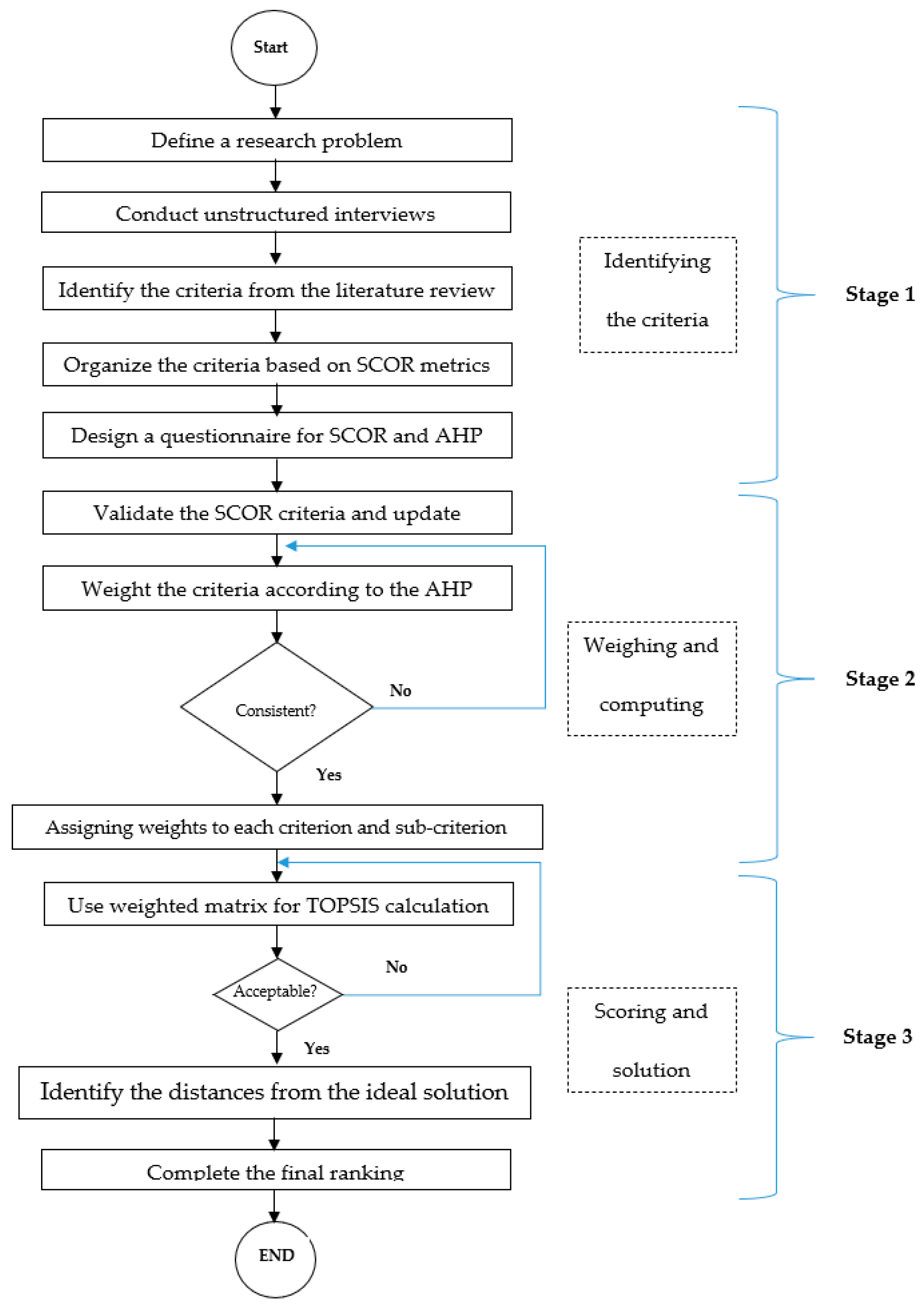
3.2. Proposed Stages
- Define a research problem
- Data Collection
- ○
- Conduct unstructured interviews
- ○
- Identify the criteria from the literature review
- ○
- Determine the criteria based on SCOR metrics
- Design a questionnaire for SCOR and AHP
- Validate the SCOR criteria and update
- Weight the criteria according to the AHP model
- Assigning weights to each criterion and sub-criterion
3.3. Equations
3.3.1. AHP Method
- 1.
- Calculate the geometric means
- 2.
- Performing a pairwise comparison of elements.
- 3.
- Calculating weights and Consistency Ratio (CR).
3.3.2. TOPSIS Method
- Normalized decision matrix ();
- Net weights ();
- Weighted normalized decision matrix ().
- is the net weight from AHP;
- is the AHP normalized decision matrix;
- is the weighted normalized decision matrix.
- 4.
- Identify the ideal best A+ and ideal worst A−
- 5.
- Separation measure for each row calculation
- 6.
- Calculate the relative closeness of each alternative to the ideal solution, as follows:
- 7.
- Rank the attributes based on Ci values
4. Results and Discussion
4.1. SCOR Model Implementation
4.2. AHP
4.3. TOPSIS
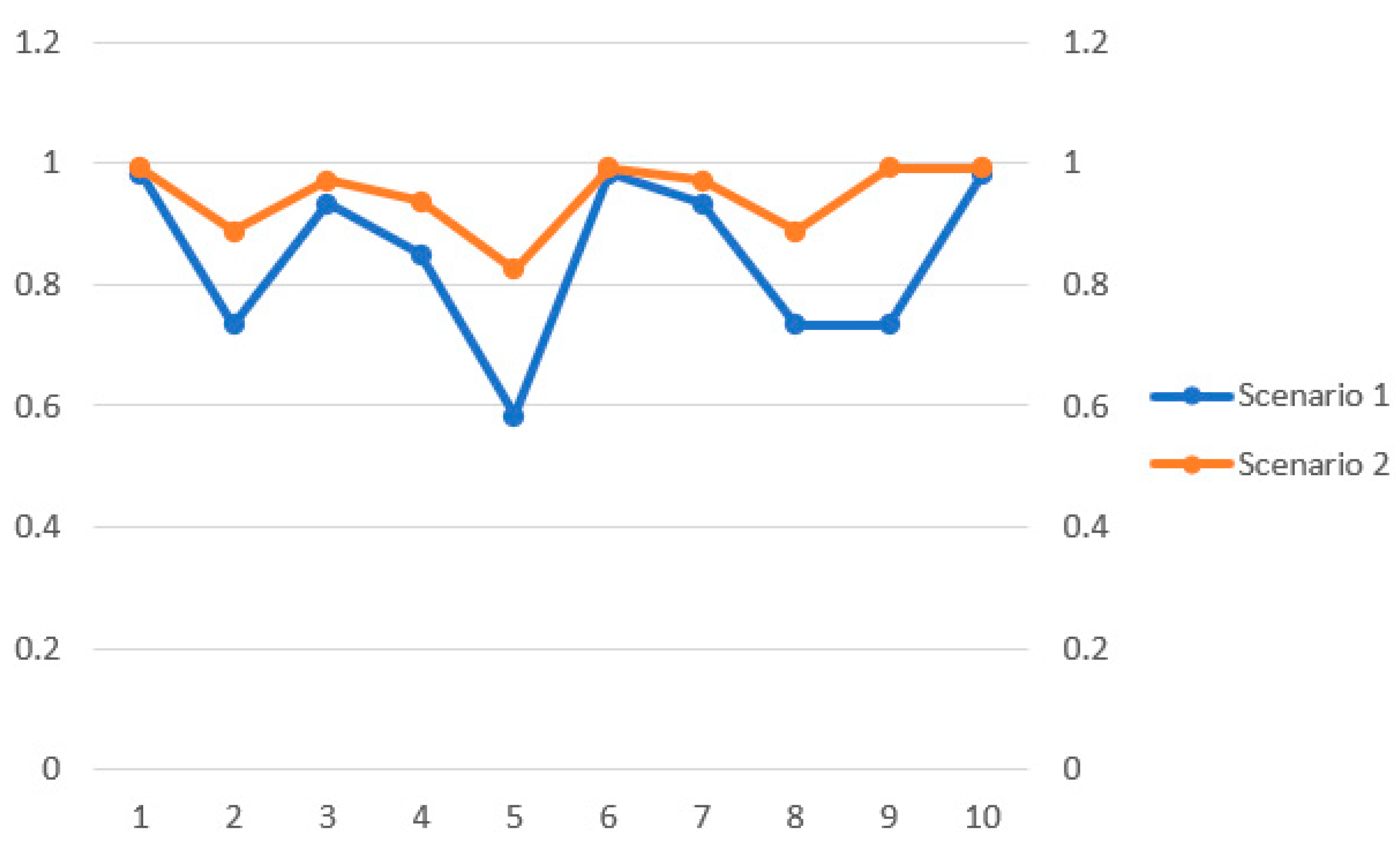
4.3.1. Scenario 1
4.3.2. Scenario 2
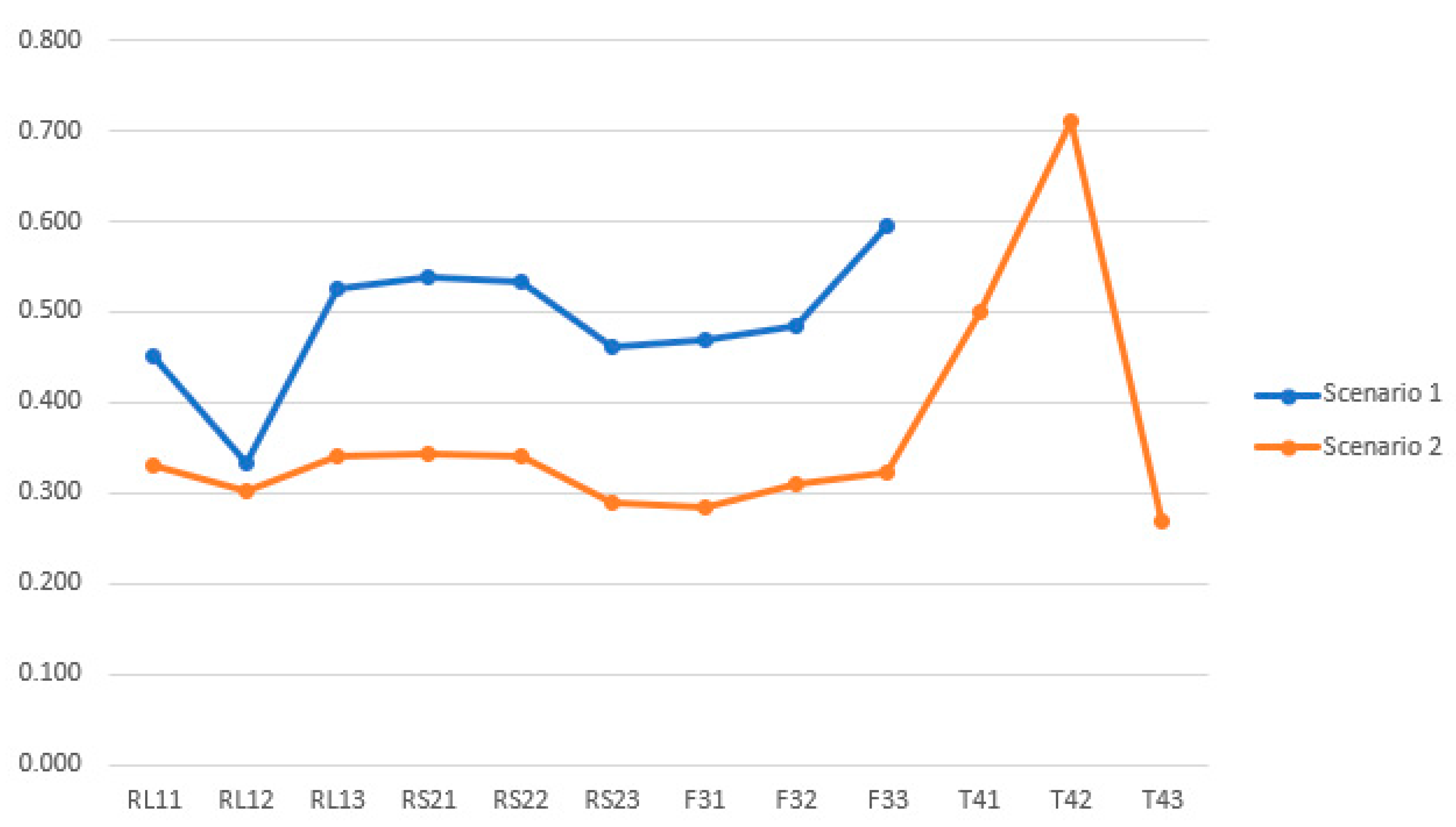
4.4. Results Comparison
4.5. Sensitivity Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Frequency | Percentage (%) | |
|---|---|---|
| Gender | ||
| Male | 62 | 66 |
| Female | 32 | 34 |
| Professional Experience | ||
| Professional in pharmaceutical supply chain management (sourcing, procurement, warehousing, distribution, retailing) | 2 | 2.1 |
| Professional in other sectors of the healthcare industry (hospitals, clinics, other medical service providers) | 1 | 1.1 |
| Experienced in supply chain management | 8 | 8.5 |
| Professional in other sectors of the healthcare industry (hospitals, clinics, other medical service providers) | 51 | 54.3 |
| Professional in pharmaceutical supply chain management (sourcing, procurement, warehousing, distribution, retailing) | 32 | 34.0 |
| Total | 94 | 100.0 |
| Activity in Pharmaceutical Supply Chain | ||
| Distributor/Wholesaler of drugs | 15 | 16.0 |
| Healthcare user | 7 | 7.4 |
| Hospitals/Clinics/Pharmacy | 45 | 47.9 |
| Manufacturer of drugs | 16 | 17.0 |
| Packager of drugs | 3 | 3.2 |
| Supplier of pharmaceuticals’ raw materials | 8 | 8.5 |
| Total | 94 | 100.0 |
| Years of Experience | ||
| >10 years | 15 | 16.0 |
| 1–3 years | 56 | 59.6 |
| 4–6 years | 12 | 12.8 |
| 7–9 years | 11 | 11.6 |
| Total | 94 | 100.0 |
Appendix B
- Which model of the pharmaceutical supply chain does your company use?
- Who are your upper and lower bounds of partners and stakeholders?
- What makes your supply chain robust, and what are the success factors for safe medications?
- What is your procedure for selecting a supplier of raw materials/manufacturer/distributor/pharmacy?
- What are the main barriers you face in ensuring the safety of the medication you receive?
- What are the major problems your company face in transporting insulin?
- What are the steps involved in recalling medications?
- Is a cold supply chain method a better transportation mechanism for transporting insulin? Is it the only one ideal for insulin?
- What traceability technologies do your company usually adopt for their products in general and, in particular, insulin?
- We will be preparing a survey. Are you willing to help us with the required information from your side and your stakeholders?
Appendix C
| Reliability | Responsiveness | Flexibility | |
|---|---|---|---|
| Reliability | 1 | 0.793 | 0.837 |
| Responsiveness | 1.2610340 | 1 | 1.2811673 |
| Flexibility | 1.1947431 | 0.7805382 | 1 |
| Sum | 3.4557772 | 2.5735382 | 3.1181673 |
| Reliability | Responsiveness | Flexibility | ||
|---|---|---|---|---|
| Reliability | 0.2893705 | 0.3081361 | 0.2684269 | 0.2886445 |
| Responsiveness | 0.3649061 | 0.3885701 | 0.4108719 | 0.3881160 |
| Flexibility | 0.3457234 | 0.3032938 | 0.3207012 | 0.3232395 |
| Sum | 1 | 1 | 1 | 1 |
- Consistency Ratio Matrix (CRM) = Average weight matrix (normalized weights of rows) × Net weight matrix
| Reliability | Responsiveness | Flexibility | |
|---|---|---|---|
| Reliability | 0.2893705 × 0.2886445 | 0.3081361 × 0.3881160 | 0.2684269 × 0.3232395 |
| Responsiveness | 0.3649061 × 0.2886445 | 0.3885701 × 0.3881160 | 0.4108719 × 0.3232395 |
| Flexibility | 0.3457234 × 0.2886445 | 0.3032938 × 0.3881160 | 0.3207012 × 0.3232395 |
- CRM =0.86697191.16623040.9710349
- Then, we need to calculate the Consistency Vector Matrix (CVM) =
- CVM =3.00359773.00485003.0040727
References
- Deisingh, A.K. Pharmaceutical counterfeiting. Analyst 2005, 130, 271–279. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Sixty-Third World Health Assembly A63/23 Provisional Agenda Item 11.20; World Health Organization: Geneva, Switzerland, 2010; Available online: https://apps.who.int/gb/ebwha/pdf_files/WHA63/A63_23-en.pdf (accessed on 2 August 2021).
- Haji, M.; Kerbache, L.; Sheriff, K.M.; Al-Ansari, T. Critical success factors and traceability technologies for establishing a safe pharmaceutical supply chain. Methods Protoc. 2021, 4, 85. [Google Scholar] [CrossRef] [PubMed]
- Cheung, H.H.; Choi, S.H. Implementation issues in RFID-based anti-counterfeiting systems. Comput. Ind. 2011, 62, 708–718. [Google Scholar] [CrossRef]
- Bottoni, P.; Caroli, S. Fake pharmaceuticals: A review of current analytical approaches. Microchem. J. 2019, 149, 104053. [Google Scholar] [CrossRef]
- Konrad, W. Black Market Insulin: What You Need to Know; CBS News: New York, NY, USA, 2017; Available online: https://www.cbsnews.com/news/black-market-insulin-what-you-need-to-know/ (accessed on 27 November 2021).
- Beran, D.; Lazo-Porras, M.; Mba, C.M.; Mbanya, J.C. A global perspective on the issue of access to insulin. Diabetologia 2021, 64, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Beran, D.; Ewen, M.; Lipska, K.; Hirsch, I.B.; Yudkin, J.S. Availability and affordability of essential medicines: Implications for global diabetes treatment. Curr. Diabetes Rep. 2018, 18, 1–10. [Google Scholar] [CrossRef]
- Chow, C.K.; Ramasundarahettige, C.; Hu, W.; AlHabib, K.F.; Avezum, A., Jr.; Cheng, X.; Chifamba, J.; Dagenais, G.; Dans, A.; Egbujie, B.A.; et al. Availability and affordability of essential medicines for diabetes across high-income, middle-income, and low-income countries: A prospective epidemiological study. Lancet Diabetes Endocrinol. 2018, 6, 798–808. [Google Scholar] [CrossRef]
- Luo, J.; Kesselheim, A.S.; Sarpatwari, A. Insulin access and affordability in the USA: Anticipating the first interchangeable insulin product. Lancet Diabetes Endocrinol. 2020, 8, 360–362. [Google Scholar] [CrossRef]
- Jacobo-Cabrera, M.; Caballero-Morales, S.O.; Martínez-Flores, J.L.; Cano-Olivos, P. Decision model for the pharmaceutical distribution of insulin. In International Conference on Applied Informatics; Springer: Cham, Switzerland, 2018; pp. 75–89. [Google Scholar] [CrossRef]
- Statista. Estimated Number of Diabetics Worldwide in 2021, 2030, and 2045 (In Millions) [Figure 1]. 2022. Available online: https://www.statista.com/statistics/271442/number-of-diabetics-worldwide/ (accessed on 12 July 2022).
- Biswas, S. Is the World Heading for an Insulin Shortage? BBC News: London, UK, 2018; Available online: https://www.bbc.com/news/world-asia-india-46354989 (accessed on 12 January 2022).
- Vanhee, C.; Janvier, S.; Moens, G.; Deconinck, E.; Courselle, P. A simple dilute and shoot methodology for the identification and quantification of illegal insulin. J. Pharm. Anal. 2016, 6, 326–334. [Google Scholar] [CrossRef]
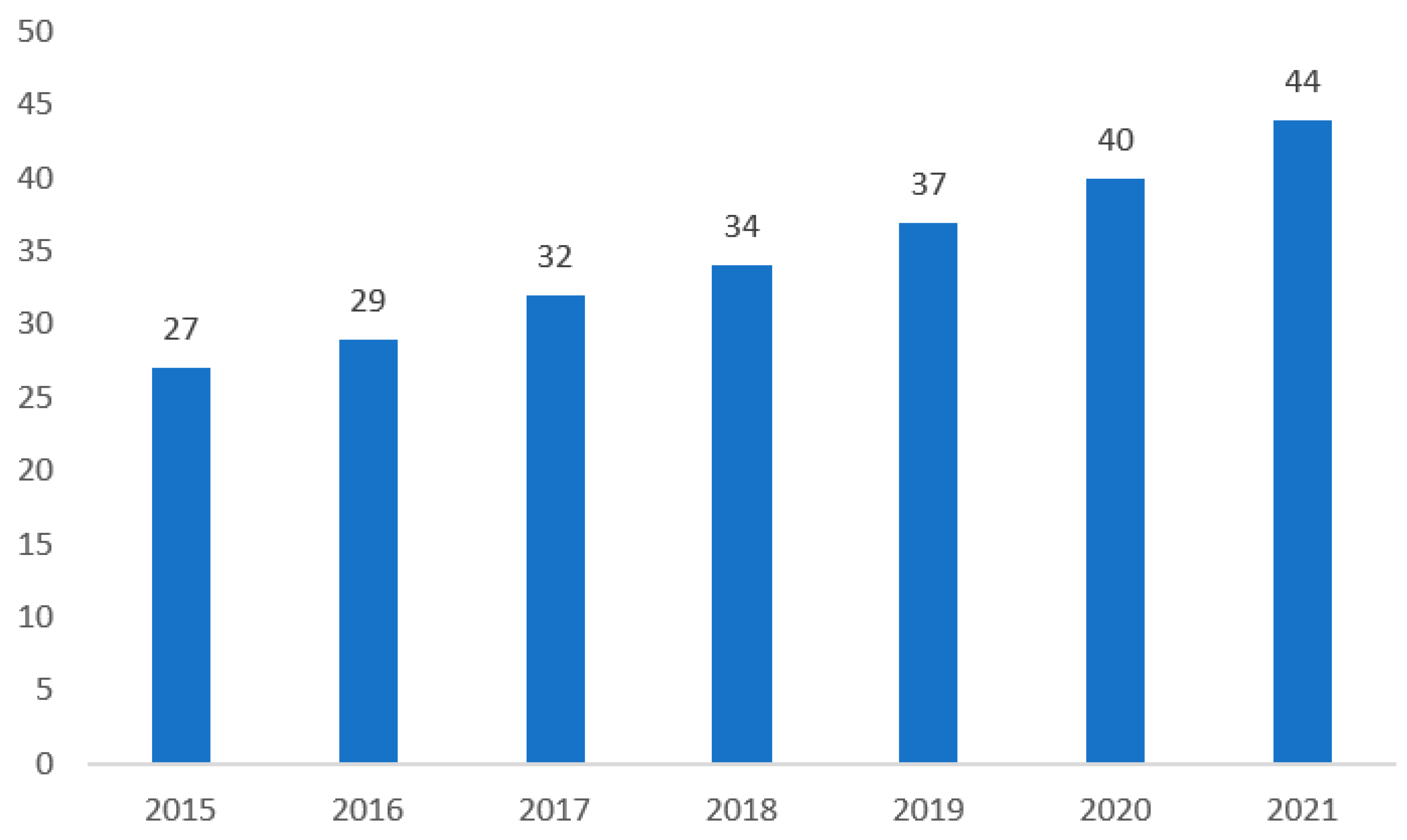
- Statista. Estimated Global Human Insulin Market Revenue from 2015 to 2021 (In Billion U.S. Dollars) [Figure 2]. 2022. Available online: https://www.statista.com/statistics/731843/human-insulin-revenue-worldwide/ (accessed on 12 July 2022).
- Godman, B.; Basu, D.; Pillay, Y.; Mwita, J.C.; Rwegerera, G.M.; Anand Paramadhas, B.D.; Tiroyakgosi, C.; Okwen, P.M.; Niba, L.L.; Nonvignon, J.; et al. Review of ongoing activities and challenges to improve the care of patients with type 2 diabetes across Africa and the implications for the future. Front. Pharmacol. 2020, 11, 108. [Google Scholar] [CrossRef] [Green Version]
- Cefalu, W.T.; Dawes, D.E.; Gavlak, G.; Goldman, D.; Herman, W.H.; Van Nuys, K.; Powers, A.C.; Taylor, S.I.; Yatvin, A.L. Insulin access and affordability working group: Conclusions and recommendations. Diabetes Care 2018, 41, 1299–1311. [Google Scholar] [CrossRef] [Green Version]
- Cameron, A.; Ewen, M.; Ross-Degnan, D.; Ball, D.; Laing, R. Medicine prices, availability, and affordability in 36 developing and middle-income countries: A secondary analysis. Lancet 2009, 373, 240–249. [Google Scholar] [CrossRef]
- Beran, D.; Ewen, M.; Laing, R. Constraints and challenges in access to insulin: A global perspective. Lancet Diabetes Endocrinol. 2016, 4, 275–285. [Google Scholar] [CrossRef]
- Ewen, M.; Joosse, H.J.; Beran, D.; Laing, R. Insulin prices, availability and affordability in 13 low-income and middle-income countries. BMJ Glob. Health 2019, 4, e001410. [Google Scholar] [CrossRef] [Green Version]
- Greene, J.A.; Riggs, K.R. Why is there no generic insulin? Historical origins of a modern problem. N. Engl. J. Med. 2015, 372, 1171–1175. [Google Scholar] [CrossRef]
- Ceriello, A.; deValk, H.W.; Guerci, B.; Haak, T.; Owens, D.; Canobbio, M.; Fritzen, K.; Stautner, C.; Schnell, O. The burden of type 2 diabetes in Europe: Current and future aspects of insulin treatment from patient and healthcare spending perspectives. Diabetes Res. Clin. Pract. 2020, 161, 108053. [Google Scholar] [CrossRef]
- Editors. New WHO Report Maps Barriers to Insulin Availability and Suggests Actions to Promote Universal Access World Health Organization. 2021. Available online: https://wwwwho.int/news/item/12-11-2021-new-who-report-maps-barriers-to-insulin-availability-and-suggests-actions-to-promote-universal-access (accessed on 12 July 2022).
- Cheng, M.M. Is the drugstore safe? Counterfeit diabetes products on the shelves. J. Diabetes Sci. Technol. 2009, 3, 1516–1520. [Google Scholar] [CrossRef] [Green Version]
- Blackstone, E.A.; Fuhr, J.P., Jr.; Pociask, S. The health and economic effects of counterfeit drugs. Am. Health Drug Benefits 2014, 7, 216. [Google Scholar]
- Godman, B.; Haque, M.; Leong, T.; Allocati, E.; Kumar, S.; Islam, S.; Charan, J.; Akter, F.; Kurdi, A.; Vassalo, C.; et al. The current situation regarding long-acting insulin analogues including biosimilars among African, Asian, European, and South American Countries; findings and implications for the future. Front. Public Health 2021, 9, 636. [Google Scholar] [CrossRef]
- Huan, S.H.; Sheoran, S.K.; Wang, G. A review and analysis of supply chain operations reference (SCOR) model. Supply Chain. Manag. Int. J. 2004, 9, 23–29. [Google Scholar] [CrossRef]
- Bolstorff, P.; Rosenbaum, R. Supply chain excellence: A handbook for dramatic improvement using the SCOR model. J. Supply Chain Manag. 2003, 39, 38. [Google Scholar]
- Lambert, D.M. Supply Chain Management: Processes, Partnerships, Performance; Supply Chain Management Inst: San Diego, CA, USA, 2008; p. 305. [Google Scholar]
- Zhou, H.; Benton, W.C., Jr.; Schilling, D.A.; Milligan, G.W. Supply chain integration and the SCOR model. J. Bus. Logist. 2011, 32, 332–344. [Google Scholar] [CrossRef]
- Erkan, T.E.; Ugur, B.A.Ç. Supply chain performance measurement: A case study about applicability of SCOR model in a manufacturing industry firm. Int. J. Bus. Manag. Stud. 2011, 3, 381–390. Available online: https://dergipark.org.tr/en/pub/ijbms/issue/26068/274713 (accessed on 2 August 2022).
- Ikasari, N.; Sutopo, W.; Zakaria, R. Performance measurement in supply chain using SCOR Model in the lithium battery factory. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020; Volume 943, p. 012049. Available online: https://iopscience.iop.org/article/10.1088/1757-899X/943/1/012049/meta (accessed on 2 August 2022).
- Wang, C.N.; Huang, Y.F.; Cheng, I.F.; Nguyen, V.T. A multi-criteria decision-making (MCDM) approach using hybrid SCOR metrics, AHP, and TOPSIS for supplier evaluation and selection in the gas and oil industry. Processes 2018, 6, 252. [Google Scholar] [CrossRef] [Green Version]
- Nazim, R.; Yahya, S.; Malim, M.R. A new approach to supplier selection problem: An introduction of AHP-SCOR integrated model. Int. J. Recent Innov. Trends Comput. Commun. 2015, 3, 338–346. Available online: https://www.researchgate.net/profile/Raja-Nazim-Abdullah/publication/329963234_A_New_Approach_to_Supplier_Selection_Problem_An_Introduction_of_AHP-SCOR_Integrated_Model/links/5c25df05299bf12be39e091d/A-New-Approach-to-Supplier-Selection-Problem-An-Introduction-of-AHP-SCOR-Integrated-Model.pdf (accessed on 2 August 2022).
- Shahin, A.; Jamshidian, M. Indicators of Supply Chain Management (SCM): Performance and Supplier Selection. Available online: https://www.researchgate.net/profile/Arash-Shahin/publication/255597414_Indicators_of_Supply_Chain_Management_SCM_Performance_and_Supplier_Selection/links/58946eaeaca27231daf8b8d1/Indicators-of-Supply-Chain-Management-SCM-Performance-and-Supplier-Selection.pdf (accessed on 12 July 2022).
- Wibowo, M.A.; Sholeh, M.N. Application of supply chain performance measurement in Scor model at building project. IPTEK J. Proc. Ser. 2017, 3, 60–64. Available online: http://iptek.its.ac.id/index.php/jps/article/view/2193 (accessed on 2 August 2022).
- Ayyildiz, E.; Taskin Gumus, A. Interval-valued Pythagorean fuzzy AHP method-based supply chain performance evaluation by a new extension of SCOR model: SCOR 4.0. Complex Intell. Syst. 2021, 7, 559–576. [Google Scholar] [CrossRef]
- Bukhori, I.B.; Widodo, K.H.; Ismoyowati, D. Evaluation of poultry supply chain performance in XYZ slaughtering house Yogyakarta using SCOR and AHP method. Agric. Agric. Sci. Procedia 2015, 3, 221–225. [Google Scholar] [CrossRef] [Green Version]
- Pundarika, A.Z. Supply Chain Performance Measurement by Using SCOR Method and Analytical Hierarchy Process in PT.XYZ. Ph.D. Thesis, President University, Bekasi Regency, Indonesia, 2018. Available online: http://repository.president.ac.id/xmlui/handle/123456789/427 (accessed on 12 January 2022).
- Pan, X.; Zhang, M. Quality and reliability improvement based on the quality function deployment method. In 2018 12th International Conference on Reliability, Maintainability, and Safety (ICRMS); IEEE: Piscataway, NJ, USA, 2018; pp. 38–42. [Google Scholar] [CrossRef]
- Li, D.; Mishra, N. Engaging suppliers for reliability improvement under outcome-based compensations. Omega 2021, 102, 102343. [Google Scholar] [CrossRef]
- Bushuev, M.A.; Guiffrida, A.L. Improving delivery performance for gamma distributed delivery time. Int. J. Bus. Perform. Supply Chain Model. 2019, 10, 195–214. [Google Scholar] [CrossRef]
- Kocaoğlu, B.; Gülsün, B.; Tanyaş, M. A SCOR based approach for measuring a benchmarkable supply chain performance. J. Intell. Manuf. 2013, 24, 113–132. [Google Scholar] [CrossRef]
- Sambasivan, M.; Mohamed, Z.A.; Nandan, T. Performance measures and metrics for e-supply chains. J. Enterp. Inf. Manag. 2009, 22, 346–360. [Google Scholar] [CrossRef]
- Sanayei, A.; Mousavi, S.F.; Abdi, M.R.; Mohaghar, A. An integrated group decision-making process for supplier selection and order allocation using multi-attribute utility theory and linear programming. J. Frankl. Inst. 2008, 345, 731–747. [Google Scholar] [CrossRef]
- Ntabe, E.N.; LeBel, L.; Munson, A.D.; Santa-Eulalia, L.A. A systematic literature review of the supply chain operations reference (SCOR) model application with special attention to environmental issues. Int. J. Prod. Econ. 2015, 169, 310–332. [Google Scholar] [CrossRef]
- Thunberg, M.; Persson, F. Using the SCOR model’s performance measurements to improve construction logistics. Prod. Plan. Control 2014, 25, 1065–1078. [Google Scholar] [CrossRef]
- Bhagwat, R.; Sharma, M.K. Performance measurement of supply chain management using the analytical hierarchy process. Prod. Plan. Control 2007, 18, 666–680. [Google Scholar] [CrossRef]
- Hall, K.D.; Guyenet, S.J.; Leibel, R.L. The carbohydrate-insulin model of obesity is difficult to reconcile with current evidence. JAMA Intern. Med. 2018, 178, 1103–1105. [Google Scholar] [CrossRef]
- Castle, J.R.; DeVries, J.H.; Kovatchev, B. Future of automated insulin delivery systems. Diabetes Technol. Ther. 2017, 19, S-67. [Google Scholar] [CrossRef]
- Xie, J.; Li, A.; Li, J. Advances in pH-sensitive polymers for smart insulin delivery. Macromol. Rapid Commun. 2017, 38, 1700413. [Google Scholar] [CrossRef]
- Bauer, D.; Göbl, M. Flexibility measurement issues in supply chain management. J. Appl. Leadersh. Manag. 2017, 5, 1–14. Available online: https://hdl.handle.net/10419/175332 (accessed on 27 August 2022).
- Sellitto, M.A.; Pereira, G.M.; Borchardt, M.; Da Silva, R.I.; Viegas, C.V. A SCOR-based model for supply chain performance measurement: Application in the footwear industry. Int. J. Prod. Res. 2015, 53, 4917–4926. [Google Scholar] [CrossRef]
- Ertay, T.; Ruan, D.; Tuzkaya, U.R. Integrating data envelopment analysis and analytic hierarchy for the facility layout design in manufacturing systems. Inf. Sci. 2006, 176, 237–262. [Google Scholar] [CrossRef]
- Barnes-Schuster, D.; Bassok, Y.; Anupindi, R. Coordination and flexibility in supply contracts with options. Manuf. Serv. Oper. Manag. 2002, 4, 171–207. [Google Scholar] [CrossRef] [Green Version]
- Ploszczuk, L.; Nolan, R. How Postponement Strategy can Reduce Cost and Lead Time for Pharma Supply Chains. 2021. Available online: https://hdl.handle.net/1721.1/130952 (accessed on 27 November 2021).
- Bollen, A.F.; Emond, J.P. Traceability in postharvest systems. In Postharvest Handling, 3rd ed.; Wojciech, J., Florkowski, R.L., Shewfelt, B., Brueckner, B., Prussia, S.E., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 485–504. [Google Scholar] [CrossRef]
- Millard, P.; Paine, S.; O’Hagan, S.; Hipkiss, J. Traceability of allergenic foods in the food chain. In Handbook of Food Allergen Detection and Control; Woodhead Publishing: Sawston, UK, 2015; pp. 19–40. [Google Scholar] [CrossRef]
- Haji, M.; Kerbache, L.; Muhammad, M.; Al-Ansari, T. Roles of technology in improving perishable food supply chains. Logistics 2020, 4, 33. [Google Scholar] [CrossRef]
- Mackey, T.K.; Nayyar, G. A review of existing and emerging digital technologies to combat the global trade in fake medicines. Expert Opin. Drug Saf. 2017, 16, 587–602. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.Q.; Qin, Z.; Qu, T.; Dai, Q. RFID-enabled pharmaceutical regulatory traceability system. In Proceedings of the 2010 IEEE International Conference on RFID-Technology and Applications, Guangzhou, China, 17–19 June 2010; IEEE: Piscataway, NJ, USA, 2010; pp. 211–216. [Google Scholar] [CrossRef]
- Li, B.H.; Zhang, L.; Wang, S.L.; Tao, F.; Cao, J.W.; Jiang, X.D.; Song, X.; Chai, X.D. Cloud manufacturing: A new service-oriented networked manufacturing model. Comput. Integr. Manuf. Syst. 2010, 16, 1–7. [Google Scholar]
- Kim, J.; Lee, J.; Kim, B.; Kim, J. Raw material criticality assessment with weighted indicators: An application of fuzzy analytic hierarchy process. Resour. Policy 2019, 60, 225–233. [Google Scholar] [CrossRef]
- Benedetti, M.; Bellman, A.; Rotunno, R.; Introna, V.; Cesarotti, V. Impact of track and trace integration on pharmaceutical production systems. Int. J. Eng. Bus. Manag. 2014, 6, 6–25. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Tripathi, R. Traceability of counterfeit medicine supply chain through Blockchain. In Proceedings of the 2019 11th International Conference on Communication Systems & Networks (COMSNETS), Bengaluru, India, 7–11 January 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 568–570. [Google Scholar] [CrossRef]
- Rahman, M.S.; Yoshida, N.; Tsuboi, H.; Tomizu, N.; Endo, J.; Miyu, O.; Akimoto, Y.; Kimura, K. The health consequences of falsified medicines—A study of the published literature. Trop. Med. Int. Health 2018, 23, 1294–1303. [Google Scholar] [CrossRef] [Green Version]
- Wazid, M.; Das, A.K.; Khan, M.K.; Al-Ghaiheb, A.A.D.; Kumar, N.; Vasilakos, A.V. Secure authentication scheme for medicine anti-counterfeiting system in IoT environment. IEEE Internet Things J. 2017, 4, 1634–1646. [Google Scholar] [CrossRef]
- Bansal, D.; Malla, S.; Gudala, K.; Tiwari, P. Anti-counterfeit technologies: A pharmaceutical industry perspective. Sci. Pharm. 2013, 81, 1–14. [Google Scholar] [CrossRef]
- Li, Y.; Marier-Bienvenue, T.; Perron-Brault, A.; Wang, X.; Paré, G. Blockchain technology in business organizations: A scoping review. In Proceedings of the 51st Hawaii International Conference on System Sciences, Waikoloa Village, HI, USA, 3–6 January 2018; Available online: https://scholarspace.manoa.hawaii.edu/handle/10125/50454/ (accessed on 14 February 2021).
- Brennan, F.; Williams, P.; Armstrong, K.; Klatman, E.; Donelan, N.; Ogle, G.D.; Eussen, A.; Jenkins, A.J. A human rights-based approach to improve access to insulin and other aspects of diabetes care. Diabetes Res. Clin. Pract. 2022, 183, 109153. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Yusuf, S.; Chow, C.K. Availability and affordability of medicines for diabetes and cardiovascular disease across countries: Information learned from the prospective urban rural epidemiological study. Diabetology 2022, 3, 236–245. [Google Scholar] [CrossRef]
- Shukar, S.; Zahoor, F.; Hayat, K.; Saeed, A.; Gillani, A.H.; Omer, S.; Hu, S.; Babar, Z.; Fang, Y.; Yang, C. Drug shortage: Causes, impact, and mitigation strategies. Front. Pharmacol. 2021, 12, 693426. [Google Scholar] [CrossRef]
- Bonsu, D.O.M.; Afoakwah, C.; Aguilar-Caballos, M.D.L.P. Counterfeit formulations: Analytical perspective on anorectics. Forensic Toxicol. 2021, 39, 1–25. [Google Scholar] [CrossRef]
- Hamidli, N.; Pajaziti, B.; Andrási, M.; Nagy, C.; Gáspár, A. Determination of human insulin and its six therapeutic analogues by capillary electrophoresis–mass spectrometry. J. Chromatogr. A 2022, 1678, 463351. [Google Scholar] [CrossRef]
- Penley, B.; Minshew, L.; Chen, H.H.; Eckel, S.; Ozawa, S. Accessibility of low-cost insulin from illegitimate internet pharmacies: Cross-sectional study. J. Med. Internet Res. 2022, 24, e25855. Available online: https://preprints.jmir.org/preprint/25855 (accessed on 27 August 2022).
- Haji, M.; Kerbache, L.; Al-Ansari, T. Food Quality, Drug Safety, and Increasing Public Health Measures in Supply Chain Management. Processes 2022, 10, 1715. [Google Scholar] [CrossRef]
- Tobias, S.; Shapiro, A.M.; Grant, C.J.; Patel, P.; Lysyshyn, M.; Ti, L. Drug checking identifies counterfeit alprazolam tablets. Drug Alcohol Depend. 2021, 218, 108300. [Google Scholar] [CrossRef]
- Uddin, M. Blockchain Medledger: Hyperledger fabric enabled drug traceability system for counterfeit drugs in pharmaceutical industry. Int. J. Pharm. 2021, 597, 120235. [Google Scholar] [CrossRef]
- White, C.M. Counterfeit drugs: A major issue for vulnerable citizens throughout the world and in the United States. J. Am. Pharm. Assoc. 2021, 61, e93–e98. [Google Scholar] [CrossRef]
- Ozsahin, D.U.; Hamidat, L.; Alimi, F.D.; Uzun, B.; Ozsahin, I. Evaluation of migraine drugs using MCDM methods. In Applications of Multi-Criteria Decision-Making Theories in Healthcare and Biomedical Engineering; Academic Press: Cambridge, MA, USA, 2021; pp. 261–275. [Google Scholar] [CrossRef]
- Hien, D.N.; Thanh, N.V. Optimization of cold chain logistics with Fuzzy MCDM Model. Processes 2022, 10, 947. [Google Scholar] [CrossRef]
- Hanugrani, N.; Setyanto, N.W.; Efranto, R.Y. Measurement of supply chain performance using Supply Chain Operation Reference (SCOR) Based on Analytical Hierarchy Process (AHP) and Objective Matrix (OMAX). J. Rekayasa Dan Manaj. Sist. Ind. 2013, 1, 127417. [Google Scholar]
- Marzouk, M.; Sabbah, M. AHP-TOPSIS social sustainability approach for selecting supplier in construction supply chain. Clean. Environ. Syst. 2021, 2, 100034. [Google Scholar] [CrossRef]
- Saaty, T.L. The Analytic Hierarchy Process; McGraw-Hill: New York, NY, USA, 1980. [Google Scholar]
- Saaty, T.L. Axiomatic foundation of the analytic hierarchy process. Manag. Sci. 1986, 32, 841–855. [Google Scholar] [CrossRef]
- Saaty, T.L. Fundamentals of Decision Making and Priority Theory with the Analytic Hierarchy Process; RWS Publications: Pittsburgh, PA, USA, 1994. [Google Scholar]
- Fichtner, J. On deriving priority vectors from matrices of pairwise comparisons. Socio-Econ. Plan. Sci. 1986, 20, 341–345. [Google Scholar] [CrossRef]
- Yoon, K.P.; Hwang, C.L. Multiple Attribute Decision Making: An Introduction; SAGE Publications: Thousand Oaks, CA, USA, 1995. [Google Scholar]
- Sidhu, S.S.; Singh, K.; Ahuja, I.S. Ranking of implementation dimensions for maintenance practices in Northern Indian SMEs using integrated AHP-TOPSIS approach. J. Small Bus. Entrep. 2022, 34, 175–194. [Google Scholar] [CrossRef]
- Chaharsooghi, S.K.; Ashrafi, M. Sustainable supplier performance evaluation and selection with neofuzzy TOPSIS method. Int. Sch. Res. Not. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Chu, T.C. Selecting plant location via a fuzzy TOPSIS approach. Int. J. Adv. Manuf. Technol. 2002, 20, 859–864. [Google Scholar] [CrossRef]
- Triantaphyllou, E.; Shu, B.; Sanchez, S.N.; Ray, T. Multi-criteria decision making: An operations research approach. Encycl. Electr. Electron. Eng. 1998, 15, 175–186. Available online: https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.471.4670&rep=rep1&type=pdf (accessed on 2 August 2022).
- Glover, D.G.; Hermans, J. Improving the Traceability of the Clinical Trial Supply Chain. 2017. Available online: https://www.appliedclinicaltrialsonline.com/view/improving-traceability-clinical-trial-supply-chain (accessed on 10 March 2022).
- Kumar, A.; Zavadskas, E.K.; Mangla, S.K.; Agrawal, V.; Sharma, K.; Gupta, D. When risks need attention: Adoption of green supply chain initiatives in the pharmaceutical industry. Int. J. Prod. Res. 2019, 57, 3554–3576. [Google Scholar] [CrossRef]
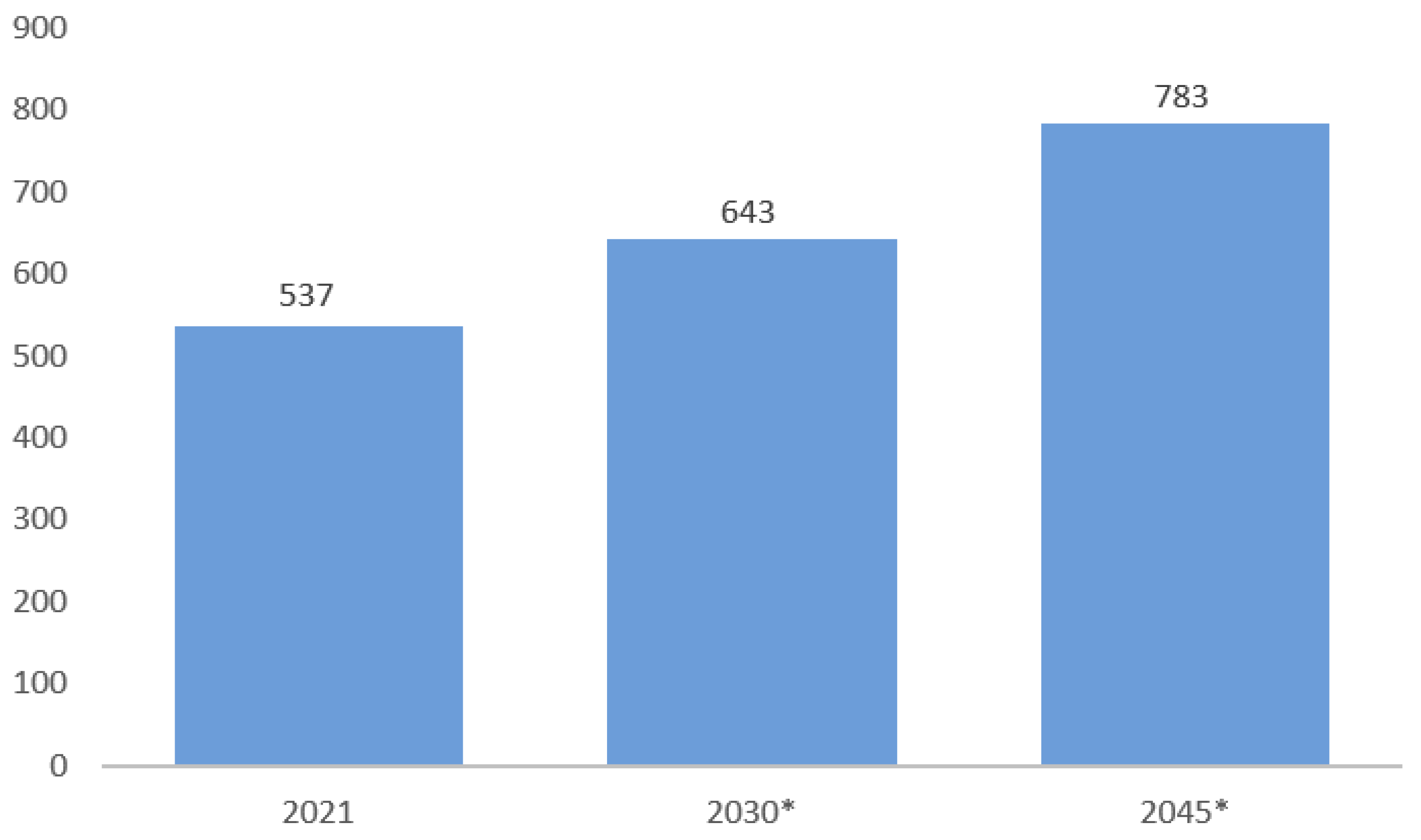


| Model | Section | Performance Attributes | Definition |
|---|---|---|---|
| SCOR performance attributes | Customer | Reliability | Efficiency of the process. Distributing supplies to the right clients at the right time, place, and quantity, and with the expected packaging and quality. |
| Responsiveness | The speed at which tasks are accomplished. Providing products to customers as quickly as possible. | ||
| Flexibility | A flexible approach to change ensures that a supply chain remains competitive by responding to market changes. | ||
| Internal | Cost | Expenses associated with the supply chain operations. Controlling and reducing all costs of the supply chain processes. | |
| Assets | Utilizing assets efficiently. Managing and optimizing assets to meet demand. |
| Level 1 KPIs | Level 2 KPIs | Codes | Scenario 1 | Scenario 2 |
|---|---|---|---|---|
| Reliability (RL) | Maximize timely delivery | RL11 |  |  |
| Maximize documentation accuracy | RL12 |  |  | |
| Maximize quality | RL13 |  |  | |
| Responsiveness (RS) | Maximize supplier assistance rate | RS21 |  |  |
| Minimize delivery lead time | RS22 |  |  | |
| Minimize time to solve a complaint | RS23 |  |  | |
| Flexibility (F) | Volume change flexibility | F31 |  |  |
| Item change flexibility | F32 |  |  | |
| Custom order flexibility | F33 |  |  | |
| Traceability (T) | Sensors, such as IOT or RFID | T41 |  | |
| Blockchain | T42 |  | ||
| Pedigrees and mass serialization | T43 |  |
| Intensity of Importance | Definition | Explanation |
|---|---|---|
| 1 | Equal importance | Two elements contribute equally to the objective. |
| 3 | Moderate importance | Experience and judgement favor moderately one element over another. |
| 5 | Strong importance | One element is favored strongly over another; its dominance is practically demonstrated. |
| 7 | Very strong importance | The evidence favoring one element over another has the highest possibility of affirmation. |
| 9 | Extreme importance | One element is favored against another at the highest possibility of the order of affirmation. |
| 2, 4, 6, and 8 were used for expressing immediate values. | ||
| L 1 KPIs | L 2 KPIs | L 1 KPIs Weights | L 2 KPIs Weights |
|---|---|---|---|
| Reliability (RL) | RL11 | 0.2886445 | 0.0921203 |
| RL12 | 0.1019035 | ||
| RL13 | 0.1034720 | ||
| Responsiveness (RS) | RS21 | 0.3881160 | 0.1025291 |
| RS22 | 0.1082195 | ||
| RS23 | 0.1165034 | ||
| Flexibility (F) | F31 | 0.3232395 | 0.1219434 |
| F32 | 0.1242532 | ||
| F33 | 0.1290556 | ||
| Sum | 1 |
| L 1 KPIs | L 2 KPIs | L 1 KPIs Weights | L 2 KPIs Weights |
|---|---|---|---|
| Reliability (RL) | RL11 | 0.2190725 | 0.0684206 |
| RL12 | 0.0735677 | ||
| RL13 | 0.0747803 | ||
| Responsiveness (RS) | RS21 | 0.2698025 | 0.0744026 |
| RS22 | 0.0774349 | ||
| RS23 | 0.0799297 | ||
| Flexibility (F) | F31 | 0.2288229 | 0.0824567 |
| F32 | 0.0846041 | ||
| F33 | 0.0865902 | ||
| Traceability (T) | T41 | 0.2823022 | 0.1018253 |
| T42 | 0.1032666 | ||
| T43 | 0.0927213 | ||
| Sum | 1 |
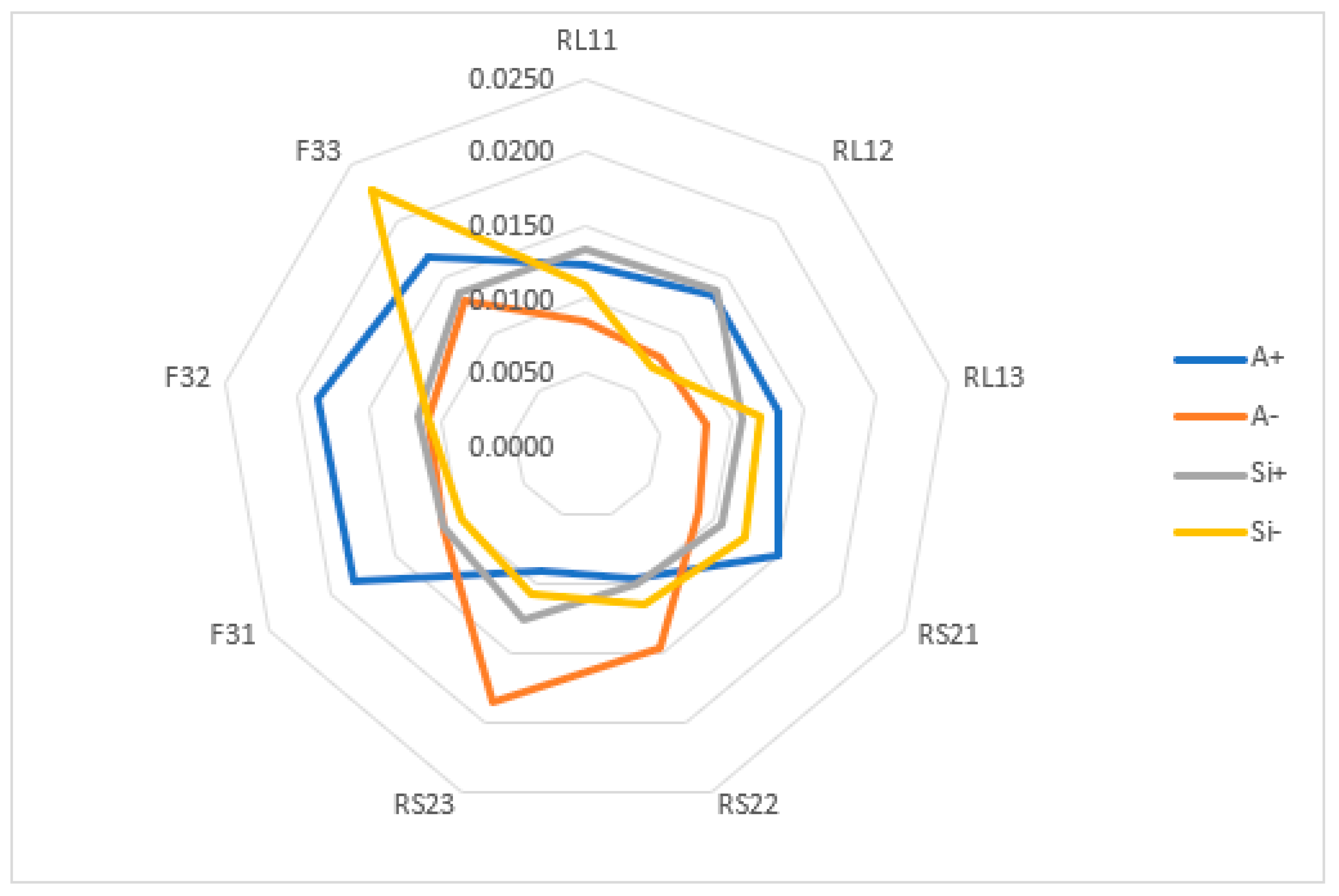
| L2 KPIs | A+ | A− | Si+ | Si− | Si+ + Si− | Ci | % | Rank |
|---|---|---|---|---|---|---|---|---|
| RL11 | 0.0123 | 0.0085 | 0.0133 | 0.0109 | 0.0242 | 0.4500 | 45.00 | 8 |
| RL12 | 0.0134 | 0.0079 | 0.0139 | 0.0069 | 0.0207 | 0.3320 | 33.20 | 9 |
| RL13 | 0.0133 | 0.0082 | 0.0108 | 0.0120 | 0.0228 | 0.5259 | 52.59 | 4 |
| RS21 | 0.0150 | 0.0088 | 0.0106 | 0.0124 | 0.0230 | 0.5388 | 53.88 | 2 |
| RS22 | 0.0096 | 0.0147 | 0.0099 | 0.0114 | 0.0213 | 0.5344 | 53.44 | 3 |
| RS23 | 0.0091 | 0.0185 | 0.0125 | 0.0107 | 0.0232 | 0.4618 | 46.18 | 7 |
| F31 | 0.0182 | 0.0112 | 0.0111 | 0.0099 | 0.0210 | 0.4698 | 46.98 | 6 |
| F32 | 0.0186 | 0.0109 | 0.0116 | 0.0108 | 0.0224 | 0.4836 | 48.36 | 5 |
| F33 | 0.0167 | 0.0128 | 0.0092 | 0.0136 | 0.0228 | 0.5956 | 59.56 | 1 |
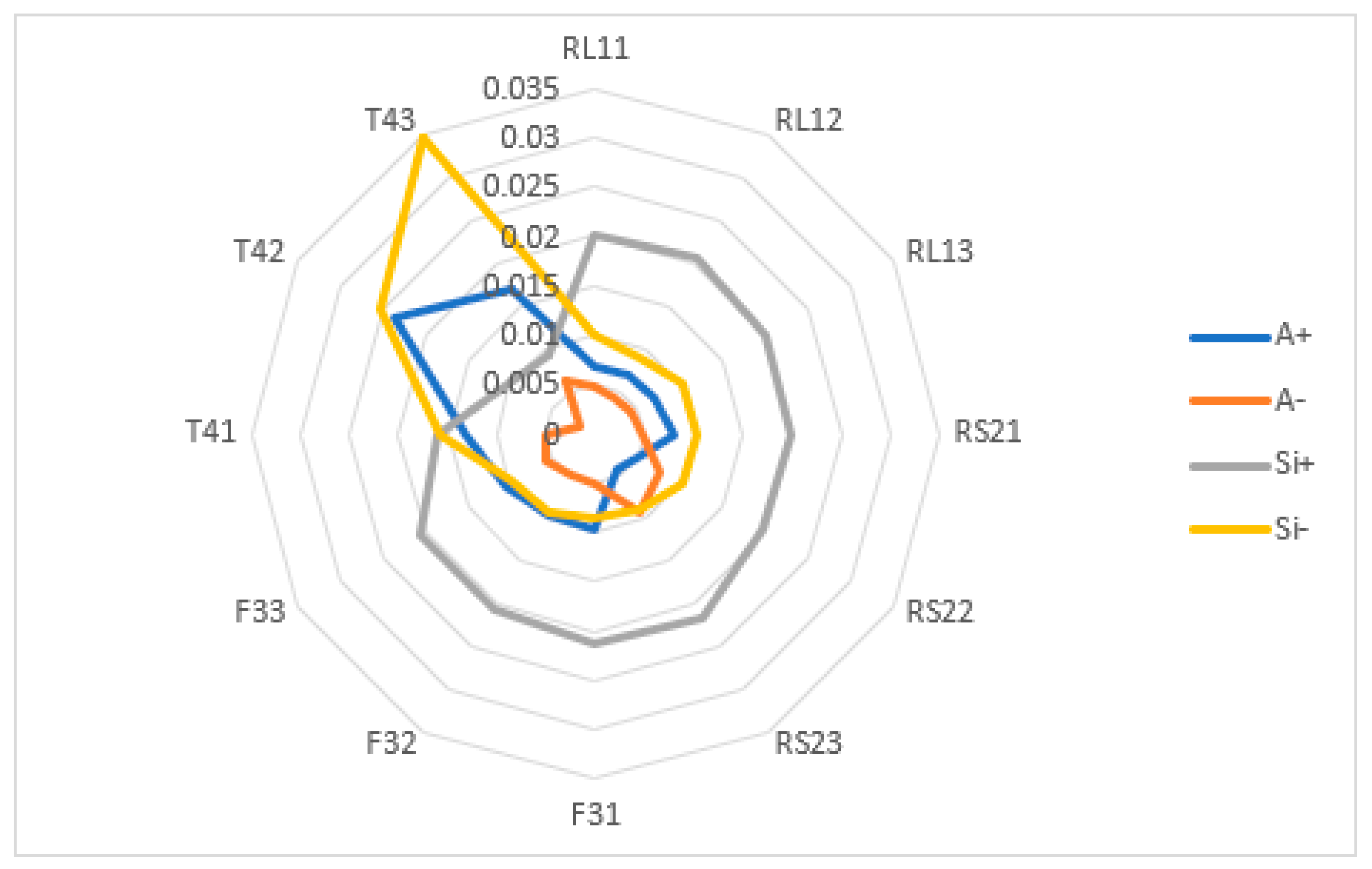
| L2 KPIs | A+ | A− | Si+ | Si− | Si+ + Si− | Ci | % | Rank |
|---|---|---|---|---|---|---|---|---|
| RL11 | 0.0068 | 0.0047 | 0.0202 | 0.0099 | 0.0301 | 0.3291 | 32.91 | 6 |
| RL12 | 0.0070 | 0.0041 | 0.0205 | 0.0089 | 0.0294 | 0.3019 | 30.19 | 9 |
| RL13 | 0.0070 | 0.0043 | 0.0198 | 0.0103 | 0.0300 | 0.3414 | 34.14 | 4 |
| RS21 | 0.0079 | 0.0047 | 0.0199 | 0.0104 | 0.0303 | 0.3427 | 34.27 | 3 |
| RS22 | 0.0049 | 0.0076 | 0.0196 | 0.0101 | 0.0297 | 0.3404 | 34.04 | 5 |
| RS23 | 0.0043 | 0.0091 | 0.0216 | 0.0088 | 0.0304 | 0.2893 | 28.93 | 10 |
| F31 | 0.0096 | 0.0050 | 0.0213 | 0.0085 | 0.0298 | 0.2839 | 28.39 | 11 |
| F32 | 0.0094 | 0.0050 | 0.0204 | 0.0092 | 0.0297 | 0.3110 | 31.10 | 8 |
| F33 | 0.0105 | 0.0057 | 0.0204 | 0.0097 | 0.0301 | 0.3226 | 32.26 | 7 |
| T41 | 0.0130 | 0.0051 | 0.0159 | 0.0158 | 0.0317 | 0.4988 | 49.88 | 2 |
| T42 | 0.0236 | 0.0017 | 0.0103 | 0.0251 | 0.0354 | 0.7097 | 70.97 | 1 |
| T43 | 0.0170 | 0.0061 | 0.0254 | 0.0093 | 0.0347 | 0.2685 | 26.85 | 12 |
| RL11 | RL12 | RL13 | RS21 | RS22 | RS23 | F31 | F32 | F33 | Ranking | |
|---|---|---|---|---|---|---|---|---|---|---|
| Original Weights | 0.09212 | 0.101904 | 0.103472 | 0.102529 | 0.108219 | 0.116503 | 0.121943 | 0.124253 | 0.129056 | F33 > F32 > F31 > RS23 > RS22 > RL13 > RS21 > RL12 > RL11 |
| Test 1 | 0.101904 | 0.09212 | 0.103472 | 0.102529 | 0.108219 | 0.116503 | 0.121943 | 0.124253 | 0.129056 | F33 > F32 > F31 > RS23 > RS22 > RL13 > RS21 > RL12 > RL11 |
| Test 2 | 0.108219 | 0.101904 | 0.103472 | 0.102529 | 0.09212 | 0.116503 | 0.121943 | 0.124253 | 0.129056 | F33 > F32 > F31 > RS23 > RL11 > RL13 > RS21 > RL12 > RS22 |
| Test 3 | 0.09212 | 0.103472 | 0.101904 | 0.102529 | 0.108219 | 0.116503 | 0.121943 | 0.124253 | 0.129056 | F33 > F32 > F31 > RS23 > RL12 > RL13 > RS21 > RS22 > RL11 |
| Test 4 | 0.09212 | 0.108219 | 0.103472 | 0.102529 | 0.101904 | 0.116503 | 0.121943 | 0.124253 | 0.129056 | F33 > F32 > RL12 > RS23 > RS22 > RL13 > RS21 > F31 > RL11 |
| Test 5 | 0.09212 | 0.121943 | 0.103472 | 0.102529 | 0.108219 | 0.116503 | 0.101904 | 0.124253 | 0.129056 | F33 > F32 > F31 > RS23 > RS22 > RS21 > RL13 > RL12 > RL11 |
| Test 6 | 0.09212 | 0.101904 | 0.102529 | 0.103472 | 0.108219 | 0.116503 | 0.121943 | 0.124253 | 0.129056 | F33 > F32 > F31 > RS23 > RS22 > RL13 > RS21 > RL12 > RL11 |
| Test 7 | 0.09212 | 0.101904 | 0.116503 | 0.102529 | 0.108219 | 0.103472 | 0.121943 | 0.124253 | 0.129056 | F33 > F32 > F31 > RL13 > RS22 > RS23 > RS21 > RL12 > RL11 |
| Test 8 | 0.09212 | 0.101904 | 0.124253 | 0.102529 | 0.108219 | 0.116503 | 0.121943 | 0.103472 | 0.129056 | F33 > RL13 > F31 > RS23 > RS22 > F32 > RS21 > RL12 > RL11 |
| Test 9 | 0.09212 | 0.101904 | 0.103472 | 0.121943 | 0.108219 | 0.116503 | 0.102529 | 0.124253 | 0.129056 | F33 > F32 > RS21 > RS23 > RS22 > RL13 > F31 > RL12 > RL11 |
| Test 10 | 0.09212 | 0.101904 | 0.103472 | 0.102529 | 0.116503 | 0.108219 | 0.121943 | 0.124253 | 0.129056 | F33 > F32 > F31 > RS22 > RS23 > RL13 > RS21 > RL12 > RL11 |
| RL11 | RL12 | RL13 | RS21 | RS22 | RS23 | F31 | F32 | F33 | T41 | T42 | T43 | Rankin | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Original Weights | 0.068421 | 0.073568 | 0.07478 | 0.074403 | 0.077435 | 0.07993 | 0.082457 | 0.084604 | 0.08659 | 0.101825 | 0.103267 | 0.092721 | T42 > T41 > T43 > F33 > F32 > F31 > RS23 > RS22 > RL13 > RS21 > RL12 > RL11 |
| Test 1 | 0.073568 | 0.068421 | 0.07478 | 0.074403 | 0.077435 | 0.07993 | 0.082457 | 0.084604 | 0.08659 | 0.101825 | 0.103267 | 0.092721 | T42 > T41 > T43 > F33 > F32 > F31 > RS23 > RS22 > RL13 > RS21 > RL11 > RL12 |
| Test 2 | 0.077435 | 0.073568 | 0.07478 | 0.074403 | 0.068421 | 0.07993 | 0.082457 | 0.084604 | 0.08659 | 0.101825 | 0.103267 | 0.092721 | T42 > T41 > T43 > F33 > F32 > F31 > RS23 > RL11 > RL13 > RS21 > RL12 > RS22 |
| Test 3 | 0.068421 | 0.07478 | 0.073568 | 0.074403 | 0.077435 | 0.07993 | 0.082457 | 0.084604 | 0.08659 | 0.101825 | 0.103267 | 0.092721 | T42 > T41 > T43 > F33 > F32 > F31 > RS23 > RS22 > RL12 > RS21 > RL13 > RL11 |
| Test 4 | 0.068421 | 0.077435 | 0.07478 | 0.074403 | 0.073568 | 0.07993 | 0.082457 | 0.084604 | 0.08659 | 0.101825 | 0.103267 | 0.092721 | T42 > T41 > T43 > F33 > F32 > F31 > RS23 > RL12 > RL13 > RS22 > RL11 > |
| Test 5 | 0.068421 | 0.082457 | 0.07478 | 0.074403 | 0.077435 | 0.07993 | 0.073568 | 0.084604 | 0.08659 | 0.101825 | 0.103267 | 0.092721 | T42 > T41 > T43 > F33 > F32 > RL12 > RS23 > RS22 > RL13 > RS21 > F31 > RL11 |
| Test 6 | 0.068421 | 0.073568 | 0.074403 | 0.07478 | 0.077435 | 0.07993 | 0.082457 | 0.084604 | 0.08659 | 0.101825 | 0.103267 | 0.092721 | T42 > T41 > T43 > F33 > F32 > F31 > RS23 > RS22 > RS21 > RL13 > RL12 > RL11 |
| Test 7 | 0.068421 | 0.073568 | 0.07993 | 0.074403 | 0.077435 | 0.07478 | 0.082457 | 0.084604 | 0.08659 | 0.101825 | 0.103267 | 0.092721 | T42 > T41 > T43 > F33 > F32 > F31 > RL13 > RS22 > RS23 > RS21 > RL12 > RL11 |
| Test 8 | 0.068421 | 0.073568 | 0.084604 | 0.074403 | 0.077435 | 0.07993 | 0.082457 | 0.07478 | 0.08659 | 0.101825 | 0.103267 | 0.092721 | T42 > T41 > T43 > F33 > RL13 > F31 > RS23 > RS22 > F32 > RS21 > RL12 > RL11 |
| Test 9 | 0.068421 | 0.073568 | 0.07478 | 0.074403 | 0.077435 | 0.103267 | 0.082457 | 0.084604 | 0.08659 | 0.101825 | 0.07993 | 0.092721 | T42 > T41 > F33 > T43 > F32 > F31 > RS23 > RS22 > RL13 > RS21 > RL12 > RL11 |
| Test 10 | 0.068421 | 0.073568 | 0.07478 | 0.074403 | 0.077435 | 0.07993 | 0.082457 | 0.084604 | 0.092721 | 0.101825 | 0.103267 | 0.08659 | T42 > T41 > F33 > T43 > F32 > F31 > RS23 > RS22 > RL13 > RS21 > RL12 > RL11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haji, M.; Kerbache, L.; Al-Ansari, T. Evaluating the Performance of a Safe Insulin Supply Chain Using the AHP-TOPSIS Approach. Processes 2022, 10, 2203. https://doi.org/10.3390/pr10112203
Haji M, Kerbache L, Al-Ansari T. Evaluating the Performance of a Safe Insulin Supply Chain Using the AHP-TOPSIS Approach. Processes. 2022; 10(11):2203. https://doi.org/10.3390/pr10112203
Chicago/Turabian StyleHaji, Mona, Laoucine Kerbache, and Tareq Al-Ansari. 2022. "Evaluating the Performance of a Safe Insulin Supply Chain Using the AHP-TOPSIS Approach" Processes 10, no. 11: 2203. https://doi.org/10.3390/pr10112203
APA StyleHaji, M., Kerbache, L., & Al-Ansari, T. (2022). Evaluating the Performance of a Safe Insulin Supply Chain Using the AHP-TOPSIS Approach. Processes, 10(11), 2203. https://doi.org/10.3390/pr10112203








