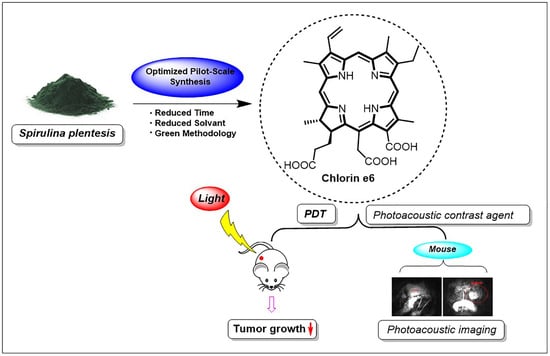
Improved Pilot-Plant-Scale Synthesis of Chlorin e6 and Its Efficacy as a Photosensitizer for Photodynamic Therapy and Photoacoustic Contrast Agent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biomass, Chemicals and Reagents
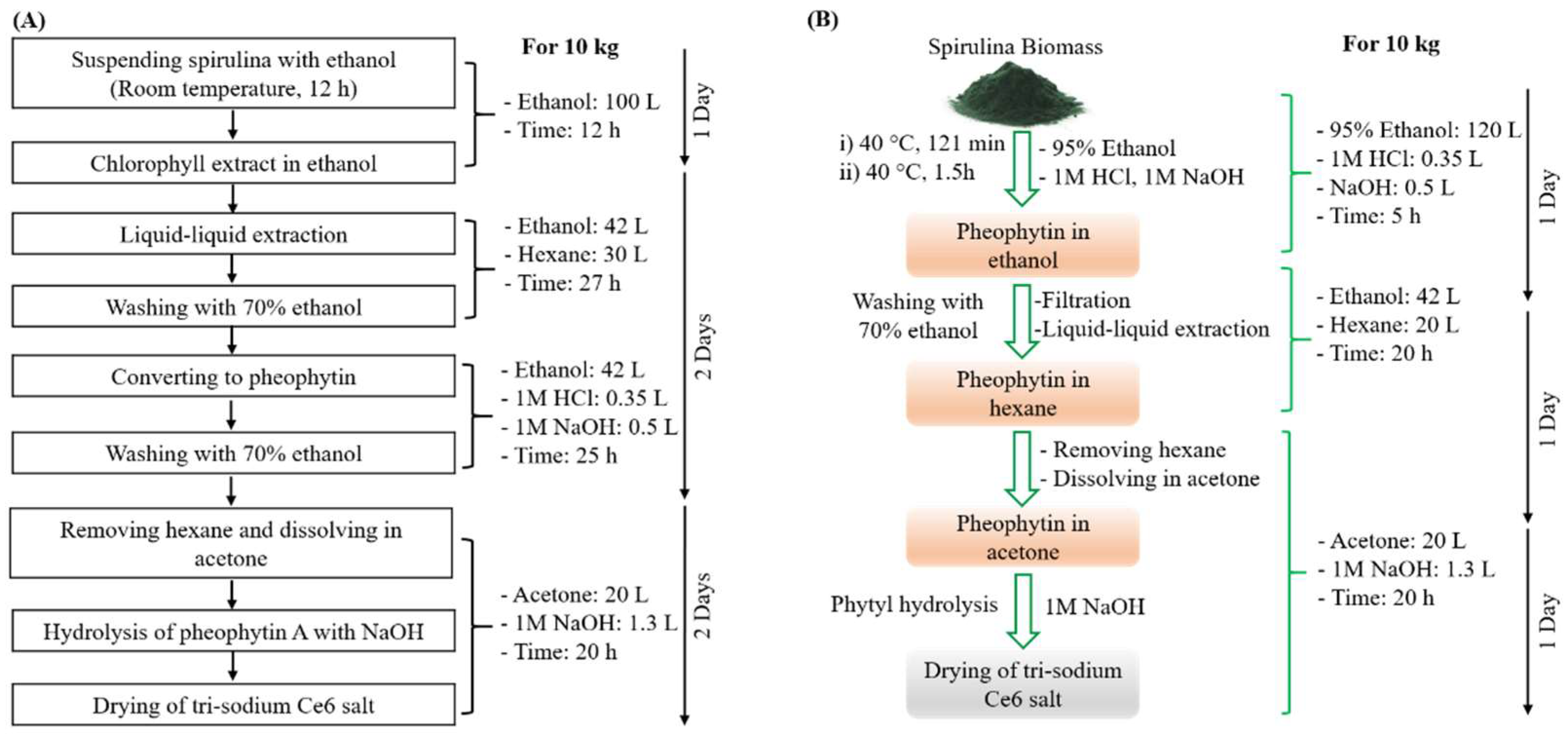
2.2. Pilot-Plant-Scale Synthesis of Ce6 by Conventional Method
2.3. Optimization of Chlorophyll a Extraction from Spirulina
2.4. Pilot-Plant-Scale Synthesis of Ce6 by Modified Method
2.5. Purification of Chlorin e6
2.6. Cell Culture
2.7. Cell Viability Assay
2.8. Animal Model
2.9. Allograft Mouse Model Using B16F10 Melanoma Cell
2.10. PDT in Animal Model
2.11. Photoacoustic (PA) Imaging
3. Results
3.1. Extraction of Chlorophyll a Using 95% Ethanol
3.2. Efficient Pilot-Plant Synthesis of Ce6
3.3. Purification of Ce6 and Its Spectral Properties
3.4. Cytotoxicity of Ce6 PDT In Vitro
3.5. Ce6 PDT Effects on Allograft Mouse Model Using B16F10 Melanoma Cells
3.6. Ce6 as a PA Contrast Agent
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Josefsen, L.B.; Boyle, R.W. Photodynamic Therapy and the Development of Metal-Based Photosensitisers. Met.-Based Drugs 2008, 2008, 276109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef]
- Plaetzer, K.; Krammer, B.; Berlanda, J.; Berr, F.; Kiesslich, T. Photophysics and Photochemistry of Photodynamic Therapy: Fundamental Aspects. Lasers Med. Sci. 2009, 24, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Sai, D.L.; Lee, J.; Nguyen, D.L.; Kim, Y.-P. Tailoring Photosensitive ROS for Advanced Photodynamic Therapy. Exp. Mol. Med. 2021, 53, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Sánchez, I.; García-Moreno, D.; Mulero, V. Zebrafish Models to Study the Crosstalk between Inflammation and NADPH Oxidase-Derived Oxidative Stress in Melanoma. Antioxidants 2022, 11, 1277. [Google Scholar] [CrossRef]
- Domingues, B.; Lopes, J.M.; Soares, P.; Pópulo, H. Melanoma Treatment in Review. ImmunoTargets Ther. 2018, 7, 35–49. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.; Favazza, C.; Wang, L.V. In Vivo Photoacoustic Tomography of Chemicals: High-Resolution Functional and Molecular Optical Imaging at New Depths. Chem. Rev. 2010, 110, 2756–2782. [Google Scholar] [CrossRef] [Green Version]
- Miyata, A.; Ishizawa, T.; Kamiya, M.; Shimizu, A.; Kaneko, J.; Ijichi, H.; Shibahara, J.; Fukayama, M.; Midorikawa, Y.; Urano, Y.; et al. Photoacoustic Tomography of Human Hepatic Malignancies Using Intraoperative Indocyanine Green Fluorescence Imaging. PLoS ONE 2014, 9, e112667. [Google Scholar] [CrossRef]
- Attia, A.B.E.; Balasundaram, G.; Moothanchery, M.; Dinish, U.S.; Bi, R.; Ntziachristos, V.; Olivo, M. A Review of Clinical Photoacoustic Imaging: Current and Future Trends. Photoacoustics 2019, 16, 100144. [Google Scholar] [CrossRef]
- Han, S.; Lee, D.; Kim, S.; Kim, H.-H.; Jeong, S.; Kim, J. Contrast Agents for Photoacoustic Imaging: A Review Focusing on the Wavelength Range. Biosensors 2022, 12, 594. [Google Scholar] [CrossRef]
- Plekhova, N.; Shevchenko, O.; Korshunova, O.; Stepanyugina, A.; Tananaev, I.; Apanasevich, V. Development of Novel Tetrapyrrole Structure Photosensitizers for Cancer Photodynamic Therapy. Bioengineering 2022, 9, 82. [Google Scholar] [CrossRef]
- Ethirajan, M.; Chen, Y.; Joshi, P.; Pandey, R.K. The Role of Porphyrin Chemistry in Tumor Imaging and Photodynamic Therapy. Chem. Soc. Rev. 2011, 40, 340–362. [Google Scholar] [CrossRef]
- Dąbrowski, J.M.; Arnaut, L.G.; Pereira, M.M.; Monteiro, C.J.P.; Urbańska, K.; Simões, S.; Stochel, G. New Halogenated Water-Soluble Chlorin and Bacteriochlorin as Photostable PDT Sensitizers: Synthesis, Spectroscopy, Photophysics, and in Vitro Photosensitizing Efficacy. ChemMedChem 2010, 5, 1770–1780. [Google Scholar] [CrossRef]
- Chen, H.; Humble, S.W.; Jinadasa, R.G.W.; Zhou, Z.; Nguyen, A.L.; Vicente, M.G.H.; Smith, K.M. Syntheses and PDT Activity of New Mono- and Di-Conjugated Derivatives of Chlorin E6. J. Porphyr. Phthalocyanines 2017, 21, 354–363. [Google Scholar] [CrossRef] [Green Version]
- Beack, S.; Kong, W.H.; Jung, H.S.; Do, I.H.; Han, S.; Kim, H.; Kim, K.S.; Yun, S.H.; Hahn, S.K. Photodynamic Therapy of Melanoma Skin Cancer Using Carbon Dot – Chlorin E6 – Hyaluronate Conjugate. Acta Biomater. 2015, 26, 295–305. [Google Scholar] [CrossRef]
- Son, J.; Yi, G.; Kwak, M.-H.; Yang, S.M.; Park, J.M.; Lee, B.-I.; Choi, M.-G.; Koo, H. Gelatin–Chlorin E6 Conjugate for in Vivo Photodynamic Therapy. J. Nanobiotechnol. 2019, 17, 50. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Yang, F.; Wu, D.; Liu, Y.; Gao, Y.; Lian, H.; Zhang, H.; Yin, Z.; Wu, A.; Zeng, L. Ce6-Conjugated and Polydopamine-Coated Gold Nanostars with Enhanced Photoacoustic Imaging and Photothermal/Photodynamic Therapy to Inhibit Lung Metastasis of Breast Cancer. Nanoscale 2020, 12, 22173–22184. [Google Scholar] [CrossRef]
- Bui, H.T.H.; Pham, T.T.; Nguyen, H.T.T.; Do, T.M.; Nga, V.T.; Bac, N.D.; Huyen, V.T.B.; Le, H.M.; Tran, Q.C. Transformation Chlorophyll a of Spirulina Platensis to Chlorin E6 Derivatives and Several Applications. Open Access Maced. J. Med. Sci. 2019, 7, 4372–4377. [Google Scholar] [CrossRef] [Green Version]
- Tong, Y.; Gao, L.; Xiao, G.; Pan, X. Microwave Pretreatment-Assisted Ethanol Extraction of Chlorophylls from Spirulina platensis. J. Food Process Eng. 2012, 35, 792–799. [Google Scholar] [CrossRef]
- Minchev, I.; Petkova, N.; Milkova-Tomova, I. Ultrasound-Assisted Extraction of Chlorophylls and Phycocyanin from Spirulina platensis. Biointerface Res. Appl. Chem. 2020, 11, 9296–9304. [Google Scholar] [CrossRef]
- Tong, Y.; Gao, L.; Xiao, G.; Pan, X. Supercritical CO2 Extraction of Chlorophyll a from Spirulina Platensis with a Static Modifier. Chem. Eng. Technol. 2011, 34, 241–248. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. In Plant Cell Membranes; Packer, L., Douce, R., Eds.; Methods in Enzymology Volume 148; Academic Press: Cambridge, MA, USA, 1987; pp. 350–382. [Google Scholar]
- Van de Sompel, D.; Sasportas, L.S.; Jokerst, J.V.; Gambhir, S.S. Comparison of Deconvolution Filters for Photoacoustic Tomography. PLoS ONE 2016, 11, e0152597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lafarga, T.; Fernández-Sevilla, J.M.; González-López, C.; Acién-Fernández, F.G. Spirulina for the Food and Functional Food Industries. Food Res. Int. 2020, 137, 109356. [Google Scholar] [CrossRef] [PubMed]
- Sartory, D.P.; Grobbelaar, J.U. Extraction of Chlorophyll a from Freshwater Phytoplankton for Spectrophotometric Analysis. Hydrobiologia 1984, 114, 177–187. [Google Scholar] [CrossRef]
- Nutrizio, M.; Maltar-Strmečki, N.; Chemat, F.; Duić, B.; Jambrak, A.R. High-Voltage Electrical Discharges in Green Extractions of Bioactives from Oregano Leaves (Origanum vulgare L.) Using Water and Ethanol as Green Solvents Assessed by Theoretical and Experimental Procedures. Food Eng. Rev. 2021, 13, 161–174. [Google Scholar] [CrossRef]
- Wasmund, N.; Topp, I.; Schories, D. Optimising the Storage and Extraction of Chlorophyll Samples. Oceanologia 2006, 48, 125–144. [Google Scholar]
- Kong, W.; Liu, N.; Zhang, J.; Yang, Q.; Hua, S.; Song, H.; Xia, C. Optimization of Ultrasound-Assisted Extraction Parameters of Chlorophyll from Chlorella vulgaris Residue after Lipid Separation Using Response Surface Methodology. J. Food Sci. Technol. 2014, 51, 2006–2013. [Google Scholar] [CrossRef] [Green Version]
- Weremfo, A.; Abassah-Oppong, S.; Adulley, F.; Dabie, K.; Seidu-Larry, S. Response Surface Methodology as a Tool to Optimize the Extraction of Bioactive Compounds from Plant Sources. J. Sci. Food Agric. 2022. [Google Scholar] [CrossRef]
- Martins, M.; Albuquerque, C.M.; Pereira, C.F.; Coutinho, J.A.P.; Neves, M.G.P.M.S.; Pinto, D.C.G.A.; Faustino, M.A.F.; Ventura, S.P.M. Recovery of Chlorophyll a Derivative from Spirulina Maxima: Its Purification and Photosensitizing Potential. ACS Sustain. Chem. Eng. 2021, 9, 1772–1780. [Google Scholar] [CrossRef]
- Lu, J.; Feng, X.; Han, Y.; Xue, C. Optimization of Subcritical Fluid Extraction of Carotenoids and Chlorophyll a from Laminaria japonica Aresch by Response Surface Methodology. J. Sci. Food Agric. 2014, 94, 139–145. [Google Scholar] [CrossRef]
- Pérez-López, P.; González-García, S.; Ulloa, R.G.; Sineiro, J.; Feijoo, G.; Moreira, M.T. Life Cycle Assessment of the Production of Bioactive Compounds from Tetraselmis suecica at Pilot Scale. J. Clean. Prod. 2014, 64, 323–331. [Google Scholar] [CrossRef]
- Yang, C.; Fu, Y.; Huang, C.; Hu, D.; Zhou, K.; Hao, Y.; Chu, B.; Yang, Y.; Qian, Z. Chlorin E6 and CRISPR-Cas9 Dual-Loading System with Deep Penetration for a Synergistic Tumoral Photodynamic-Immunotherapy. Biomaterials 2020, 255, 120194. [Google Scholar] [CrossRef]
- Potez, M.; Trappetti, V.; Bouchet, A.; Fernandez-Palomo, C.; Güç, E.; Kilarski, W.W.; Hlushchuk, R.; Laissue, J.; Djonov, V. Characterization of a B16-F10 Melanoma Model Locally Implanted into the Ear Pinnae of C57BL/6 Mice. PLoS ONE 2018, 13, e0206693. [Google Scholar] [CrossRef]
- Wu, D.; Huang, L.; Jiang, M.S.; Jiang, H. Contrast Agents for Photoacoustic and Thermoacoustic Imaging: A Review. Int. J. Mol. Sci. 2014, 15, 23616–23639. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Gillman, P.K. CNS Toxicity Involving Methylene Blue: The Exemplar for Understanding and Predicting Drug Interactions That Precipitate Serotonin Toxicity. J. Psychopharmacol. 2011, 25, 429–436. [Google Scholar] [CrossRef]
- Hu, D.; Zhong, L.; Wang, M.; Li, H.; Qu, Y.; Liu, Q.; Han, R.; Yuan, L.; Shi, K.; Peng, J.; et al. Perfluorocarbon-Loaded and Redox-Activatable Photosensitizing Agent with Oxygen Supply for Enhancement of Fluorescence/Photoacoustic Imaging Guided Tumor Photodynamic Therapy. Adv. Funct. Mater. 2019, 29, 1806199. [Google Scholar] [CrossRef]
- Jiao, X.; Zhang, W.; Zhang, L.; Cao, Y.; Xu, Z.; Kang, Y.; Xue, P. Rational Design of Oxygen Deficient TiO2−x Nanoparticles Conjugated with Chlorin E6 (Ce6) for Photoacoustic Imaging-Guided Photothermal/Photodynamic Dual Therapy of Cancer. Nanoscale 2020, 12, 1707–1718. [Google Scholar] [CrossRef]






| Parameters | Conventional Method | Efficient Pilot Method |
|---|---|---|
| Solvent | Ethanol | 95% Ethanol |
| Extraction time | 12 h | 2 h |
| Demetallation time | 3 h | 1.5 h |
| Ethanol | 184 L | 160 L |
| Hexane | 30 L | 20 L |
| Total time | 5 days | 3 days |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thapa Magar, T.B.; Shrestha, R.; Gurung, P.; Lim, J.; Kim, Y.-W. Improved Pilot-Plant-Scale Synthesis of Chlorin e6 and Its Efficacy as a Photosensitizer for Photodynamic Therapy and Photoacoustic Contrast Agent. Processes 2022, 10, 2215. https://doi.org/10.3390/pr10112215
Thapa Magar TB, Shrestha R, Gurung P, Lim J, Kim Y-W. Improved Pilot-Plant-Scale Synthesis of Chlorin e6 and Its Efficacy as a Photosensitizer for Photodynamic Therapy and Photoacoustic Contrast Agent. Processes. 2022; 10(11):2215. https://doi.org/10.3390/pr10112215
Chicago/Turabian StyleThapa Magar, Til Bahadur, Rajeev Shrestha, Pallavi Gurung, Junmo Lim, and Yong-Wan Kim. 2022. "Improved Pilot-Plant-Scale Synthesis of Chlorin e6 and Its Efficacy as a Photosensitizer for Photodynamic Therapy and Photoacoustic Contrast Agent" Processes 10, no. 11: 2215. https://doi.org/10.3390/pr10112215