Research on Co-Combustion Behaviors of Binary and Ternary Blends of Coal, Walnut Shell, and Biochar by TGA
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Experiment Design and Procedure
2.3. Kinetic Model
2.4. Interaction between Materials
3. Results and Discussion
3.1. Thermal Behavior of Individual Material
3.2. Thermal Behavior of Binary Blends
3.3. Thermal Behavior of Ternary Blends
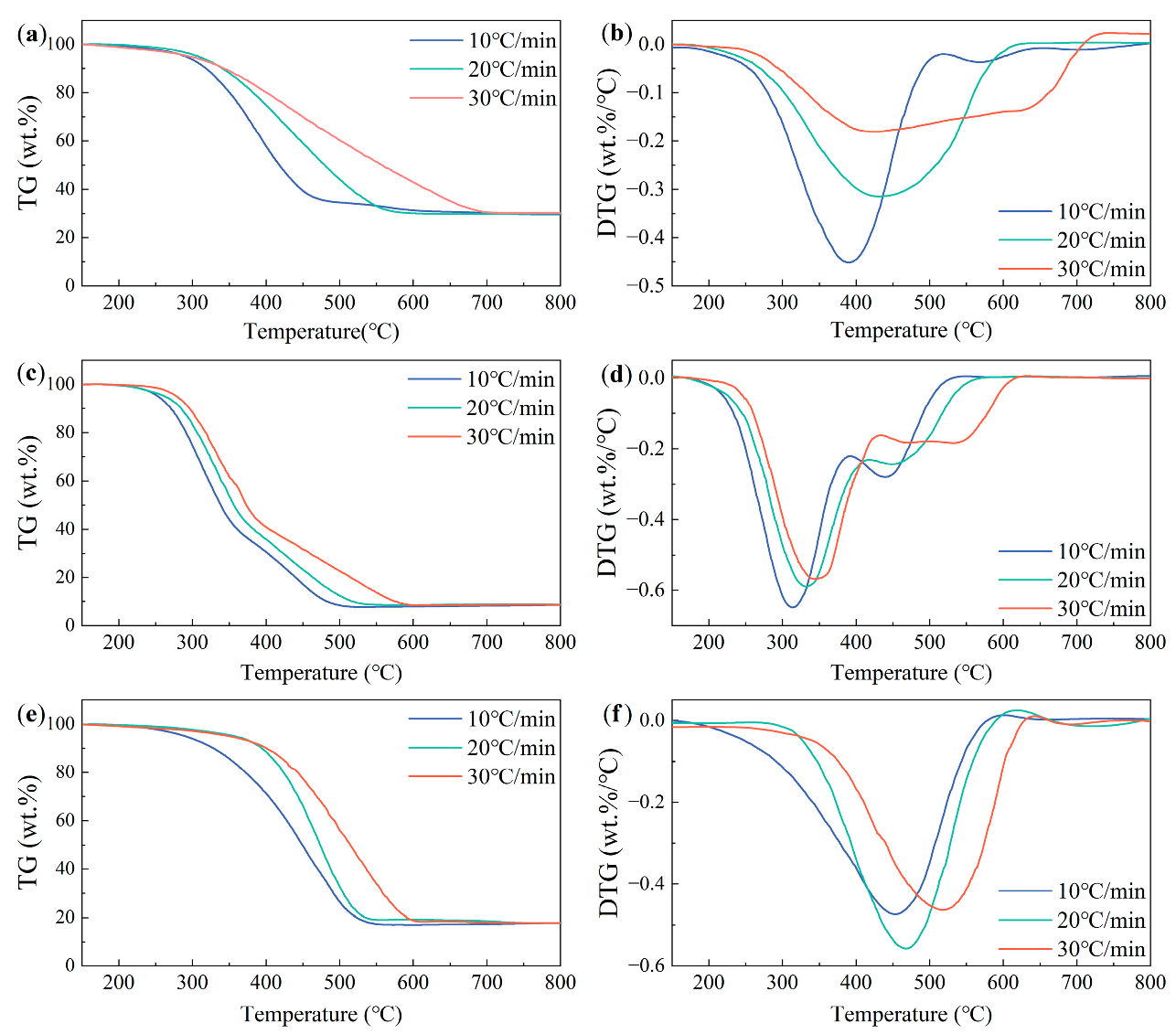
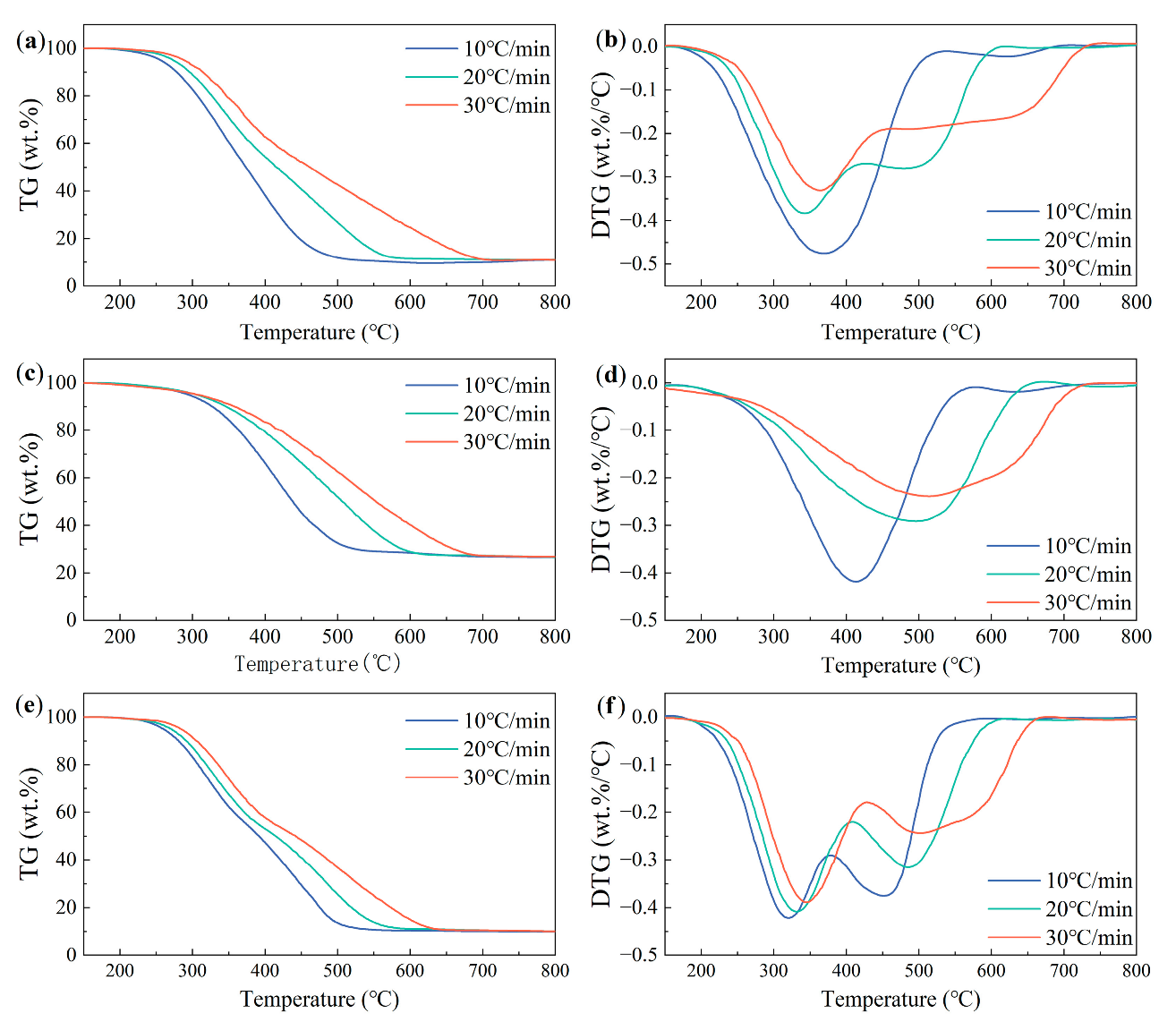
3.4. Influence of the Heating Rate on the Combustion Behaviors of the Samples
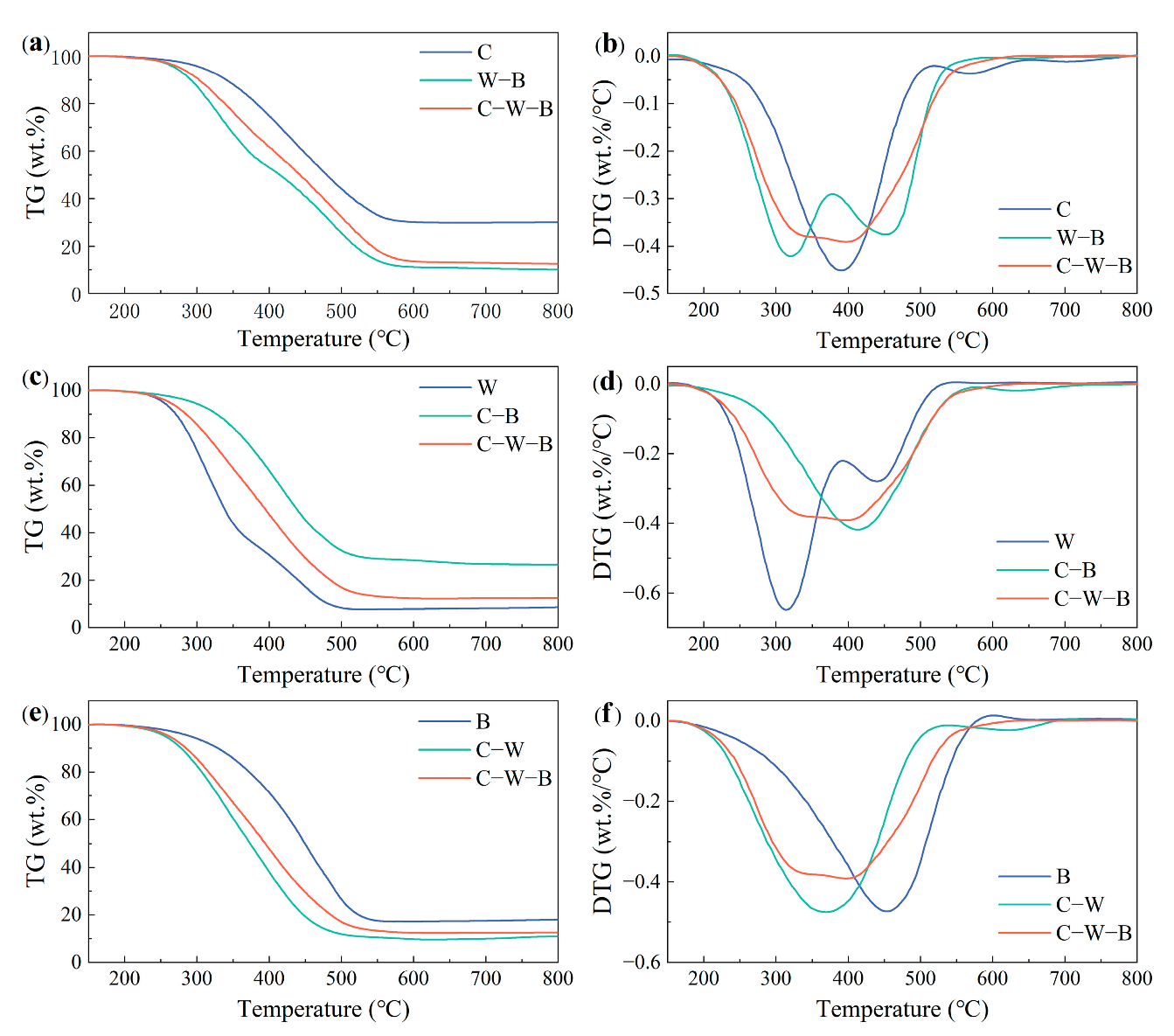
3.5. Interactions between Materials
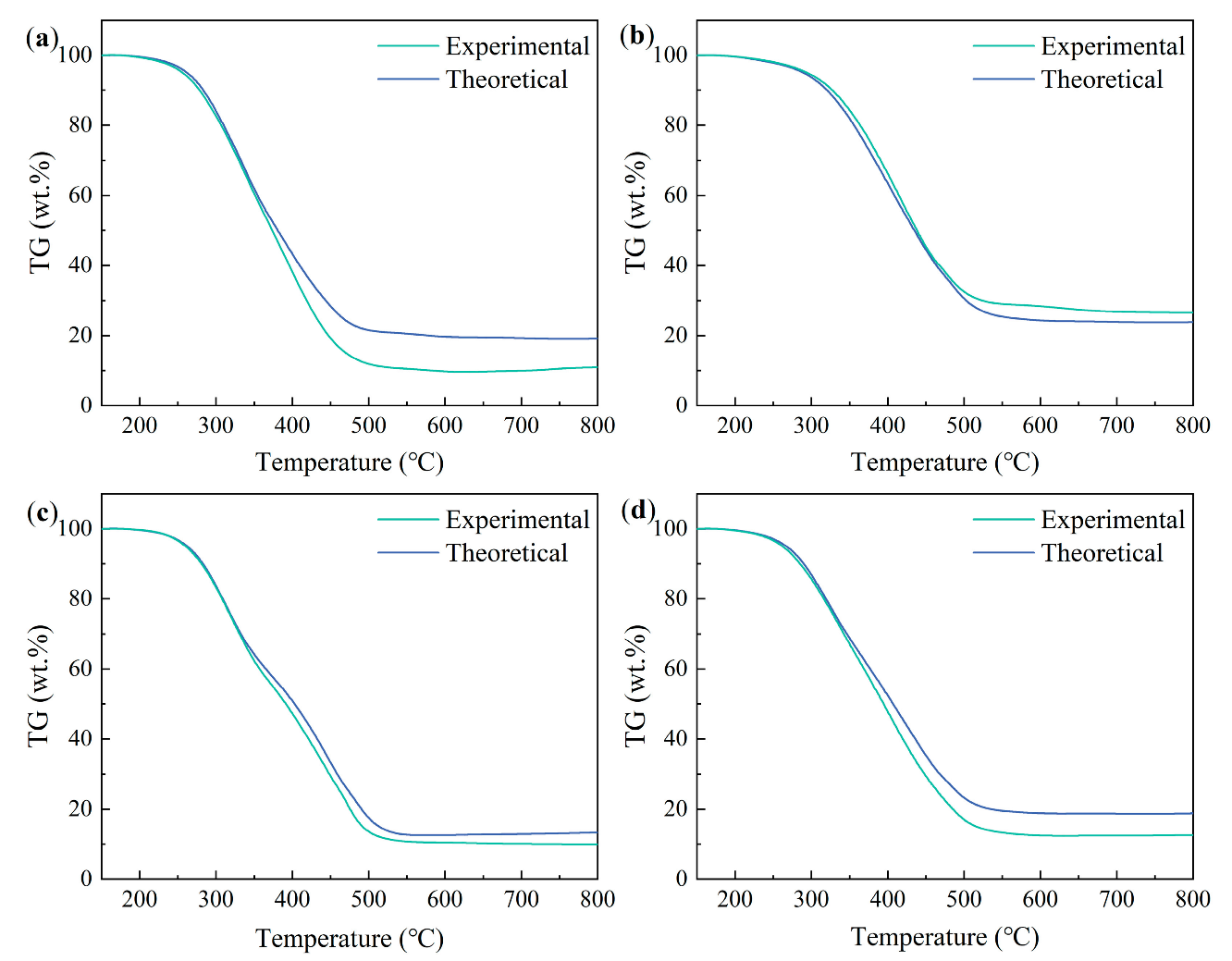
3.6. Impact of the Third Material Addition on the Binary Blends
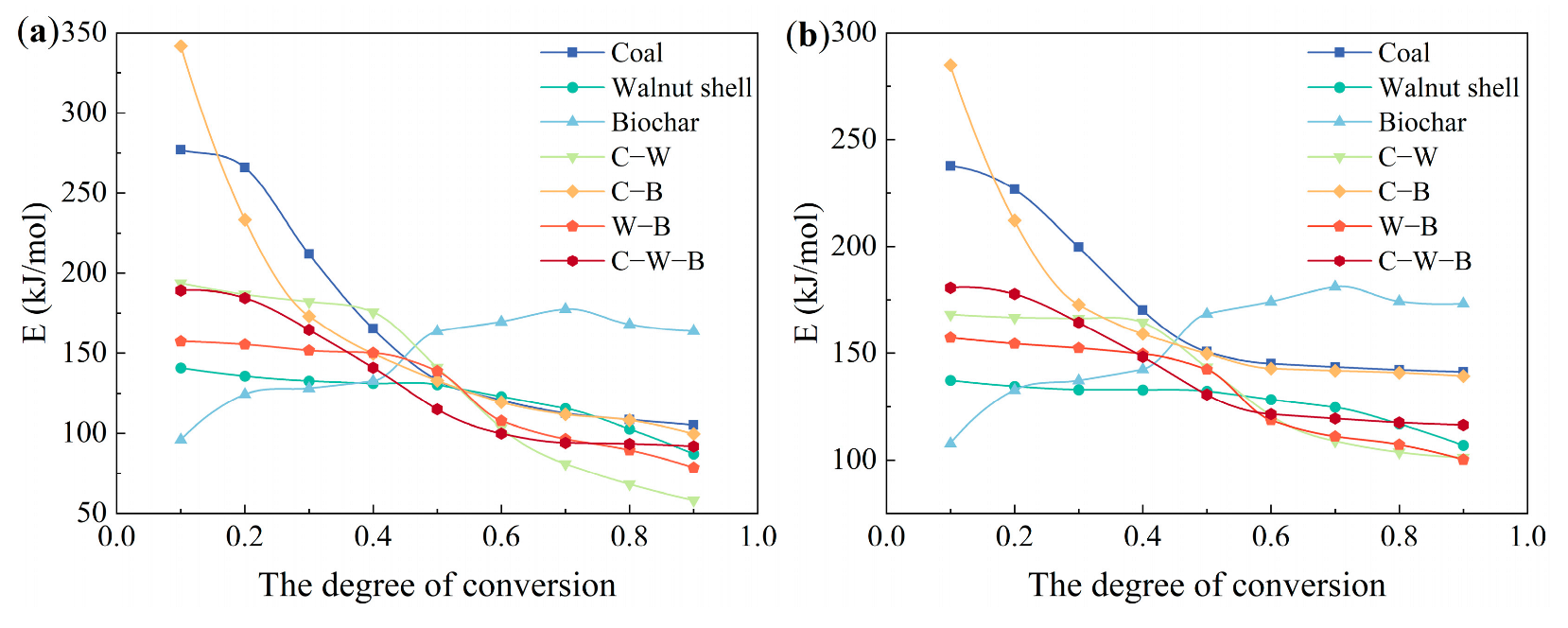
3.7. Kinetic Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, Z.-Y.; Cao, F.; Fan, M.-Y.; Zhang, W.-Q.; Zhai, X.-Y.; Wang, Q.; Zhang, Y.-L. Coal and Biomass Burning as Major Emissions of NOx in Northeast China: Implication from Dual Isotopes Analysis of Fine Nitrate Aerosols. Atmos. Environ. 2020, 242, 117762. [Google Scholar] [CrossRef]
- Munir, S.; Nimmo, W.; Gibbs, B.M. The Effect of Air Staged, Co-Combustion of Pulverised Coal and Biomass Blends on NOx Emissions and Combustion Efficiency. Fuel 2011, 90, 126–135. [Google Scholar] [CrossRef]
- Picciano, P.; Aguilar, F.X.; Burtraw, D.; Mirzaee, A. Environmental and Socio-Economic Implications of Woody Biomass Co-Firing at Coal-Fired Power Plants. Resour. Energy Econ. 2022, 68, 101296. [Google Scholar] [CrossRef]
- Toptas, A.; Yildirim, Y.; Duman, G.; Yanik, J. Combustion Behavior of Different Kinds of Torrefied Biomass and Their Blends with Lignite. Bioresour. Technol. 2015, 177, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Gohar, H.; Khoja, A.H.; Ansari, A.A.; Naqvi, S.R.; Liaquat, R.; Hassan, M.; Hasni, K.; Qazi, U.Y.; Ali, I. Investigating the Characterisation, Kinetic Mechanism, and Thermodynamic Behaviour of Coal-Biomass Blends in Co-Pyrolysis Process. Process Saf. Environ. Prot. 2022, 163, 645–658. [Google Scholar] [CrossRef]
- Liu, Z.; Balasubramanian, R. A Comparison of Thermal Behaviors of Raw Biomass, Pyrolytic Biochar and Their Blends with Lignite. Bioresour. Technol. 2013, 146, 371–378. [Google Scholar] [CrossRef]
- Wei, X.; Huang, S.; Wu, Y.; Wu, S. Effects of Washing Pretreatment on Properties and Pyrolysis Biochars of Penicillin Mycelial Residues. Biomass Bioenergy 2022, 161, 106477. [Google Scholar] [CrossRef]
- Qi, S.; Wang, Z.; Costa, M.; He, Y.; Cen, K. Ignition and Combustion of Single Pulverized Biomass and Coal Particles in N2/O2 and CO2/O2 Environments. Fuel 2021, 283, 118956. [Google Scholar] [CrossRef]
- Zou, H.; Liu, C.; Evrendilek, F.; He, Y.; Liu, J. Evaluation of Reaction Mechanisms and Emissions of Oily Sludge and Coal Co-Combustions in O2/CO2 and O2/N2 Atmospheres. Renew. Energy 2021, 171, 1327–1343. [Google Scholar] [CrossRef]
- Riaza, J.; Gil, M.V.; Álvarez, L.; Pevida, C.; Pis, J.J.; Rubiera, F. Oxy-Fuel Combustion of Coal and Biomass Blends. Energy 2012, 41, 429–435. [Google Scholar] [CrossRef]
- Xiao, Z.; Wang, S.; Luo, M.; Cai, J. Combustion Characteristics and Synergistic Effects during Co-Combustion of Lignite and Lignocellulosic Components under Oxy-Fuel Condition. Fuel 2022, 310, 122399. [Google Scholar] [CrossRef]
- Gil, M.V.; Riaza, J.; Álvarez, L.; Pevida, C.; Pis, J.J.; Rubiera, F. Kinetic Models for the Oxy-Fuel Combustion of Coal and Coal/Biomass Blend Chars Obtained in N2 and CO2 Atmospheres. Energy 2012, 48, 510–518. [Google Scholar] [CrossRef]
- Rago, Y.P.; Collard, F.-X.; Görgens, J.F.; Surroop, D.; Mohee, R. Co-Combustion of Torrefied Biomass-Plastic Waste Blends with Coal through TGA: Influence of Synergistic Behaviour. Energy 2022, 239, 121859. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, S.; Zhao, J.; Chen, L.; Meng, H. Synergistic Effect on Thermal Behavior during Co-Pyrolysis of Lignocellulosic Biomass Model Components Blend with Bituminous Coal. Bioresour. Technol. 2014, 169, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Vuthaluru, H.B. Thermal Behaviour of Coal/Biomass Blends during Co-Pyrolysis. Fuel Process. Technol. 2004, 85, 141–155. [Google Scholar] [CrossRef]
- Ashraf, A.; Sattar, H.; Munir, S. A Comparative Performance Evaluation of Co-Combustion of Coal and Biomass in Drop Tube Furnace. J. Energy Inst. 2022, 100, 55–65. [Google Scholar] [CrossRef]
- Galina, N.R.; Romero Luna, C.M.; Arce, G.L.A.F.; Ávila, I. Comparative Study on Combustion and Oxy-Fuel Combustion Environments Using Mixtures of Coal with Sugarcane Bagasse and Biomass Sorghum Bagasse by the Thermogravimetric Analysis. J. Energy Inst. 2019, 92, 741–754. [Google Scholar] [CrossRef]
- Keivani, B.; Gungor, A. Techno-Economic Assessment of Coal and Torrefied Biomass Co-Combustion: A Case Study of Oxy-Combustion Carbon Capture Power Plants in Turkey. J. CO2 Util. 2022, 62, 102103. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Wang, G.; Wu, Y.; Yang, H.; Jin, L.; Hu, S.; Hu, H. Effect of Fe Components in Red Mud on Catalytic Pyrolysis of Low Rank Coal. J. Energy Inst. 2022, 100, 1–9. [Google Scholar] [CrossRef]
- Hu, J.; Shao, J.; Yang, H.; Lin, G.; Chen, Y.; Wang, X.; Zhang, W.; Chen, H. Co-Gasification of Coal and Biomass: Synergy, Characterization and Reactivity of the Residual Char. Bioresour. Technol. 2017, 244, 1–7. [Google Scholar] [CrossRef]
- Tong, W.; Liu, Q.; Ran, G.; Liu, L.; Ren, S.; Chen, L.; Jiang, L. Experiment and Expectation: Co-Combustion Behavior of Anthracite and Biomass Char. Bioresour. Technol. 2019, 280, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Jiao, H.; Cai, J.; Wang, J.; Yang, Y.; Bridgwater, A.V. Co-Pyrolysis of Miscanthus Sacchariflorus and Coals: A Systematic Study on the Synergies in Thermal Decomposition, Kinetics and Vapour Phase Products. Fuel 2020, 262, 116603. [Google Scholar] [CrossRef]
- Tran, K.-Q.; Werle, S.; Trinh, T.T.; Magdziarz, A.; Sobek, S.; Pogrzeba, M. Fuel Characterization and Thermal Degradation Kinetics of Biomass from Phytoremediation Plants. Biomass Bioenergy 2020, 134, 105469. [Google Scholar] [CrossRef]
- Chansa, O.; Luo, Z.; Yu, C. Study of the Kinetic Behaviour of Biomass and Coal during Oxyfuel Co-Combustion. Chin. J. Chem. Eng. 2020, 28, 1796–1804. [Google Scholar] [CrossRef]
- Magdziarz, A.; Wilk, M. Thermogravimetric Study of Biomass, Sewage Sludge and Coal Combustion. Energy Convers. Manag. 2013, 75, 425–430. [Google Scholar] [CrossRef]
- Liang, W.; Jiang, C.; Wang, G.; Ning, X.; Zhang, J.; Guo, X.; Xu, R.; Wang, P.; Ye, L.; Li, J.; et al. Research on the Co-Combustion Characteristics and Kinetics of Agricultural Waste Hydrochar and Anthracite. Renew. Energy 2022, 194, 1119–1130. [Google Scholar] [CrossRef]
- Wani Likun, P.K.; Zhang, H. Insights into Pyrolysis of Torrefied-Biomass, Plastics/Tire and Blends: Thermochemical Behaviors, Kinetics and Evolved Gas Analyses. Biomass Bioenergy 2020, 143, 105852. [Google Scholar] [CrossRef]
- Zhu, H.; Lang, L.; Fang, G.; Na, D.; Yin, X.; Yu, X.; Wu, C.; Bridgwater, A.V. Thermogravimetric Characteristics and Kinetics of Herb Residues Catalyzed by Potassium Carbonate. J. Anal. Appl. Pyrolysis 2021, 156, 105170. [Google Scholar] [CrossRef]
- Feng, P.; Li, X.; Wang, J.; Li, J.; Wang, H.; He, L. The Mixtures of Bio-Oil Derived from Different Biomass and Coal/Char as Biofuels: Combustion Characteristics. Energy 2021, 224, 120132. [Google Scholar] [CrossRef]
- Muigai, H.H.; Choudhury, B.J.; Kalita, P.; Moholkar, V.S. Co–Pyrolysis of Biomass Blends: Characterization, Kinetic and Thermodynamic Analysis. Biomass Bioenergy 2020, 143, 105839. [Google Scholar] [CrossRef]
- Yuan, Y.; Zuo, H.; Wang, J.; Gao, Y.; Xue, Q.; Wang, J. Co-Combustion Behavior, Kinetic and Ash Melting Characteristics Analysis of Clean Coal and Biomass Pellet. Fuel 2022, 324, 124727. [Google Scholar] [CrossRef]
- Xinjie, L.; Singh, S.; Yang, H.; Wu, C.; Zhang, S. A Thermogravimetric Assessment of the Tri-Combustion Process for Coal, Biomass and Polyethylene. Fuel 2021, 287, 119355. [Google Scholar] [CrossRef]
- Jiang, L.; Zhou, Z.; Xiang, H.; Yang, Y.; Tian, H.; Wang, J. Characteristics and Synergistic Effects of Co-Pyrolysis of Microalgae with Polypropylene. Fuel 2022, 314, 122765. [Google Scholar] [CrossRef]
- Mundike, J.; Collard, F.-X.; Görgens, J.F. Co-Combustion Characteristics of Coal with Invasive Alien Plant Chars Prepared by Torrefaction or Slow Pyrolysis. Fuel 2018, 225, 62–70. [Google Scholar] [CrossRef]
- Hou, Y.; Feng, Z.; He, Y.; Gao, Q.; Ni, L.; Su, M.; Ren, H.; Liu, Z.; Hu, W. Co-Pyrolysis Characteristics and Synergistic Interaction of Bamboo Residues and Disposable Face Mask. Renew. Energy 2022, 194, 415–425. [Google Scholar] [CrossRef]
- Lv, P.; Bai, Y.; Wang, J.; Song, X.; Su, W.; Yu, G.; Ma, Y. Investigation into the Interaction of Biomass Waste with Industrial Solid Waste during Co-Pyrolysis and the Synergetic Effect of Its Char Gasification. Biomass Bioenergy 2022, 159, 106414. [Google Scholar] [CrossRef]
- Bi, H.; Wang, C.; Lin, Q.; Jiang, X.; Jiang, C.; Bao, L. Combustion Behavior, Kinetics, Gas Emission Characteristics and Artificial Neural Network Modeling of Coal Gangue and Biomass via TG-FTIR. Energy 2020, 213, 118790. [Google Scholar] [CrossRef]
- Ma, L.; Goldfarb, J.L.; Song, J.; Chang, C.; Ma, Q. Enhancing Cleaner Biomass-Coal Co-Combustion by Pretreatment of Wheat Straw via Washing versus Hydrothermal Carbonization. J. Clean. Prod. 2022, 366, 132991. [Google Scholar] [CrossRef]
- Riaza, J.; Ajmi, M.; Gibbins, J.; Chalmers, H. Ignition and Combustion of Single Particles of Coal and Biomass under O2/CO2 Atmospheres. Energy Procedia 2017, 114, 6067–6073. [Google Scholar] [CrossRef]
- Ma, L.; Yu, S.; Chen, X.; Fang, Q.; Yin, C.; Zhang, C.; Chen, G. Combustion Interactions in Oxy-Fuel Firing of Coal Blends: An Experimental and Numerical Study. J. Energy Inst. 2021, 94, 11–21. [Google Scholar] [CrossRef]
- Magalhaes, D.; Kazanc, F. Influence of Biomass Thermal Pre-Treatment on the Particulate Matter Formation during Pulverized Co-Combustion with Lignite Coal. Fuel 2022, 308, 122027. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, W.; Jiang, Z.; Mi, B.; Fei, B. Investigating Combustion Behaviors of Bamboo, Torrefied Bamboo, Coal and Their Respective Blends by Thermogravimetric Analysis. Renew. Energy 2016, 87, 346–352. [Google Scholar] [CrossRef]
- Azizi, K.; Moshfegh Haghighi, A.; Keshavarz Moraveji, M.; Olazar, M.; Lopez, G. Co-Pyrolysis of Binary and Ternary Mixtures of Microalgae, Wood and Waste Tires through TGA. Renew. Energy 2019, 142, 264–271. [Google Scholar] [CrossRef]
- Boumanchar, I.; Chhiti, Y.; M’hamdi Alaoui, F.E.; Elkhouakhi, M.; Sahibed-dine, A.; Bentiss, F.; Jama, C.; Bensitel, M. Investigation of (Co)-Combustion Kinetics of Biomass, Coal and Municipal Solid Wastes. Waste Manag. 2019, 97, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Zhang, B.; Liu, C.; Shujaa aldeen, A. Mechanism of Synergistic Effects and Kinetics Analysis in Catalytic Co-Pyrolysis of Water Hyacinth and HDPE. Energy Convers. Manag. 2021, 228, 113717. [Google Scholar] [CrossRef]
- Singh, S.; Patil, T.; Tekade, S.P.; Gawande, M.B.; Sawarkar, A.N. Studies on Individual Pyrolysis and Co-Pyrolysis of Corn Cob and Polyethylene: Thermal Degradation Behavior, Possible Synergism, Kinetics, and Thermodynamic Analysis. Sci. Total Environ. 2021, 783, 147004. [Google Scholar] [CrossRef]
- Buyukada, M. Uncertainty Estimation by Bayesian Approach in Thermochemical Conversion of Walnut Hull and Lignite Coal Blends. Bioresour. Technol. 2017, 232, 87–92. [Google Scholar] [CrossRef]
- Zhuang, X.; Song, Y.; Zhan, H.; Yin, X.; Wu, C. Synergistic Effects on the Co-Combustion of Medicinal Biowastes with Coals of Different Ranks. Renew. Energy 2019, 140, 380–389. [Google Scholar] [CrossRef]


| Coal | Walnut Shell | Biochar | |
|---|---|---|---|
| Ultimate analysis (wt.%) | |||
| C | 74.44 | 49.87 | 68.73 |
| H | 4.68 | 6.53 | 5.92 |
| O * | 19.43 | 42.97 | 24.18 |
| N | 1.18 | 0.49 | 1.05 |
| S | 0.27 | 0.14 | 0.12 |
| Proximate analysis (wt.%) | |||
| Volatile matter | 29.44 | 77.05 | 46.85 |
| Fixed carbon | 49.48 | 18.64 | 43.27 |
| Moisture | 9.87 | 2.94 | 0.32 |
| Ash | 11.21 | 1.37 | 9.56 |
| Stages | Parameters | Coal | Walnut Shell | Biochar |
|---|---|---|---|---|
| Stage 1 | Temperature range (°C) | 150–258 | 150–226 | 150-243 |
| Weight loss (wt.%) | 1.62 | 1.54 | 1.49 | |
| Stage 2 | Temperature range (°C) | 258–689 | 226–603 | 243-621 |
| Weight loss (wt.%) | 67.22 | 89.87 | 80.05 | |
| Stage 3 | Temperature range (°C) | 689–800 | 603–800 | 621-800 |
| Weight loss (wt.%) | 0.78 | 0.14 | 0.51 | |
| Residual weight at 800 °C (wt.%) | 30.38 | 8.45 | 17.95 | |
| Stages | Parameters | C-W | C-B | W-B |
|---|---|---|---|---|
| Stage 1 | Temperature range (°C) | 150–221 | 150–238 | 150–226 |
| Weight loss (wt.%) | 1.49 | 1.33 | 0.93 | |
| Stage 2 | Temperature range (°C) | 221–625 | 238–645 | 226–637 |
| Weight loss (wt.%) | 87.10 | 71.28 | 88.11 | |
| Stage 3 | Temperature range (°C) | 625–800 | 645–800 | 637–800 |
| Weight loss (wt.%) | 0.47 | 0.61 | 0.92 | |
| Residual weight at 800 °C (wt.%) | 10.94 | 26.78 | 10.04 | |
| KAS | FWO | KAS | FWO | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| α | E | R2 | E | R2 | α | E | R2 | E | R2 | ||
| (kJ/mol) | (kJ/mol) | (kJ/mol) | (kJ/mol) | ||||||||
| Coal | 0.1 | 277.00 | 0.999 | 237.75 | 0.998 | C-W | 0.1 | 193.60 | 0.989 | 168.12 | 0.993 |
| 0.2 | 266.01 | 0.998 | 226.80 | 0.986 | 0.2 | 186.60 | 0.981 | 166.63 | 0.983 | ||
| 0.3 | 211.89 | 0.999 | 199.66 | 0.999 | 0.3 | 182.08 | 0.999 | 166.23 | 0.987 | ||
| 0.4 | 165.32 | 0.997 | 170.17 | 0.994 | 0.4 | 175.66 | 0.987 | 164.40 | 0.983 | ||
| 0.5 | 133.19 | 0.985 | 150.75 | 0.996 | 0.5 | 141.09 | 0.983 | 143.48 | 0.984 | ||
| 0.6 | 120.67 | 0.983 | 145.11 | 0.993 | 0.6 | 103.30 | 0.989 | 120.79 | 0.988 | ||
| 0.7 | 112.54 | 0.992 | 143.56 | 0.999 | 0.7 | 81.00 | 0.997 | 108.87 | 0.987 | ||
| 0.8 | 108.53 | 0.987 | 142.15 | 0.998 | 0.8 | 68.16 | 0.991 | 103.60 | 0.988 | ||
| 0.9 | 105.08 | 0.998 | 141.28 | 0.987 | 0.9 | 58.20 | 0.987 | 101.14 | 0.986 | ||
| average | 166.69 | 173.03 | average | 132.19 | 138.14 | ||||||
| Walnut shell | 0.1 | 140.85 | 0.999 | 137.33 | 0.999 | C-B | 0.1 | 341.61 | 0.999 | 284.87 | 0.999 |
| 0.2 | 135.73 | 0.990 | 134.51 | 0.993 | 0.2 | 233.31 | 0.994 | 212.21 | 0.999 | ||
| 0.3 | 132.62 | 0.999 | 132.95 | 0.999 | 0.3 | 173.09 | 0.999 | 172.71 | 0.989 | ||
| 0.4 | 131.12 | 0.989 | 132.81 | 0.993 | 0.4 | 149.77 | 0.992 | 159.02 | 0.983 | ||
| 0.5 | 130.25 | 0.981 | 132.22 | 0.981 | 0.5 | 132.89 | 0.998 | 149.77 | 0.997 | ||
| 0.6 | 122.89 | 0.991 | 128.36 | 0.989 | 0.6 | 119.49 | 0.997 | 142.83 | 0.995 | ||
| 0.7 | 115.57 | 0.985 | 124.64 | 0.991 | 0.7 | 111.86 | 0.998 | 141.74 | 0.988 | ||
| 0.8 | 102.64 | 0.997 | 116.92 | 0.994 | 0.8 | 108.21 | 0.998 | 140.82 | 0.989 | ||
| 0.9 | 87.09 | 0.993 | 106.83 | 0.993 | 0.9 | 99.56 | 0.992 | 139.30 | 0.999 | ||
| average | 122.08 | 127.39 | average | 163.31 | 171.47 | ||||||
| Biochar | 0.1 | 95.93 | 0.992 | 107.76 | 0.995 | W-B | 0.1 | 157.42 | 0.990 | 157.31 | 0.990 |
| 0.2 | 124.25 | 0.997 | 132.73 | 0.998 | 0.2 | 155.41 | 0.983 | 154.51 | 0.986 | ||
| 0.3 | 128.03 | 0.997 | 137.17 | 0.998 | 0.3 | 151.73 | 0.990 | 152.39 | 0.988 | ||
| 0.4 | 132.57 | 0.995 | 142.42 | 0.998 | 0.4 | 150.10 | 0.984 | 149.66 | 0.987 | ||
| 0.5 | 163.59 | 0.983 | 168.37 | 0.985 | 0.5 | 138.94 | 0.984 | 142.43 | 0.981 | ||
| 0.6 | 169.59 | 0.987 | 174.09 | 0.987 | 0.6 | 107.79 | 0.988 | 118.63 | 0.987 | ||
| 0.7 | 177.54 | 0.989 | 181.09 | 0.994 | 0.7 | 96.32 | 0.985 | 111.01 | 0.983 | ||
| 0.8 | 167.86 | 0.987 | 174.30 | 0.988 | 0.8 | 89.42 | 0.990 | 107.23 | 0.987 | ||
| 0.9 | 163.92 | 0.988 | 173.28 | 0.989 | 0.9 | 78.61 | 0.982 | 100.19 | 0.982 | ||
| average | 147.03 | 154.58 | average | 125.08 | 132.59 | ||||||
| C-W-B | 0.1 | 189.12 | 0.986 | 180.65 | 0.989 | ||||||
| 0.2 | 184.49 | 0.998 | 177.73 | 0.998 | |||||||
| 0.3 | 164.53 | 0.994 | 164.39 | 0.996 | |||||||
| 0.4 | 140.97 | 0.999 | 148.37 | 0.999 | |||||||
| 0.5 | 114.94 | 0.986 | 130.59 | 0.998 | |||||||
| 0.6 | 99.91 | 0.991 | 121.41 | 0.996 | |||||||
| 0.7 | 93.96 | 0.998 | 119.35 | 0.991 | |||||||
| 0.8 | 93.30 | 0.991 | 117.48 | 0.999 | |||||||
| 0.9 | 91.79 | 0.986 | 116.34 | 0.997 | |||||||
| average | 130.33 | 141.81 | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Song, X.; Liu, S.; Liu, Z. Research on Co-Combustion Behaviors of Binary and Ternary Blends of Coal, Walnut Shell, and Biochar by TGA. Processes 2022, 10, 2264. https://doi.org/10.3390/pr10112264
Wang R, Song X, Liu S, Liu Z. Research on Co-Combustion Behaviors of Binary and Ternary Blends of Coal, Walnut Shell, and Biochar by TGA. Processes. 2022; 10(11):2264. https://doi.org/10.3390/pr10112264
Chicago/Turabian StyleWang, Rui, Xianglei Song, Shanjian Liu, and Zhuwei Liu. 2022. "Research on Co-Combustion Behaviors of Binary and Ternary Blends of Coal, Walnut Shell, and Biochar by TGA" Processes 10, no. 11: 2264. https://doi.org/10.3390/pr10112264
APA StyleWang, R., Song, X., Liu, S., & Liu, Z. (2022). Research on Co-Combustion Behaviors of Binary and Ternary Blends of Coal, Walnut Shell, and Biochar by TGA. Processes, 10(11), 2264. https://doi.org/10.3390/pr10112264









