Providing Antimicrobial Properties to Cardboard Food Packaging by Coating with ZnO, TiO2, and SiO2—Water-Based Varnish Nanocomposites
Abstract
:1. Introduction
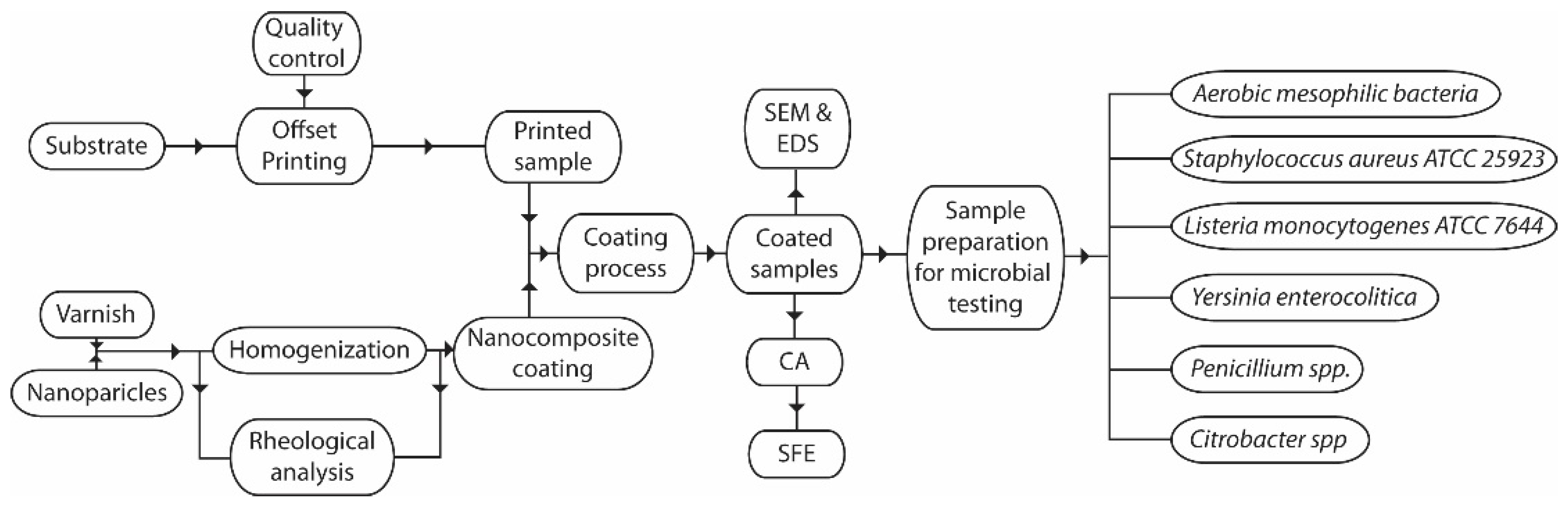
2. Materials and Methods
2.1. Sample Preparation
2.2. SEM and EDS Analysis of Samples
2.3. Surface Free Energy Determination
2.4. Testing of Antimicrobial Properties of Nanocomposite Coatings
2.4.1. Strains Used for Artificial Inoculation of Samples
2.4.2. Testing of Antimicrobial Capacity of Nanocomposite Coatings during Environmental Contamination of Cardboard Samples
2.4.3. Testing the Growth Inhibition of Artificially Inoculated Pathogens by Nanocomposite Coatings
2.4.4. Testing the Reduction in Bacterial Population on Nanocomposite-Coated Cardboard Samples
2.5. Statistical Analysis
3. Results and Discussion
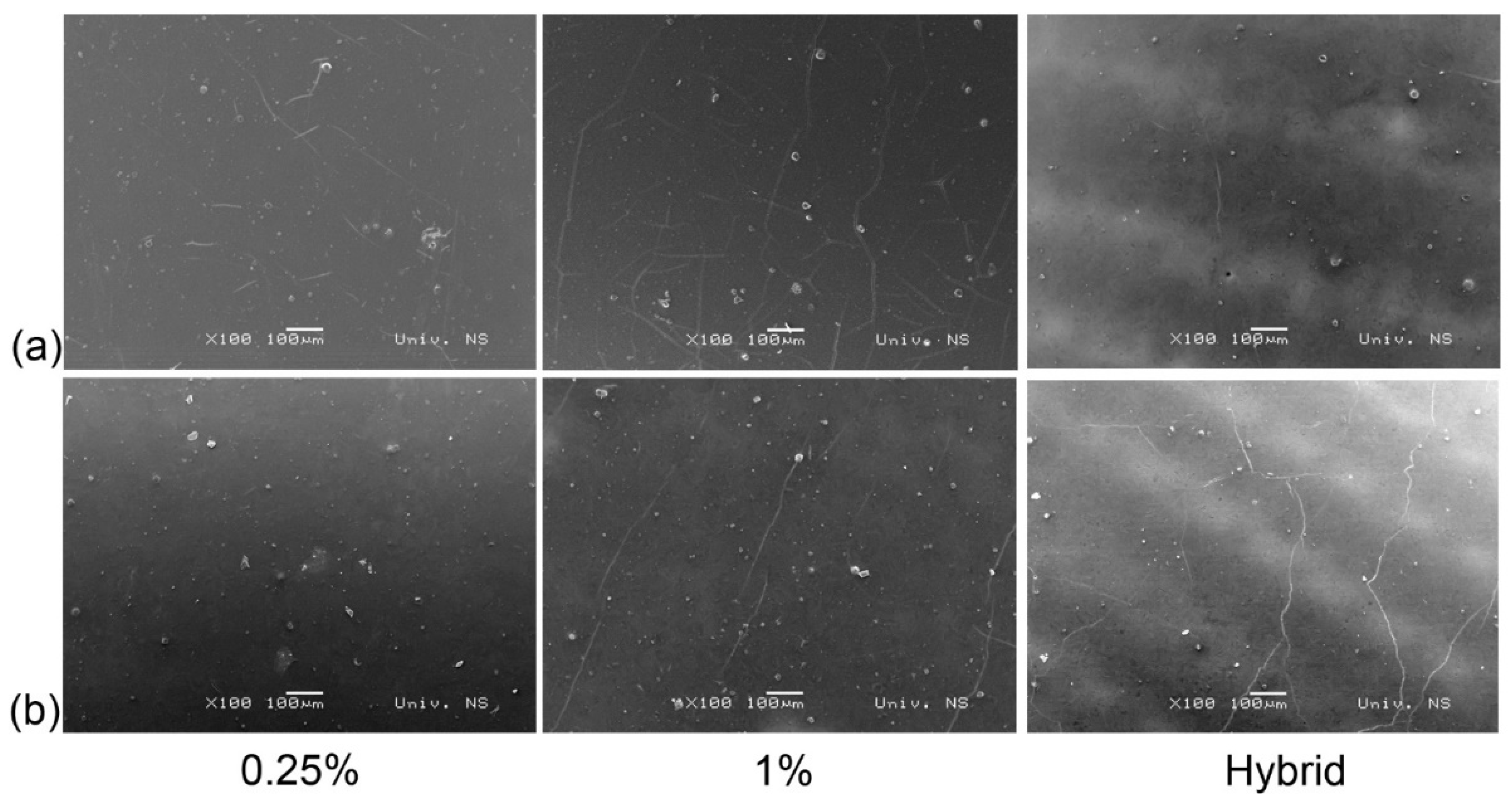
3.1. SEM and EDS Analysis
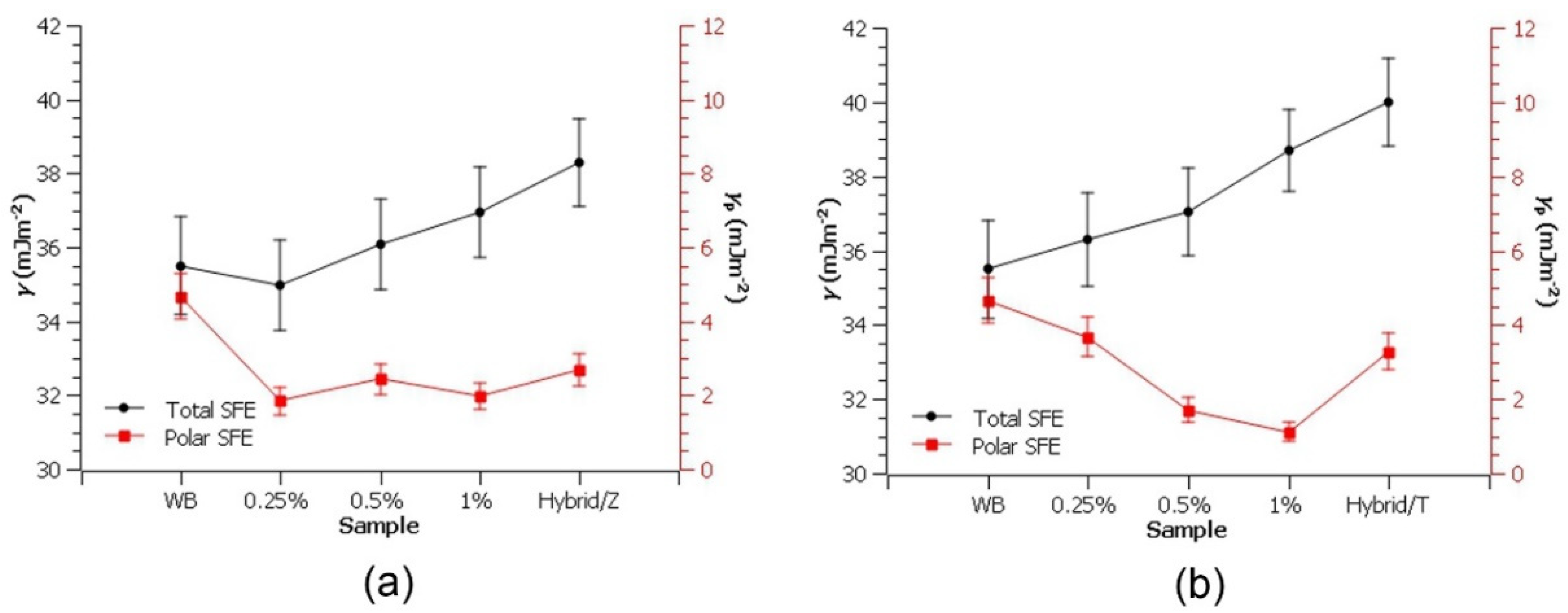
3.2. SFE Analysis
3.3. Microbiological Analyses
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rastogi, V.K.; Samyn, P. Bio-Based Coatings for Paper Applications. Coatings 2015, 5, 887–930. [Google Scholar] [CrossRef] [Green Version]
- Soroka, W. Fundamentals of Packaging Technology. In Paper and Paperboard; Institute of Packaging Professionals: Herndon, VA, USA, 2002; pp. 109–127. [Google Scholar]
- Rawdkuen, S.; Punbusayakul, N.; Lee, D.S. Antimicrobial Packaging for Meat Products. In Antimicrobial Food Packaging; Academic Press: Cambridge, MA, USA, 2016; pp. 229–241. [Google Scholar] [CrossRef]
- Jung, J.; Zhao, Y. Antimicrobial Packaging for Fresh and Minimally Processed Fruits and Vegetables. In Antimicrobial Food Packaging; Academic Press: Cambridge, MA, USA, 2016; pp. 243–256. [Google Scholar] [CrossRef]
- Malhotra, B.; Keshwani, A.; Kharkwal, H. Antimicrobial Food Packaging: Potential and Pitfalls. Front. Microbiol. 2015, 6, 611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koelsch Sand, C. Antimicrobial Packaging on the Rise Again. Food Technol. 2020, 74, 80–82. [Google Scholar]
- Moradi, M.; Guimarães, J.T.; Sahin, S. Current Applications of Exopolysaccharides from Lactic Acid Bacteria in the Development of Food Active Edible Packaging. Curr. Opin. Food Sci. 2021, 40, 33–39. [Google Scholar] [CrossRef]
- Salathé, M.; Kazandjieva, M.; Lee, J.W.; Levis, P.; Feldman, M.W.; Jones, J.H. A High-Resolution Human Contact Network for Infectious Disease Transmission. Proc. Natl. Acad. Sci. USA 2010, 107, 22020–22025. [Google Scholar] [CrossRef] [Green Version]
- Coronavirus Disease (COVID-19): How Is It Transmitted? Available online: https://www.who.int/news-room/questions-and-answers/item/coronavirus-disease-covid-19-how-is-it-transmitted (accessed on 12 September 2022).
- Hudika, T.; Cigula, T.; Vukoje, M. Antimicrobial Properties of TiO2 Nanocomposite Coating. In Proceedings of the Proceedings 13th International Conference on Nanomaterials—Research & Application, Brno, Czech Republic, 20–22 October 2021; pp. 351–358. [Google Scholar]
- Cigula, T.; Hudika, T.; Vukoje, M. Modulation of Water Based Commercial Varnish by Adding ZnO and SO2 Nanoparticles to Enhance Protective Function on Printed Packaging. In Proceedings of the 2nd International Circular Packaging Conference, ICP, FTPO Slovenia, Ljubljana, Slovenia, 9–10 September 2021; pp. 249–260. [Google Scholar]
- The Importance of Secondary Packaging. Available online: https://www.alcaminow.com/blog/the-importance-of-secondary-packaging (accessed on 25 January 2022).
- Zvekic, D.; Srdic, V.; Karaman, M.; Matavulj, M. Antimicrobial Properties of ZnO Nanoparticles Incorporated in Polyurethane Varnish. Process. Appl. Ceram. 2011, 5, 41–45. [Google Scholar] [CrossRef]
- Al-Tayyar, N.A.; Youssef, A.M.; Al-Hindi, R.R. Antimicrobial Packaging Efficiency of ZnO-SiO2 Nanocomposites Infused into PVA/CS Film for Enhancing the Shelf Life of Food Products. Food Packag. Shelf. Life 2020, 25, 100523. [Google Scholar] [CrossRef]
- Kubacka, A.; Diez, M.S.; Rojo, D.; Bargiela, R.; Ciordia, S.; Zapico, I.; Albar, J.P.; Barbas, C.; Martins Dos Santos, V.A.P.; Fernández-García, M.; et al. Understanding the Antimicrobial Mechanism of TiO2-Based Nanocomposite Films in a Pathogenic Bacterium. Sci. Rep. 2014, 4, 4134. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Umar, A.; Kumar, G.; Nalwa, H.S. Antimicrobial Properties of ZnO Nanomaterials: A Review. Ceram. Int. 2017, 43, 3940–3961. [Google Scholar] [CrossRef]
- Meng, D.; Liu, X.; Xie, Y.; Du, Y.; Yang, Y.; Xiao, C. Antibacterial Activity of Visible Light-Activated TiO2 Thin Films with Low Level of Fe Doping. Adv. Mater. Sci. Eng. 2019, 2019, 5819805. [Google Scholar] [CrossRef] [Green Version]
- Espitia, P.J.P.; Otoni, C.G.; Soares, N.F.F. Zinc Oxide Nanoparticles for Food Packaging Applications. In Antimicrobial Food Packaging; Academic Press: Cambridge, MA, USA, 2016; pp. 425–431. [Google Scholar] [CrossRef]
- Ghule, K.; Ghule, A.V.; Chen, B.J.; Ling, Y.C. Preparation and Characterization of ZnO Nanoparticles Coated Paper and Its Antibacterial Activity Study. Green Chem. 2006, 8, 1034–1041. [Google Scholar] [CrossRef] [Green Version]
- Petković, G.; Vukoje, M.; Bota, J.; Pasanec Preprotić, S. Enhancement of Polyvinyl Acetate (PVAc) Adhesion Performance by SiO2 and TiO2 Nanoparticles. Coatings 2019, 9, 707. [Google Scholar] [CrossRef] [Green Version]
- Vukoje, M.; Kulčar, R.; Itrić Ivanda, K.; Bota, J.; Cigula, T. Improvement in Thermochromic Offset Print UV Stability by Applying PCL Nanocomposite Coatings. Polymers 2022, 14, 1484. [Google Scholar] [CrossRef]
- Pietrzyk, B.; Porȩbska, K.; Jakubowski, W.; Miszczak, S. Antibacterial Properties of Zn Doped Hydrophobic SiO2 Coatings Produced by Sol-Gel Method. Coatings 2019, 9, 362. [Google Scholar] [CrossRef] [Green Version]
- Kadhum, S.A. The Effect of Two Types of Nano-Particles (ZnO & SiO2) on Different Types of Bacterial Growth. Biomed. Pharmacol. J. 2017, 10, 1701–1708. [Google Scholar] [CrossRef]
- Yang, D.L.; Cui, Y.N.; Sun, Q.; Liu, M.; Niu, H.; Wang, J.X. Antibacterial Activity and Reinforcing Effect of SiO2-ZnO Complex Cluster Fillers for Dental Resin Composites. Biomater. Sci. 2021, 9, 1795–1804. [Google Scholar] [CrossRef]
- Bota, J.; Hrnjak-Murgić, Z.; Brozović, M. The Effect of Film Thickness and Concentration of SIO2 Nanoparticles in PCL Coatings on Color Change of Tonal Value Increase. Acta Graph. 2016, 27, 15–22. [Google Scholar]
- Nguyen, T.V.; Nguyen, T.A.; Nguyen, T.H. The Synergistic Effects of SiO2 Nanoparticles and Organic Photostabilizers for Enhanced Weathering Resistance of Acrylic Polyurethane Coating. J. Compos. Sci. 2020, 4, 23. [Google Scholar] [CrossRef] [Green Version]
- Mills, D.J.; Jamali, S.S.; Paprocka, K. Investigation into the Effect of Nano-Silica on the Protective Properties of Polyurethane Coatings. Surf. Coat. Technol. 2012, 209, 137–142. [Google Scholar] [CrossRef]
- Malaki, M.; Hashemzadeh, Y.; Karevan, M. Effect of Nano-Silica on the Mechanical Properties of Acrylic Polyurethane Coatings. Prog. Org. Coat. 2016, 101, 477–485. [Google Scholar] [CrossRef]
- Wang, F.; Feng, L.; Ma, H.; Zhai, Z.; Liu, Z. Influence of Nano-SiO 2 on the Bonding Strength and Wear Resistance Properties of Polyurethane Coating. Sci. Eng. Compos. Mater. 2019, 26, 77–83. [Google Scholar] [CrossRef]
- Downey, A. COVID-19: Do I Need to Wash My Shopping and Groceries? | Patient. Available online: https://patient.info/news-and-features/covid-19-do-i-need-to-wash-my-shopping-and-groceries (accessed on 25 January 2022).
- Gloves Won’t Reduce Your Risk of COVID-19 at the Grocery Store. Available online: https://www.healthline.com/health-news/wearing-gloves-while-grocery-shopping-doesnt-prevent-covid-19#Why-gloves-arent-medically-necessary (accessed on 14 September 2021).
- Antimicrobial Packaging Market—Growth, Trends, COVID-19 Impact, and Forecasts (2021–2026). Available online: https://www.mordorintelligence.com/industry-reports/antimicrobial-packaging-market (accessed on 15 April 2021).
- King, A. More Bacteria Are Becoming Resistant to Antibiotics—Here’s How Viruses and Vaccines Could Help | Research and Innovation. Available online: https://ec.europa.eu/research-and-innovation/en/horizon-magazine/more-bacteria-are-becoming-resistant-antibiotics-heres-how-viruses-and-vaccines-could-help (accessed on 25 January 2022).
- Russell, C.D.; Fairfield, C.J. Co-Infections, Secondary Infections, and Antimicrobial Use in Patients Hospitalised with COVID-19 during the First Pandemic Wave from the ISARIC WHO CCP-UK Study: A Multicentre, Prospective Cohort Study. Lancet Microbe 2021, 2, e354–e365. [Google Scholar] [CrossRef]
- Guzińska, K.; Owczarek, M.; Dymel, M. Investigation in the Microbiological Purity of Paper and Board Packaging Intended for Contact with Food. Fibres Text. East. Eur. 2012, 96, 186–190. [Google Scholar]
- Skrivanek, T. Determination of the Surface Free Energy of Electronic Components. Metal Finish. 2003, 101, 87. [Google Scholar] [CrossRef]
- van Oss, C.J.; Giese, R.F.; Wu, W. On the Predominant Electron-Donicity of Polar Solid Surfaces. J. Adhes. 1997, 63, 71–88. [Google Scholar] [CrossRef]
- Zdolec, N.; Kiš, M.; Jankuloski, D.; Blagoevska, K.; Kazazić, S.; Pavlak, M.; Blagojević, B.; Antić, D.; Fredriksson-Ahomaa, M.; Pažin, V. Prevalence and Persistence of Multidrug-Resistant Yersinia enterocolitica 4/O:3 in Tonsils of Slaughter Pigs from Different Housing Systems in Croatia. Foods 2022, 11, 1459. [Google Scholar] [CrossRef]
- Bernd Schwegmann GmbH & Co. Anti Set-off Powders for the Printing Industry. Available online: https://www.schwegmannnet.de/PDF/Broschueren/Druck/EN/Wirbelwind_Broschuere_EN.pdf (accessed on 9 September 2022).
- Shamsudin, S.; Ahmad, M.K.; Aziz, A.N.; Fakhriah, R.; Mohamad, F.; Ahmad, N.; Nafarizal, N.; Soon, C.F.; Ameruddin, A.S.; Faridah, A.B.; et al. Hydrophobic Rutile Phase TiO2 Nanostructure and Its Properties for Self-Cleaning Application. AIP Conf. Proc. 2017, 1883, 020030. [Google Scholar]
- Nuruddin, A.; Yuliarto, B.; Kurniasih, S.; Setiyanto, H.; Ramelan, A. Preparation of Superhydrophobic Zinc Oxide Nanorods Coating on Stainless Steel via Chemical Bath Deposition. IOP Conf. Ser. Mater. Sci. Eng. 2019, 547, 012052. [Google Scholar] [CrossRef] [Green Version]
- Mardosaitė, R.; Jurkevičiū Tė, A.; Račkauskas, S. Superhydrophobic ZnO Nanowires: Wettability Mechanisms and Functional Applications. Cryst. Growth Des. 2021, 21, 4765–4779. [Google Scholar] [CrossRef]
- Akhidime, I.D.; Saubade, F.; Benson, P.S.; Butler, J.A.; Olivier, S.; Kelly, P.; Verran, J.; Whitehead, K.A. The Antimicrobial Effect of Metal Substrates on Food Pathogens. Food Bioprod. Process. 2019, 113, 68–76. [Google Scholar] [CrossRef]
- Liu, C.; Tao, Y.; Gout, J.; Qin, D. Antimicrobial Characteristic and Mechanisam of Nano-Fumed Silica Salt Grafted N,N-Dimethly-n-Tetradecylamine. Life Sci. J. 2009, 6, 52–54. [Google Scholar]
- Yeo, S.Y.; Lee, H.J.; Jeong, S.H. Preparation of Nanocomposite Fibers for Permanent Antibacterial Effect. J. Mater. Sci. 2003, 38, 2143–2147. [Google Scholar] [CrossRef]
- Jiang, J.; Oberdörster, G.; Elder, A.; Gelein, R.; Mercer, P.; Biswas, P. Does Nanoparticle Activity Depend upon Size and Crystal Phase? Nanotoxicology 2008, 2, 33–42. [Google Scholar] [CrossRef] [PubMed]

| Nanoparticle | Weight Ratio (%) | Homogenization Time (min) | Viscosity (mPa·s) | Denomination |
|---|---|---|---|---|
| Pure WB | - | - | 105 | WB |
| ZnO | 0.25 | 15 | 168 | 0.25% ZnO nanoclusters |
| ZnO | 0.5 | 20 | 272 | 0.5% ZnO nanoclusters |
| ZnO | 1 | 30 | 376 | 1% ZnO nanoclusters |
| TiO2 | 0.25 | 15 | 213 | 0.25% TiO2 nanoclusters |
| TiO2 | 0.5 | 20 | 310 | 0.5% TiO2 nanoclusters |
| TiO2 | 1 | 30 | 357 | 1% TiO2 nanoclusters |
| SiO2 + TiO2 | 0.5 + 0.5 | 40 | 690 | Hybrid/T |
| SiO2 + ZnO | 0.5 + 0.5 | 40 | 710 | Hybrid/Z |
| Liquid | Surface Tension (mNm−1) | Dispersive Part (mNm−1) | Polar Part (mNm−1) | Author |
|---|---|---|---|---|
| Water γ = 2.0 µS cm−1 | 72.8 | 21.8 | 51 | Strӧm et al. [36] |
| Glycerol | 60 | 34 | 51 | Strӧm et al. [36] |
| Diiodomethane | 50.8 | 50.8 | 0 | Strӧm et al. [36] |
| Formamide | 58 | 39 | 19 | Van Oss et al. [37] |
| Added Weight Ratio of NP | Staphylococcus aureus ATCC 25923 | Listeria monocytogenes ATCC 7644 (Serogroup 1/2c) | Aerobic Mesophilic Bacteria |
|---|---|---|---|
| Cardboard without coating 1 | 6.12 ± 0.42 | 5.77 ± 0.34 ABC | 2.45 ± 0.04 ABCDE |
| Pure WB 2 | 5.23 ± 0.94 | 6.59 ± 0.17 ADEFG | 2.00 ± 0.05 AG |
| 1% TiO2 | 5.56 ± 0.45 | 7.00 ± 0.08 Bab | 2.39 ± 0.03 |
| 0.5% TiO2 | 5.09 ± 0.29 | 5.3 ± 0.26 Dac | 2.10 ± 0.11 a |
| 0.25% TiO2 | 4.97 ± 0.48 | 6.21 ± 0.27 bc | 2.53 ± 0.05 Ga |
| 1% ZnO | 5.65 ± 0.61 | 5.89 ± 0.09 F | 1.95 ± 0.04 D |
| 0.5% ZnO | 5.06 ± 0.61 | 6.17 ± 0.18 | 1.99 ± 0.09 B |
| 0.25% ZnO | 5.71 ± 0.12 | 5.49 ± 0.07 E | 1.68 ± 0.10 C |
| 0.5%TiO2 + 0.5% SiO2 (Hybrid/T) | 5.74 ± 0.27 | 6.71 ± 0.36 Cd | 2.20 ± 0.20 b |
| 0.5% ZnO + 0.5% SiO2 (Hybrid/Z) | 5.00 ± 0.09 | 5.56 ± 0.23 Gd | 1.73 ± 0.32 Eb |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hudika, T.; Zdolec, N.; Kiš, M.; Cigula, T. Providing Antimicrobial Properties to Cardboard Food Packaging by Coating with ZnO, TiO2, and SiO2—Water-Based Varnish Nanocomposites. Processes 2022, 10, 2285. https://doi.org/10.3390/pr10112285
Hudika T, Zdolec N, Kiš M, Cigula T. Providing Antimicrobial Properties to Cardboard Food Packaging by Coating with ZnO, TiO2, and SiO2—Water-Based Varnish Nanocomposites. Processes. 2022; 10(11):2285. https://doi.org/10.3390/pr10112285
Chicago/Turabian StyleHudika, Tomislav, Nevijo Zdolec, Marta Kiš, and Tomislav Cigula. 2022. "Providing Antimicrobial Properties to Cardboard Food Packaging by Coating with ZnO, TiO2, and SiO2—Water-Based Varnish Nanocomposites" Processes 10, no. 11: 2285. https://doi.org/10.3390/pr10112285
APA StyleHudika, T., Zdolec, N., Kiš, M., & Cigula, T. (2022). Providing Antimicrobial Properties to Cardboard Food Packaging by Coating with ZnO, TiO2, and SiO2—Water-Based Varnish Nanocomposites. Processes, 10(11), 2285. https://doi.org/10.3390/pr10112285








