Resource Utilization of Lake Sediment to Prepare “Sponge” Light Aggregate: Pore Structure and Water Retention Mechanism Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
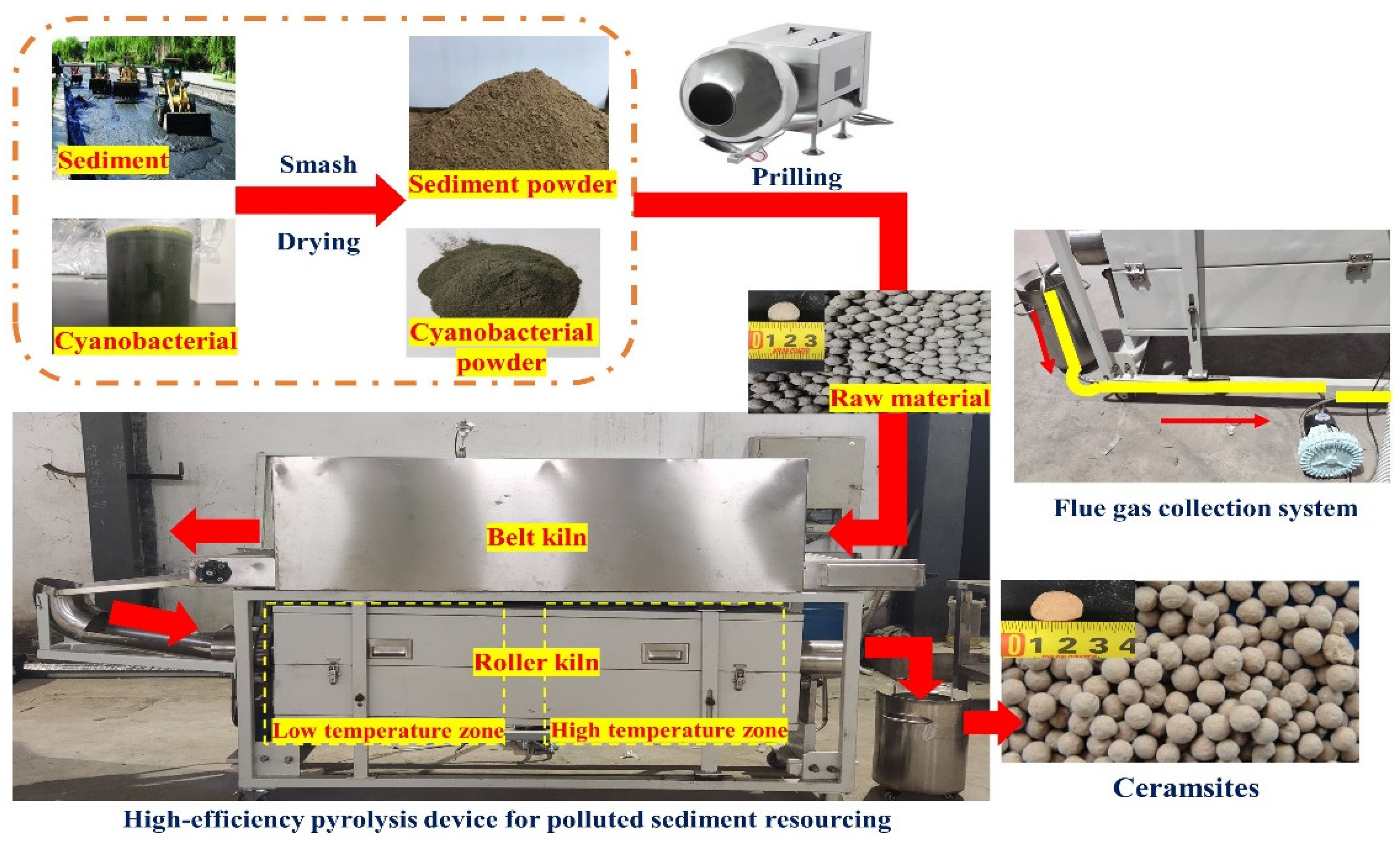
2.2. Ceramsite Preparation Method and Experimental Equipment
2.3. Research Content
2.4. Analytical Methods
2.5. Water Absorption and Release Effect Test of Ceramsite
2.6. Environmental Risk Test of Ceramsite
3. Results and Discussion
3.1. Effect of Raw Material Ratio on Pore-Forming Characteristics of Ceramsite
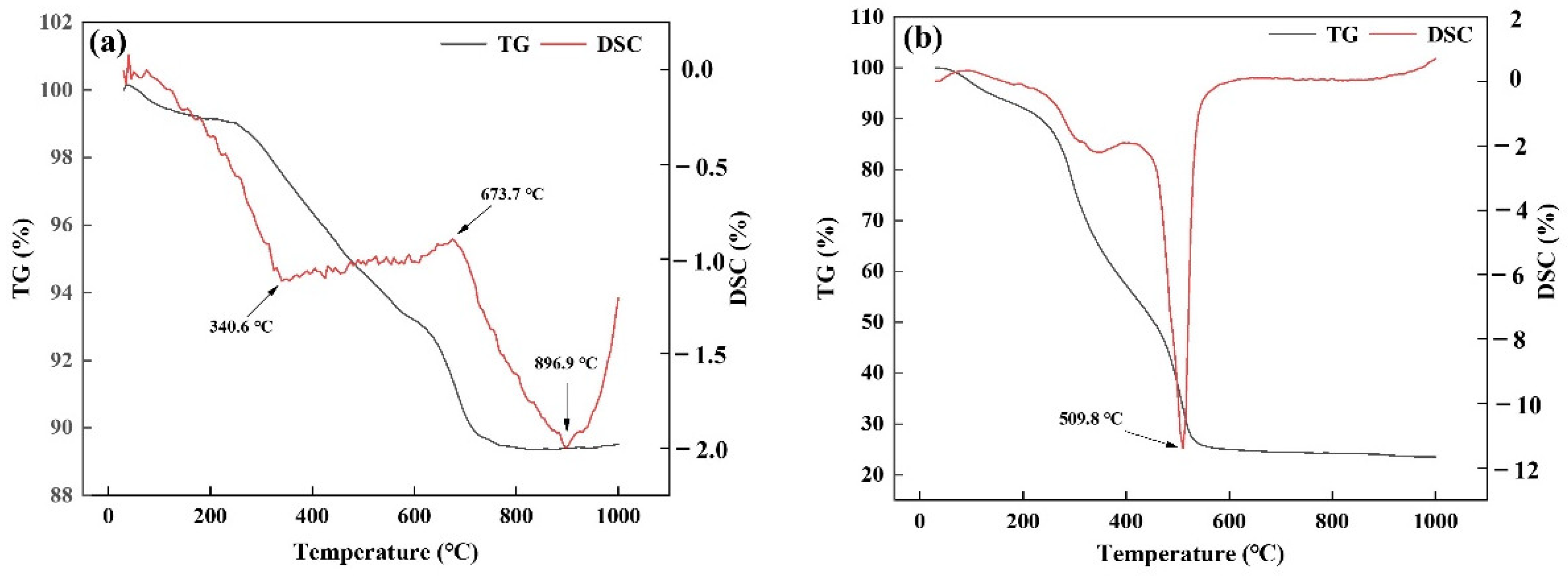
3.2. Effect of Low Temperature Pyrolysis Temperature on Pore-Forming Characteristics of Ceramsite
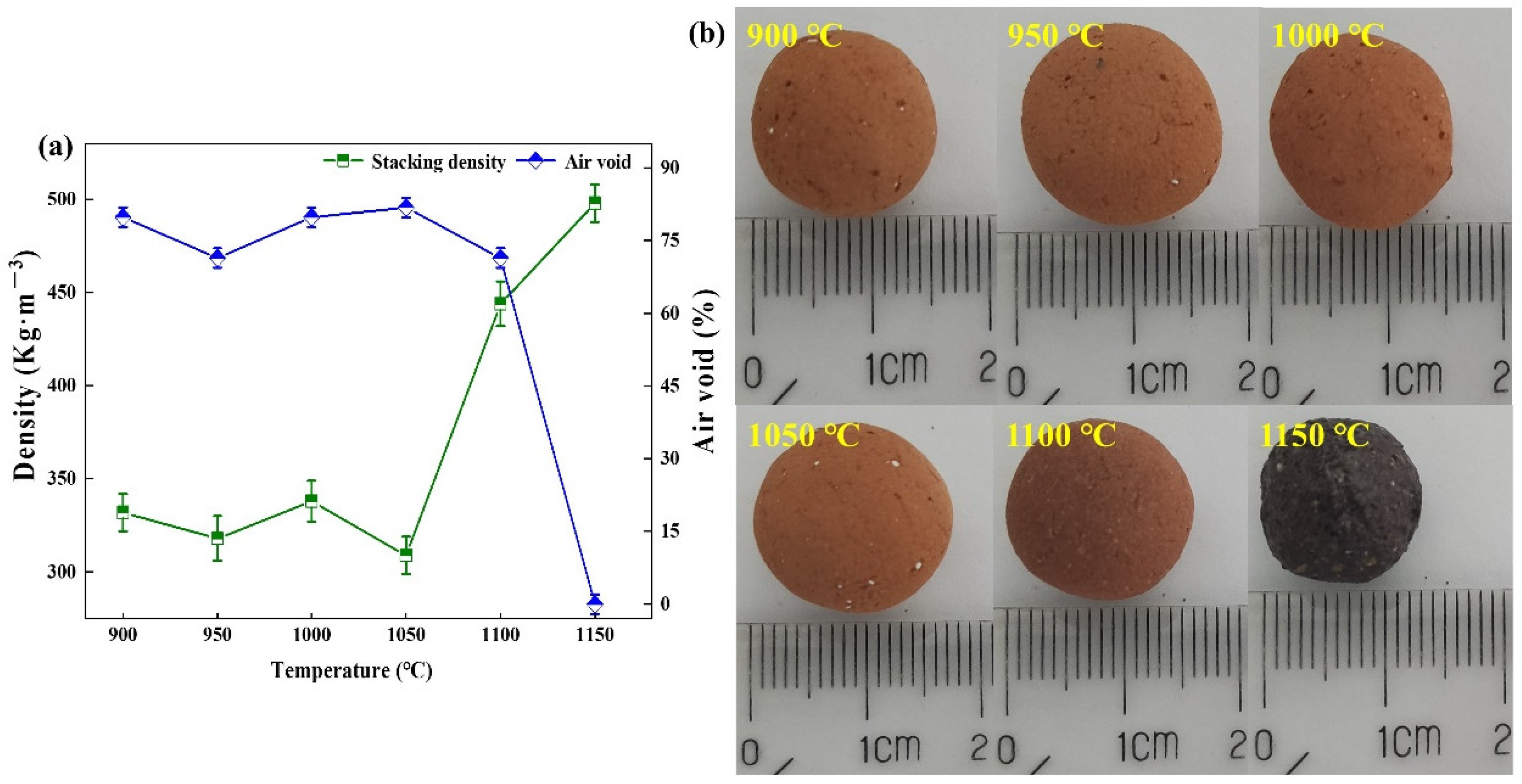
3.3. Effect of High Temperature Pyrolysis Temperature on Pore-Forming Characteristics of Ceramsite
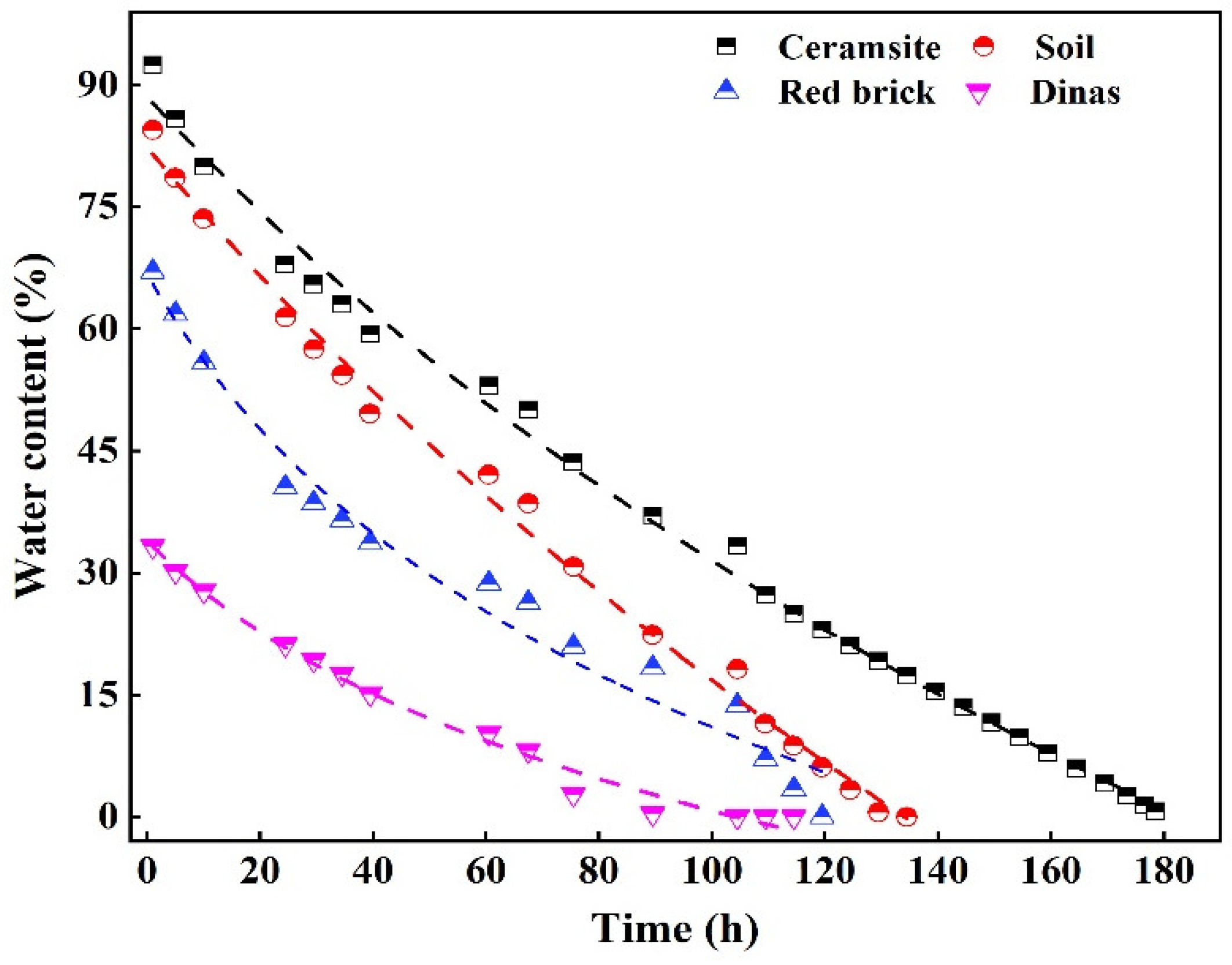
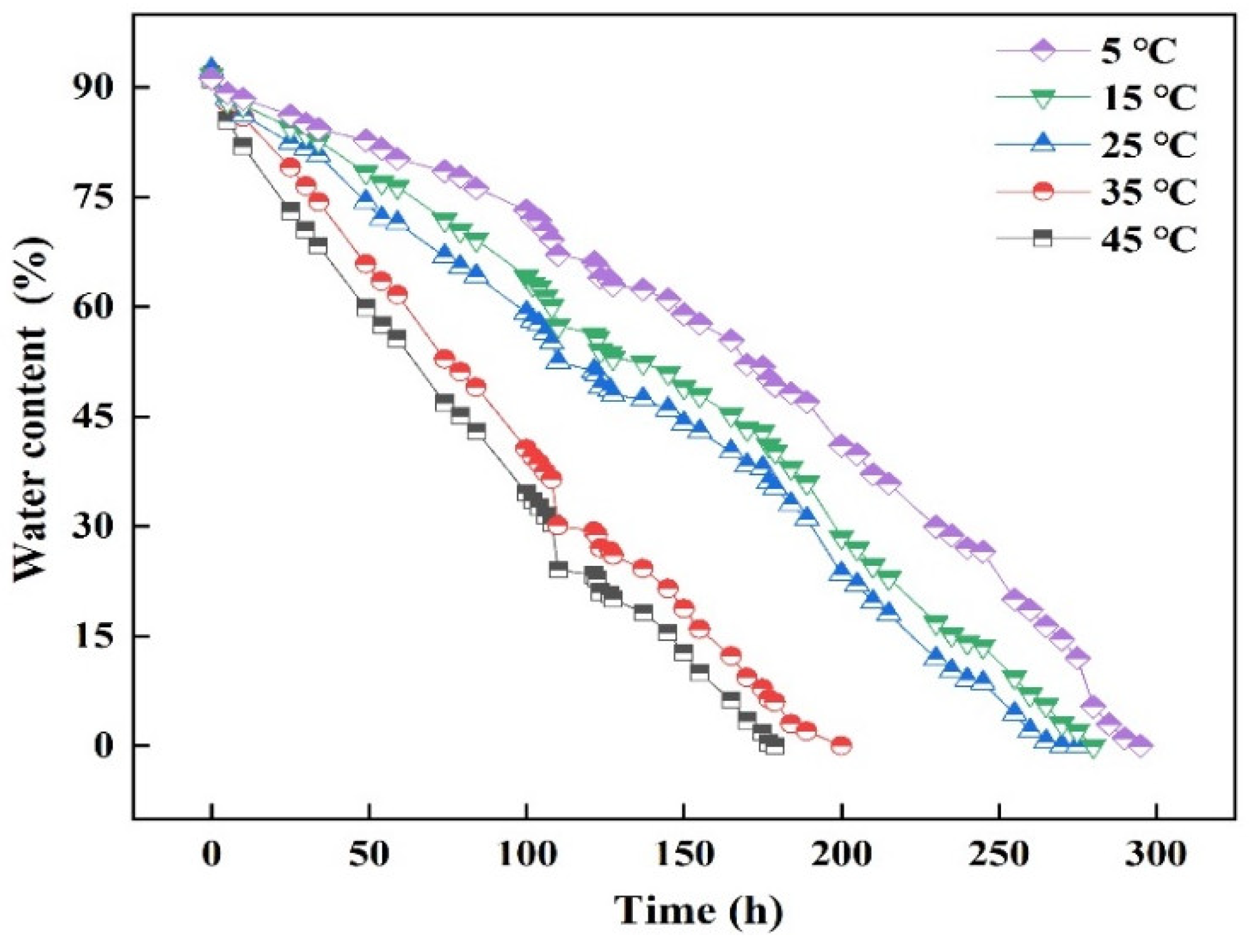
3.4. Study on Water Absorption and Release of Ceramsite
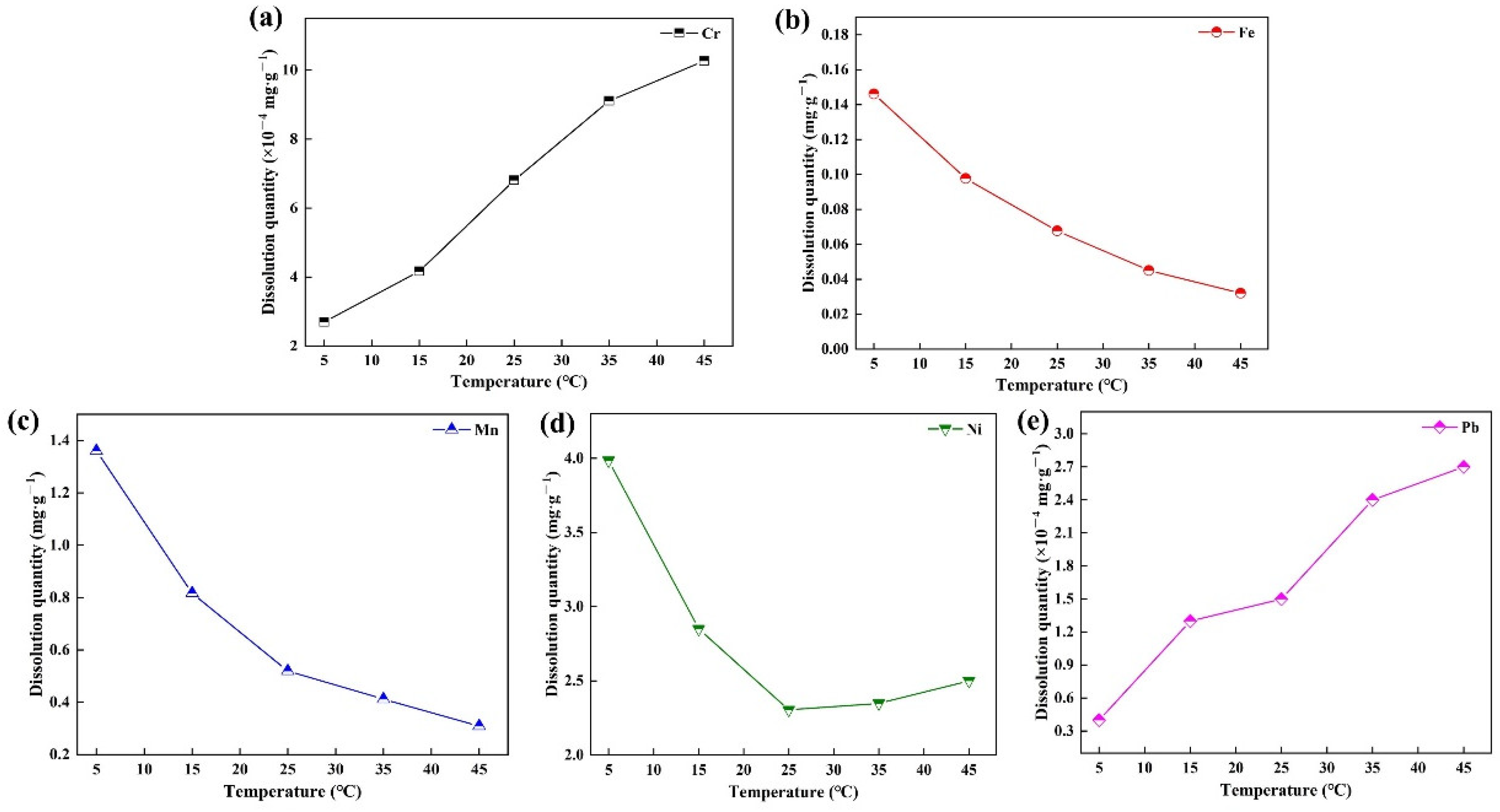
3.5. Risk Assessment of Metals’ Dissolution in Ceramsite
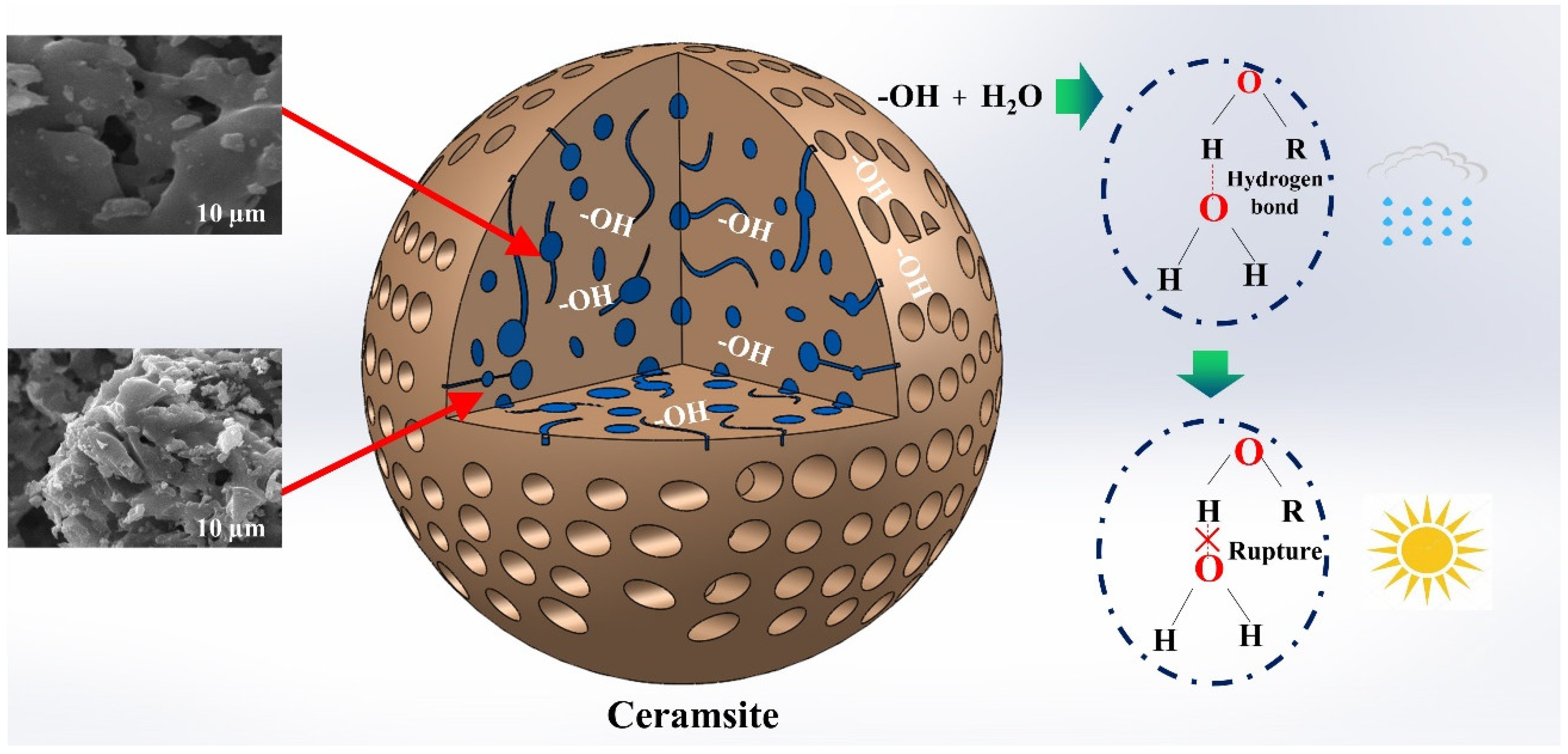
3.6. Water Absorption and Release Mechanism of Ceramsite
4. Conclusions
- The pore formation of ceramsite is mainly classified into two stages: low temperature organic matter pyrolysis and high temperature decomposable salt pyrolysis. When the low temperature pyrolysis temperature is 500 °C and the high temperature pyrolysis temperature is 1050 °C, the pores of ceramsite are abundant and the proportion of pores per unit area is 80.12%.
- Through BET characterization analysis, it is found that the proportion of macropores is the determinant of the water absorption performance of ceramsite and mesopores are the main reason for the excellent water release effect of ceramsite.
- XRD characterization analysis: CaAl2Si2O8 and MgAl2O4 in the ceramsite is the reason for the material’s pore structure stability and the decisive factor for the recycling of the material.
- The water release experiment found that the ceramsite pore structure would be destroyed under acidic and alkaline conditions and the water release time would be reduced.
- Through the analysis of metals’ dissolution, it is found that the retention rate of Fe, Ni, Mn, Cr, and Pb is more than 99% under different conditions and the environmental application is safe. It is applied to sponge city construction, highway slope protection, and community landscape.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, H.; Song, C.; Yang, L.; Qin, H.; Cao, X.; Zhou, Y. Nutrients regeneration pathway, release potential, transformation pattern and algal utilization strategies jointly drove cyanobacterial growth and their succession. J. Environ. Sci. 2021, 103, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Liu, W.; Shi, Y.; Wang, B.; Huang, C.; Liu, C.; Wang, A. Microbial electrolysis enhanced bioconversion of waste sludge lysate for hydrogen production compared with anaerobic digestion. Sci. Total Environ. 2020, 767, 144344. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Zhao, P.; Yue, Q.; Gao, B.; Yue, D.; Li, Q. A novel polynary fatty acid/sludge ceramsite composite phase change materials and its applications in building energy conservation. Renew. Energy 2015, 76, 45–52. [Google Scholar] [CrossRef]
- Yang, N.; Xiao, H.; Pi, K.; Fang, J.; Liu, S.; Chen, Y.; Shi, Y.; Zhang, H.; Gerson, A.R.; Liu, D. Synchronization of dehydration and phosphorous immobilization for river sediment by calcified polyferric sulfate pretreatment. Chemosphere 2021, 269, 129403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hu, M.; Bian, B.; Yang, Z.; Yang, W.; Zhang, L. Full-scale thermophilic aerobic co-composting of blue-green algae sludge with livestock faeces and straw. Sci. Total Environ. 2021, 753, 142079. [Google Scholar] [CrossRef]
- Zeng, X.; Twardowska, I.; Wei, S.; Sun, L.; Wang, J.; Zhu, J.; Cai, J. Removal of trace metals and improvement of dredged sediment dewaterability by bioleaching combined with Fenton-like reaction. J. Hazard. Mater. 2015, 288, 51–59. [Google Scholar] [CrossRef]
- Xu, Y.; Luo, C.; Sang, H.; Lu, B.; Wu, F.; Li, X.; Zhang, L. Structure and surface insight into a temperature-sensitive CaO-based CO2 sorbent. Chem. Eng. J. 2022, 435, 1385–8947. [Google Scholar] [CrossRef]
- Seidel, H.; Löser, C.; Zehnsdorf, A.; Hoffmann, P.; Schmerold, R. Bioremediation process for sediments contaminated by metals: Feasibility study on a pilot scale. Environ. Sci. Technol. 2004, 38, 1582–1588. [Google Scholar] [CrossRef]
- Mulligan, C.N.; Yong, R.N.; Gibbs, B.F. An evaluation of technologies for the metals remediation of dredged sediments. J. Hazard. Mater. 2001, 85, 145–163. [Google Scholar] [CrossRef]
- Maiguizo-Diagne, H.; Ndiaye, N.A.; Ndour-Badiane, Y.; Masse, D.; Torrijos, M.; Sousbie, P.; Gaye, M.L.; Ndoye, I.; Hamelin, J.; Fall, S. The use of green macroalgae (Ulva lactuca and Codium tomentosum) that have a high methane potential, as a source of biogas in Senegal. J. Appl. Biosci. 2019, 132, 13404. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Periyasamy, M.; Prathima, A. Biodiesel from microalgae: Environmental aspects. Mater. Today Proc. 2020, 33, 3664–3667. [Google Scholar] [CrossRef]
- Kumar, M.D.; Kavitha, S.; Tyagi, V.K.; Rajkumar, M.; Bhatia, S.K.; Kumar, G.; Banu, J.R. Macroalgae-derived biohydrogen production: Biorefinery and circular bioeconomy. Biomass Convers. Biorefin. 2021, 12, 769–791. [Google Scholar] [CrossRef]
- Riley, C.M. Relation of Chemical Properties to the Bloating of Clays. J. Am. Ceram. Soc. 1951, 34, 121–128. [Google Scholar] [CrossRef]
- Luo, W.; Lu, J. Inhibition of in situ coating of sediment ceramsite on sediment nutrient release of eutrophic lakes. Environ. Geochem. Health 2020, 44, 1471–1485. [Google Scholar] [CrossRef]
- Jin, Y.; Huang, S.; Wang, Q.; Gao, M.; Ma, H. Ceramsite production from sediment in Beian River: Characterization and parameter optimization. R. Soc. Open Sci. 2019, 6, 190–197. [Google Scholar] [CrossRef] [Green Version]
- Cheng, G.; Li, Q.; Su, Z.; Sheng, S.; Fu, J. Preparation, optimization, and application of sustainable ceramsite substrate from coal fly ash/waterworks sludge/oyster shell for phosphorus immobilization in constructed wetlands. J. Clean. Prod. 2018, 175, 572–581. [Google Scholar] [CrossRef]
- Li, P.; Luo, S.; Zhang, L.; Wang, Q.; Huang, X.; Zhang, Y.; Liu, X.; Liang, J.; Duan, X. Study on preparation and performance of iron tailings-based porous ceramsite filter materials for water treatment. Sep. Purif. Technol. 2021, 276, 119380. [Google Scholar] [CrossRef]
- Mi, H.; Yi, L.; Wu, Q.; Xia, J.; Zhang, B. Preparation of high-strength ceramsite from red mud, fly ash, and bentonite. Ceram. Int. 2021, 47, 18218–18229. [Google Scholar] [CrossRef]
- Cai, Y.; Gao, H.; Qu, G.; Ning, P.; Hu, Y.; Zou, H.; Ren, N. Research on the efficient water-absorbing ceramsite generated by dredged sediments in Dian Lake-China and coal fly ash. Water Environ. Res. 2021, 93, 2769–2779. [Google Scholar] [CrossRef]
- Zhang, J.H.; Hu, G.J.; Xu, T.X.; Guo, Q.; Wang, Y.; Tian, C. Effect of biomass addition on preparation of ceramsite made by fly ash. Metalurgija 2018, 57, 189375. [Google Scholar]
- Zhong, L.; Yang, X.Q.; Song, J.; Liu, Y.; Zhu, W.L.; Wen, H.B.; Liu, Y.X. Technology of Preparing Ceramsite from Electric Ceramic Waste. Solid State Phenom. 2022, 335, 73–78. [Google Scholar] [CrossRef]
- Zhao, L.; Hu, M.; Muslim, H.; Hou, T.; Bian, B.; Yang, Z.; Yang, W.; Zhang, L. Co-utilization of lake sediment and blue-green algae for porous lightweight aggregate (ceramsite) production. Chemosphere 2022, 287, 132145. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, G.K.; Cordes, L.N.; Patterson, M.B.J.G. Laboratory-based characterization of plutonium in soil particles using micro-XRF and 3D confocal XRF. J. Anal. Atom. Spectrom. 2015, 30, 1511–1517. [Google Scholar] [CrossRef]
- Arif, A.T.; Maschowski, C.; Garra, P.; Garcia-Käufer, M.; Petithory, T.; Trouvé, G.; Dieterlen, A.; Mersch-Sundermann, V.; Khanaqa, P.; Nazarenko, I.; et al. Cytotoxic and genotoxic responses of human lung cells to combustion smoke particles of Miscanthus straw, softwood and beech wood chips. Atmos. Environ. 2017, 163, 138–154. [Google Scholar] [CrossRef] [PubMed]
- Merikallio, T. Drying of lightweight concrete produced from crushed expanded clay aggregates. Cem. Concr. Res. 1996, 26, 1423–1433. [Google Scholar] [CrossRef]
- Li, B.; Jian, S.; Zhu, J.; Gao, X.; Gao, W. Effect of sintering temperature on lightweight aggregates manufacturing from copper contaminated soil. Ceram. Int. 2021, 47, 31319–31328. [Google Scholar] [CrossRef]
- Yang, Y.; Wei, Z.; Chen, Y.; Li, Y.; Li, X. Utilizing phosphate mine tailings to produce ceramisite. Constr. Buil. Mater. 2017, 155, 1081–1090. [Google Scholar] [CrossRef]
- Bu, H.; Yuan, P.; Liu, H.; Liu, D.; Liu, J.; He, H.; Zhou, J.; Song, H.; Li, Z. Effects of complexation between organic matter (OM) and clay mineral on OM pyrolysis. Geochim. Cosmochim. Acta 2017, 212, 1–15. [Google Scholar] [CrossRef]
- Wang, L.; Shao, Y.; Zhao, Z.; Chen, S.; Shao, X. Optimized utilization studies of dredging sediment for making water treatment ceramsite based on an extreme vertex design. J. Water Process Eng. 2020, 38, 101603. [Google Scholar] [CrossRef]
- Mohan, V.B.; Jayaraman, K.; Bhattacharyya, D. Brunauer–Emmett–Teller (BET) specific surface area analysis of different graphene materials: A comparison to their structural regularity and electrical properties. Solid State Commun. 2020, 320, 114004. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, S.C.; Guan, Y. Efficient removal of selenate in water by cationic poly(allyltrimethylammonium) grafted chitosan and biochar composite. Environ. Res. 2021, 194, 110667. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wang, J.; Ma, J.; Gao, D.; Bi, Y.; Luo, Y.; Chen, X. Effect of Municipal Sludge on the Performance of Coal Gasification Slag-Based Ceramsite. Ceram. Silik. 2021, 65, 1–9. [Google Scholar] [CrossRef]
- Xiong, D.; Wang, C.; Huang, X. Particular pollutants, physical properties, and environmental performance of porous ceramsite materials containing oil-based drilling cuttings residues. Environ. Sci. Pollut. Res. Int. 2022, 29, 7202–7213. [Google Scholar] [CrossRef]
- Shen, Y.; Yang, J.; Chen, X.; Chen, Y.; Xu, X.; Zhan, M.; He, Y.; Jiao, W.; Yin, Y. Kill two birds with one stone: Ceramisite production using organic contaminated soil. J. Hazard. Mater. 2022, 436, 129062. [Google Scholar] [CrossRef]
- Shao, Y.; Shao, Y.; Zhang, W.; Zhu, Y.; Dou, T.; Chu, L.; Liu, Z. Preparation of municipal solid waste incineration fly ash-based ceramsite and its mechanisms of metals immobilization. Waste Manag. 2022, 143, 54–56. [Google Scholar] [CrossRef]
- Chen, R.; Huang, J.; Leung, A.K.; Chen, Z.; Chen, Z. Experimental investigation on water release and gas emission of evapotranspirative capillary barrier landfill covers. Soil Sci. Soc. Am. J. 2022, 86, 311–323. [Google Scholar] [CrossRef]
- Rudd, T.; Sterritt, R.M.; Lester, J.N. Complexation of Metals by Extracellular Polymers in the Activated Sludge Process. J. Water Pollut. Control Fed. 1984, 56, 1260–1268. [Google Scholar]
- Sun, X.P.; Wang, A.T.; Li, X.H.; He, Z.H. Migration of metals in sludge treatment by thermal hydrolysis process. China Water Wastewater 2010, 26, 66–72. [Google Scholar]
- Johnson, W.P.; Logan, B.E. Enhanced transport of bacteria in porous media by sediment-phase and aqueous-phase natural organic matter. Water Res. 1996, 30, 923–931. [Google Scholar] [CrossRef]
- Novoa, J.J.; Sosa, C. Evaluation of the Density Functional Approximation on the Computation of Hydrogen Bond Interactions. J. Phys. Chem. 1995, 99, 15837–15845. [Google Scholar] [CrossRef]
- Ping, Z. The Adsorption of VOCs by Honeycomb Ceramics Loaded with Molecular Sieves. J. Chem. 2022, 2022, 7207403. [Google Scholar]






| Raw materials | SiO2 | Al2O3 | Fe2O3 | MgO | CaO | K2O | Ni | Mn | Cr | Pb |
| Dredging sediment | 57.95 | 16.66 | 9.00 | 2.37 | 6.66 | 3.61 | 0.024 | 0.224 | 0.051 | 0.017 |
| Cyanobacterial slurry | 18.93 | 35.89 | 10.54 | 1.77 | 10.92 | 5.56 | 0.059 | 0.384 | 0.101 | 0.040 |
| Experiments | Cyanobacteria-to-Sediment Ratios | Low Temperature Pyrolysis (°C) | High Temperature Pyrolysis (°C) |
|---|---|---|---|
| Set Ⅰ | 0:10 | 500 | 1050 |
| 1:9 | |||
| 2:8 | |||
| 3:7 | |||
| 4:6 | |||
| 5:5 | |||
| Set Ⅱ | 3:7 | 400 | 1050 |
| 450 | |||
| 500 | |||
| 550 | |||
| 600 | |||
| 650 | |||
| Set Ⅲ | 3:7 | 500 | 900 |
| 950 | |||
| 1000 | |||
| 1050 | |||
| 1100 | |||
| 1150 |
| Experiments | Materials | Mass (g) | Ambient Temperature (°C) | Water Ambient Temperature (°C) | pH |
|---|---|---|---|---|---|
| Set Ⅰ | Ceramsite | 2 | 40 | - | - |
| Soil | 2 | 40 | - | - | |
| Red brick | 2 | 40 | - | - | |
| Dinas | 2 | 40 | - | - | |
| Set Ⅱ | Ceramsite | 1 | 45 | - | 3 |
| 1 | 45 | - | 5 | ||
| 1 | 45 | - | 7 | ||
| 1 | 45 | - | 9 | ||
| 1 | 45 | - | 11 | ||
| Set Ⅲ | Ceramsite | 1 | 5 | - | 9 |
| 1 | 15 | - | 9 | ||
| 1 | 25 | - | 9 | ||
| 1 | 35 | - | 9 | ||
| 1 | 45 | - | 9 | ||
| Set Ⅳ | Ceramsite | 2 | 40 | - | 9 |
| Set Ⅴ | Ceramsite | 1 | 25 | - | 3 |
| 1 | 25 | - | 5 | ||
| 1 | 25 | - | 7 | ||
| 1 | 25 | - | 9 | ||
| 1 | 25 | - | 11 | ||
| Set Ⅵ | Ceramsite | 1 | 5 | - | 9 |
| 1 | 15 | - | 9 | ||
| 1 | 25 | - | 9 | ||
| 1 | 35 | - | 9 | ||
| 1 | 45 | - | 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Li, K.; Zhou, C.; Du, X.; Peng, J.; Liang, B.; Ding, Z.; Xiong, W. Resource Utilization of Lake Sediment to Prepare “Sponge” Light Aggregate: Pore Structure and Water Retention Mechanism Study. Processes 2022, 10, 2331. https://doi.org/10.3390/pr10112331
Huang Y, Li K, Zhou C, Du X, Peng J, Liang B, Ding Z, Xiong W. Resource Utilization of Lake Sediment to Prepare “Sponge” Light Aggregate: Pore Structure and Water Retention Mechanism Study. Processes. 2022; 10(11):2331. https://doi.org/10.3390/pr10112331
Chicago/Turabian StyleHuang, Yu, Kunpeng Li, Chi Zhou, Xiaotian Du, Jiangnan Peng, Baowen Liang, Ziyi Ding, and Wen Xiong. 2022. "Resource Utilization of Lake Sediment to Prepare “Sponge” Light Aggregate: Pore Structure and Water Retention Mechanism Study" Processes 10, no. 11: 2331. https://doi.org/10.3390/pr10112331




