Activated Carbon Aerogel as an Electrode with High Specific Capacitance for Capacitive Deionization
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Electrode Preparation
2.3. Design of CDI System and Measurement of NaCl Removal
2.4. Characterization of Material
2.5. Characterization of Electrode
2.6. CDI Experiments
3. Results and Discussion
3.1. Characterization of CA and ACA
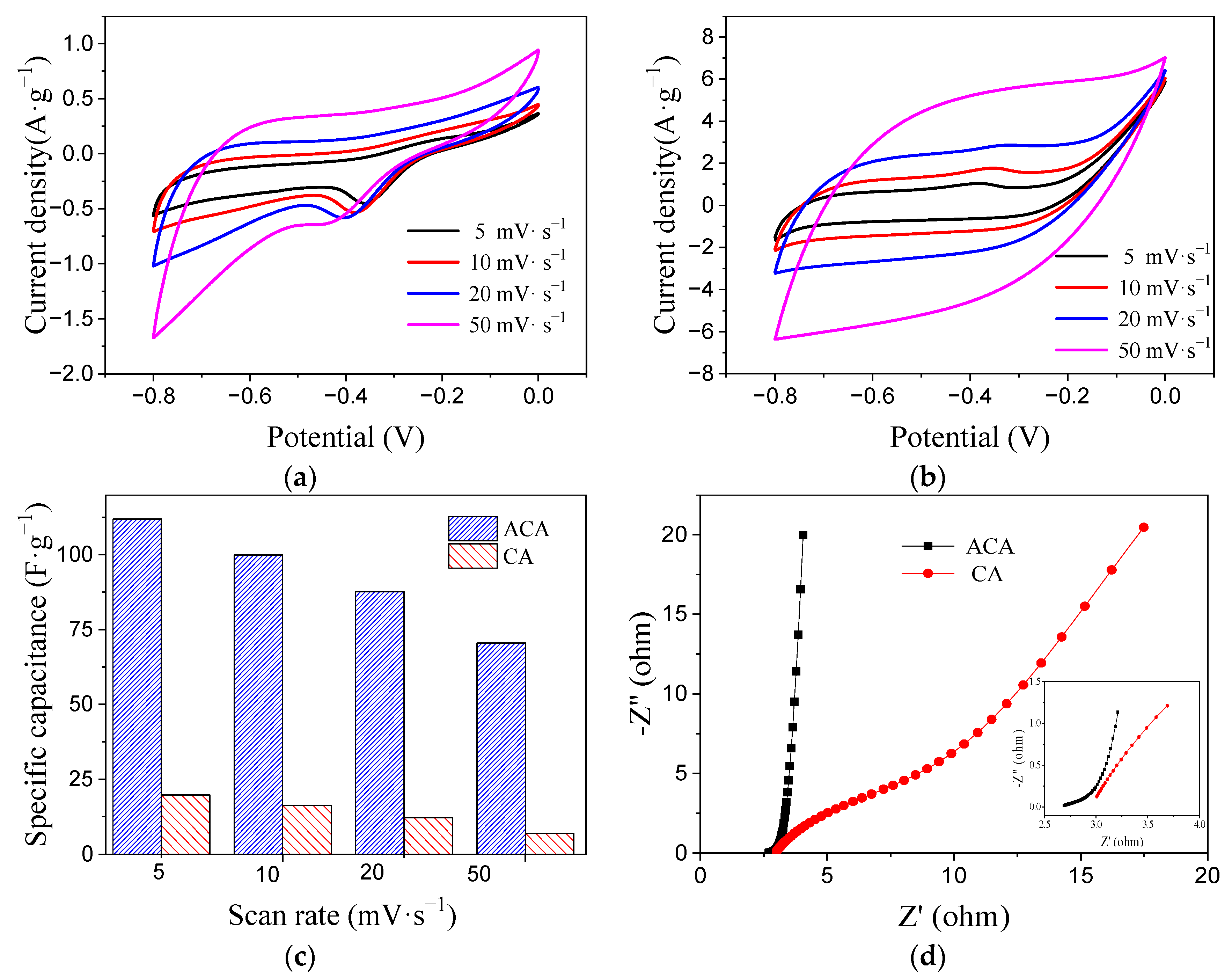
3.2. Electrode Characterization
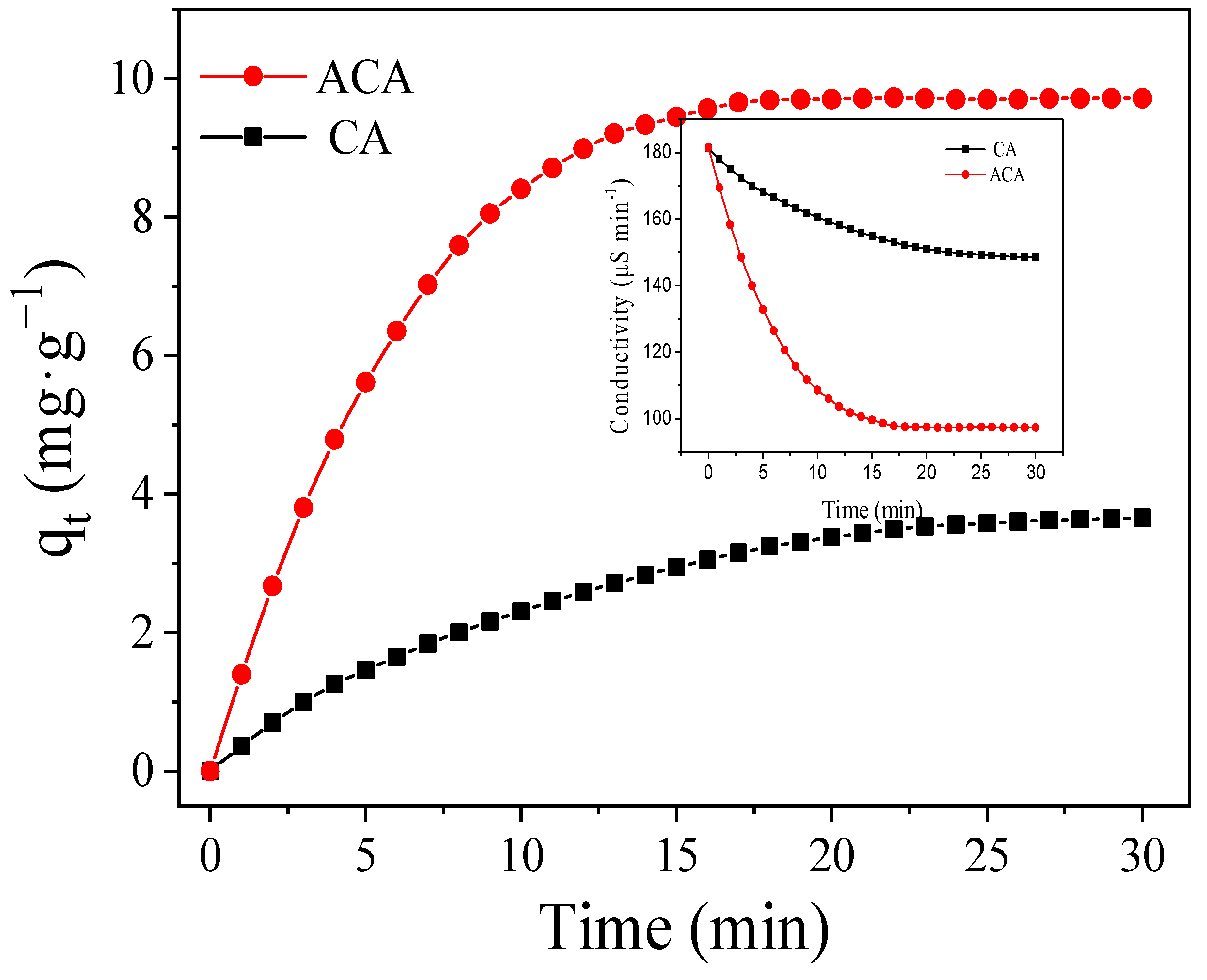
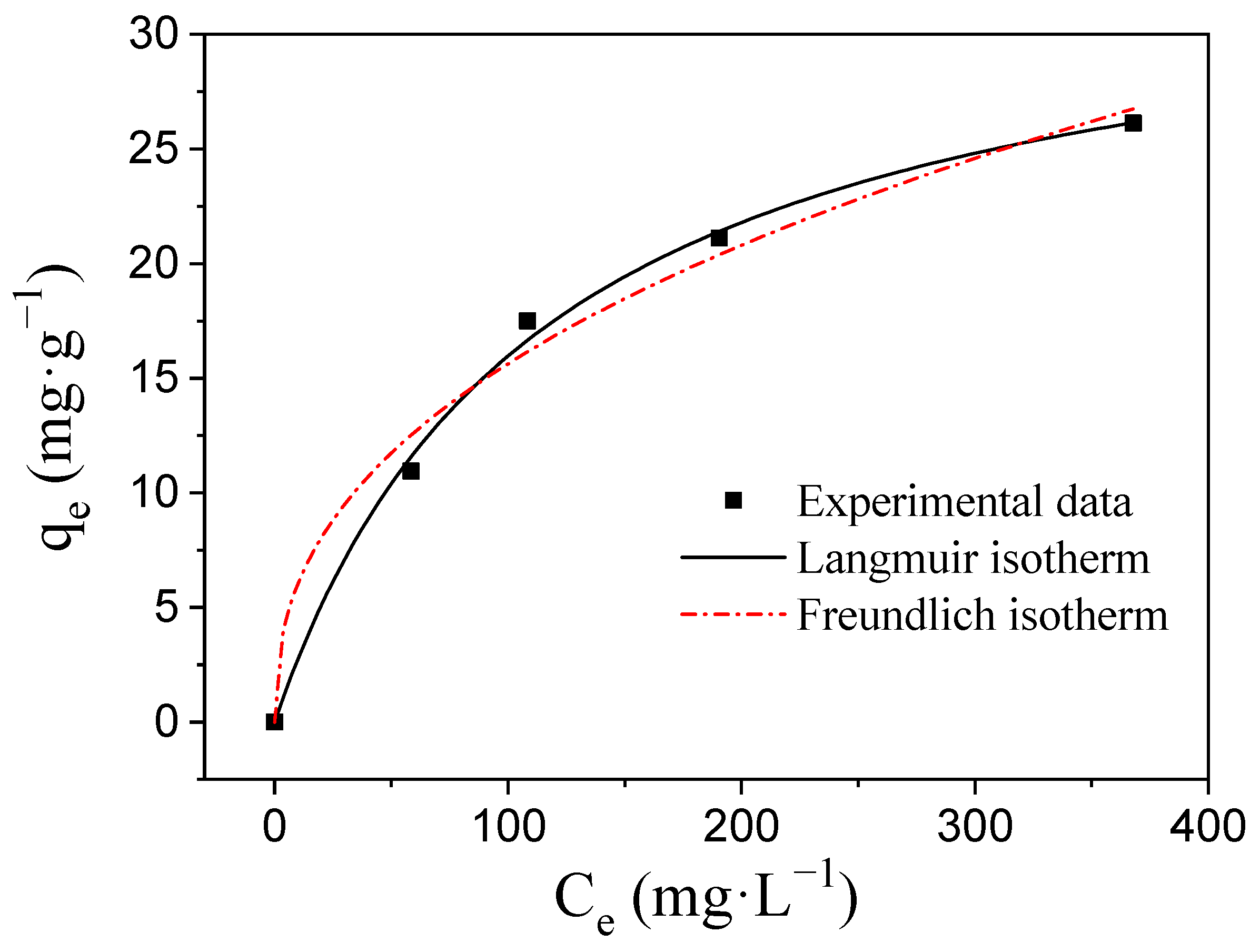
3.3. Desalination Performance of CDI Electrodes
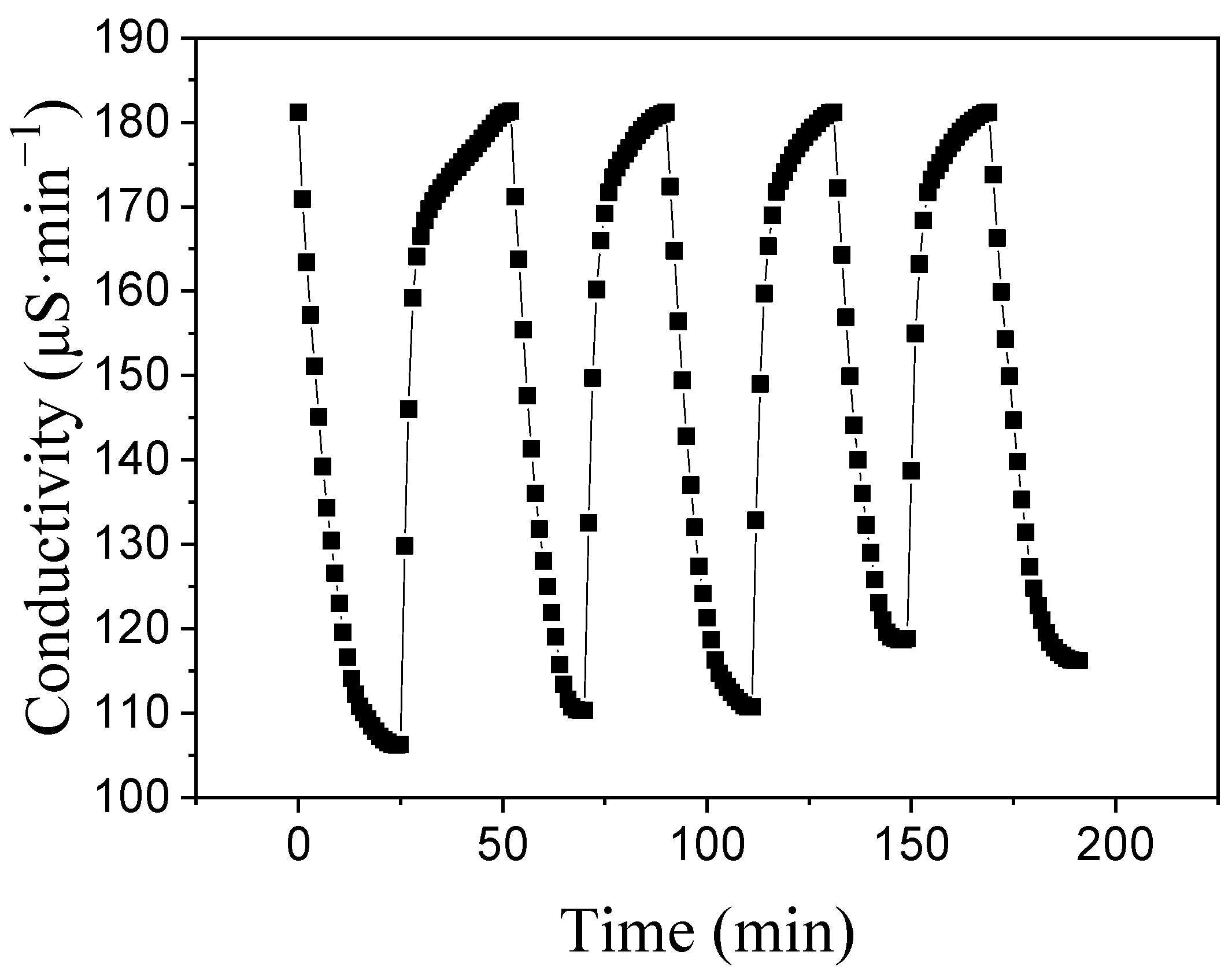
3.4. Regeneration Property of ACA Electrode
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dong, Y.X.; Poredoš, P.; Ma, Q.M.; Wang, R.Z. High-yielding and stable desalination via photothermal membrane distillation with free-flow evaporation channel. Desalination 2022, 543, 116103. [Google Scholar] [CrossRef]
- Brogioli, D.; Yip, N.Y. Energy efficiency analysis of membrane distillation for thermally regenerative salinity gradient power technologies. Desalination 2022, 531, 115694. [Google Scholar] [CrossRef]
- Fajardo-Diaz, J.L.; Morelos-Gomez, A.; Cruz-Silva, R.; Ishii, K.; Yasuike, T.; Kawakatsu, T.; Yamanaka, A.; Tejima, S.; Izu, K.; Saito, S.; et al. Low-pressure reverse osmosis membrane made of cellulose nanofiber and carbon nanotube polyamide nano-nanocomposite for high purity water production. Chem. Eng. J. 2022, 448, 137359. [Google Scholar] [CrossRef]
- Biesheuvel, P.M.; Dykstra, J.E.; Porada, S.; Elimelech, M. New parametrization method for salt permeability of reverse osmosis desalination membranes. J. Membr. Sci. 2022, 2, 100010. [Google Scholar] [CrossRef]
- Chen, Q.B.; Wang, J.Y.; Liu, Y.; Zhao, J.L.; Li, P.F. Novel energy-efficient electrodialysis system for continuous brackish water desalination: Innovative stack configurations and optimal inflow modes. Water Res. 2020, 179, 115847. [Google Scholar] [CrossRef]
- Chen, Q.B.; Xu, Y.; Li, P.F.; Wang, J.; Dong, L.; Zhao, J.L.; Wang, J.Y. An emerging pilot-scale electrodialysis system for desalination of SWNF permeate: Evaluating the role of typical factors. Desalination 2022, 542, 116064. [Google Scholar] [CrossRef]
- Wang, Z.D.; Tian, S.H.; Niu, J.J.; Kong, W.; Lin, J.Y.; Hao, X.G.; Guan, G.Q. An electrochemically switched ion exchange process with self-electrical-energy recuperation for desalination. Sep. Purif. Technol. 2020, 239, 116521. [Google Scholar] [CrossRef]
- Subban, C.V.; Gadgil, A.J. Electrically regenerated ion-exchange technology for desalination of low-salinity water sources. Desalination 2019, 465, 38–43. [Google Scholar] [CrossRef]
- Wu, Q.H.; Liang, D.W.; Lu, S.F.; Wang, H.N.; Xiang, Y.; Aurbach, D.; Avraham, E.; Cohen, I. Advances and perspectives in integrated membrane capacitive deionization for water desalination. Desalination 2022, 542, 116043. [Google Scholar] [CrossRef]
- Toledo-Carrillo, E.; Zhang, X.Y.; Laxman, K.; Dutta, J. Asymmetric electrode capacitive deionization for energy efficient desalination. Electrochim. Acta 2020, 358, 136939. [Google Scholar] [CrossRef]
- Zhang, C.Y.; He, D.; Ma, J.X.; Tang, W.W.; Waite, T.D. Faradaic reactions in capacitive deionization (CDI)-problems and possibilities: A review. Water Res. 2018, 128, 314–330. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.Y.; Haddad, A.Z.; Kumar, A.; Wang, S.W. Brackish water desalination using reverse osmosis and capacitive deionization at the water-energy nexus. Water Res. 2020, 183, 116064. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Deshmukh, A.; Epsztein, R.; Patel, S.K.; Owoseni, O.M.; Walker, W.S.; Elimelech, M. Comparing energy efficiency of capacitive deionization and reverse osmosis. Desalination 2019, 455, 100–114. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Tewari, S. Capacitive deionization: Processes, materials and state of the technology. J. Electroanal. Chem. 2018, 813, 178–192. [Google Scholar] [CrossRef]
- Gupta, S.S.; Islam, M.R.; Pradeep, T. Capacitive Deionization (CDI): An alternative cost-efficient desalination technique. In Advances in Water Purification Techniques, Meeting the Needs of Developed and Developing Countries; Ahuja, S., Ed.; Elsevier/Ahuja Consulting: Calabash, NC, USA, 2019; Chapter 7; pp. 165–202. [Google Scholar]
- Wu, L.; Liu, M.; Huo, S.; Zang, X.; Xua, M.; Ni, W.; Yang, Z.; Yan, Y.M. Mold-casting prepared free-standing activated carbon electrodes for capacitive deionization. Carbon 2019, 149, 627–636. [Google Scholar] [CrossRef]
- Atoufi1, H.D.; Hasheminejad, H.; Lampert, D.J. Performance of activated carbon coated graphite bipolar electrodes on capacitive deionization method for salinity reduction. Front. Environ. Sci. Eng. 2020, 14, 99. [Google Scholar] [CrossRef]
- Han, J.L.; Yan, T.T.; Shen, J.J.; Shi, L.Y.; Zhang, J.P.; Zhang, D.S. Capacitive deionization of saline water by using MoS2–graphene hybrid electrodes with high volumetric adsorption capacity. Environ. Sci. Technol. 2019, 53, 12668–12676. [Google Scholar] [CrossRef]
- Xu, P.; Drewes, J.E.; Heil, D.; Wang, G. Treatment of brackish produced water using carbon aerogel-based capacitive deionization technology. Water Res. 2008, 42, 2605–2617. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Wang, H.; Wang, S.; Hayat, T.; Alsaedi, A.; Wang, X. Functionalization of biomass carbonaceous aerogels and their application as electrode materials for electro-enhanced recovery of metal ions. Environ. Sci. Nano 2017, 4, 1114–1123. [Google Scholar] [CrossRef]
- Kumar, R.; Sen Gupta, S.; Katiyar, S.; Raman, V.K.; Varigala, S.K.; Pradeep, T.; Sharma, A. Carbon aerogels through organo-inorganic co-assembly and their application in water desalination by capacitive deionization. Carbon 2016, 99, 375–383. [Google Scholar] [CrossRef]
- Law, K.Y. Definitions for hydrophilicity, hydrophobicity, and superhydrophobicity: Getting the basics right. J. Phys. Chem. Lett. 2014, 5, 686–688. [Google Scholar] [CrossRef]
- Wang, J.; Chen, S.; Xu, J.Y.; Liu, L.C.; Zhou, J.C.; Cai, J.J. High-surface-area porous carbons produced by the mild KOH activation of a chitosan hydrochar and their CO2 capture. Carbon 2022, 188, 545. [Google Scholar] [CrossRef]
- Acosta, R.; Fierro, V.; Martinez de Yuso, A.; Nabarlatz, D.; Celzard, A. Tetracycline adsorption onto activated carbons produced by KOH activation of tyre pyrolysis char. Chemosphere 2016, 149, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Forghani, M.; Donne, S.W. Method comparison for deconvoluting capacitive and pseudo-capacitive contributions to electrochemical capacitor electrode behavior. J. Electrochem. Soc. 2018, 165, A664. [Google Scholar] [CrossRef]
- Lee, K.M.; Kim, K. Electrode potentials in electrochemical double-layer capacitors with asymmetric electrode thicknesses. Electrochem. Electrochim. Acta 2022, 435, 141364. [Google Scholar] [CrossRef]
- Quan, X.; Fu, Z.; Yuan, L.; Zhong, M.; Mi, R.; Yang, X.; Yi, Y.; Wang, C. Capacitive deionization of NaCl solutions with ambient pressure dried carbon aerogel microsphere electrodes. RSC Adv. 2017, 7, 35875–35882. [Google Scholar] [CrossRef] [Green Version]
- Kuo, H.A.; Ramachandran, A.; Oyarzun, D.I.; Clevenger, E.C.; Santiago, J.G.; Stadermann, M.; Campbell, P.G.; Hawks, S.A. Understanding resistances in capacitive deionization devices. Environ. Sci. Water Res. Technol. 2020, 6, 1842. [Google Scholar] [CrossRef]
- Wang, H.; Yan, T.; Liu, P.; Chen, G.; Shi, L.; Zhang, J.; Zhong, Q.; Zhang, D. In situ creating interconnected pores across 3D graphene architectures and their application as high performance electrodes for flow-through deionization capacitors. J. Mater. Chem. A 2015, 4, 4908–4919. [Google Scholar] [CrossRef]
- Zhao, S.; Yan, T.; Wang, H.; Zhang, J.; Shi, L.; Zhang, D. Creating 3D hierarchical carbon architectures with micro-, meso-, and macropores via a simple self-blowing strategy for a flow-through deionization capacitor. ACS App. Mater. Interfaces 2016, 8, 18027–18035. [Google Scholar] [CrossRef]
- Maheshwari, K.; Agarwal, M.; Solanki, Y.S. Electrode material effect on electrochemical characterization, properties and operational parameters in capacitive deionization. Mater. Today Proc. 2021, 43, 1204–1209. [Google Scholar] [CrossRef]
- Dahiya, S.; Mishra, B.K. Enhancing understandability and performance of flow electrode capacitive deionisation by optimizing configurational and operational parameters: A review on recent progress. Sep. Purif. Technol. 2020, 240, 116660. [Google Scholar] [CrossRef]
- Liu, P.; Wang, H.; Yan, T.; Zhang, J.; Shi, L.; Zhang, D. Grafting sulfonic and amine functional groups on 3D graphene for improved capacitive deionization. J. Mater. Chem. A 2016, 4, 5303–5313. [Google Scholar] [CrossRef]
- Luo, G.; Wang, Y.; Gao, L.; Zhang, D.; Lin, T. Graphene bonded carbon nanofiber aerogels with high capacitive deionization capability. Electrochim. Acta 2018, 260, 656–663. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Shen, J.; Qi, J.; Li, J.; Sun, X.; Shen, J.; Han, W.; Wang, L. Design of nitrogen-doped cluster-like porous carbons with hierarchical hollow nanoarchitecture and their enhanced performance in capacitive deionization. Desalination 2018, 430, 45–55. [Google Scholar] [CrossRef]
- Xie, J.; Xue, Y.; He, M.; Luo, W.; Wang, H.; Wang, R.; Yan, Y.M. Organic-inorganic hybrid binder enhances capacitive deionization performance of activated-carbon electrode. Carbon 2017, 123, 574–582. [Google Scholar] [CrossRef]
- Xu, X.; Tang, H.; Wang, M.; Liu, Y.; Li, Y.; Lu, T.; Pan, L. Carbon spheres with hierarchical micro/mesopores for water desalination by capacitive deionization. J. Mater. Chem. A 2016, 4, 16094–16100. [Google Scholar] [CrossRef]
- Li, G.X.; Hou, P.X.; Zhao, S.Y.; Liu, C.; Cheng, H.M. A flexible cotton-derived carbon sponge for high-performance capacitive deionization. Carbon 2016, 101, 1–8. [Google Scholar] [CrossRef]
- Wang, G.; Dong, Q.; Wu, T.; Zhan, F.; Zhou, M.; Qiu, J. Ultrasound-assisted preparation of electrospun carbon fiber/graphene electrodes for capacitive deionization: Importance and unique role of electrical conductivity. Carbon 2016, 103, 311–317. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Lu, T.; Sun, Z.; Chu, D.C.; Pan, L. Ultra-thin carbon nanofiber networks derived from bacterial cellulose for capacitive deionization. J. Mater. Chem. A 2015, 3, 8693–8700. [Google Scholar] [CrossRef]
- Xu, X.; Pan, L.; Liu, Y.; Lu, T.; Sun, Z. Enhanced capacitive deionization performance of graphene by nitrogen doping. J. Colloid Interface Sci. 2015, 445, 143–150. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, L.; Chen, T.; Xu, X.; Lu, T.; Sun, Z.; Chua, D.H.C. Porous carbon spheres via microwave-assisted synthesis for capacitive deionization. Electrochim. Acta 2015, 151, 489–496. [Google Scholar] [CrossRef]
- Wang, H.; Yan, T.; Shen, J.; Zhang, J.; Shi, L.; Zhang, D. Efficient removal of metal ions by capacitive deionization with straw waste derived graphitic porous carbon nanosheets. Environ. Sci. Nano 2020, 7, 317–326. [Google Scholar] [CrossRef]
- Li, C.P.; Wu, Y.Q.; Zhang, F.Y.; Gao, L.X.; Zhang, D.Q.; An, Z.X. Capacitive deionization of NaCl solution with hierarchical porous carbon materials derived from Mg-MOFs. Sep. Purif. Technol. 2021, 277, 119618. [Google Scholar] [CrossRef]

| Electrode Materials | Adsorption Conditions | qm (mg·g−1) | Reference |
|---|---|---|---|
| Carbon aerogel | 1.2 V, 500 mg·L−1, 50 mL | 15.7 | [34] |
| Porous carbons | 1.2 V, 500 mg·L−1, 30 mL | 17.2 | [35] |
| Activated carbon | 1.5 V, 1000 mg·L−1, 50 mL | 14.6 | [36] |
| Porous carbon spheres | 1.2 V, 500 mg·L−1, 20 mL | 15.8 | [37] |
| Graphene | 1.4 V, 500 mg·L−1, 35 mL | 13.7 | [33] |
| Carbon sponge | 1.2 V, 500 mg·L−1, 80 mL | 16.1 | [38] |
| Graphene | 1.2 V, 100 mg·L−1 | 9.2 | [39] |
| Carbon nanofiber | 1.2 V, 1000 mg·L−1, 50 mL | 12.8 | [40] |
| Graphene | 1.8 V, 100 mg·L−1, 50 mL | 4.8 | [41] |
| Porous carbon spheres | 1.6 V, 500 mg·L−1, 50 mL | 5.8 | [42] |
| Graphitic porous carbon nanosheets | 1.2 V, 500 mg·L−1 | 19.3 | [43] |
| MoS2−graphene | 1.2 V, 500 mg·L−1, 50 mL | 19.4 | [18] |
| ACA | 1.4 V, 500 mg·L−1, 35 mL | 26.1 | This study |
| C0 (mg·L−1) | qe (mg·g−1) | Pseudo-First Order | Pseudo-Second Order | |||||
|---|---|---|---|---|---|---|---|---|
| qe,cal (mg·g−1) | k1 (min−1) | R2 | qe,cal (mg·g−1) | k2 (g·mg−1·min−1) | h0 (mg·g−1·min−1) | R2 | ||
| 100 | 10.95 | 10.77 | 0.50 | >0.99 | 13.09 | 0.0018 | 0.31 | 0.99 |
| 200 | 17.50 | 20.68 | 0.30 | 0.97 | 29.44 | 0.0006 | 0.56 | >0.99 |
| 300 | 21.12 | 32.32 | 0.63 | 0.95 | 27.34 | 0.0003 | 0.22 | 0.98 |
| 500 | 26.12 | 27.27 | 0.33 | >0.99 | 37.08 | 0.0002 | 0.27 | 0.98 |
| Models | Parameters | Values |
|---|---|---|
| Langmuir | qm (mg·g−1) | 34.32 |
| KL (L·mg−1) | 0.01 | |
| RL | 0.54 | |
| R2 | 0.99 | |
| Freundlich | KF ((mg·g−1) (L·mg−1)1/n) | 2.34 |
| n | 2.42 | |
| R2 | 0.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Li, K.; Song, G.; Zhou, M.; Tan, P. Activated Carbon Aerogel as an Electrode with High Specific Capacitance for Capacitive Deionization. Processes 2022, 10, 2330. https://doi.org/10.3390/pr10112330
Wang W, Li K, Song G, Zhou M, Tan P. Activated Carbon Aerogel as an Electrode with High Specific Capacitance for Capacitive Deionization. Processes. 2022; 10(11):2330. https://doi.org/10.3390/pr10112330
Chicago/Turabian StyleWang, Wei, Kerui Li, Ge Song, Minghua Zhou, and Peng Tan. 2022. "Activated Carbon Aerogel as an Electrode with High Specific Capacitance for Capacitive Deionization" Processes 10, no. 11: 2330. https://doi.org/10.3390/pr10112330
APA StyleWang, W., Li, K., Song, G., Zhou, M., & Tan, P. (2022). Activated Carbon Aerogel as an Electrode with High Specific Capacitance for Capacitive Deionization. Processes, 10(11), 2330. https://doi.org/10.3390/pr10112330






