Abstract
The application of the plant growth regulator 1-(2-chloro-4-pyridyl)-3-phenylurea (CPPU) is extensively used for red-fleshed kiwifruits or ‘Donghong’, but it has toxicological properties. Extra plant growth regulators (PGRs) were screened for partial substitution of CPPU (10 mg L−1) to the crops to minimize the CPPU content. The results showed that CPPU at a concentration of 5 mg L−1 plus 14-hydroxylated brassinosteroid (14-OH BR) at a concentration of 0.15 mg L−1 has a nearly equal effect to CPPU at a concentration of 10 mg L−1; it maintains the kiwifruit yields and quality as well as increases the postharvest time. Transcriptome sequencing data revealed that the regulation of 14-OH BR on kiwifruit growth acts mainly by activating Brassinosteroid (BR) signaling to synergistically and antagonistically stimulate the signaling of other endogenous growth regulators, including auxin (IAA), abscisic acid (ABA), cytokinin (CK), gibberellin (GA), jasmonic acid (JA) and ethylene (ET).
1. Introduction
Being of Chinese origin, kiwifruit (Actinidia chinensis) is an edible berry rich in vitamins, minerals, and amino acids. The red-fleshed cultivars have an attractive appeal and unique flavor, approved by consumers. As the predominant cultivar, ‘Hongyang’ was first released from Cangxi County of Sichuan Province in China [1], but it is susceptible to Pseudomonas syringae pv. Actinidiae (Psa); it is a bacterial pathogen which can cause huge economic losses to kiwifruit plants [2]. Kiwifruit ‘Donghong’ is a red-fleshed kiwifruit cultivar selected from the F1 offspring of open-pollinated kiwifruit ‘Hongyang’ at Wuhan Botanical Garden, Chinese Academy of Sciences [3]. Maturation of ‘Donghong’ kiwifruit accompanied by a complicated bioactive component [4]. A non-destructive device called Kiwi-Meter™, was recently developed to assess its maturation and ripening [5]. ‘Donghong’ is popular because of its disease resistance and longer shelf life, as well as the particularly sweet taste. However, like ‘Hongyang’, the small fruit size of the red-fleshed kiwifruit limits its commercial benefit, whose average fruit weight is only 65~75 g.
To increase the fruit size and yield, 1-(2-chloro-4-pyridyl)-3-phenylurea (CPPU), a plant growth regulator (PGR), has been widely applied in the initial stages of red-fleshed kiwifruit development [6,7], it was found that a dose-dependent CPPU regulatory model exists in ‘Hongyang’, and 5 mg L−1 was the optimal concentration for CPPU to enhance fruit enlargement by stimulating cell division and cell expansion [8]. The underlying CPPU regulation mechanism for red-fleshed ‘Hongyang’ kiwifruit development was found to involve influencing the biosynthesis of gibberellin (GA), cytokinin (CK), abscisic acid (ABA) and auxin (IAA) and enhancing the energy supply [7]. As a Phenylurea-derived cytokinin, CPPU has also been applied to various horticultural crops, such as grape [9], apple [10] and peach [11], to enhance the fruit set and improve the fruit size, yield and quality.
In fact, so far, a number of PGRs are being widely applied in a variety of plant species to induce a particular plant response. For example, with the proper application of CKs and GAs in the cultivation of Zantedeschia, PGRs can improve the flowering intensity, extend the postharvest life and enhance the leaves’ greenness index and protein quantity [12,13,14,15]. A study of gibberellic acid (GA3) and arbuscular mycorrhizal fungi (AMF) on the flowering and quality of Zantedeschia albomaculata (Hook.) Baill ‘Albomaculata’ plants indicated that AMF and GA3 had a favorable effect on the quality of inflorescences [16]. Janowska et al. also reported that benzyladenine (BA) and GA3 affect the elongation of inflorescences and spike, respectively, as well as the content of micro- and macronutrients in its leaves in the Gladiolus hybridus cv. Black Velvet [17,18]. The study conducted on the influence of gibberellic acid (GA3) on the content of biologically active substances in Crocosmia × crocosmiiflora ‘Lucifer’ tubers suggested that the antioxidant activity of the extracts from tubers increased consistently with the GA3 concentration [19]. Andrzejak et al. assessed the influence of the Trichoderma spp. on the flowering and nutritional status of Begonia × tuberhybrida Voss. ‘Picotee Sunburst’ and Gladiolus hybridus L. ‘Advances Red’ plants. These results displayed that Trichoderma spp. enhanced the flowering of the ‘Picotee Sunburst’ cultivar by 2.7–8.7 days, encouraged the development of buds and flowers and altered their size [20], improved the nutrient uptake, chlorophyll a + b, carotenoids and flowering in the plant of Gladiolus hybridus L. ‘Advances Red’ [21].
Even though the dosage-controlled agricultural use of CPPU is legal, the potential toxicity associated with CPPU is still unclear [22]; a repeated-dose toxicity study of CPPU in rats indicated the potential danger of CPPU on the ovaries and steroid production [23] and animal studies in zebrafish also indicated that CPPU adversely affects cardiac morphology and function [24]. Many environmental and animal-friendly PGRs were reported to have growth-promoting functions, such as 14-hydroxylated brassinosteroid (14-OH BR); a brassinolide analog derived from natural extracts [25], Zeatin [26], GA4+7 [27] and Jianda 990.
In the present study, we combine low-dosage CPPU (5 mg L−1) with different biological PGRs for fruit enlargement testing via a standard fruit dipping method. We measured the fruit characteristics of each treatment, trying to minimize the CPPU application contents in kiwifruit ‘Donghong’ without detectable yield trade-offs.
2. Materials and Methods
2.1. Plant Materials
The fruits examined were from five-year-old mature Donghong (Actinidia chinensis) vines grown at the “8S” kiwifruit demonstration garden in Daxing Town, Pujiang County, Chengdu city, China.
2.2. Experimental PGRs
In this study, apart from CPPU (AS 0.1%, Sichuan Lanyue Technology Co., Ltd., Chengdu, China), 4 other biological PGRs were exploited, including 14-hydroxylated brassinosteroid (14-OH BR, AS, 0.007 5%, Chengdu Newsun Crop Science Co., Ltd., Chengdu, China), Jianda 990 (SG, 39.998% GA3 + 0.002% 14-OH BR, Chengdu Newsun Crop Science Co., Ltd., Chengdu, China), Zeatin (WP, 0.1%, Zhengzhou Zhengshi Chemical Products Co., Ltd., Zhengzhou, China), GA4+7 (AS, 40%, Jiangxi New Reyphon Biochemical Co., Ltd., Ji’an, China). In addition, sugar, alcohol, and a range of minerals (calcium and magnesium, each at a concentration of 150 g L−1, iron, manganese, copper, zinc and boron, each at a concentration of 2–6 g L−1, AS, Chengdu Newsun Crop Science Co., Ltd., Chengdu, China) were used as an extra supplement of all treatments.
2.3. Experimental Treatments
There were twelve treatments performed 14 days after anthesis (DAA, on 2 May): CPPU at a concentration of 10 mg L−1 (control), CPPU at a concentration of 5 mg L−1 + 14-OH BR at a concentration of 0.15, 0.075 and 0.0375 mg L−1 (T1, T2 and T3, respectively), CPPU at a concentration of 5 mg L−1 + Jianda 990 diluted by 3 × 103, 6 × 103 and 9 × 103 (T4, T5 and T6, respectively), CPPU at a concentration of 5 mg L−1 + Zeatin at a concentration of 1, 0.1 and 0.01 mg L−1 (T7, T8 and T9, respectively), CPPU at a concentration of 5 mg L−1 + GA4+7 at a concentration of 30, 60 and 120 mg L−1 (as T10,T11 and T12, respectively), a 500-fold dilution of sugar, alcohol, calcium and magnesium was also applied for each treatment.
In all the treatments, the test solution was applied as a 3s fruit dip. Each treatment is repeated 3 times, and each replication includes 2 kiwi trees. Except for the different experimental treatments, the fertilization, watering and pest control during the entire growth period are all consistent.
2.4. Evaluation of Fruit Quality
One hundred fruits were randomly selected for each treatment after the standard harvesting period (20 September for ‘Donghong’). The fruits were first measured to determine the horizontal and vertical diameter (for shape index) and single fruit weight, then 50 fruits were randomly selected to determine the hardness; the remaining 50 fruits were tested for the Vc content, total solubility solids (TSS), Titratable acids (TA) and TSS/TA ratio, as well as the mineral and trace element content. The vitamin C concentration was determined by High-Performance Liquid Chromatography [28], the TSS was measured with a digital refractometer (PAL-1, Atago, Tokyo, Japan) with juice drops, the TA were determined by using an acid-base titration method based on CNS GB/T 12456-2008 [29], and mineral and trace element content were determined by the national standard GB 5009.268-2016 [30].
2.5. Fruit Postharvest Storage Quality
For postharvest quality, 150 kiwifruits for each treatment were randomly harvested at the standard harvesting period; the covered bags were taken off, and fruits were packed in plastic baskets and placed in the refrigerator (temperature 0.5–1 °C, humidity 90–95%) for 3 months. Afterwards, 50 fruits were randomly picked to test hardness, soluble solids and dry matter on days 3, 7 and 12.
2.6. Transcriptome Profiling
Transcriptome profiling was carried out by Wuhan Metware Biotechnology Co., Ltd. (Wuhan, China) (19 October 2020; http://www.metware.cn/), following their standard procedures.
2.6.1. RNA Extraction and RNA-Seq
The total RNA of the samples was extracted with an RNA prep Pure Plant kit (DP441, Tiangen, China). Illumina RNA-Seq was performed by Metware Biotechnology Co., Ltd. (Wuhan, China). The RNA quality was detected by a NanoPhotometer spectrophotometer (IMPLEN, Westlake Village, CA, USA), Qubit 2.0 Fluorometer (Life Technologies, Carlsbad, CA, USA), and Agilent Bioanalyzer 2100 system (Agilent Technologies, Santa Clara, CA, USA). The poly(A) mRNA was enriched by magnetic beads with oligo (dT). The mRNA was randomly fragmented. First-strand cDNA was synthesized using the M-MuLV reverse transcriptase system. The RNA strand was then degraded by RNase H, and the second-strand cDNA was synthesized using DNA polymerase. The double-stranded cDNAs were ligated to sequencing adapters. The cDNAs (~200 bp) were screened using AMPure XP beads. After amplification and purification, cDNA libraries were obtained and sequenced using the Illumina Nova seq6000 system.
2.6.2. Sequence Data Processing
The raw reads were transformed from the sequencing raw image data by CASAVA base recognition. To obtain high-quality data, adapters of sequences were cut, and low-quality reads with ≥5 uncertain bases or with over 50% Qphred ≤ 20 bases were removed using fastp. The GC content of clean reads was calculated. The Q20 and Q30 values were also produced by FastQC to evaluate the base quality. Then, the clean reads were mapped to the kiwifruit reference genome using HISAT2 with default parameters. Gene expression levels were determined using the FPKM (Fragments Per Kilobase of transcript per Million fragments mapped) method.
Differentially expressed genes (DEGs) were defined with a threshold of |log2fold change| ≥1 and FDR ≤ 0.05. The identified DEGs were subjected to enrichment analysis of KEGG pathway analysis.
2.7. Statistical Analysis
Data analysis was performed with Graph Prism software. The Student’s t-test was used to compare the means of average fruit weight between CPPU concentrations at 10 and 5 mg L−1 (p ≤ 0.01). One-way ANOVA was performed for each treatment to obtain a statistical assessment. The Tukey’s test was used for mean comparisons of twelve treatments on fruit quality during kiwifruit (Actinidia chinensis) ripening (p ≤ 0.05).
3. Results
3.1. The Effect of Different Treatments on Fruit Development of A. chinensis ‘Donghong’
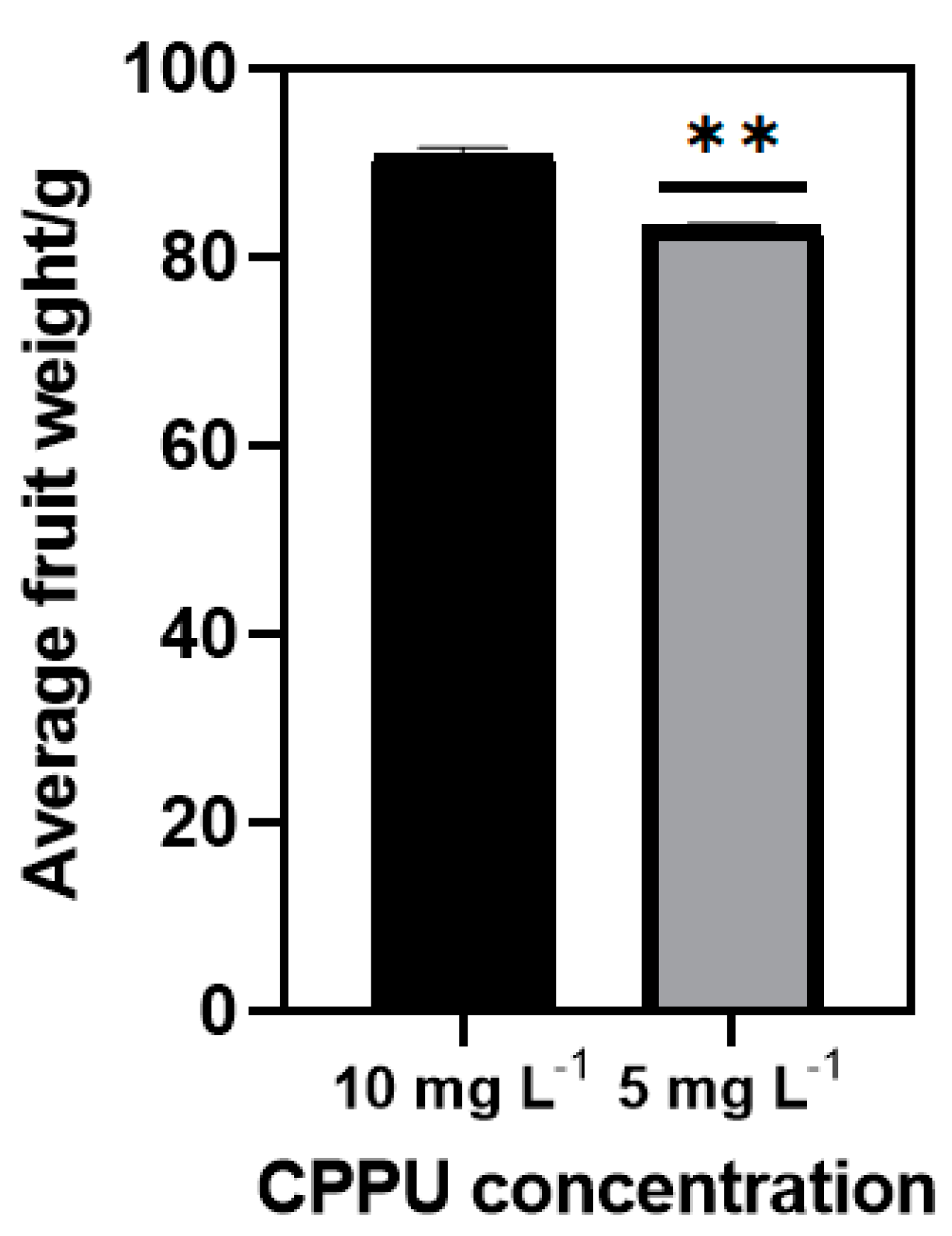
The optimal concentration of CPPU to enhance ‘Donghong’ fruit enlargement is 10 mg L−1 in our experience; halving the concentration would cause a severe reduction in the average fruit weight (Figure 1). To find alternative kiwifruit enlargement stimuli packages, we took CPPU at a concentration of 10 mg L−1 as the control. We tested different biological PGRs plus CPPU at a concentration of 5 mg L−1 to assess whether any other PGRs can partially replace CPPU’s function and compensate for the yield loss.
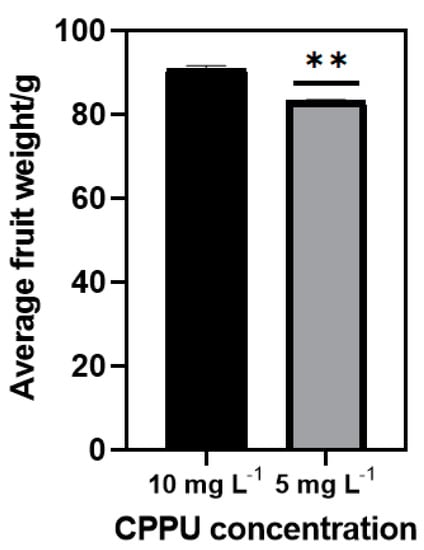
Figure 1.
The effect of halving the concentration of CPPU on kiwifruit (Actinidia chinensis) ‘Donghong’ enlargement. A ** in the column means that it is significantly different to the control by t-test at p ≤ 0.01.
Among the twelve treatments, our results showed that, apart from T3 and T4, which demonstrated a significant weight decrease in comparison to the control (Table 1), all other treatments recovered the weight loss caused by reduced CPPU usage and maintained a similar shape index as the control, suggesting that the application of a correct concentration of different PGRs can reduce the content of CPPU needed for the kiwifruit ‘Donghong’. Besides, all twelve treatments considerably increased the fruit hardness while they tended to decrease the TSS at the same time, implying that the reduction of CPPU can prolong fruit storage time further. Specifically, we found that T1, T4 and T12 have the smallest varying degrees in terms of TSS, which resulted in a relatively higher TSS/TA ratio. Further data showed that these three treatments have insignificant Vitamin C content loss.

Table 1.
The effect of different treatments on fruit quality during kiwifruit (Actinidia chinensis) ‘Donghong’ ripening.
We further investigated the effects of different treatment on the mineral and trace element content. As shown in the Table 2, T1 was found to have an amazing boost effect on the calcium and magnesium synthesis during kiwifruit ripening, suggested its promising function in fruit mineral content improvement. Whereas, other treatments did not show noticeable variation. Besides, all treatments declined the copper content because of the lower CPPU, meanwhile, T10 and T12 were found to have a stimulating effect on the iron and zinc generation, respectively.

Table 2.
The effect of different treatments on the mineral and trace element content during kiwifruit (Actinidia chinensis) ‘Donghong’ ripening.
3.2. Evaluation of Fruit Postharvest Quality of Different Treatments
Concerning the effect of different treatments on the postharvest quality of the fruit under cold storage, as storage time was prolonged, all treatments showed a tendency of decreased firmness of fruits; we found T3 and T7 possessed the highest and lowest hardness degree, respectively, compared to the non-treated control at day 12 (Table 3). The other treatments did not show a significant difference in the fruit-softening level. Regarding the TSS and dry matter, no significant variation was obtained when storage was finished (Table 3).

Table 3.
Effect of different treatments on the kiwifruit (Actinidia chinensis) ‘Donghong’ postharvest quality under cold storage.
3.3. Comprehensive Evaluation of Different PGRs on Regulation of Fruits Characteristics during Substitution of CPPU in Kiwifruit ‘Donghong’
To screen out the optimal PGR in maintaining fruit quality when the substitution of CPPU is carried out, a method of modified Z-score, z’ = (x − μ)/σ was used to standardize all tested terms, where x is the raw value of T1–T12, μ is the control value, and σ is the population standard deviation. Except for the negative contributor of Titratable acids, all corresponding standardized data of each term were added to represent the total score of each treatment; the higher the value, the better the treatment could be.
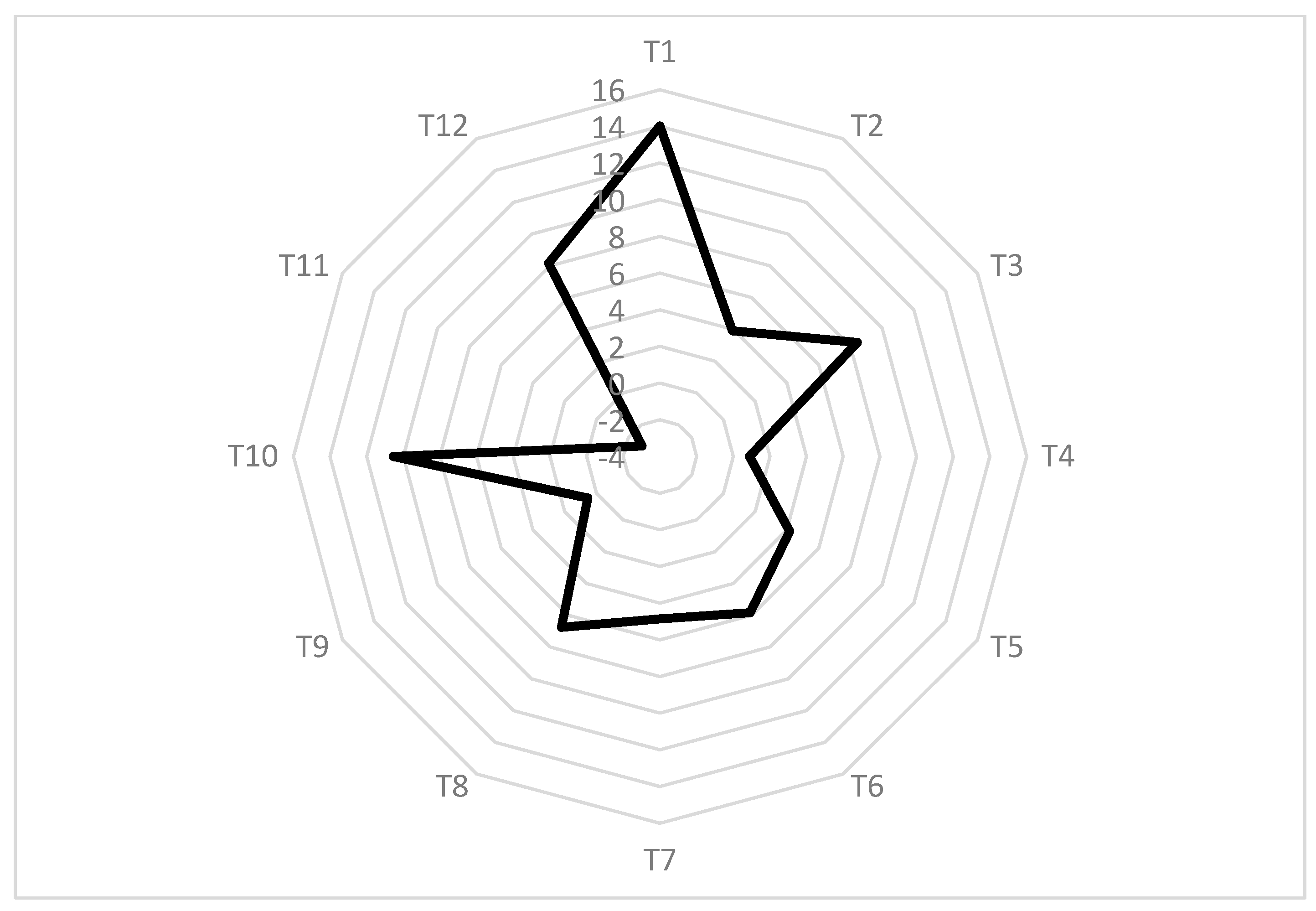
As shown in Figure 2, T1 and T10 clearly have the greatest potential to improve both harvest and postharvest fruit quality. Assuming that they could be applied in the field to minimize the application content of CPPU in kiwifruit ‘Donghong’, we selected T1, which has the highest z’ score, to clarify the underlying mechanism for its successful substitution in more detail. T1 consists of CPPU at a concentration of 5 mg L−1 and 14-OH BR at a concentration of 0.15 mg L−1, which means a PGR of 14-OH BR plays a critical role in the promotion of fruit enlargement (Figure 3) as well as maintains the impressive fruit characteristics. 14-OH BR is a natural brassinolide analogue derived from rape pollen; it plays an important role in accelerating cell division and growth, nutrient absorption and transportation, as well as improving stress resistance; it also proved to be highly safe [31,32]. To elucidate the mechanisms underlying the role of 14-OH BR in the regulation of kiwifruit development further, RNA-seq was conducted.
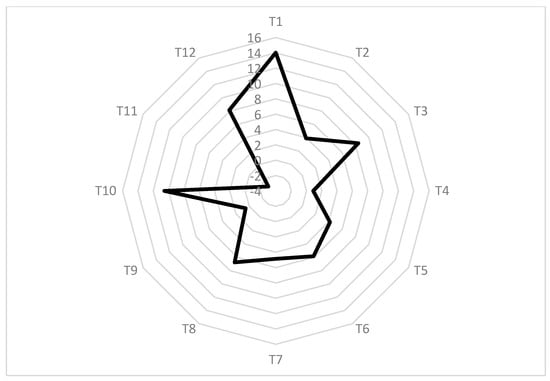
Figure 2.
Radar chart for competitive analysis of different treatments.

Figure 3.
The phenotype of kiwifruit (Actinidia chinensis) ‘Donghong’ throughout fruit development. Coin diameter= 30 mm.
3.4. Transcriptional Enrichment Analysis of 14-OH BR Regulation of Kiwifruit Development
The flesh of 3 groups of fruit samples 6 days after dipping (on 15 May) were used: fruits induced by water (6DCK), fruits induced by CPPU at a concentration of 5 mg L−1 (6DUP) and fruits induced by CPPU at a concentration of 5 mg L−1 and 14-OH BR at a concentration of 0.15 mg L−1 (6D3). Using the Illumina HiSeq sequencing platform, a total of 58.19 Gb of paired-end short read data (after quality control and removal of reads containing adapter, reads containing ploy-N, and low-quality reads from raw data) were obtained. The percentages of Q30 and GC were 95.35–95.79% and 46.13–46.46%, respectively, indicating that the quality of transcriptome sequencing data is high.
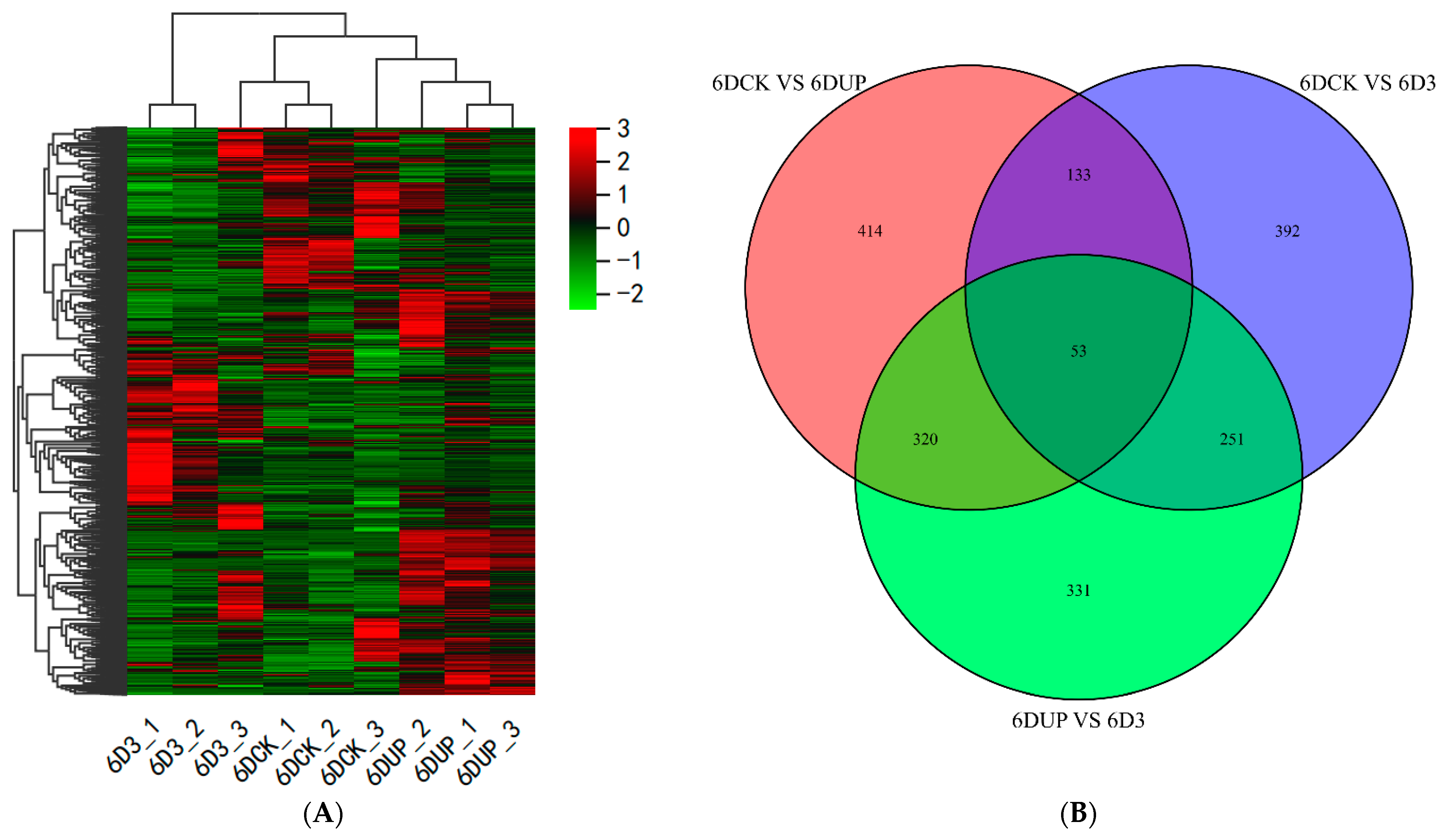
A total of 31,308 genes were functionally annotated in the databases (Figure 4A, Supplementary file S1). Moreover, 920 (701 up and 219 down-regulated), 829 (503 up and 326 down-regulated) and 955 (374 up and 581 down-regulated) DEGs (|log2Fold Change| ≥ 1, and FDR < 0.05) were identified in the pairwise comparison of 6DCK versus 6DUP, 6DCK versus 6D3 and 6DUP versus. 6D3, respectively (Figure 4B, Supplementary file S2). The results indicated that 14-OH BR could induce the changes in transcripts at the very beginning of kiwifruit enlargement.
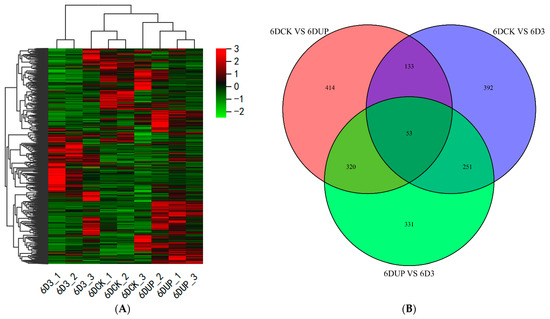
Figure 4.
Differentially expressed genes (DEG) in kiwifruit (Actinidia chinensis) ‘Donghong’ in response to different treatments during fruit ripening stages. (A) The heatmap of DEGs from different treatments. (B) Venn enrichment analysis of DEGs.
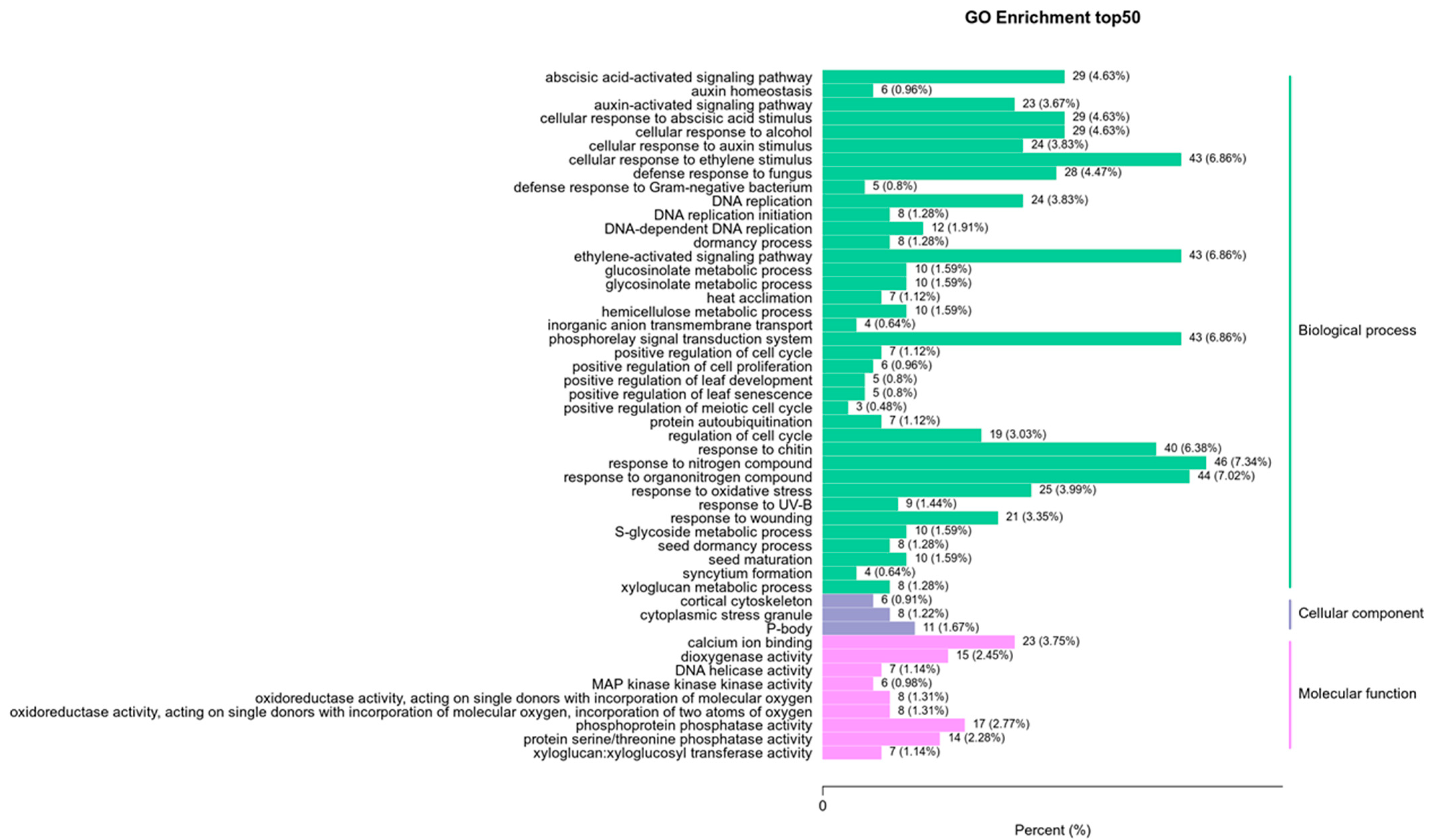
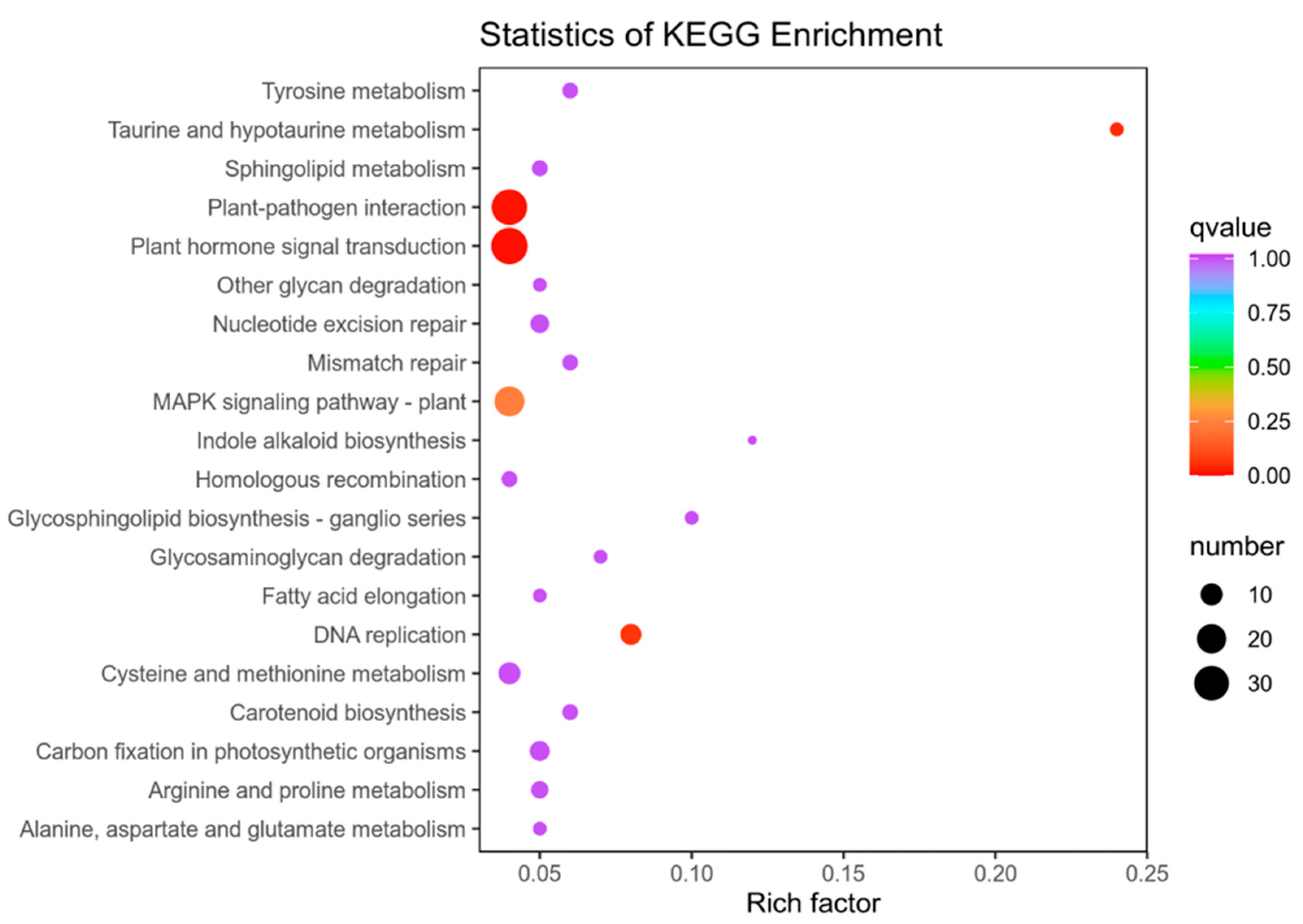
To dissect how 14-OH BR affects kiwifruit development further, we focused on the enrichment analysis of KEGG between 6D3 and 6DUP. GO and KEGG enrichment analysis was performed on the differential genes. The GO results showed that the differential genes were mainly concentrated in plant-activated biological processes, including abscisic acid, auxin and the ethylene-activated signaling pathway (Figure 5).
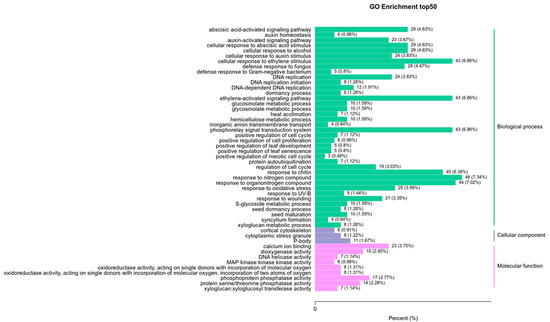
Figure 5.
GO pathway enrichment analysis of DEGs between 6D3 and 6DUP.
The results of KEGG enrichment analysis also showed that hormone transduction activation plays an important role in this process (Figure 6), suggesting that 14-OH BR regulation of kiwifruit development occurs mainly through changes to endogenous phytohormone signaling.
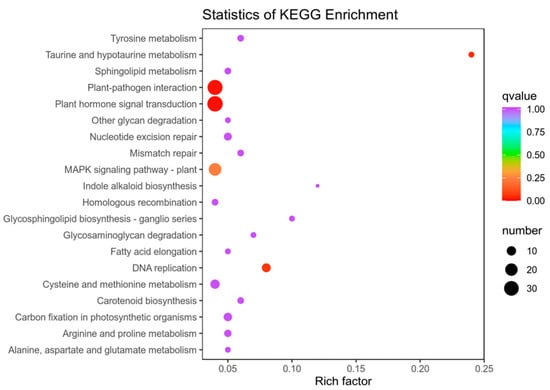
Figure 6.
KEGG pathway enrichment analysis of DEGs between 6D3 and 6DUP.
3.5. Candidate Genes Related to Phytohormone Signaling Transduction Involved in 14-OH BR-Regulated Kiwifruit Development
To unravel the molecular details regarding gene functioning in the regulation of phytohormone biosynthesis and signaling, we screened our RNA-seq datasets. The results exhibited induced gene downregulation of auxin signaling transduction in kiwifruits due to 14-OH BR treatment (Table 4). These results were supported by the decreased expression of most auxin-responsive genes, such as auxin-amido synthetases GH3, as well as various auxin-responsive proteins (such as SAUR36, IAA4, SAUR24, 22D and ARG7). Furthermore, 14-OH BR treatment also repressed the expression of the CK signaling pathway positive regulator of ARR5.

Table 4.
List of DEGs involved in phytohormone biosynthetic and signaling in kiwifruit (Actinidia chinensis) ‘Donghong’ in response to 14-OH BR treatment.
For the induced signaling transduction, we found protein phosphatase 2C (PP2C), which is an ABA signaling negative regulator, the GA signaling negative regulator DELLA protein as well as jasmonate acid (JA)signaling repressor JAZ1. They were downregulated at the same time. We also found that receptors of ethylene, GA and JA were induced by 14-OH BR treatment. Besides, the increased expression of JAR1 suggested the biosynthesis of JA was also stimulated.
Besides, as expected, genes involved in brassinosteroid (BR) signaling were upregulated, including the BR receptor BRI1, positive regulator BSK and corn transcription factor BES1/BZR1 (Table 4).
Collectively, these results suggested that 14-OH BR compensate the reduced content of CPPU to promote the kiwifruit ‘Donghong’ enlargement mainly through enhanced signaling transduction of BR, GA, ABA and JA, along with repressed signaling of auxin and CK. Additionally, it looks like the regulation of 14-OH BR treatment also involved with the JA biosynthesis and the involvement of ethylene biosynthesis and signaling are quite limited at the early stage of kiwifruit development.
4. Discussion
Plant growth regulators (PGRs) are natural or synthetic compounds that can positively modify plant growth and development by mimicking naturally occurring plant hormones in very small concentrations [33]. Forchlorfenuron (CPPU, N-(2-Chloro-4-pyridyl)-N’-phenylurea) is a synthetic cytokinin extensively used to increase the berry size of grapes and kiwifruit. It can stimulate the periclinal cell division leading to more round-shaped berries and increasing fruit quality. However, the incidence of CPPU has caused an adverse impact on the kiwifruit industry; its toxicological properties may persist in their transformation products (TPs), which were speculated to have an even higher toxicity than the parent molecule [34]. In this study, we found that the application of 14-OH BR, Jianda 990, Zeatin and GA4+7 in the right concentration (T1, T4 and T12) could further minimize the CPPU content from 10 mg L−1 to 5 mg L−1 without significant fruit quality decreasing in comparison to the control of CPPU at a concentration of 10 mg L−1 use alone (Table 1). Moreover, most treatments increased the hardness of the fruit and decreased the TSS, thus prolonging the kiwifruit ‘Donghong’ storage time (Table 3). GAs constitute a group of plant hormones that control developmental processes such as germination, shoot elongation, tuber formation, flowering, fruit set, and growth in diverse species [35]. It was reported that early growth in tomatoes depends on GAs [36], and GA treatment has the potential to increase fruit firmness and size, and delays the maturity of ‘sweetheart’ sweet cherry [37]. In our data, both GA4+7 and GA3 (the main component of Jianda 990) were found to stimulate kiwifruit growth. In detail, GA4+7 at a high concentration and GA3 at a low concentration have the best effects (Table 1 and Table 3). Zeatin and its derivatives occur in many plant extracts and are the active ingredient in coconut milk, which causes plant growth [38]; applications of zeatin riboside stimulated cell growth (cell division or/and cell elongation) in banana fruit [39]. Our data also showed that it has a fruit-prompting function (Table 1). In terms of the mineral and trace element content, we found T1 can increase the calcium and magnesium content of kiwifruit, which might be because 14-OH BR can increase nutrient absorption. At the same time, all twelve treatments displayed a depressed copper content. This could because less CPPU was applied; the underlying mechanism was not clear. Besides, no noticeable changes of fruit quality were found postharvest. Nevertheless, among the 12 treatments, T1, which consisted of CPPU at a concentration of 5 mg L−1 and 14-OH BR at a concentration of 0.15 mg L−1 was finally chosen for further detailed molecular analysis, because it fully maintained the characteristics of kiwifruit ‘Donghong’ when CPPU (10 mg L−1) alone is used (Table 1, Table 2 and Table 3).
BRs have been collectively referred to as ‘pleotropic phytohormones’. They are essential for many biological processes in plants, such as initiation and cessation of flowering, plant canopy architecture, micropropagation, cell division and elongation, vegetative growth, flowering, fruit set, fruit ripening, quality and yield [40]. It was found that the application of BRs can boost the ripening of tomatoes and grapes [41,42]. In early fruit development, the application of exogenous 24-epibrassinolide can induce the expression of cell cycle-related genes in cucumbers [43]. Transcriptomic sequencing was conducted to elucidate the molecular mechanisms underlying 14-OH BR regulation of red flesh kiwifruit development at the initial stage. A total of 1894 DEGs were identified due to CPPU and 14-OH BR treatment. It was reported that both the initial cell division and the later cell expansion synergistically determine the final maximum size of fruits, where different phytohormones play a critical role in these processes [44,45]. In terms of the CPPU regulation of red flesh kiwifruit development, it was recorded that the GA and CKs contents were strongly increased, while the ABA and auxin contents were clearly decreased. Corresponding transcriptomic research has also consistently shown that expression levels of genes encoding GA and CK biosynthetic key enzymes, such as GA20-oxidase 1 (GA20ox1) and cytokinin phosphoribohydrolase ‘Lonely guy’ (LOG3), were significantly increased. In contrast, gene transcripts encoding auxin and ABA biosynthetic critical enzymes including flavin monooxygenase (YUCC2 and YUCCA2-like) and nine-cis-epoxycarotenoid dioxygenase (NCED3) were repressed [7,46]. When extra 14-OH BR was applied, our data showed that BR signaling was initiated as expected, and the expression of most auxin-responsive genes, such as SAUR36, IAA4, SAUR24, 22D and ARG7 decreased further.
In contrast, ABA signaling was enhanced by repressing the negative regulator PP2Cs (Table 4). These data suggested that, apart from the reduced content, the low-intensity response of auxin and, by contrast, increased ABA signaling transduction were indispensable in the 14-OH BR regulation of fruit development during the fastest growth stage. This finding was consistent with the finding that ABA was essential to the fruit size [47], whereas auxin signaling was repressed [7]. Furthermore, we also found that a transcription factor of ARR5 involved in CK signaling was repressed, whereas GA signaling was more stimulated via the regulation of the core components. These data indicate that 14-OH BR regulation of kiwifruits ‘Donghong’ growth is more dependent on the ABA and GA signaling transduction than the auxins and CKs during the early growth phase.
Interestingly, in our case, JA biosynthesis and signaling were also promoted under the stimulation of 14-OH BR. It was reported that, in strawberries, the bioactive JA jasmonoyl-isoleucine (JA-Ile) accumulated at the immature fruit stages and then decreased as the fruit ripens [48]. However, as a stress hormone, the way JA signaling functions in the fruit growth stage was still mostly unclear. Besides, as a climacteric fruit, the ripening of kiwifruit is tightly regulated by ethylene [49]. It has been reported that CPPU treatment increases ethylene biosynthesis [50], which did not agree with our data, possibly because of the different fruit growth testing stages.
5. Conclusions
In the present study, by screening effects of different concentrations of four PGRs plus lowered content (5 mg L−1) of CPPU on the red flesh kiwifruit ‘Donghong’ enlargement, we screened 14-OH BR as a promising safer and effective PGR to minimize the use content of CPPU without detectable kiwifruits yield trade-offs. Moreover, 14-OH BR controls kiwifruit development mainly through the activation of BR signaling transduction, which can further synergistically and antagonistically regulate signaling of other endogenous growth regulators, including auxin, ABA, CKs, GA, JA and ethylene.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr10112345/s1, Supplementary file S1; Supplementary file S2.
Author Contributions
Methodology, Y.L. and D.W.; software, B.D.; formal analysis, H.W.; investigation, Y.W. and B.M.; resources, D.R.; data curation, P.Y.; writing—Y.W. and B.M.; writing—review and editing, X.W.; supervision, J.H.; project administration, D.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to acknowledge technician Fei Xu in Datang district for his technical support during our experiment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wu, B.-L. Some new kiwifruit cultivars. J. Fruit Sci. 1992, 9, 56–58. [Google Scholar]
- Balestra, G.M.; Mazzaglia, A.; Quattrucci, A.; Renzi, M.; Rossetti, A. Occurrence of Pseudomonas syringae pv. Actinidiae in Jin Tao kiwi plants in Italy. Phytopathol. Mediterr. 2009, 48, 299–301. [Google Scholar]
- Zhong, C.H.; Han, F.; Li, D.W.; Liu, X.L.; Zhang, Q.; Jiang, Z.W.; Huang, H.W. Breeding of red-fleshed kiwifruit cultivar ‘Donghong’. J. Fruit Sci. 2016, 33, 1596–1599. [Google Scholar] [CrossRef]
- Nie, X.-R.; Li, H.-Y.; Wei, S.-Y.; Han, Q.-H.; Zhao, L.; Zhang, Q.; Li, S.-Q.; Qin, W.; Wu, D.-T. Changes of phenolic compounds, antioxidant capacities, and inhibitory effects on digestive enzymes of kiwifruits (Actinidia chinensis) during maturation. J. Food Meas. 2020, 14, 1765–1774. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Z.; Zhang, Q.; Feng, S.; Burdon, J.; Zhong, C.J.H. Maturity, Ripening and Quality of ‘Donghong’ Kiwifruit Evaluated by the Kiwi-Meter™. Horticulturae 2022, 8, 852. [Google Scholar] [CrossRef]
- Blank, R.H.; Richardson, A.C.; Oshima, K.; Hampton, R.E.; Olson, M.H.; Dawson, T.E. Effect of a forchlorfenuron dip on kiwifruit fruit size. N. Z. J. Crop Hortic. Sci. 1992, 20, 73–78. [Google Scholar] [CrossRef]
- Wu, L.; Lan, J.; Xiang, X.; Xiang, H.; Jin, Z.; Khan, S.; Liu, Y. Transcriptome sequencing and endogenous phytohormone analysis reveal new insights in CPPU controlling fruit development in kiwifruit (Actinidia chinensis). PLoS ONE 2020, 15, e0240355. [Google Scholar] [CrossRef]
- Kim, J.G.; Takami, Y.; Mizugami, T.; Beppu, K.; Fukuda, T.; Kataoka, I. CPPU application on size and quality of hardy kiwifruit. Sci. Hortic. 2006, 110, 219–222. [Google Scholar] [CrossRef]
- Zabadal, T.J.; Bukovac, M.J. Effect of CPPU on Fruit Development of Selected Seedless and Seeded Grape Cultivars. HortScience 2006, 41, 154–157. [Google Scholar] [CrossRef]
- Stern, R.A.; Ben-Arie, R.; Neria, O.; Flaishman, M. CPPU and BA increase fruit size of ‘Royal Gala’ (Malus domestica) apple in a warm climate. J. Hortic. Sci. Biotechnol. 2003, 78, 297–302. [Google Scholar] [CrossRef]
- Ying, H.; Shi, J.; Zhang, S.; Pingcuo, G.; Wang, S.; Zhao, F.; Cui, Y.; Zeng, X. Transcriptomic and metabolomic profiling provide novel insights into fruit development and flesh coloration in Prunus mira Koehne, a special wild peach species. BMC Plant Biol. 2019, 19, 463. [Google Scholar] [CrossRef] [PubMed]
- Janowska, B.; Andrzejak, R. Cytokinins and Gibberellins Stimulate the Flowering and Post-Harvest Longevity of Flowers and Leaves of Calla Lilies (Zantedeschia Spreng.) with Colourful Inflorescence Spathes. Agronomy 2022, 12, 1859. [Google Scholar] [CrossRef]
- Janowska, B. Effect of growth regulators on flower and leaf yield of the calla lily (Zantedeschia Spreng.). Hortic. Sci. 2013, 40, 78–82. [Google Scholar] [CrossRef]
- Kozłowska, M.; Rybus-Zając, M.; Stachowiak, J.; Janowska, B. Changes in carbohydrate contents of Zantedeschia leaves under gibberellin-stimulated flowering. Acta Physiol. Plant. 2007, 29, 27–32. [Google Scholar] [CrossRef]
- Janowska, B.; Stanecki, M. Effect of rhizome soaking in a mixture of BA and GA3 on the earliness of flowering and quality of the yield of flowers and leaves in the calla lily (Zantedeschia Spreng.). Acta Sci. Polonorum. Hortorum Cultus 2013, 12, 3–12. [Google Scholar]
- Andrzejak, R.; Janowska, B. Yield and Quality of Inflorescences in the Zantedeschia albomaculata (Hook.) Baill. ‘Albomaculata’ after the Treatment with AMF and GA3. Agronomy 2021, 11, 644. [Google Scholar] [CrossRef]
- Janowska, B.; Andrzejak, R.; Kosiada, T.; Kwiatkowska, M.; Smolinska, D. The flowering and nutritional status of Gladiolus hybridus cv. Black Velvet following a cytokinin treatment. J. Elem. 2018, 23, 1119–1128. [Google Scholar] [CrossRef]
- Janowska, B.; Andrzejak, R.; Kosiada, T.; Kwiatkowska, M.; Smolińska, D.J.H.S. Flowering and nutritional status of Gladiolus hybridus L. ‘Black Velvet’ following gibberellin treatment. Hortic. Sci. 2018, 45, 205–210. [Google Scholar] [CrossRef]
- Janowska, B.; Andrzejak, R.; Szwajkowska-Michałek, L.; Stuper-Szablewska, K.J.A. The Content of Biologically Active Substances in Crocosmia× crocosmiiflora ‘Lucifer’ Tubers after Treatment with GA3. Agronomy 2021, 11, 553. [Google Scholar] [CrossRef]
- Andrzejak, R.; Janowska, B.; Reńska, B.; Kosiada, T. Effect of Trichoderma spp. and fertilization on the flowering of Begonia× tuberhybrida Voss. ‘Picotee Sunburst’. Agronomy 2021, 11, 1278. [Google Scholar] [CrossRef]
- Andrzejak, R.; Janowska, B. Flowering, Nutritional Status, and Content of Chloroplast Pigments in Leaves of Gladiolus hybridus L. ‘Advances Red’ after Application of Trichoderma spp. Sustainability 2022, 14, 4576. [Google Scholar] [CrossRef]
- Dong, J.; Guo, W.; Zhao, F.; Liu, D. Discrimination of “Hayward” Kiwifruits Treated with Forchlorfenuron at Different Concentrations Using Hyperspectral Imaging Technology. Food Anal. Methods 2017, 10, 477–486. [Google Scholar] [CrossRef]
- Bu, Q.; Wang, X.; Xie, H.; Zhong, K.; Wu, Y.; Zhang, J.; Wang, Z.; Gao, H.; Huang, Y. 180 Day Repeated-Dose Toxicity Study on Forchlorfenuron in Sprague–Dawley Rats and Its Effects on the Production of Steroid Hormones. J. Agric. Food Chem. 2019, 67, 10207–10213. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Kam, H.; Tse, Y.; Lee, S.M. Cardiotoxicity of forchlorfenuron (CPPU) in zebrafish (Danio rerio) and H9c2 cardiomyocytes. Chemosphere 2019, 235, 153–162. [Google Scholar] [CrossRef]
- He, Q. Preparation Method, Agricultural Composition and Applications of Natural Brassinolide Analogs. U.S. Patent 9,326,506, 3 May 2016. [Google Scholar]
- Cebalo, T.; Letham, D.S. Synthesis of Zeatin, a Factor inducing Cell Division. Nature 1967, 213, 86. [Google Scholar] [CrossRef]
- Ito, A.; Sakamoto, D.; Itai, A.; Nishijima, T.; Oyama-Okubo, N.; Nakamura, Y.; Moriguchi, T.; Nakajima, I. Effects of GA3+4 and GA4+7 Application Either Alone or Combined with Prohexadione-Ca on Fruit Development of Japanese Pear ’Kosu’. Hortic. J. 2016, 85, 201–208. [Google Scholar] [CrossRef]
- Kvesitadze, G.I.; Kalandiya, A.G.; Papunidze, S.G.; Vanidze, M.R. Identification and Quantification of Ascorbic Acid in Kiwi Fruit by High-Performance Liquid Chromatography. Appl. Biochem. Microbiol. 2001, 37, 215–218. [Google Scholar] [CrossRef]
- GB/T 12456-2008; Determination of Total Acid in Food. National Standards Organization: Beijing, China, 2008.
- GB 5009.268-2016; National Food Safety Standard—Determination of Multi-elements in Foods. National Standards Organization: Beijing, China, 2016.
- Jiang, Y. Plant growth regulator 14-hydroxylated brassinosteroid. Pestic. Sci. Admin. 2018, 39, 67. [Google Scholar]
- WANG, Z. Brassinosteroid regulated kinases (BRKs) that mediate brassinosteroid signal transduction and uses thereof. U.S. Patent 8,541,649, 24 September 2013. [Google Scholar]
- Végvári, G.; Vidéki, E. Plant hormones, plant growth regulators. Orvosi Hetil. 2014, 155, 1011–1018. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, Z.; Wang, Y.; Yuan, Y.; Dong, J.; Yue, T. Transformation products elucidation of forchlorfenuron in postharvest kiwifruit by time-of-flight mass spectrometry. PLoS ONE 2017, 12, e0184021. [Google Scholar] [CrossRef]
- Olszewski, N.; Sun, T.-P.; Gubler, F. Gibberellin signaling: Biosynthesis, catabolism, and response pathways. Plant Cell 2002, 14, S61–S80. [Google Scholar] [CrossRef] [PubMed]
- Serrani, J.C.; Sanjuán, R.; Ruiz-Rivero, O.; Fos, M.; García-Martínez, J.L. Gibberellin regulation of fruit set and growth in tomato. Plant Physiol. 2007, 145, 246–257. [Google Scholar] [CrossRef]
- Kappel, F.; MacDonald, R.A. Gibberellic acid increases fruit firmness, fruit size, and delays maturity of ‘Sweetheart’ sweet cherry. J. Am. Pomol. Soc. 2002, 56, 219. [Google Scholar]
- Mok, D.W.; Mok, M.C. Cytokininschemistry, Activity, and Function; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Schiller, L.G.; Magnitskiy, S. Effect of trans-zeatin riboside application on growth of banana (Musa AAA Simmonds) cv. Williams in the juvenile phase. Rev. Colomb. De Cienc. Hortícolas 2019, 13, 161–170. [Google Scholar] [CrossRef]
- Baghel, M.; Nagaraja, A.; Srivastav, M.; Meena, N.K.; Senthil Kumar, M.; Kumar, A.; Sharma, R.R. Pleiotropic influences of brassinosteroids on fruit crops: A review. Plant Growth Regul. 2019, 87, 375–388. [Google Scholar] [CrossRef]
- Vardhini, B.V.; Rao, S.R. Acceleration of ripening of tomato pericarp discs by brassinosteroids. Phytochemistry 2002, 61, 843–847. [Google Scholar] [CrossRef]
- Symons, G.M.; Davies, C.; Shavrukov, Y.; Dry, I.B.; Reid, J.B.; Thomas, M.R. Grapes on steroids. Brassinosteroids are involved in grape berry ripening. Plant Physiol. 2006, 140, 150–158. [Google Scholar] [CrossRef]
- Fu, F.Q.; Mao, W.H.; Shi, K.; Zhou, Y.H.; Asami, T.; Yu, J.Q. A role of brassinosteroids in early fruit development in cucumber. J. Exp. Bot. 2008, 59, 2299–2308. [Google Scholar] [CrossRef]
- Pabón-Mora, N.; Wong, G.K.-S.; Ambrose, B.A. Evolution of fruit development genes in flowering plants. Front. Plant Sci. 2014, 5, 300. [Google Scholar] [CrossRef]
- Renaudin, J.-P.; Deluche, C.; Cheniclet, C.; Chevalier, C.; Frangne, N. Cell layer-specific patterns of cell division and cell expansion during fruit set and fruit growth in tomato pericarp. J. Exp. Bot. 2017, 68, 1613–1623. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Zeng, S.; Xiao, G.; Wang, G.; Wang, Y.; Peng, M.; Huang, H. Gene Expression Profiling of Development and Anthocyanin Accumulation in Kiwifruit (Actinidia chinensis) Based on Transcriptome Sequencing. PLoS ONE 2015, 10, e01364399. [Google Scholar] [CrossRef]
- Nitsch, L.; Kohlen, W.; Oplaat, C.; Charnikhova, T.; Cristescu, S.; Michieli, P.; Wolters-Arts, M.; Bouwmeester, H.; Mariani, C.; Vriezen, W.H.; et al. ABA-deficiency results in reduced plant and fruit size in tomato. J. Plant Physiol. 2012, 169, 878–883. [Google Scholar] [CrossRef]
- Gansser, D.; Latza, S.; Berger, R.G. Methyl jasmonates in developing strawberry fruit (Fragaria ananassa Duch. Cv. Kent). J. Agric. Food Chem. 1997, 45, 2477–2480. [Google Scholar] [CrossRef]
- Fei, L.; Yuan, X.; Chen, C.; Wan, C.; Fu, Y.; Chen, J.; Gan, Z.J.A. Exogenous application of sucrose promotes postharvest ripening of kiwifruit. Agronomy 2020, 10, 245. [Google Scholar] [CrossRef]
- Bangerth, F.K. Can regulatory mechanism in fruit growth and development be elucidated through the study of endogenous hormone concentrations? In Proceedings of the VIII International Symposium on Plant Bioregulation in Fruit Production 463, Valencia, Spain, 1–4 April 1997; pp. 77–88. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).