Limited Phosphorous Supply Improved Lipid Content of Chlorella vulgaris That Increased Phenol and 2-Chlorophenol Adsorption from Contaminated Water with Acid Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Chlorella vulgaris Growth Conditions
2.2. C. vulgaris Growth at Different Phosphorus Concentrations & Biomass Harvesting
2.3. Acid Treatment of Dried Powder of C. vulgaris
2.4. Adsorption Experiment with C. vulgaris Dried Biomass
2.5. Statistical Analysis
3. Results
3.1. Effect of Phosphorous Starvation on the Lipid and Protein Content of C. vulgaris
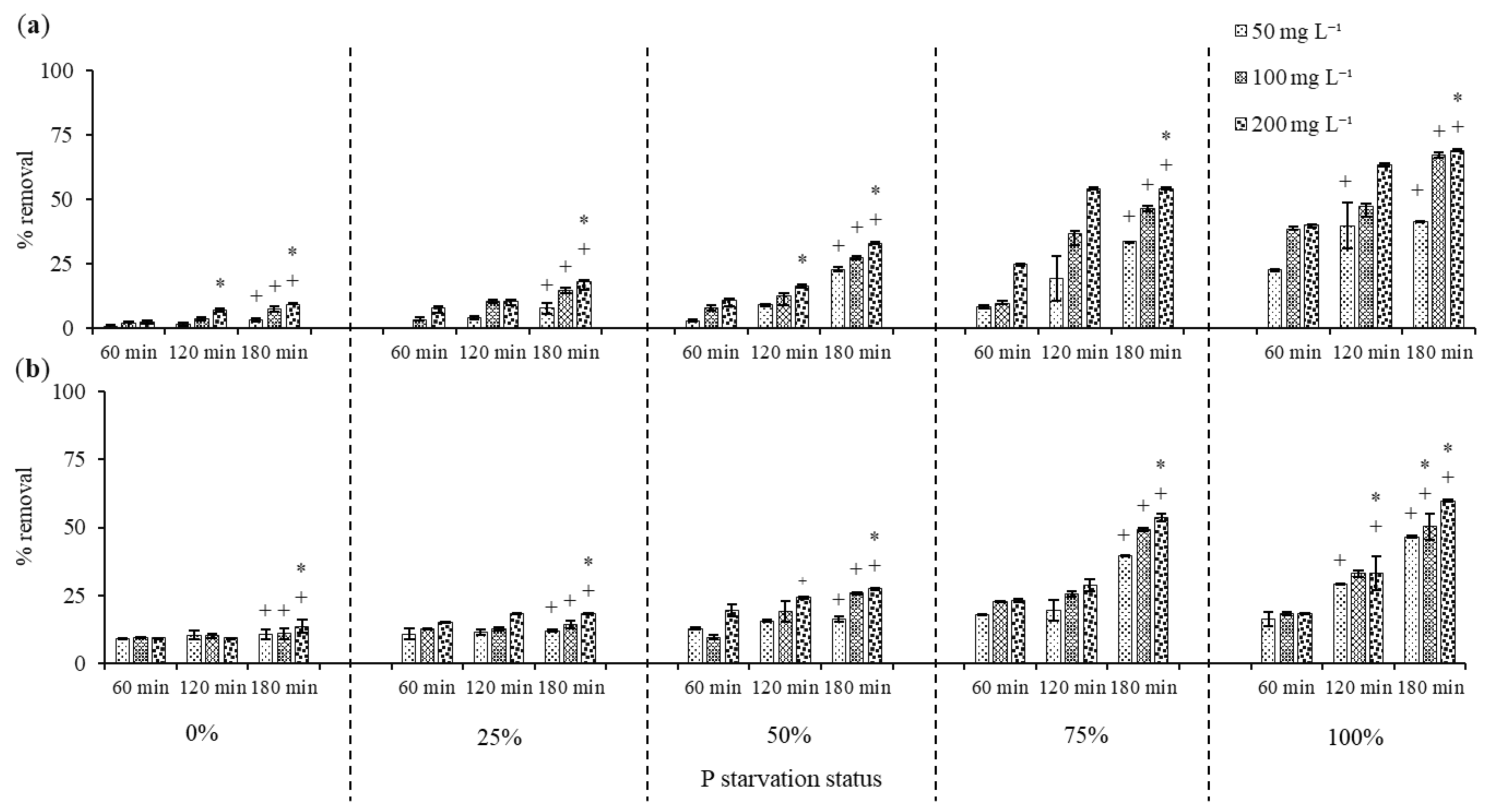
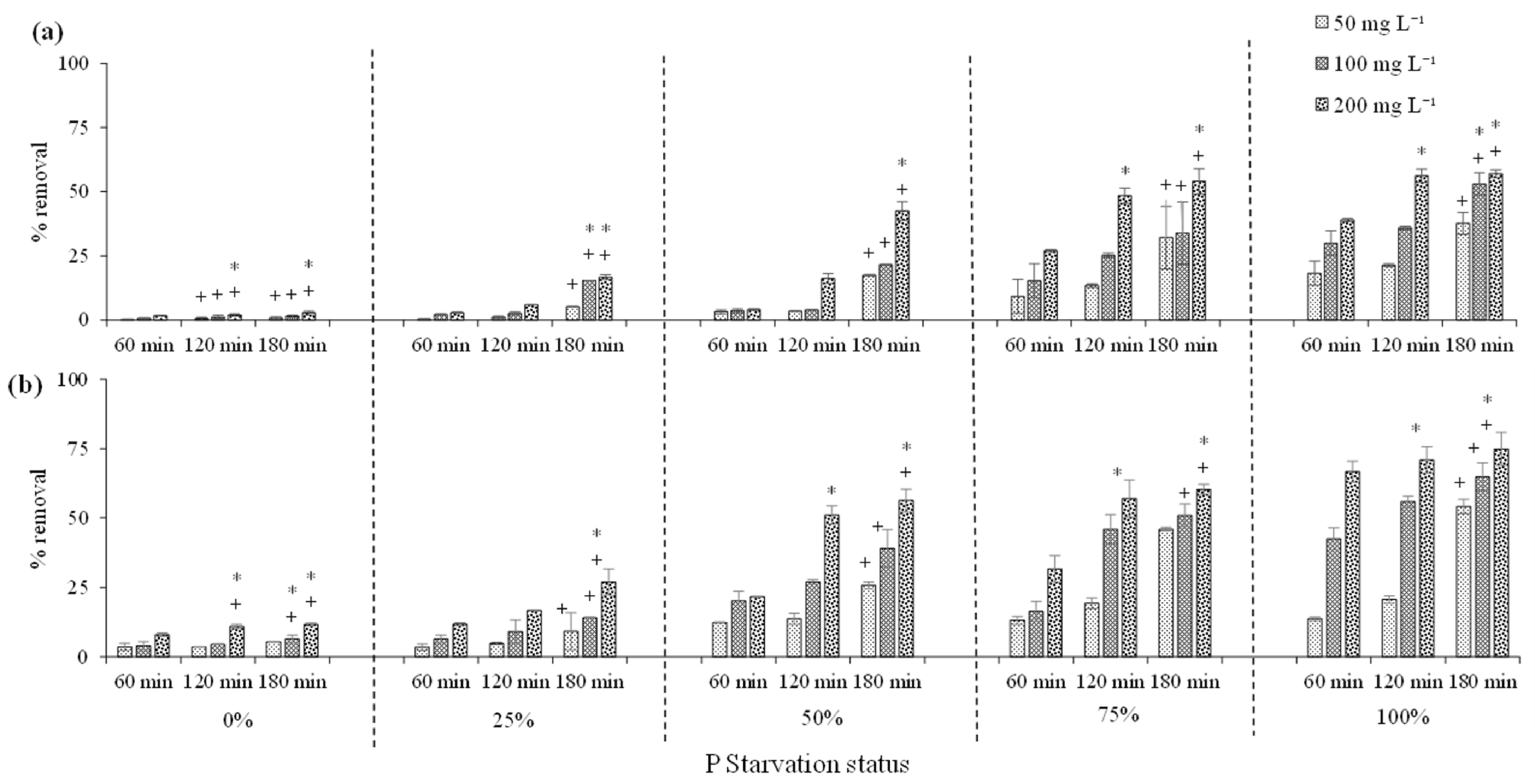
3.2. Phenol Removal by Dried C. vulgaris
3.2.1. Effect of Phosphorus Starvation on Phenol Removal
3.2.2. Phenol Removal by Acid Treated C. vulgaris Biomass
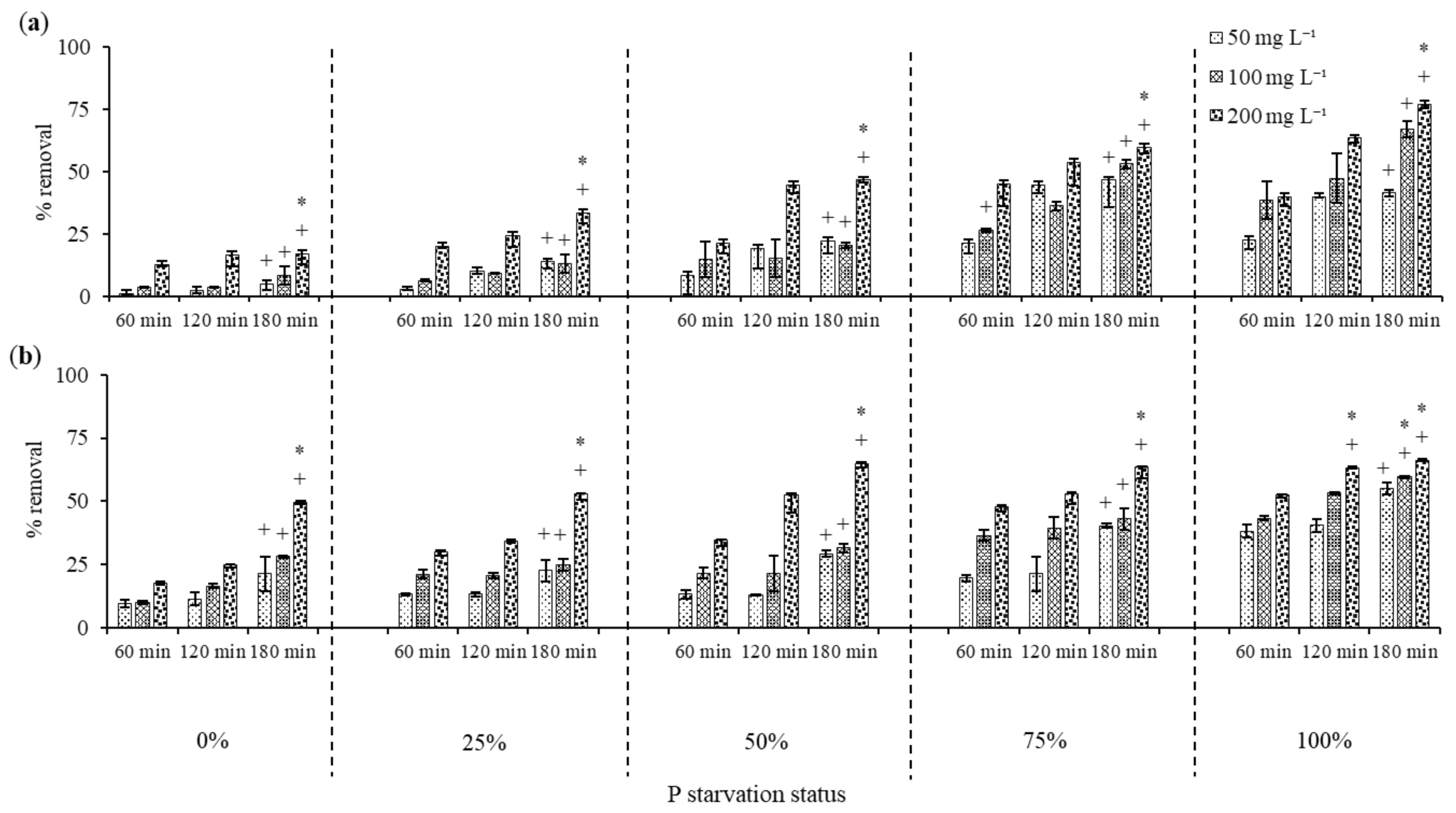
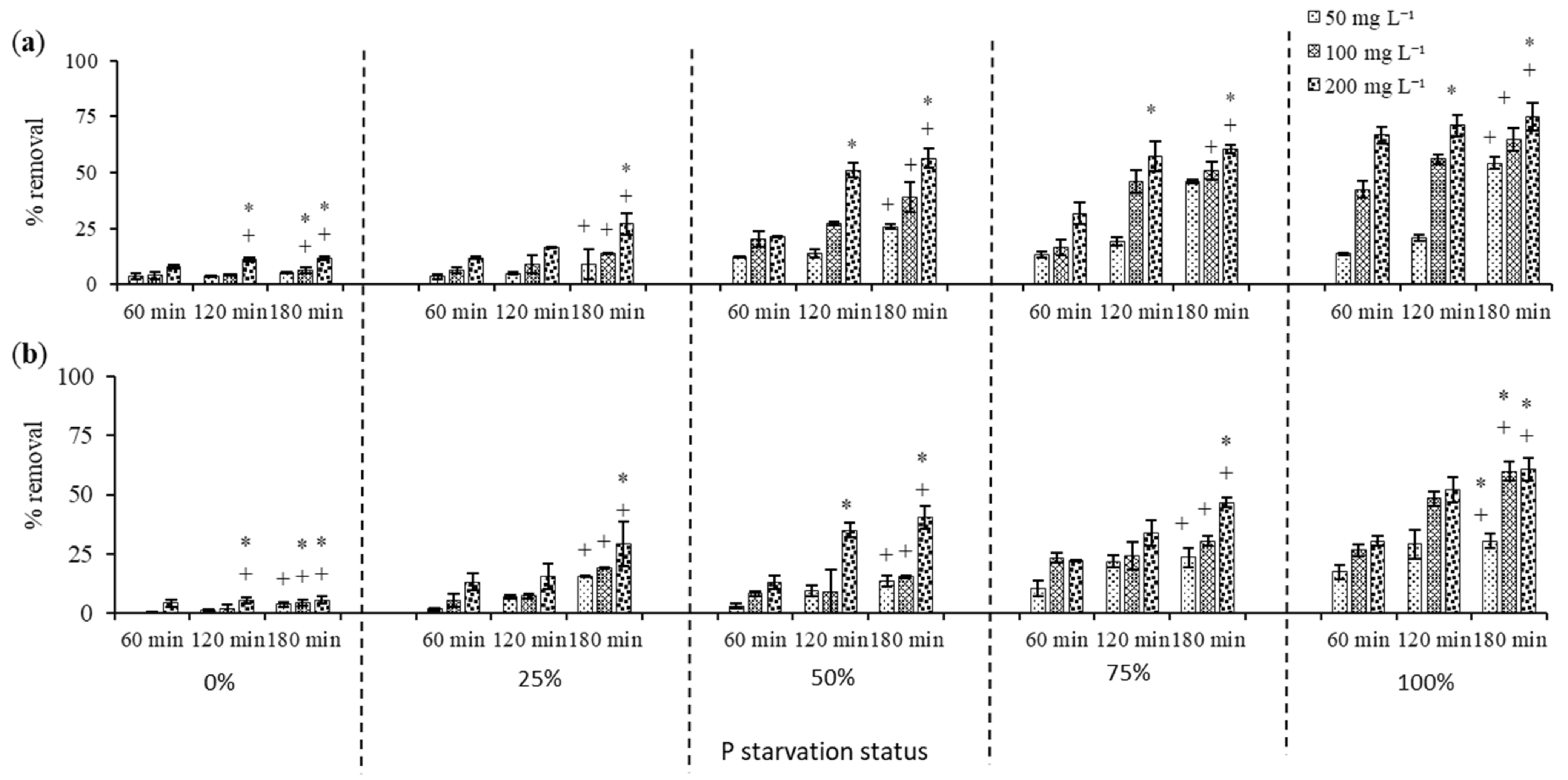
3.3. 2-Chlorophenol Removal by Dried C. vulgaris
3.3.1. Effect of Phosphorus Starvation on 2-Chlorophenol Removal
3.3.2. 2-Chlorophenol Removal by Acid Treated C. vulgaris Biomass
3.4. Interactive Effect of Applied Factors on Contaminant Removal and Data Clustering
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, G.; Duan, R. A new method to determine the scale range of environmental damage caused by water pollution accidents. Water Supply 2022, 22, 3968–3979. [Google Scholar] [CrossRef]
- Hussain, F.; Hussain, I.; Khan, A.H.A.; Muhammad, Y.S.; Iqbal, M.; Soja, G.; Reichenauer, T.G.; Yousaf, S. Combined application of biochar, compost, and bacterial consortia with Italian ryegrass enhanced phytoremediation of petroleum hydrocarbon contaminated soil. Environ. Exp. Bot. 2018, 153, 80–88. [Google Scholar] [CrossRef]
- Raza, A.; Khan, A.H.A.; Nawaz, I.; Qu, Z.; Yousaf, S.; Ali, M.A.; Sayal, A.U.; Iqbal, M. Evaluation of arsenic-induced stress in Dahlia pinnata Cav.: Morphological and physiological response. Soil Sediment Contam. 2019, 28, 716–728. [Google Scholar] [CrossRef]
- Manzoor, M.; Khan, A.H.A.; Ullah, R.; Khan, M.Z.; Ahmad, I. Environmental epidemiology of cancer in South Asian population: Risk assessment against exposure to polycyclic aromatic hydrocarbons and volatile organic compounds. Arab. J. Sci. Eng. 2016, 41, 2031–2043. [Google Scholar] [CrossRef]
- Salaudeen, T.; Okoh, O.; Okoh, A. Performance assessment of wastewater treatment plants with special reference to phenol removal. Int. J. Environ. Sci. Technol. 2019, 16, 401–412. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Phenolic compounds: Current industrial applications, limitations, and future challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef]
- Khan, A.H.A.; Ayaz, M.; Arshad, M.; Yousaf, S.; Khan, M.A.; Anees, M.; Sultan, A.; Nawaz, I.; Iqbal, M. Biogeochemical cycle, occurrence and biological treatments of polycyclic aromatic hydrocarbons (PAHs). Iran. J. Sci. Technol. A 2019, 43, 1393–1410. [Google Scholar] [CrossRef]
- Hussain, F.; Khan, A.H.A.; Hussain, I.; Farooqi, A.; Muhammad, Y.S.; Iqbal, M.; Arslan, M.; Yousaf, S. Soil conditioners improve rhizodegradation of aged petroleum hydrocarbons and enhance the growth of Lolium multiflorum. Environ. Sci. Pollut. R 2021, 29, 9097–9109. [Google Scholar] [CrossRef]
- Qurban, M.; Mirza, C.R.; Khan, A.H.A.; Khalifa, W.; Boukendakdji, M.; Achour, B.; Yousaf, S.; Nawaz, I.; Butt, T.A.; Iqbal, M. Metal accumulation profile of Catharanthus roseus (L.) G.Don and Celosia argentea L. with EDTA co-Application. Processes 2021, 9, 598. [Google Scholar] [CrossRef]
- Khan, A.H.A.; Anees, M.; Arshad, M.; Muhammad, Y.S.; Iqbal, M.; Yousaf, S. Effects of illuminance and nutrients on bacterial photo-physiology of hydrocarbon degradation. Sci. Total Environ. 2016, 557, 705–711. [Google Scholar] [CrossRef]
- Narayanan, M.; Prabhakaran, M.; Natarajan, D.; Kandasamy, S.; Raja, R.; Carvalho, I.S.; Ashokkumar, V.; Chinnathambi, A.; Alharbi, S.A.; Devarayan, K.; et al. Phycoremediation potential of Chlorella sp. on the polluted Thirumanimutharu river water. Chemosphere 2021, 277, 130246. [Google Scholar] [CrossRef] [PubMed]
- El-Gendy, N.S.; Nassar, H.N. Phycoremediation of phenol-polluted petro-industrial effluents and its techno-economic values as a win-win process for a green environment, sustainable energy and bioproducts. J. Appl. Microbiol. 2021, 131, 1621–1638. [Google Scholar] [CrossRef] [PubMed]
- Lee, X.J.; Ong, H.C.; Ooi, J.; Yu, K.L.; Tham, T.C.; Chen, W.H.; Ok, Y.S. Engineered macroalgal and microalgal adsorbents: Synthesis routes and adsorptive performance on hazardous water contaminants. J. Hazard. Mater. 2022, 423, 126921. [Google Scholar] [CrossRef] [PubMed]
- Spain, O.; Plöhn, M.; Funk, C. The cell wall of green microalgae and its role in heavy metal removal. Physiol. Plant. 2021, 173, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Gondi, R.; Kavitha, S.; Kannah, R.Y.; Karthikeyan, O.P.; Kumar, G.; Tyagi, V.K.; Banu, J.R. Algal-based system for removal of emerging pollutants from wastewater: A review. Bioresour. Technol. 2022, 344, 126245. [Google Scholar] [CrossRef]
- Habibzadeh, M.; Chaibakhsh, N.; Naeemi, A.S. Optimized treatment of wastewater containing cytotoxic drugs by living and dead biomass of the freshwater microalga, Chlorella vulgaris. Ecol. Eng. 2018, 111, 85–93. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Kumar, P.S.; Saravanan, A.; Vo, D.V.N. Advances in biosorbents for removal of environmental pollutants: A review on pretreatment, removal mechanism and future outlook. J. Hazard. Mater. 2021, 420, 126596. [Google Scholar] [CrossRef]
- Song, X.; Liu, B.-F.; Kong, F.; Ren, N.Q.; Ren, H.-Y. Overview on stress-induced strategies for enhanced microalgae lipid production: Application, mechanisms and challenges. Resour. Conserv. Recycl. 2022, 183, 106355. [Google Scholar] [CrossRef]
- Yaakob, M.A.; Mohamed, R.M.S.R.; Al-Gheethi, A.; Ravishankar, G.A.; Ambati, R.R. Influence of nitrogen and phosphorus on microalgal growth, biomass, lipid, and fatty acid production: An overview. Cells 2021, 10, 393. [Google Scholar] [CrossRef]
- Ghafari, M.; Rashidi, B.; Haznedaroglu, B.Z. Effects of macro and micronutrients on neutral lipid accumulation in oleaginous microalgae. Biofuels 2016, 9, 147–156. [Google Scholar] [CrossRef]
- Obulisamy, P.K.; Verma, P.; Seoane, R.; Santaeufemia, S.; Abalde, J.; Torres, E. Efficient removal of methylene blue using living biomass of the microalga Chlamydomonas moewusii: Kinetics and equilibrium studies. Int. J. Environ. Res. Public Health 2022, 19, 2653. [Google Scholar]
- Lavrinovičs, A.; Mežule, L.; Juhna, T. Microalgae starvation for enhanced phosphorus uptake from municipal wastewater. Algal Res. 2020, 52, 102090. [Google Scholar] [CrossRef]
- Rajalakshmi, A.M.; Silambarasan, T.; Dhandapani, R. Biosorption of heavy metals from tannery effluents by using green unicellular microalga, Tetradesmus obliquus RDRL01. Appl. Biol. Res. 2022, 24, 28–37. [Google Scholar] [CrossRef]
- Mota, M.F.S.; Souza, M.F.; Bon, E.P.S.; Rodrigues, M.A.; Freitas, S.P. Colorimetric protein determination in microalgae (Chlorophyta): Association of milling and SDS treatment for total protein extraction. J. Phycol. 2018, 54, 577–580. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.; Song, L.; Sommerfeld, M.; Hu, Q. Automated accelerated solvent extraction method for total lipid analysis of microalgae. Algal Res. 2020, 51, 102080. [Google Scholar] [CrossRef]
- Kong, W.; Yang, S.; Guo, B.; Wang, H.; Huo, H.; Zhang, A.; Niu, S. Growth behavior, glucose consumption and phenol removal efficiency of Chlorella vulgaris under the synergistic effects of glucose and phenol. Ecotox. Environ. Saf. 2019, 186, 109762. [Google Scholar] [CrossRef]
- Liu, X.; Chen, G.; Tao, Y.; Wang, J. Application of effluent from WWTP in cultivation of four microalgae for nutrients removal and lipid production under the supply of CO2. Renew. Energy 2020, 149, 708–715. [Google Scholar] [CrossRef]
- Fawzy, M.A.; Alharthi, S. Cellular responses and phenol bioremoval by green alga Scenedesmus abundans: Equilibrium, kinetic and thermodynamic studies. Environ. Technol. Innov. 2021, 22, 101463. [Google Scholar] [CrossRef]
- Abdel-Raouf, N.; Sholkamy, E.N.; Bukhari, N.; Al-Enazi, N.M.; Alsamhary, K.I.; Al-Khiat, S.H.A.; Ibraheem, I.B.M. Bioremoval capacity of Co+2 using Phormidium tenue and Chlorella vulgaris as biosorbents. Environ. Res. 2022, 204, 111630. [Google Scholar] [CrossRef]
- Vazirzadeh, A.; Jafarifard, K.; Ajdari, A.; Chisti, Y. Removal of nitrate and phosphate from simulated agricultural runoff water by Chlorella vulgaris. Sci. Total Environ. 2022, 802, 149988. [Google Scholar] [CrossRef]
- Benavente-Valdés, J.R.; Aguilar, C.; Contreras-Esquivel, J.C.; Méndez-Zavala, A.; Montañez, J. Strategies to enhance the production of photosynthetic pigments and lipids in Chlorophycae species. Biotechnol. Rep. 2016, 10, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Xiang, W.; Li, T.; Long, L. Transcriptome analysis for phosphorus starvation-induced lipid accumulation in Scenedesmus sp. Sci. Rep. 2018, 8, 16420. [Google Scholar] [CrossRef] [PubMed]
- Anto, S.; Pugazhendhi, A.; Mathimani, T. Lipid enhancement through nutrient starvation in Chlorella sp. and its fatty acid profiling for appropriate bioenergy feedstock. Biocatal. Agric. Biotechnol. 2019, 20, 101179. [Google Scholar] [CrossRef]
- Rocha, G.S.; Lombardi, A.T.; Espíndola, E.L.G. Combination of P-limitation and cadmium in photosynthetic responses of the freshwater microalga Ankistrodesmus densus (Chlorophyceae). Environ. Pollut. 2021, 275, 116673. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, D. Growth, lipid content, and fatty acid profile of freshwater cyanobacteria Dolichospermum affine (Lemmermann) Wacklin, Hoffmann, & Komárek by using modified nutrient media. Aquacult. Int. 2020, 28, 1371–1388. [Google Scholar]
- Senturk, I.; Buyukgungor, H. Equilibrium and kinetic studies on the biosorption of 2-chlorophenol and 4-chlorophenol by live Aspergillus niger. Ekoloji 2013, 22, 1–12. [Google Scholar] [CrossRef]
- Surkatti, R.; Al-Zuhair, S. Effect of cresols treatment by microalgae on the cells’ composition. J. Water Process. Eng. 2018, 26, 250–256. [Google Scholar] [CrossRef]
- Li, F.; Zhao, L.; Jinxu, Y.; Shi, W.; Zhou, S.; Yuan, K.; Sheng, G.D. Removal of dichlorophenol by Chlorella pyrenoidosa through self-regulating mechanism in air-tight test environment. Ecotox. Environ. Saf. 2018, 164, 109–117. [Google Scholar] [CrossRef]
- Agrawal, P.R.; Sharma, R.; Agrawal, A. Biosorption for eliminating organic contaminants from wastewater. In Biosorption for Wastewater Contaminants; Selvasembian, R., Singh, P., Eds.; John Wiley & Sons Ltd.: New York, NY, USA, 2021; pp. 63–78. [Google Scholar]
- Touliabah, H.E.S.; El-Sheekh, M.M.; Ismail, M.M.; El-Kassas, H. A review of microalgae- and cyanobacteria-based biodegradation of organic pollutants. Molecules 2022, 27, 1141. [Google Scholar] [CrossRef]
- Khan, A.A.; Gul, J.; Naqvi, S.R.; Ali, I.; Farooq, W.; Liaqat, R.; AlMohamadi, H.; Štěpanec, L.; Juchelková, D. Recent progress in microalgae-derived biochar for the treatment of textile industry wastewater. Chemosphere 2022, 306, 135565. [Google Scholar] [CrossRef]
- Rangabhashiyam, S.; Suganya, E.; Selvaraju, N.; Varghese, L.A. Significance of exploiting non-living biomaterials for the biosorption of wastewater pollutants. World J. Microb. Biot. 2014, 30, 1669–1689. [Google Scholar] [CrossRef]
- Zheng, H.; Guo, W.; Li, S.; Chen, Y.; Wu, Q.; Feng, X.; Yin, R.; Ho, S.H.; Ren, N.; Chang, J.S. Adsorption of p-nitrophenols (PNP) on microalgal biochar: Analysis of high adsorption capacity and mechanism. Bioresour. Technol. 2017, 244, 1456–1464. [Google Scholar] [CrossRef]
- Ata, A.; Nalcaci, O.O.; Ovez, B. Macro algae Gracilaria verrucosa as a biosorbent: A study of sorption mechanisms. Algal Res. 2021, 1, 194–204. [Google Scholar] [CrossRef]
- Rubín, E.; Rodríguez, P.; Herrero, R.; Sastre de Vicente, M. Biosorption of phenolic compounds by the brown alga Sargassum muticum. J. Chem. Technol. Biot. 2006, 81, 1093–1099. [Google Scholar] [CrossRef]
- Aravindhan, R.; Rao, J.R.; Nair, B.U. Application of a chemically modified green macro alga as a biosorbent for phenol removal. J. Environ. Manag. 2009, 90, 1877–1883. [Google Scholar] [CrossRef]

| Phosphorus Starvation | Lipid Content (mg g−1) | Protein Content (% w/w) |
|---|---|---|
| 0% | 15 ± 0.003 | 0.123 * ± 0.005 |
| 25% | 23 ± 0.002 | 0.102 ± 0.014 |
| 50% | 67 ± 0.001 | 0.061 ± 0.007 |
| 75% | 78 ± 0.003 | 0.021 ± 0.003 |
| 100% | 117 * ± 0.005 | 0.017 ± 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Javed, S.; Mirza, C.R.; Khan, A.H.A.; Khalifa, W.; Achour, B.; Barros, R.; Yousaf, S.; Butt, T.A.; Iqbal, M. Limited Phosphorous Supply Improved Lipid Content of Chlorella vulgaris That Increased Phenol and 2-Chlorophenol Adsorption from Contaminated Water with Acid Treatment. Processes 2022, 10, 2435. https://doi.org/10.3390/pr10112435
Javed S, Mirza CR, Khan AHA, Khalifa W, Achour B, Barros R, Yousaf S, Butt TA, Iqbal M. Limited Phosphorous Supply Improved Lipid Content of Chlorella vulgaris That Increased Phenol and 2-Chlorophenol Adsorption from Contaminated Water with Acid Treatment. Processes. 2022; 10(11):2435. https://doi.org/10.3390/pr10112435
Chicago/Turabian StyleJaved, Sidra, Cyrus Raza Mirza, Aqib Hassan Ali Khan, Walid Khalifa, Belkacem Achour, Rocio Barros, Sohail Yousaf, Tayyab Ashfaq Butt, and Mazhar Iqbal. 2022. "Limited Phosphorous Supply Improved Lipid Content of Chlorella vulgaris That Increased Phenol and 2-Chlorophenol Adsorption from Contaminated Water with Acid Treatment" Processes 10, no. 11: 2435. https://doi.org/10.3390/pr10112435
APA StyleJaved, S., Mirza, C. R., Khan, A. H. A., Khalifa, W., Achour, B., Barros, R., Yousaf, S., Butt, T. A., & Iqbal, M. (2022). Limited Phosphorous Supply Improved Lipid Content of Chlorella vulgaris That Increased Phenol and 2-Chlorophenol Adsorption from Contaminated Water with Acid Treatment. Processes, 10(11), 2435. https://doi.org/10.3390/pr10112435











