Azithromycin Adsorption onto Different Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soils
2.2. Chemical Reagents
2.3. Sorption and Desorption Experiments
2.4. Data Treatment
3. Results and Discussion
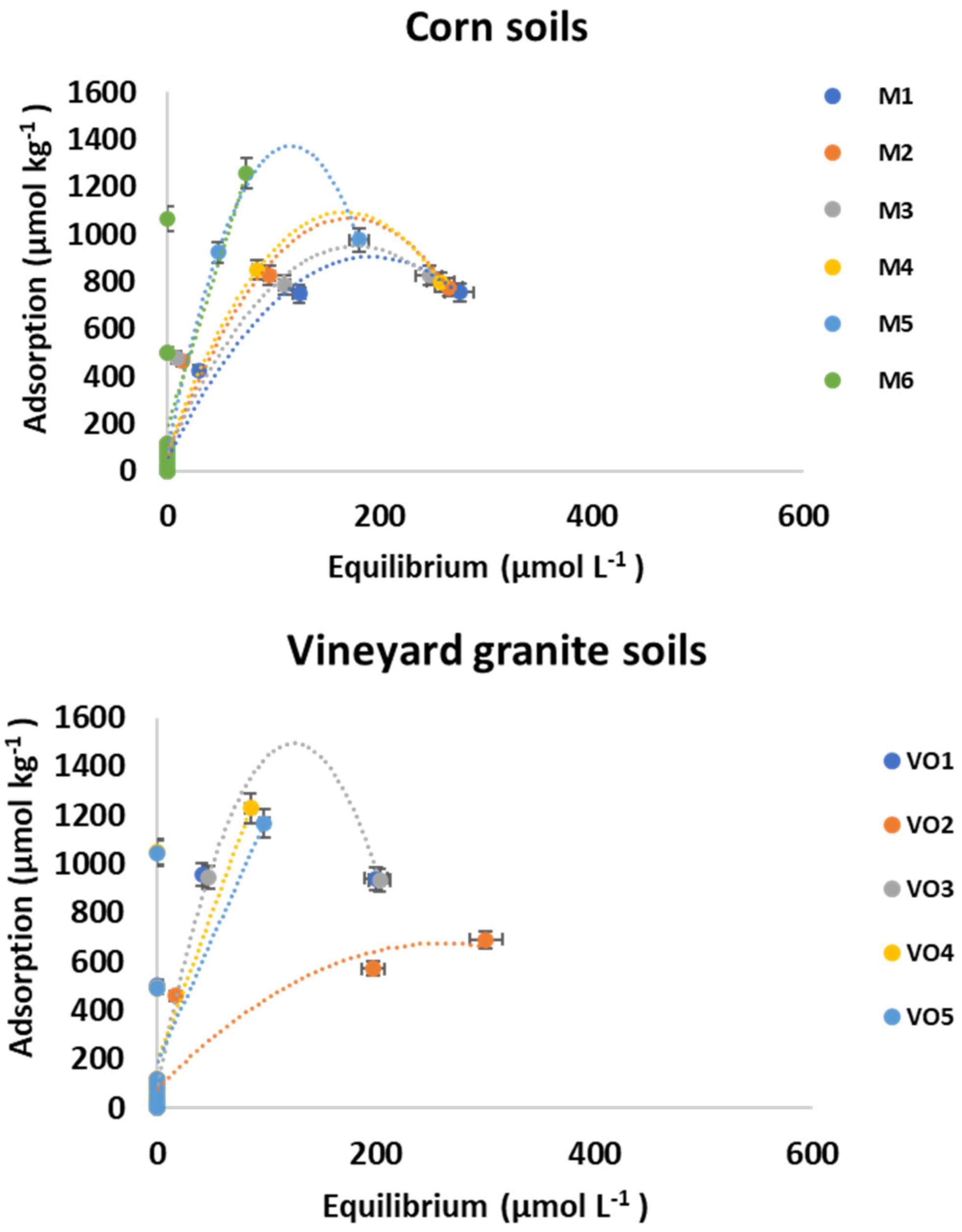
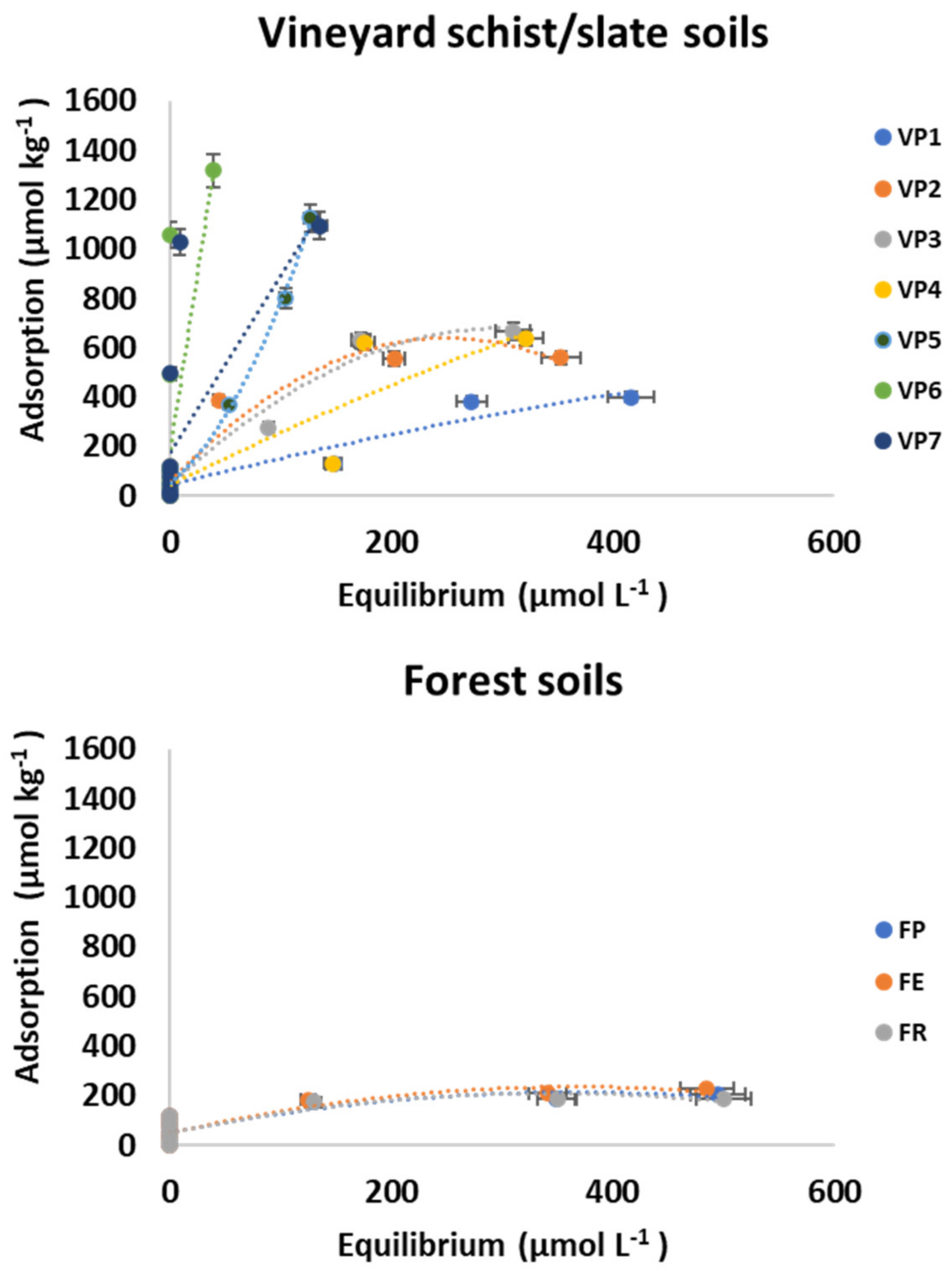
3.1. Adsorption
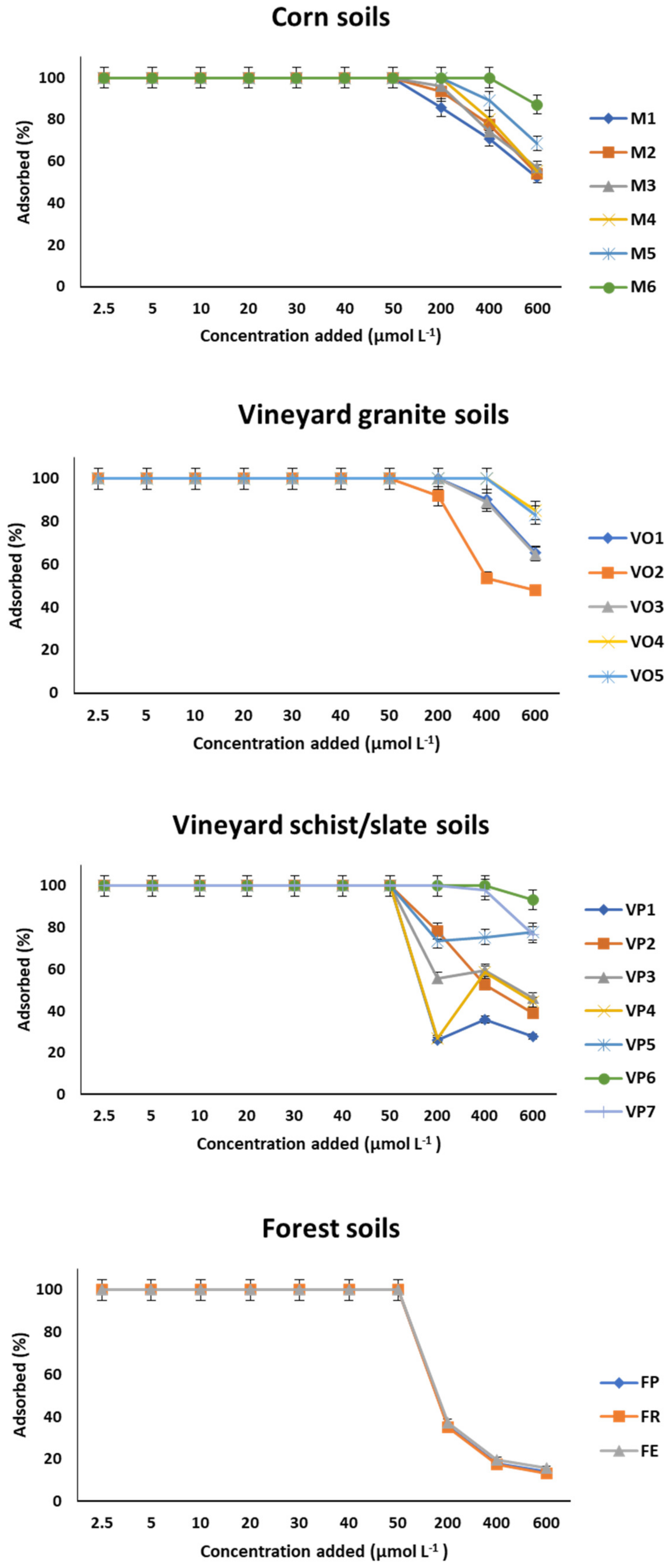
3.2. Desorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maier, M.L.V.; Tjeerdema, R.S. Azithromycin sorption and biodegradation in a simulated California river system. Chemosphere 2018, 190, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Bakheit, A.H.H.; Al-Hadiya, B.M.H.; Abd-Elgalil, A.A. Chapter One—Azithromycin. In Profiles of Drug Substances, Excipients and Related Methodology; Brittain, H.G., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 39, pp. 1–40. [Google Scholar] [CrossRef]
- Goossens, H. Antibiotic consumption and link to resistance. Clin. Microbiol. Infect. 2009, 15, 12–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ECDC Antimicrobial Consumption Interactive Database (ESAC-Net). Available online: http://ecdc.europa.eu/en/healthtopics/antimicrobial_resistance/esac-net-database/Pages/database.aspx (accessed on 29 July 2022).
- World Health Organization. WHO Report on Surveillance of Antibiotic Consumption: 2016–2018 Early Implementation; World Health Organization: Geneva, Switzerland, 2018; Licence: CC BY-NC-SA 3.0 IGO.
- Gonzalez-Zorn, B. Antibiotic use in the COVID-19 crisis in Spain. Clin. Microbiol. Infect. 2021, 27, 646–647. [Google Scholar] [CrossRef] [PubMed]
- Zuccato, E.; Gastiglioni, S.; Bagnati, R.; Melis, M.; Fanelli, R. Source, occurrence and fate of antibiotics in the Italian aquatic environment. J. Hazard. Mater. 2010, 179, 1042–1048. [Google Scholar] [CrossRef]
- Cardoso, O.; Porcher, J.M.; Sanchez, W. Factory-discharged pharmaceuticals could be a relevant source of aquatic environment contamination: Review of evidence and need for knowledge. Chemosphere 2014, 115, 20–30. [Google Scholar] [CrossRef]
- Santás-Miguel, V.; Díaz-Raviña, M.; Martín, A.; García-Campos, E.; Barreiro, A.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Arias-Estévez, M.; Fernández-Calviño, D. Medium-term influence of tetracyclines on total and specific microbial biomass in cultivated soils of Galicia (NW Spain). Span. J. Soil Sci. 2020, 10, 218–232. [Google Scholar] [CrossRef]
- Barreiro, A.; Cela-Dablanca, R.; Nebot, C.; Rodríguez-López, L.; Santás-Miguel, V.; Arias-Estévez, M.; Fernández-Sanjurjo, M.; Núñez-Delgado, A.; Álvarez-Rodríguez, E. Occurrence of Nine Antibiotics in Different Kinds of Sewage Sludge, Soils, Corn and Grapes After Sludge Spreading. Span. J. Soil Sci. 2022, 12, 10741–10753. [Google Scholar] [CrossRef]
- Rizzo, L.; Manaia, C.; Merlin, C.; Schwartz, T.; Dagot, C.; Ploy, M.C.; Michael, I.; FattaKassinos, D. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: A review. Sci. Total Environ. 2013, 447, 345–360. [Google Scholar] [CrossRef] [Green Version]
- Kümmerer, K. The presence of pharmaceuticals in the environment due to human use–present knowledge and future challenges. J. Environ. Manag. 2009, 90, 2354–2366. [Google Scholar] [CrossRef]
- Zuckerman, J.M. The newer macrolides: Azithromycin and clarithromycin. Infect. Dis. Clin. N. Am. 2000, 14, 449–462. [Google Scholar] [CrossRef]
- Martínez-Polanco, M.P.; Valderrama-Rincón, J.A.; Martínez-Rojas, A.J.; Luna-Wandurraga, H.J.; Díaz-Báez, M.C.; Bustos-López, M.C.; Valderrama-Rincon, J.D. Degradation of high concentrations of azithromycin when present in a high organic content wastewater by using a continuously fed laboratory-scale UASB bioreactor. Chemosphere 2022, 287, 132191. [Google Scholar] [CrossRef]
- Mirzaei, R.; Mesdaghinia, A.; Hoseini, S.S.; Yunesian, M. Antibiotics in urban wastewater and rivers of Tehran, Iran: Consumption, mass load, occurrence, and ecological risk. Chemosphere 2019, 221, 55–66. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, H.; Dú, J.; Qu, Y.; Shen, C.; Tan, F.; Chen, J.; Quan, X. Ocurrence, removal, and risk assessment of antibiotics in 12 wastewater treatment plants from Dalian, China. Environ. Sci. Pollut. Res. 2017, 24, 16478–16487. [Google Scholar] [CrossRef]
- Topp, E.; Renaud, J.; Sumarah, M.; Sabourin, L. Reduced persistence of the macrolide antibiotics erythromycin, clarithromycin and azithromycin in agricultural soil following several years of exposure in the field. Sci. Total Environ. 2016, 562, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mozaz, S.; Vaz-Moreira, I.; Varela Della Giustina, S.; Llorca, M.; Barceló, D.; Schubert, S.; Berendonk, T.U.; Michael-Kordatou, I.; Fatta-Kassinos, D.; Martinez, J.L.; et al. Antibiotic residues in final effluents of European wastewater treatment plants and their impact on the aquatic environment. Environ. Int. 2020, 140, 105733. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, G.A.; Elliot, H.A.; Basta, N.T.; Bastian, R.K.; Pierzynski, G.M.; Sims, R.C.; Smith, J.E. Sustainable Land Application. J. Environ. Qual. 2005, 34, 7–17. [Google Scholar] [CrossRef] [Green Version]
- Kelessidis, A.; Stasinakis, A.S. Comparative study of the methods used for treatment and final disposal of sewage sludge in European countries. Waste Manag. 2012, 32, 1186–1195. [Google Scholar] [CrossRef] [PubMed]
- Milieu Ltd.; WRc; RPA. Environmental, Economic and Social Impacts of the Use of Sewage Sludge on Land. Final Report, Part III: Project Interim Reports. DG ENV.G.4./ ETU/2008/0076r. 2010. Available online: http://ec.europa.eu/environment/archives/waste/sludge/pdf/part_iii_report.pdf (accessed on 2 May 2022).
- Pan, M.; Chu, L.M. Fate of antibiotics in soil and their uptake by edible crops. Sci. Total Environ. 2017, 599–600, 500–512. [Google Scholar] [CrossRef]
- Walters, E.; McClellan, K.; Halden, R.U. Occurrence and loss over three years of 72 pharmaceuticals and personal care products from biosolids-soil mixtures in outdoor mesocosms. Water Res. 2010, 44, 6011–6020. [Google Scholar] [CrossRef]
- Arun, S.; Kumar, R.M.; Ruppa, J.; Mukhopadhyay, M.; Ilango, K.; Chakraborty, P. Occurrence, sources and risk assessment of fluoroquinolones in dumpsite soil and sewage sludge from Chennai, India. Environ. Toxicol. Pharmacol. 2020, 79, 103410. [Google Scholar] [CrossRef] [PubMed]
- Berthod, L.; Roberts, G.; Sharpe, A.; Whitley, D.C.; Greenwood, R.; Mills, G.A. Effect of sewage sludge type on the partitioning behaviour of pharmaceuticals: A meta-analysis. Environ. Sci. Water Res. Technol. 2016, 2, 154–163. [Google Scholar] [CrossRef] [Green Version]
- OECD Guideline for the Testing of Chemicals: Adsorption–Desorption Using a Batch Equilibrium Method. Available online: http://www.epa.gov/scipoly/sap/meetings/2008/october/106_adsorption_desorption_using.pdf. (accessed on 16 June 2022).
- Kemper, N. Veterinary antibiotics in the aquatic and terrestrial environment. Ecol. Indic. 2008, 8, 1–13. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Schmidt, T.C. Determination of acid dissociation constants (pKa) of cephalosporin antibiotics: Computational and experimental approaches. Chemosphere 2017, 169, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Demiralay, E.Ç.; Koç, D.; Daldal, Y.D.; Çakır, C. Determination of chromatographic and spectrophotometric dissociation constants of some beta lactam antibiotics. J. Pharm. Biomed. Anal. 2012, 71, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, H.; D′Angelo, E.; O′Connor, G. Retention-release of ciprofloxacin and azithromycin in biosolids and biosolids-amended soils. Sci. Total Environ. 2019, 650, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Babić, S.; Horvat, A.J.M.; Pavlović, D.M.; Kaštelan-Macan, M. Determination of pKa values of active pharmaceutical ingredients. TrAC Trends Anal. Chem. 2007, 26, 1043–1061. [Google Scholar] [CrossRef]
- Zrnčić, M.; Babić, S.; Pavlović, D.M. Determination of thermodynamic pKa values of pharmaceuticals from five different groups using capillary electrophoresis. J. Sep. Sci. 2015, 38, 1232–1239. [Google Scholar] [CrossRef]
- Derendorf, H. Excessive lysosomal ion-trapping of hydroxychloroquine and azithromycin. Int. J. Antimicrob. Agents 2020, 55, 106007. [Google Scholar] [CrossRef]
- Rodríguez-López, L.; Santás-Miguel, V.; Núñez-Delgado, A.; Álvarez-Rodríguez, E.; Pérez-Rodríguez, P.; Arias-Estévez, M. Influence of pH, Humic Acids, and Salts on the Dissipation of Amoxicillin and Azithromycin Under Simulated Sunlight. Span. J. Soil Sci. 2022, 12, 10438. [Google Scholar] [CrossRef]
- Cela-Dablanca, R.; Nebot, C.; López, L.R.; Fernández-Calviño, D.; Arias-Estévez, M.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E. Efficacy of different waste and by-products from forest and food industries in the removal/retention of the antibiotic cefuroxime. Processes 2021, 9, 1151. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef] [Green Version]
- Chu, B.; Goyne, K.W.; Anderson, S.H.; Lin, C.-H.; Udawatta, R.P. Veterinary antibiotic sorption to agroforestry buffer, grass buffer and cropland soils. Agrofor. Syst. 2010, 79, 67–80. [Google Scholar] [CrossRef]
- Figueroa-Diva, R.A.; Vasudevan, D.; MacKay, A.A. Trends in soil sorption coefficients within common antimicrobial families. Chemosphere 2010, 79, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Shi, X.; Mao, J.; Zhu, D. Tetracycline sorption to coal and soil humic acids: An examination of humic structural heterogeneity. Environ. Toxicol. Chem. 2010, 29, 1934–1942. [Google Scholar] [CrossRef]
- Balarak, D.; Mahvi, A.H.; Shahbaksh, S.; Wahab, A.; Abdala, A. Adsorptive removal of azithromycin antibiotic from aqueous solution by Azolla Filiculoides-based activated porous carbon. Nanomaterials 2021, 11, 3281. [Google Scholar] [CrossRef]
- Peterson, J.W.; O´Meara, T.A.; Seymour, M.D.; Wang, W.; Gu, B. Sorption mechanisms of cephapirin, a veterinary antibiotic, onto quartz and feldspar minerals as detected by Raman spectroscopy. Environ. Pollut. 2009, 157, 1849–1856. [Google Scholar] [CrossRef]
- Chen, H.; Ma, L.Q.; Gao, B.; Gu, C. Effects of Cu and Ca cations and Fe/Al coating on ciprofloxacin sorption onto sand media. J. Hazard. Mater. 2013, 252–253, 375–381. [Google Scholar] [CrossRef]
- Gravesen, C.; Judy, J.D. Effect of biosolids characteristics on retention and release behavior of azithromycin and ciprofloxacin. Environ. Res. 2020, 184, 109333. [Google Scholar] [CrossRef]
- Sidhu, H.; O′Connor, G.; Ogram, A.; Kumar, K. Bioavailability of biosolids-borne ciprofloxacin and azithromycin to terrestrial organisms: Microbial toxicity and earthworm responses. Sci. Total Environ. 2019, 650, 18–26. [Google Scholar] [CrossRef]
- Sidhu, H.; O′Connor, G.; Kruse, J. Plant toxicity and accumulation of biosolidsborne ciprofloxacin and azithromycin. Sci. Total Environ. 2019, 648, 1219–1226. [Google Scholar] [CrossRef]
- Lemić, J.; Kovačević, D.; Tomašević-Čanović, M.; Kovačević, D.; Stanić, T.; Pfend, R. Removal of atrazine, lindane and diazinone from water by organo-zeolites. Water Res. 2006, 40, 1079–1085. [Google Scholar] [CrossRef]
- Deng, J.-C.; Jiang, X.; Lü, X.; Yu, G.-F.; Wang, F.; Zhang, B. Atrazine Adsorption Behavior on a Fluvo-Aquic Soil as Influenced by Contact Periods. Pedosphere 2007, 17, 786–791. [Google Scholar] [CrossRef]
- Conde-Cid, M.; Ferreira-Coelho, G.; Fernández-Calviño, D.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Arias-Estévez, M.; Álvarez-Rodríguez, E. Single and simultaneous adsorption of three sulfonamides in agricultural soils: Effects of pH and organic matter content. Sci. Total Environ. 2020, 744, 140872. [Google Scholar] [CrossRef] [PubMed]
- Conde-Cid, M.; Fernández-Calviño, D.; Nóvoa-Muñoz, J.C.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Arias-Estévez, M.; Álvarez-Rodríguez, E. Experimental data and model prediction of tetracycline adsorption and desorption in agricultural soils. Environ. Res. 2019, 177, 108607. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Li, C.; Dolhi, J.M.; Li, S.; He, J.-Z.; Qiao, M. Characteristics of oxytetracycline sorption and potential bioavailability in soils with various physical–chemical properties. Chemosphere 2012, 87, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, M.; Rout, K.; Mohapatra, B.; Anand, S. Sorption behavior of Pb(II) and Cd(II) on iron ore slime and characterization of metal ion loaded sorbent. J. Hazard. Mater. 2009, 166, 1506–1513. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Zhou, Q.; Wan, Y.; Yu, Q.; Xie, X. Effects of Soil/Solution Ratios and Cation Types on Adsorption and Desorption of Tetracycline in Soils. Soil Sci. Soc. Am. J. 2010, 74, 1553–1561. [Google Scholar] [CrossRef]
| Soil Property | Correlation Coefficient (r) | Significance Level (p) |
|---|---|---|
| pH | 0.562 | 0.01 |
| OM | 0.530 | 0.05 |
| Alox | −0.43 | 0.05 |
| Freundlich | Langmuir | Linear | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soil | KF | Error | n | Error | R2 | KL | Error | qm | Error | R2 | Kd | Error | R2 |
| M1 | 95.986 | 50.522 | 0.387 | 0.103 | 0.89 | 0.028 | 0.012 | 901.315 | 100.849 | 0.94 | 3.403 | 0.55 | 0.67 |
| M2 | 341.346 | 94.081 | 0.161 | 0.057 | 0.95 | 0.097 | 0.037 | 852.124 | 60.454 | 0.96 | 3.653 | 0.77 | 0.52 |
| M3 | 354.449 | 72.119 | 0.159 | 0.042 | 0.97 | 0.167 | 0.058 | 837.409 | 49.227 | 0.97 | 4.026 | 0.72 | 0.62 |
| M4 | - | - | - | - | - | - | - | - | - | - | 3.787 | 0.9 | 0.44 |
| M5 | - | - | - | - | - | - | - | 995.768 | 258.357 | 0.8 | 6.309 | 1.42 | 0.49 |
| M6 | 9.471 | 0 | 1.136 | 0.073 | 0.31 | - | - | 1600 | 0 | 0.31 | 16.97 | 5.08 | 0.31 |
| VO1 | - | - | - | - | - | - | - | - | - | - | 5.456 | 1.43 | 0.39 |
| VO2 | 314.979 | 91.637 | 0.128 | 0.057 | 0.94 | 0.143 | 0.085 | - | - | 0.94 | 2.532 | 0.41 | 0.66 |
| VO3 | - | - | - | - | - | - | - | - | - | - | 5.376 | 1.35 | 0.42 |
| VO4 | 9.219 | 0 | 1.101 | 0.072 | 0.31 | - | - | 1600 | 0 | 0.31 | 14.46 | 4.36 | 0.31 |
| VO5 | 8.87 | 0 | 1.066 | 0.073 | 0.27 | - | - | 1554.29 | 0 | 0.27 | 11.99 | 3.78 | 0.27 |
| VP1 | - | - | 0.829 | 0.365 | 0.79 | - | - | - | - | - | 1.08 | 0.13 | 0.79 |
| VP2 | 203.771 | 95.973 | 0.178 | 0.089 | 0.93 | 0.041 | 0.026 | 608.522 | 66.977 | 0.93 | 1.952 | 0.32 | 0.65 |
| VP3 | - | - | 0.552 | 0.182 | 0.91 | - | - | - | - | - | 2.546 | 0.26 | 0.86 |
| VP4 | - | - | 0.872 | 0.418 | 0.77 | - | - | - | - | - | 2.149 | 0.29 | 0.77 |
| VP5 | - | - | 1.326 | 0.213 | 0.98 | - | - | - | - | - | 8.274 | 0.4 | 0.97 |
| VP6 | 10.24 | 0 | 1.327 | 0.082 | 0.36 | - | - | 1600 | 0 | 0.36 | 33.87 | 9.59 | 0.36 |
| VP7 | - | - | - | - | -- | - | - | 1099.382 | 190.379 | 0.84 | 8.581 | 2.56 | 0.3 |
| FP | - | - | - | - | -- | - | - | 209.883 | 80.292 | 0.44 | 0.493 | 0.11 | 0.2 |
| FR | - | - | - | - | -- | - | - | 192.928 | 73.297 | 0.4 | 0.466 | 0.11 | 0.1 |
| FE | - | - | - | - | -- | - | - | 242.635 | 87.674 | 0.54 | 0.559 | 0.11 | 0.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cela-Dablanca, R.; Barreiro, A.; Rodríguez-López, L.; Pérez-Rodríguez, P.; Arias-Estévez, M.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A. Azithromycin Adsorption onto Different Soils. Processes 2022, 10, 2565. https://doi.org/10.3390/pr10122565
Cela-Dablanca R, Barreiro A, Rodríguez-López L, Pérez-Rodríguez P, Arias-Estévez M, Fernández-Sanjurjo MJ, Álvarez-Rodríguez E, Núñez-Delgado A. Azithromycin Adsorption onto Different Soils. Processes. 2022; 10(12):2565. https://doi.org/10.3390/pr10122565
Chicago/Turabian StyleCela-Dablanca, Raquel, Ana Barreiro, Lucía Rodríguez-López, Paula Pérez-Rodríguez, Manuel Arias-Estévez, María J. Fernández-Sanjurjo, Esperanza Álvarez-Rodríguez, and Avelino Núñez-Delgado. 2022. "Azithromycin Adsorption onto Different Soils" Processes 10, no. 12: 2565. https://doi.org/10.3390/pr10122565








