From Synthetic Route of Silica Nanoparticles to Theranostic Applications
Abstract
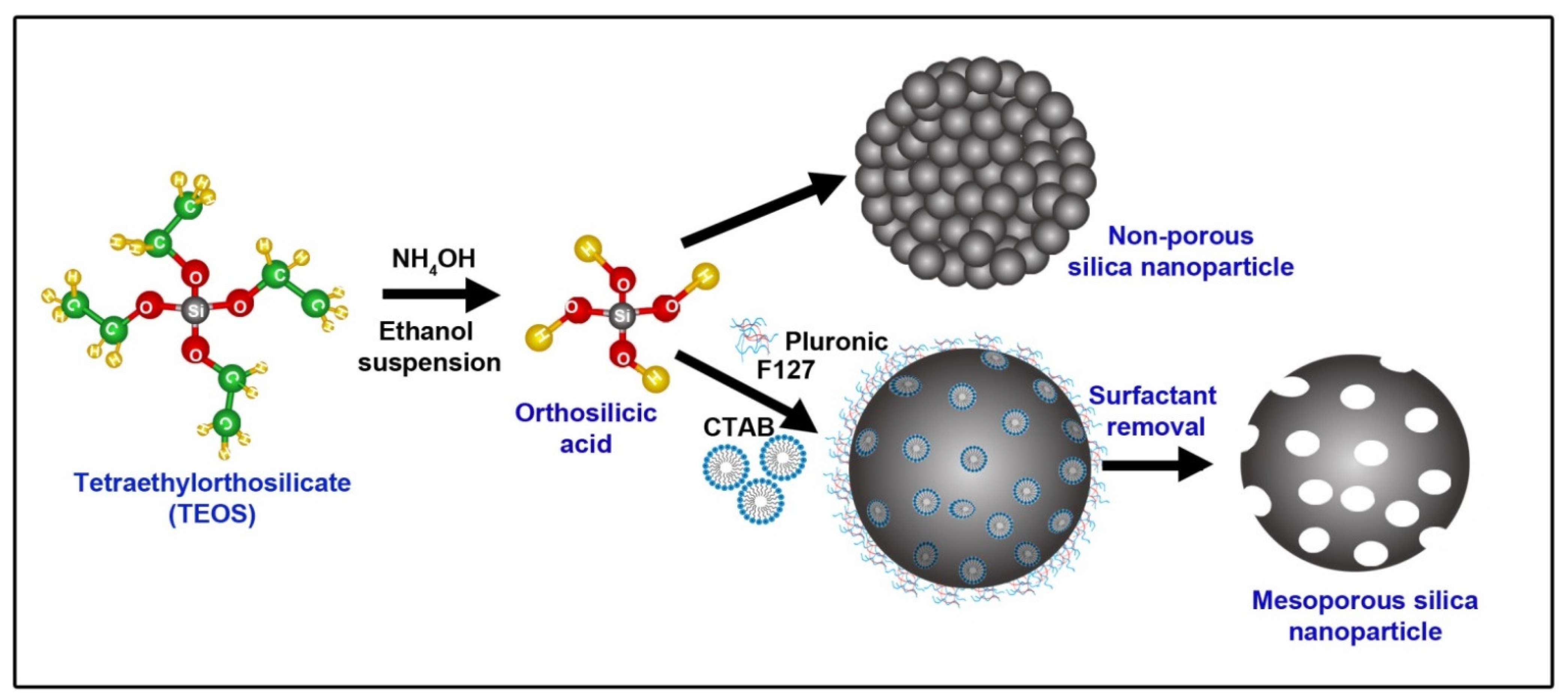
:1. Introduction
2. Different Methods to Prepare SNPs
2.1. Microemulsion Method
2.2. Gas-Phase Method
2.3. Stöber’s Method
2.4. Precipitation Method
2.5. Green Synthesis
3. Mesoporous SNPs
4. Core-Shell SNPs
5. Biomedical Applications
5.1. Drug Delivery System
5.2. Photodynamic Therapy
5.3. Photothermal Therapy
5.4. Imaging and Monitoring
5.5. Protein Recognition and Isolation
5.6. Nucleic Acid Detection and Purification
5.7. Gene Therapy
5.8. Vaccine Delivery
5.9. Other Applications
6. Biocompatibility
Toxicological Studies
7. Clinical Trials of Silica Nanoparticles
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Decuzzi, P.; Peer, D.; Di Mascolo, D.; Palange, A.L.; Manghnani, P.N.; Moghimi, S.M.; Farhangrazi, Z.S.; Howard, K.A.; Rosenblum, D.; Liang, T. Roadmap on nanomedicine. Nanotechnology 2020, 32, 12001. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Girigoswami, K.; Girigoswami, A. Membrane-encapsulated camouflaged nanomedicines in drug delivery. Nanomedicine 2019, 14, 2067–2082. [Google Scholar] [CrossRef] [PubMed]
- Sharmiladevi, P.; Girigoswami, K.; Haribabu, V.; Girigoswami, A. Nano-enabled theranostics for cancer. Mater. Adv. 2021, 2, 2876–2891. [Google Scholar] [CrossRef]
- Haribabu, V.; Farook, A.S.; Goswami, N.; Murugesan, R.; Girigoswami, A. Optimized Mn-doped iron oxide nanoparticles entrapped in dendrimer for dual contrasting role in MRI. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Haribabu, V.; Sharmiladevi, P.; Akhtar, N.; Farook, A.S.; Girigoswami, K.; Girigoswami, A. Label free ultrasmall fluoromagnetic ferrite-clusters for targeted cancer imaging and drug delivery. Curr. Drug Del. 2019, 16, 233–241. [Google Scholar] [CrossRef]
- Kazi, M.; Alhajri, A.; Alshehri, S.M.; Elzayat, E.M.; Al Meanazel, O.T.; Shakeel, F.; Noman, O.; Altamimi, M.A.; Alanazi, F.K. Enhancing oral bioavailability of apigenin using a bioactive self-nanoemulsifying drug delivery system (Bio-SNEDDS): In vitro, in vivo and stability evaluations. Pharmaceutics 2020, 12, 749. [Google Scholar] [CrossRef]
- Hussain, A.; Altamimi, M.A.; Alshehri, S.; Imam, S.S.; Shakeel, F.; Singh, S.K. Novel approach for transdermal delivery of rifampicin to induce synergistic antimycobacterial effects against cutaneous and systemic tuberculosis using a cationic nanoemulsion gel. Int. J. Nanomed. 2020, 15, 1073. [Google Scholar] [CrossRef] [Green Version]
- Shakeel, F.; Salem-Bekhit, M.M.; Haq, N.; Alshehri, S. Nanoemulsification Improves the Pharmaceutical Properties and Bioactivities of Niaouli Essential Oil (Melaleuca quinquenervia L.). Molecules 2021, 26, 4750. [Google Scholar] [CrossRef]
- Rahamathulla, M.; Bhosale, R.R.; Osmani, R.A.; Mahima, K.C.; Johnson, A.P.; Hani, U.; Ghazwani, M.; Begum, M.Y.; Alshehri, S.; Ghoneim, M.M. Carbon Nanotubes: Current Perspectives on Diverse Applications in Targeted Drug Delivery and Therapies. Materials 2021, 14, 6707. [Google Scholar] [CrossRef]
- Perween, N.; Alshehri, S.; Easwari, T.; Verma, V.; Faiyazuddin, M.; Alanazi, A.; Shakeel, F. Investigating the feasibility of mefenamic acid nanosuspension for pediatric delivery: Preparation, characterization, and role of excipients. Processes 2021, 9, 574. [Google Scholar] [CrossRef]
- Javed, S.; Alshehri, S.; Shoaib, A.; Ahsan, W.; Sultan, M.H.; Alqahtani, S.S.; Kazi, M.; Shakeel, F. Chronicles of Nanoerythrosomes: An Erythrocyte-Based Biomimetic Smart Drug Delivery System as a Therapeutic and Diagnostic Tool in Cancer Therapy. Pharmaceutics 2021, 13, 368. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Mu, Y.; Peng, C.; Lavin, M.F.; Shao, H.; Du, Z. Understanding the mechanisms of silica nanoparticles for nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2021, 13, e1658. [Google Scholar] [CrossRef] [PubMed]
- Amsaveni, G.; Farook, A.S.; Haribabu, V.; Murugesan, R.; Girigoswami, A. Engineered multifunctional nanoparticles for DLA cancer cells targeting, sorting, MR imaging and drug delivery. Adv. Sci. Eng. Med. 2013, 5, 1340–1348. [Google Scholar] [CrossRef]
- Sharmiladevi, P.; Haribabu, V.; Girigoswami, K.; Farook, A.S.; Girigoswami, A. Effect of mesoporous nano water reservoir on MR relaxivity. Sci. Rep. 2017, 7, 11179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharmiladevi, P.; Akhtar, N.; Haribabu, V.; Girigoswami, K.; Chattopadhyay, S.; Girigoswami, A. Excitation wavelength independent carbon-decorated ferrite nanodots for multimodal diagnosis and stimuli responsive therapy. ACS Appl. Bio. Mater. 2019, 2, 1634–1642. [Google Scholar] [CrossRef] [PubMed]
- Haribabu, V.; Girigoswami, K.; Sharmiladevi, P.; Girigoswami, A. Water–Nanomaterial Interaction to Escalate Twin-Mode Magnetic Resonance Imaging. ACS Biomater. Sci. Eng. 2020, 6, 4377–4389. [Google Scholar] [CrossRef] [PubMed]
- Croissant, J.G.; Butler, K.S.; Zink, J.I.; Brinker, C.J. Synthetic amorphous silica nanoparticles: Toxicity, biomedical and environmental implications. Nat. Rev. Mater. 2020, 5, 886–909. [Google Scholar] [CrossRef]
- Fu, J.; Jiao, J.; Song, H.; Gu, Z.; Liu, Y.; Geng, J.; Jack, K.S.; Du, A.; Tang, J.; Yu, C. Fractal-in-a-sphere: Confined self-assembly of fractal silica nanoparticles. Chem. Mater. 2019, 32, 341–347. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133. [Google Scholar] [CrossRef] [Green Version]
- He, Q.; Shi, J. Mesoporous silica nanoparticle based nano drug delivery systems: Synthesis, controlled drug release and delivery, pharmacokinetics and biocompatibility. J. Mater. Chem. 2011, 21, 5845–5855. [Google Scholar] [CrossRef]
- Girija, A.R.; Balasubramanian, S. Theragnostic potentials of core/shell mesoporous silica nanostructures. Nanotheranostics 2019, 3, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as drug delivery systems. Pharmacol. Rep. 2012, 64, 1020–1037. [Google Scholar] [CrossRef] [PubMed]
- Kankala, R.K.; Han, Y.H.; Na, J.; Lee, C.H.; Sun, Z.; Wang, S.B.; Kimura, T.; Ok, Y.S.; Yamauchi, Y.; Chen, A.Z. Nanoarchitectured structure and surface biofunctionality of mesoporous silica nanoparticles. Adv. Mater. 2020, 32, 1907035. [Google Scholar] [CrossRef] [PubMed]
- Bimbo, L.M.; Sarparanta, M.; Santos, H.A.; Airaksinen, A.J.; Makila, E.; Laaksonen, T.; Peltonen, L.; Lehto, V.-P.; Hirvonen, J.; Salonen, J. Biocompatibility of thermally hydrocarbonized porous silicon nanoparticles and their biodistribution in rats. ACS Nano 2010, 4, 3023–3032. [Google Scholar] [CrossRef] [PubMed]
- Rosenholm, J.M.; Sahlgren, C.; Lindén, M. Towards multifunctional, targeted drug delivery systems using mesoporous silica nanoparticles—Opportunities & challenges. Nanoscale 2010, 2, 1870–1883. [Google Scholar]
- Kumar, R.; Roy, I.; Ohulchanskky, T.Y.; Vathy, L.A.; Bergey, E.J.; Sajjad, M.; Prasad, P.N. In vivo biodistribution and clearance studies using multimodal organically modified silica nanoparticles. ACS Nano 2010, 4, 699–708. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.-H.; Liu, T.-Y.; Huang, H.-Y.; Liu, D.-M.; Chen, S.-Y. Magnetic-sensitive silica nanospheres for controlled drug release. Langmuir 2008, 24, 239–244. [Google Scholar] [CrossRef]
- Knopp, D.; Tang, D.; Niessner, R. Bioanalytical applications of biomolecule-functionalized nanometer-sized doped silica particles. Anal. Chim. Acta 2009, 647, 14–30. [Google Scholar] [CrossRef]
- Na, M.; Chen, Y.; Han, Y.; Ma, S.; Liu, J.; Chen, X. Determination of potassium ferrocyanide in table salt and salted food using a water-soluble fluorescent silicon quantum dots. Food Chem. 2019, 288, 248–255. [Google Scholar] [CrossRef]
- Maity, A.; Polshettiwar, V. Dendritic fibrous nanosilica for catalysis, energy harvesting, carbon dioxide mitigation, drug delivery, and sensing. ChemSusChem 2017, 10, 3866. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Glackin, C.A.; Horwitz, M.A.; Zink, J.I. Nanomachines and other caps on mesoporous silica nanoparticles for drug delivery. Acc. Chem. Res. 2019, 52, 1531–1542. [Google Scholar] [CrossRef] [PubMed]
- Paris, J.L.; Baeza, A.; Vallet-Regí, M. Overcoming the stability, toxicity, and biodegradation challenges of tumor stimuli-responsive inorganic nanoparticles for delivery of cancer therapeutics. Expert Opin. Drug Deliv. 2019, 16, 1095–1112. [Google Scholar] [CrossRef]
- Ren, D.; Xu, J.; Chen, N.; Ye, Z.; Li, X.; Chen, Q.; Ma, S. Controlled synthesis of mesoporous silica nanoparticles with tunable architectures via oil-water microemulsion assembly process. Colloids Surf. Physicochem. Eng. Asp. 2021, 611, 125773. [Google Scholar] [CrossRef]
- Rosenberg, D.J.; Alayoglu, S.; Kostecki, R.; Ahmed, M. Synthesis of microporous silica nanoparticles to study water phase transitions by vibrational spectroscopy. Nanoscale Adv. 2019, 1, 4878–4887. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Fan, H.; Stump, A.; Ward, T.L.; Rieker, T.; Brinker, C.J. Aerosol-assisted self-assembly of mesostructured spherical nanoparticles. Nature 1999, 398, 223–226. [Google Scholar] [CrossRef] [Green Version]
- Mahtabani, A.; La Zara, D.; Anyszka, R.; He, X.; Paajanen, M.; Van Ommen, J.R.; Dierkes, W.; Blume, A. Gas Phase Modification of Silica Nanoparticles in a Fluidized Bed: Tailored Deposition of Aminopropylsiloxane. Langmuir 2021, 37, 4481–4492. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-H.; Mou, C.-Y.; Lin, H.-P. Synthesis of mesoporous silica nanoparticles. Chem. Soc. Rev. 2013, 42, 3862–3875. [Google Scholar] [CrossRef] [PubMed]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Liu, X.; Lu, X.; Wen, P.; Shu, X.; Chi, F. Synthesis of ultrasmall silica nanoparticles for application as deep-ultraviolet antireflection coatings. Appl. Surf. Sci. 2017, 420, 180–185. [Google Scholar] [CrossRef]
- Fernandes, R.S.; Raimundo, I.M., Jr.; Pimentel, M.F. Revising the synthesis of Stöber silica nanoparticles: A multivariate assessment study on the effects of reaction parameters on the particle size. Colloids Surf. Physicochem. Eng. Aspects 2019, 577, 1–7. [Google Scholar] [CrossRef]
- Nele, M.; Vidal, A.; Bhering, D.L.; Pinto, J.C.; Salim, V.M.M. Preparation of high loading silica supported nickel catalyst: Simultaneous analysis of the precipitation and aging steps. Appl. Catal. A Gen. 1999, 178, 177–189. [Google Scholar] [CrossRef]
- Alvarez-Toral, A.; Fernández, B.; Malherbe, J.; Claverie, F.; Pecheyran, C.; Pereiro, R. Synthesis of amino-functionalized silica nanoparticles for preparation of new laboratory standards. Spectrochim. Acta Part B At. Spectrosc. 2017, 138, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, U.; Nagalakshmi, M.; Sadhukhan, R.; Girigoswami, A. Biocapped nanoparticles are more bioactive to HeLa cells than its chemical counterpart. Adv. Sci. Eng. Med. 2013, 5, 783–788. [Google Scholar] [CrossRef]
- Karande, S.D.; Jadhav, S.A.; Garud, H.B.; Kalantre, V.A.; Burungale, S.H.; Patil, P.S. Green and sustainable synthesis of silica nanoparticles. Nanotechnol. Environ. Eng. 2021, 6, 1–14. [Google Scholar] [CrossRef]
- Huang, R.; Shen, Y.-W.; Guan, Y.-Y.; Jiang, Y.-X.; Wu, Y.; Rahman, K.; Zhang, L.-J.; Liu, H.-J.; Luan, X. Mesoporous silica nanoparticles: Facile surface functionalization and versatile biomedical applications in oncology. Acta Biomater. 2020, 116, 1–15. [Google Scholar] [CrossRef]
- Yin, Q.; Shen, J.; Zhang, Z.; Yu, H.; Li, Y. Reversal of multidrug resistance by stimuli-responsive drug delivery systems for therapy of tumor. Adv. Drug Del. Rev. 2013, 65, 1699–1715. [Google Scholar] [CrossRef]
- Su, S.; Kang, P.M. Recent advances in nanocarrier-assisted therapeutics delivery systems. Pharmaceutics 2020, 12, 837. [Google Scholar] [CrossRef]
- Juneja, R.; Vadarevu, H.; Halman, J.; Tarannum, M.; Rackley, L.; Dobbs, J.; Marquez, J.; Chandler, M.; Afonin, K.; Vivero-Escoto, J.L. Combination of nucleic acid and mesoporous silica nanoparticles: Optimization and therapeutic performance in vitro. ACS Appl. Mater. Interfaces 2020, 12, 38873–38886. [Google Scholar] [CrossRef]
- Karaman, D.Ş.; Pamukçu, A.; Karakaplan, M.B.; Kocaoglu, O.; Rosenholm, J.M. Recent Advances in the Use of Mesoporous Silica Nanoparticles for the Diagnosis of Bacterial Infections. Int. J. Nanomed. 2021, 16, 6575. [Google Scholar] [CrossRef]
- Ianăşi, C.; Picioruş, E.-M.; Nicola, R.; Putz, A.-M.; Negrea, A.; Ciopec, M.; Len, A.; Almásy, L. Synthesis and characterization of magnetic iron oxide—silica nanocomposites used for adsorptive recovery of palladium (II). Soft Mater. 2021, 1–8. [Google Scholar] [CrossRef]
- Deepika, R.; Girigoswami, K.; Murugesan, R.; Girigoswami, A. Influence of divalent cation on morphology and drug delivery efficiency of mixed polymer nanoparticles. Curr. Drug Del. 2018, 15, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Vimaladevi, M.; Divya, K.C.; Girigoswami, A. Liposomal nanoformulations of rhodamine for targeted photodynamic inactivation of multidrug resistant gram negative bacteria in sewage treatment plant. J. Photochem. Photobiol. B Biol. 2016, 162, 146–152. [Google Scholar] [CrossRef]
- Niu, Y.; Yu, M.; Hartono, S.B.; Yang, J.; Xu, H.; Zhang, H.; Zhang, J.; Zou, J.; Dexter, A.; Gu, W. Nanoparticles mimicking viral surface topography for enhanced cellular delivery. Adv. Mater. 2013, 25, 6233–6237. [Google Scholar] [CrossRef] [PubMed]
- Harun, S.N.; Ahmad, H.; Lim, H.N.; Chia, S.L.; Gill, M.R. Synthesis and optimization of mesoporous silica nanoparticles for ruthenium polypyridyl drug delivery. Pharmaceutics 2021, 13, 150. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wang, H.; Wang, H.; Han, Y.; Zheng, Z.; Liu, X.; Feng, B.; Zhang, H. Light-responsive dual-functional biodegradable mesoporous silica nanoparticles with drug delivery and lubrication enhancement for the treatment of osteoarthritis. Nanoscale 2021, 13, 6394–6399. [Google Scholar] [CrossRef] [PubMed]
- Gou, K.; Wang, Y.; Guo, X.; Wang, Y.; Bian, Y.; Zhao, H.; Guo, Y.; Pang, Y.; Xie, L.; Li, S. Carboxyl-functionalized mesoporous silica nanoparticles for the controlled delivery of poorly water-soluble non-steroidal anti-inflammatory drugs. Acta Biomater. 2021, 134, 576–592. [Google Scholar] [CrossRef]
- Xu, D.; Song, X.; Zhou, J.; Ouyang, X.; Li, J.; Deng, D. Virus-like hollow mesoporous silica nanoparticles for cancer combination therapy. Colloids Surf. B Biointerfaces 2021, 197, 111452. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Q.; Han, N.; Bai, L.; Li, J.; Liu, J.; Che, E.; Hu, L.; Zhang, Q.; Jiang, T. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 313–327. [Google Scholar] [CrossRef]
- Firmansyah, A.; Nugrahani, I.; Wirasutisna, K.; Ibrahim, S. Formation of boron-silica based mesoporous and studies of its adsorption ability for curcuminoids. Biointerface Res. Appl. Chem. 2020, 10, 7977–7981. [Google Scholar]
- Shadjou, N.; Hasanzadeh, M. Bone tissue engineering using silica-based mesoporous nanobiomaterials: Recent progress. Mater. Sci. Eng. C 2015, 55, 401–409. [Google Scholar] [CrossRef]
- Roggers, R.A.; Lin, V.S.-Y.; Trewyn, B.G. Chemically reducible lipid bilayer coated mesoporous silica nanoparticles demonstrating controlled release and HeLa and normal mouse liver cell biocompatibility and cellular internalization. Mol. Pharm. 2012, 9, 2770–2777. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Bouramtane, S.; Bretin, L.; Pinon, A.; Leger, D.; Liagre, B.; Richard, L.; Brégier, F.; Sol, V.; Chaleix, V. Porphyrin-xylan-coated silica nanoparticles for anticancer photodynamic therapy. Carbohydr. Polym. 2019, 213, 168–175. [Google Scholar] [CrossRef]
- Zhou, Y.; Chang, C.; Liu, Z.; Zhao, Q.; Xu, Q.; Li, C.; Chen, Y.; Zhang, Y.; Lu, B. Hyaluronic acid-functionalized hollow mesoporous silica nanoparticles as pH-sensitive nanocarriers for cancer chemo-photodynamic therapy. Langmuir 2021, 37, 2619–2628. [Google Scholar] [CrossRef]
- Prieto-Montero, R.; Prieto-Castañeda, A.; Katsumiti, A.; Cajaraville, M.P.; Agarrabeitia, A.R.; Ortiz, M.J.; Martínez-Martínez, V. Functionalization of Photosensitized Silica Nanoparticles for Advanced Photodynamic Therapy of Cancer. Int. J. Mol. Sci. 2021, 22, 6618. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Gao, D.; Shen, J.; Wang, Q. A review of mesoporous silica nanoparticle delivery systems in chemo-based combination cancer therapies. Front. Chem. 2020, 8, 1086. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Niu, C.; Fan, S.; Li, Y.; Li, X.; Dai, Y.; Shi, J.; Wang, X. Indocyanine Green Loaded Modified Mesoporous Silica Nanoparticles as an Effective Photothermal Nanoplatform. Int. J. Mol. Sci. 2020, 21, 4789. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chang, Z.-M.; Shao, D.; Zhang, F.; Chen, F.; Li, L.; Ge, M.-F.; Hu, R.; Zheng, X.; Wang, Y. Janus gold triangle-mesoporous silica nanoplatforms for hypoxia-activated radio-chemo-photothermal therapy of liver cancer. ACS Appl. Mater. Interfaces 2019, 11, 34755–34765. [Google Scholar] [CrossRef]
- Cha, B.G.; Kim, J. Functional mesoporous silica nanoparticles for bio-imaging applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2019, 11, e1515. [Google Scholar] [CrossRef] [Green Version]
- Song, B.; Liu, Q.; Ma, H.; Tang, Z.; Liu, C.; Zou, J.; Tan, M.; Yuan, J. Tumor-targetable magnetoluminescent silica nanoparticles for bimodal time-gated luminescence/magnetic resonance imaging of cancer cells in vitro and in vivo. Talanta 2020, 220, 121378. [Google Scholar] [CrossRef]
- Khatik, R.; Wang, Z.; Li, F.; Zhi, D.; Kiran, S.; Dwivedi, P.; Xu, R.X.; Liang, G.; Qiu, B.; Yang, Q. “Magnus nano-bullets” as T1/T2 based dual-modal for in vitro and in vivo MRI visualization. Nanomed. Nanotechnol. Biol. Med. 2019, 15, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Ma, M.; Wang, J.; Wang, F.; Chern, S.-X.; Zhao, E.R.; Jhunjhunwala, A.; Darmadi, S.; Chen, H.; Jokerst, J.V. Exosome-like silica nanoparticles: A novel ultrasound contrast agent for stem cell imaging. Nanoscale 2017, 9, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Kempen, P.J.; Greasley, S.; Parker, K.A.; Campbell, J.L.; Chang, H.-Y.; Jones, J.R.; Sinclair, R.; Gambhir, S.S.; Jokerst, J.V. Theranostic mesoporous silica nanoparticles biodegrade after pro-survival drug delivery and ultrasound/magnetic resonance imaging of stem cells. Theranostics 2015, 5, 631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Zhang, F.; Lee, S.; Swierczewska, M.; Kiesewetter, D.O.; Lang, L.; Zhang, G.; Zhu, L.; Gao, H.; Choi, H.S. Long-term multimodal imaging of tumor draining sentinel lymph nodes using mesoporous silica-based nanoprobes. Biomaterials 2012, 33, 4370–4378. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Shin, T.-H.; Cheon, J.; Weissleder, R. Recent developments in magnetic diagnostic systems. Chem. Rev. 2015, 115, 10690–10724. [Google Scholar] [CrossRef] [Green Version]
- Pham, X.-H.; Hahm, E.; Kim, H.-M.; Son, B.S.; Jo, A.; An, J.; Tran Thi, T.A.; Nguyen, D.Q.; Jun, B.-H. Silica-coated magnetic iron oxide nanoparticles grafted onto graphene oxide for protein isolation. Nanomaterials 2020, 10, 117. [Google Scholar] [CrossRef] [Green Version]
- Mostafaei, M.; Hosseini, S.N.; Khatami, M.; Javidanbardan, A.; Sepahy, A.A.; Asadi, E. Isolation of recombinant Hepatitis B surface antigen with antibody-conjugated superparamagnetic Fe3O4/SiO2 core-shell nanoparticles. Protein Expr. Purif. 2018, 145, 1–6. [Google Scholar] [CrossRef]
- Choi, J.R.; Hu, J.; Tang, R.; Gong, Y.; Feng, S.; Ren, H.; Wen, T.; Li, X.; Abas, W.A.B.W.; Pingguan-Murphy, B. An integrated paper-based sample-to-answer biosensor for nucleic acid testing at the point of care. Lab. Chip 2016, 16, 611–621. [Google Scholar] [CrossRef]
- Melzak, K.A.; Sherwood, C.S.; Turner, R.F.; Haynes, C.A. Driving forces for DNA adsorption to silica in perchlorate solutions. J. Colloid Interface Sci. 1996, 181, 635–644. [Google Scholar] [CrossRef]
- Min, J.H.; Woo, M.-K.; Yoon, H.Y.; Jang, J.W.; Wu, J.H.; Lim, C.-S.; Kim, Y.K. Isolation of DNA using magnetic nanoparticles coated with dimercaptosuccinic acid. Anal. Biochem. 2014, 447, 114–118. [Google Scholar] [CrossRef]
- Ma, C.; Li, C.; He, N.; Wang, F.; Ma, N.; Zhang, L.; Lu, Z.; Ali, Z.; Xi, Z.; Li, X. Preparation and characterization of monodisperse core–shell Fe3O4@ SiO2 microspheres and its application for magnetic separation of nucleic acids from E. coli BL21. J. Biomed. Nanotechnol. 2012, 8, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Shin, J.M.; Tegafaw, T.; Han, H.S.; Chae, K.-S.; Chang, Y.; Lee, G.H. Magnetic separation of nucleic acids from various biological samples using silica-coated iron oxide nanobeads. J. Nanopart. Res. 2020, 22, 1–12. [Google Scholar] [CrossRef]
- Zhou, Y.; Quan, G.; Wu, Q.; Zhang, X.; Niu, B.; Wu, B.; Huang, Y.; Pan, X.; Wu, C. Mesoporous silica nanoparticles for drug and gene delivery. Acta Pharm. Sin. B 2018, 8, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Bharali, D.J.; Klejbor, I.; Stachowiak, E.K.; Dutta, P.; Roy, I.; Kaur, N.; Bergey, E.J.; Prasad, P.N.; Stachowiak, M.K. Organically modified silica nanoparticles: A nonviral vector for in vivo gene delivery and expression in the brain. Proc. Natl. Acad. Sci. USA 2005, 102, 11539–11544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, T.; Kovochich, M.; Liong, M.; Meng, H.; Kabehie, S.; George, S.; Zink, J.I.; Nel, A.E. Polyethyleneimine coating enhances the cellular uptake of mesoporous silica nanoparticles and allows safe delivery of siRNA and DNA constructs. ACS Nano 2009, 3, 3273–3286. [Google Scholar] [CrossRef]
- Roy, I.; Ohulchanskyy, T.Y.; Bharali, D.J.; Pudavar, H.E.; Mistretta, R.A.; Kaur, N.; Prasad, P.N. Optical tracking of organically modified silica nanoparticles as DNA carriers: A nonviral, nanomedicine approach for gene delivery. Proc. Natl. Acad. Sci. USA 2005, 102, 279–284. [Google Scholar] [CrossRef] [Green Version]
- Hartono, S.B.; Phuoc, N.T.; Yu, M.; Jia, Z.; Monteiro, M.J.; Qiao, S.; Yu, C. Functionalized large pore mesoporous silica nanoparticles for gene delivery featuring controlled release and co-delivery. J. Mater. Chem. B 2014, 2, 718–726. [Google Scholar] [CrossRef]
- Mody, K.T.; Popat, A.; Mahony, D.; Cavallaro, A.S.; Yu, C.; Mitter, N. Mesoporous silica nanoparticles as antigen carriers and adjuvants for vaccine delivery. Nanoscale 2013, 5, 5167–5179. [Google Scholar] [CrossRef]
- Gordon, S.; Teichmann, E.; Young, K.; Finnie, K.; Rades, T.; Hook, S. In vitro and in vivo investigation of thermosensitive chitosan hydrogels containing silica nanoparticles for vaccine delivery. Eur. J. Pharm. Sci. 2010, 41, 360–368. [Google Scholar] [CrossRef]
- Qiao, L.; Chen, M.; Li, S.; Hu, J.; Gong, C.; Zhang, Z.; Cao, X. A peptide-based subunit candidate vaccine against SARS-CoV-2 delivered by biodegradable mesoporous silica nanoparticles induced high humoral and cellular immunity in mice. Biomater. Sci. 2021, 9, 7287–7296. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, M.K.; Nguyen, T.L.; Kim, J. Hollow mesoporous silica nanoparticles with extra-large mesopores for enhanced cancer vaccine. ACS Appl. Mater. Interfaces 2020, 12, 34658–34666. [Google Scholar] [CrossRef] [PubMed]
- Montalvo-Quirós, S.; Vallet-Regí, M.; Palacios, A.; Anguita, J.; Prados-Rosales, R.C.; González, B.; Luque-Garcia, J.L. Mesoporous Silica Nanoparticles as a Potential Platform for Vaccine Development against Tuberculosis. Pharmaceutics 2020, 12, 1218. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, W.M.; Riffey, A.; Buhl, C.; Johnson, C.; Ryter, K.; Evans, J.T.; Burkhart, D.J. Co-adsorption of synthetic Mincle agonists and antigen to silica nanoparticles for enhanced vaccine activity: A formulation approach to co-delivery. Int. J. Pharm. 2021, 593, 120119. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ru, J.; Sun, S.; Teng, Z.; Dong, H.; Song, P.; Yang, Y.; Guo, H. Uniform dendrimer-like mesoporous silica nanoparticles as a nano-adjuvant for foot-and-mouth disease virus-like particle vaccine. J. Mater. Chem. B 2019, 7, 3446–3454. [Google Scholar] [CrossRef]
- Song, H.; Yang, Y.; Tang, J.; Gu, Z.; Wang, Y.; Zhang, M.; Yu, C. DNA vaccine mediated by rambutan-like mesoporous silica nanoparticles. Adv. Ther. 2020, 3, 1900154. [Google Scholar] [CrossRef]
- Cha, B.G.; Jeong, J.H.; Kim, J. Extra-large pore mesoporous silica nanoparticles enabling co-delivery of high amounts of protein antigen and toll-like receptor 9 agonist for enhanced cancer vaccine efficacy. ACS Cent. Sci. 2018, 4, 484–492. [Google Scholar] [CrossRef] [Green Version]
- Soares, D.C.F.; Soares, L.M.; de Goes, A.M.; Melo, E.M.; de Barros, A.L.B.; Bicalho, T.C.A.S.; Leao, N.M.; Tebaldi, M.L. Mesoporous SBA-16 silica nanoparticles as a potential vaccine adjuvant against Paracoccidioides brasiliensis. Microporous Mesoporous Mater. 2020, 291, 109676. [Google Scholar] [CrossRef]
- Amin, M.K.; Boateng, J.S. Surface modification of mobile composition of matter (MCM)-41 type silica nanoparticles for potential oral mucosa vaccine delivery. J. Pharm. Sci. 2020, 109, 2271–2283. [Google Scholar] [CrossRef]
- Hajizade, A.; Salmanian, A.H.; Amani, J.; Ebrahimi, F.; Arpanaei, A. EspA-loaded mesoporous silica nanoparticles can efficiently protect animal model against enterohaemorrhagic E. coli O157: H7. Artif. Cells Nanomed. Biotechnol. 2018, 46, S1067–S1075. [Google Scholar] [CrossRef] [Green Version]
- Injorhor, P.; Ruksakulpiwat, Y.; Ruksakulpiwat, C. Effect of shrimp shell chitosan loading on antimicrobial, absorption and morphological properties of natural rubber composites reinforced with silica-chitosan hybrid filler. Biointerface Res. Appl. Chem 2020, 10, 5656–5659. [Google Scholar]
- Alghuthaymi, M. Magnetic-silica nanoshell for extraction of fungal genomic DNA from Rhizopus oryzae. Biointerface Res. Appl. Chem. 2020, 10, 4972–4976. [Google Scholar]
- Miller, K.P. Bacterial Communication and Its Role As a Target for Nanoparticlebased Antimicrobial Therapy. Doctoral Dissertation, University of South Carolina, Columbia, SC, USA, 2015. [Google Scholar]
- Masood, A.; Maheen, S.; Khan, H.U.; Shafqat, S.S.; Irshad, M.; Aslam, I.; Rasul, A.; Bashir, S.; Zafar, M.N. Pharmaco-Technical Evaluation of Statistically Formulated and Optimized Dual Drug-Loaded Silica Nanoparticles for Improved Antifungal Efficacy and Wound Healing. ACS Omega 2021, 6, 8210–8225. [Google Scholar] [CrossRef] [PubMed]
- Abolghasemzade, S.; Pourmadadi, M.; Rashedi, H.; Yazdian, F.; Kianbakht, S.; Navaei-Nigjeh, M. PVA based nanofiber containing CQDs modified with silica NPs and silk fibroin accelerates wound healing in a rat model. J. Mater. Chem. B 2021, 9, 658–676. [Google Scholar] [CrossRef]
- Quignard, S.; Coradin, T.; Powell, J.J.; Jugdaohsingh, R. Silica nanoparticles as sources of silicic acid favoring wound healing in vitro. Colloids Surf. B Biointerfaces 2017, 155, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Li, Y.; Coradin, T. Magnetically-oriented type I collagen-SiO2@ Fe3O4 rods composite hydrogels tuning skin cell growth. Colloids Surf. B Biointerfaces 2020, 185, 110597. [Google Scholar] [CrossRef] [PubMed]
- Naruphontjirakul, P.; Porter, A.E.; Jones, J.R. In vitro osteogenesis by intracellular uptake of strontium containing bioactive glass nanoparticles. Acta Biomater. 2018, 66, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Yang, M.; Jing, L.; Wang, J.; Yu, Y.; Li, Y.; Duan, J.; Zhou, X.; Li, Y.; Sun, Z. Amorphous silica nanoparticles trigger vascular endothelial cell injury through apoptosis and autophagy via reactive oxygen species-mediated MAPK/Bcl-2 and PI3K/Akt/mTOR signaling. Int. J. Nanomedicine 2016, 11, 5257. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.-Y.; Joachim, E.; Choi, H.; Kim, K. Toxicity of silica nanoparticles depends on size, dose, and cell type. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1407–1416. [Google Scholar] [CrossRef]
- Duan, J.; Yu, Y.; Li, Y.; Yu, Y.; Li, Y.; Zhou, X.; Huang, P.; Sun, Z. Toxic effect of silica nanoparticles on endothelial cells through DNA damage response via Chk1-dependent G2/M checkpoint. PLoS ONE 2013, 8, e62087. [Google Scholar] [CrossRef] [Green Version]
- Decan, N.; Wu, D.; Williams, A.; Bernatchez, S.; Johnston, M.; Hill, M.; Halappanavar, S. Characterization of in vitro genotoxic, cytotoxic and transcriptomic responses following exposures to amorphous silica of different sizes. Mutat. Res. /Genet. Toxicol. Environ. Mutagen. 2016, 796, 8–22. [Google Scholar] [CrossRef]
- Guo, C.; Wang, J.; Jing, L.; Ma, R.; Liu, X.; Gao, L.; Cao, L.; Duan, J.; Zhou, X.; Li, Y. Mitochondrial dysfunction, perturbations of mitochondrial dynamics and biogenesis involved in endothelial injury induced by silica nanoparticles. Environ. Pollut. 2018, 236, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, L.; Liu, T.; Hao, N.; Liu, H.; Chen, D.; Tang, F. The shape effect of mesoporous silica nanoparticles on biodistribution, clearance, and biocompatibility in vivo. ACS Nano 2011, 5, 5390–5399. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpour, R.; Cheney, D.L.; Grunberger, J.W.; Yazdimamaghani, M.; Jedrzkiewicz, J.; Isaacson, K.J.; Dobrovolskaia, M.A.; Ghandehari, H. One-year chronic toxicity evaluation of single dose intravenously administered silica nanoparticles in mice and their Ex vivo human hemocompatibility. J. Control. Release 2020, 324, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Azouz, R.A.; Korany, R. Toxic impacts of amorphous silica nanoparticles on liver and kidney of male adult rats: An in vivo study. Biol. Trace Elem. Res. 2021, 199, 2653–2662. [Google Scholar] [CrossRef]
- Deng, Y.-D.; Zhang, X.-D.; Yang, X.-S.; Huang, Z.-L.; Wei, X.; Yang, X.-F.; Liao, W.-Z. Subacute toxicity of mesoporous silica nanoparticles to the intestinal tract and the underlying mechanism. J. Hazard. Mater. 2021, 409, 124502. [Google Scholar] [CrossRef]
- Yu, Y.; Duan, J.; Li, Y.; Li, Y.; Jing, L.; Yang, M.; Wang, J.; Sun, Z. Silica nanoparticles induce liver fibrosis via TGF-β1/Smad3 pathway in ICR mice. Int. J. Nanomed. 2017, 12, 6045. [Google Scholar] [CrossRef] [Green Version]
- Mohammadpour, R.; Dobrovolskaia, M.A.; Cheney, D.L.; Greish, K.F.; Ghandehari, H. Subchronic and chronic toxicity evaluation of inorganic nanoparticles for delivery applications. Adv. Drug Del. Rev. 2019, 144, 112–132. [Google Scholar] [CrossRef]
- Wu, J.; Wang, C.; Sun, J.; Xue, Y. Neurotoxicity of silica nanoparticles: Brain localization and dopaminergic neurons damage pathways. ACS Nano 2011, 5, 4476–4489. [Google Scholar] [CrossRef]
- Diab, R.; Canilho, N.; Pavel, I.A.; Haffner, F.B.; Girardon, M.; Pasc, A. Silica-based systems for oral delivery of drugs, macromolecules and cells. Adv. Colloid. Interface Sci. 2017, 249, 346–362. [Google Scholar] [CrossRef]
- Janjua, T.I.; Cao, Y.; Yu, C.; Popat, A. Clinical translation of silica nanoparticles. Nat. Rev. Mater. 2021, 6, 1072–1074. [Google Scholar] [CrossRef]
- Tan, A.; Eskandar, N.G.; Rao, S.; Prestidge, C.A. First in man bioavailability and tolerability studies of a silica–lipid hybrid (Lipoceramic) formulation: A Phase I study with ibuprofen. Drug Deliv. Transl. Res. 2014, 4, 212–221. [Google Scholar] [CrossRef]
- Meola, T.R.; Abuhelwa, A.Y.; Joyce, P.; Clifton, P.; Prestidge, C.A. A safety, tolerability, and pharmacokinetic study of a novel simvastatin silica-lipid hybrid formulation in healthy male participants. Drug Deliv. Transl. Res. 2021, 11, 1261–1272. [Google Scholar] [CrossRef] [PubMed]
- Kharlamov, A.N.; Feinstein, J.A.; Cramer, J.A.; Boothroyd, J.A.; Shishkina, E.V.; Shur, V. Plasmonic photothermal therapy of atherosclerosis with nanoparticles: Long-term outcomes and safety in NANOM-FIM trial. Future Cardiol. 2017, 13, 345–363. [Google Scholar] [CrossRef] [PubMed]
- Kharlamov, A. Plasmonic Photothermal Therapy of Atherosclerosis And Preparation Of Target Lesion In Patients With Arterial Remodeling: Subanalysis Of Nanom-Fim Trial. Atherosclerosis 2019, 287, e34. [Google Scholar] [CrossRef]
- Han, H.; Choi, K. Advances in Nanomaterial-Mediated Photothermal Cancer Therapies: Toward Clinical Applications. Biomedicines 2021, 9, 305. [Google Scholar] [CrossRef]
- Tyagi, P. Harnessing the nano-bio interface: Application of membrane coating to long acting silica particles. Eur. J. Pharm. Biopharm. 2021, 158, 382–389. [Google Scholar] [CrossRef]
- Carvalho, M.; Reis, R.; Oliveira, J.M. Dendrimer nanoparticles for colorectal cancer applications. J. Mater. Chem. B 2020, 8, 1128–1138. [Google Scholar] [CrossRef]
- Phillips, E.; Penate-Medina, O.; Zanzonico, P.B.; Carvajal, R.D.; Mohan, P.; Ye, Y.; Humm, J.; Gönen, M.; Kalaigian, H.; Schöder, H. Clinical translation of an ultrasmall inorganic optical-PET imaging nanoparticle probe. Sci. Transl. Med. 2014, 6, 260ra149. [Google Scholar] [CrossRef] [Green Version]
- Ma, K.; Mendoza, C.; Hanson, M.; Werner-Zwanziger, U.; Zwanziger, J.; Wiesner, U. Control of ultrasmall sub-10 nm ligand-functionalized fluorescent core—Shell silica nanoparticle growth in water. Chem. Mater. 2015, 27, 4119–4133. [Google Scholar] [CrossRef]
- Gidwani, B.; Sahu, V.; Shukla, S.S.; Pandey, R.; Joshi, V.; Jain, V.K.; Vyas, A. Quantum dots: Prospectives, toxicity, advances and applications. J. Drug Deliv. Sci. Technol. 2021, 61, 102308. [Google Scholar] [CrossRef]


| Sl.No. | Methods | Advantages | Disadvantages |
|---|---|---|---|
| 1. | Microemulsion |
|
|
| 2. | Gas-phase |
|
|
| 3. | Stöber’s |
|
|
| 4. | Precipitation |
|
|
| 5. | Green Synthesis |
|
|
| Sl.No | Type of Silica | Modification | Disease/Disorder | Ref. |
|---|---|---|---|---|
| 1. | Biodegradable mesoporous silica nanoparticles | Encapsulated vaccines | SARS-CoV-2 | [90] |
| 2. | Hollow mesoporous silica nanoparticles with extra-large mesopores | Core-shell with Poly(ethylenimine) coating (PEI) | Malignancy | [91] |
| 3. | Mesoporous Silica Nanoparticles | Carboxylic acids | Tuberculosis | [92] |
| 4. | Silica nanoparticles | Mincle agonists | tuberculosis | [93] |
| 5. | Dendrimer-like mesoporous silica nanoparticles | Foot-and-mouth disease VLPs (virus-like particles) | Foot-and-mouth disease | [94] |
| 6. | Rambutan-Like Mesoporous Silica Nanoparticles | DNA vaccine with PEI coating | Chronic infections and cancers. | [95] |
| 7. | Extra-large pore MSNs (XL-MSNs) | Cancer antigen with Amine modification | Malignancy | [96] |
| 8. | Mesoporous SBA-16 and silanized SBA-16 (APTES-SBA-16) nanoparticles | (3-Aminopropyl) triethoxysilane (APTES) | Paracoccidioidomycosis | [97] |
| 9. | MCM-41 type silica nanoparticles | Polymer and amine | Oral protein-based vaccine | [98] |
| 10. | Spherical MSNs | Recombinant EspA loaded | Enterohemorrhagic Escherichia coli | [99] |
| Types of SNPs/Synthetic Routes | Surface Chemistry/Modifications | Biomedical Applications | Ref. |
|---|---|---|---|
| Fractal SNP/surfactant-free Stöber method | Fractal silica nanoparticles | Sharp edge and rough surface with enhanced adhesion towards enzyme | [18] |
| Solid SNP/Stöber method | Amine group/ polyethylenimine | Mimicking virus surface topology and surface roughness enhances the binding of biomolecules | [53] |
| MSN/co-condensation | - | Ruthenium polypyridyl delivery for cancer treatment | [54] |
| MSN | Azobenzene-modified | Lubrication enhancement and drug release for osteoarthritis | [55] |
| MSN | Carboxyl-functionalized | Controlled delivery of NSAIDs | [56] |
| Virus like hollow MSN/self-consuming perovskite | Electrostatic adsorption of doxorubicin | Combination of chemotherapy and immunotherapy | [57] |
| MSN/co-condensation | Covalently bound dipalmitoyl | Controlled release of fluorescein | [61] |
| Core-shell hybrid SNPs/sol-gel process following modified Stöber method | Silica core with xylan linked 5-(4-hydroxyphenyl)-10,15,20-triphenylporphyrin (TPPOH) | PDT against colorectal cancer cells | [63] |
| Hollow-MSN/Stöber method | Hyaluronic acid | Cancer chemo-PDT | [64] |
| MSN/ modified Stöber method | haloBODIPYs, PEG and FA | Targeted cancer PDT | [65] |
| Silica nanospheres/sol-gel method | Indocyanine green to amino modified surface | PTT against drug resistant tumors | [67] |
| Janus-structured gold triangle-MSN/ sol-gel method | Amino functionalization and attachment of tirapazamine (TPZ) | Extrinsic radiosensitization, local PTT and hypoxia-specific chemotherapy | [68] |
| FA-Gd-Tb@SiO2/covalent conjugation | Covalent conjugation of luminescent Tb3+ to Si-O-Si framework followed by attachment of Gd and FA (folic acid) | Targeted cellular time-gated luminescence (TGL) and cancer cell MR imaging | [70] |
| Janus magnus nano-bullets (Mn-DTPA-F-MSNs)/sol-gel process | Mn-DTPA-functionalized Fe3O4-MSNs | GSH (glutathione) responsive T1/T2 MRI | [71] |
| Exosomes-like cup-shaped SNP/emulsion template method | - | Ultra sound contrast for stem cell imaging and drug delivery | [72] |
| Theranostic MSN | Amino-silane conjugation of fluorescein followed by Gd-DOTA | Ultra sound and MR imaging of stem cells and slow-release reservoir of insulin-like growth factor (IGF) | [73] |
| MSN/sol-gel method | Salicylic acid and ketoconazole | Antifungal and wound healing | [103] |
| SNP | Carbon quantum dots/silica nanoparticles/silk fibroin nanocomposites | Wound repair | [104] |
| Rod-like silica nanoparticles/one pot method in presence of PVP | Type I collagen-SiO2@Fe3O4 | Cell guidance and drug delivery | [106] |
| Types of Particles | Mode of Study | Probable Reasons | Ref. |
|---|---|---|---|
| MSN (20–200 nm) | In vitro | Size and dose dependency, ROS generation, and changes in membrane integrity induced by cellular uptake. | [109] |
| SNP (62 nm) | In vitro | Induced ROS formation and DNA damage response and caused toxicity to endothelial cells through Chk1-dependant G2/M DNA damage checkpoint. | [110] |
| Fluorescent MSN | In vivo | More than 80% intravenously administered MSNs deposited in liver, spleen, and lung and shape of the particles play a major role. | [113] |
| SNPs (46 ± 4.9 & 432.0 ± 18.7 nm) and MSNs (466.0 ± 86.0 nm) | In vivo & ex vivo | Microscopic lesions in liver, kidney, spleen, and lungs. Physicochemical properties, dose, and frequency of administration is responsible. | [114] |
| SNP | In vivo | Dose-dependent hepatotoxicity and nephrotoxicity in male rats due to oxidative stress and apoptosis. | [115] |
| MSN (75 nm) | In vivo | Induced intestinal oxidative stress and colonic epithelial cell apoptosis in mice | [116] |
| SNP (57.66 ± 7.30 nm) | In vitro | Impairment in mitochondrial dynamics and biogenesis leading mitochondrial dysfunction, oxidative stress, and, finally, cardiovascular disease. | [112] |
| SNP (64.43 ± 10.50) | In vivo | Induce hepatic dysfunction and granuloma formation in liver. | [117] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pallavi, P.; Harini, K.; Alshehri, S.; Ghoneim, M.M.; Alshlowi, A.; Gowtham, P.; Girigoswami, K.; Shakeel, F.; Girigoswami, A. From Synthetic Route of Silica Nanoparticles to Theranostic Applications. Processes 2022, 10, 2595. https://doi.org/10.3390/pr10122595
Pallavi P, Harini K, Alshehri S, Ghoneim MM, Alshlowi A, Gowtham P, Girigoswami K, Shakeel F, Girigoswami A. From Synthetic Route of Silica Nanoparticles to Theranostic Applications. Processes. 2022; 10(12):2595. https://doi.org/10.3390/pr10122595
Chicago/Turabian StylePallavi, Pragya, Karthick Harini, Sultan Alshehri, Mohammed M. Ghoneim, Areej Alshlowi, Pemula Gowtham, Koyeli Girigoswami, Faiyaz Shakeel, and Agnishwar Girigoswami. 2022. "From Synthetic Route of Silica Nanoparticles to Theranostic Applications" Processes 10, no. 12: 2595. https://doi.org/10.3390/pr10122595
APA StylePallavi, P., Harini, K., Alshehri, S., Ghoneim, M. M., Alshlowi, A., Gowtham, P., Girigoswami, K., Shakeel, F., & Girigoswami, A. (2022). From Synthetic Route of Silica Nanoparticles to Theranostic Applications. Processes, 10(12), 2595. https://doi.org/10.3390/pr10122595









