Temperature Control of Exothermic Reactions Using n-Octadecane@MF Resin microPCMs Based on Esterification Reactions
Abstract
:1. Introduction
2. Preparation and Characterization of the microPCMs
2.1. Experimental Materials
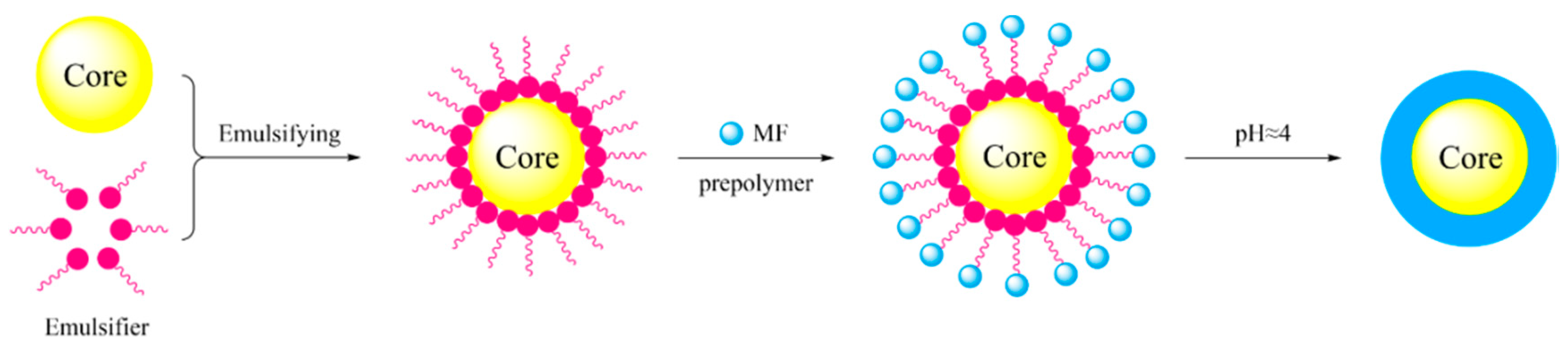
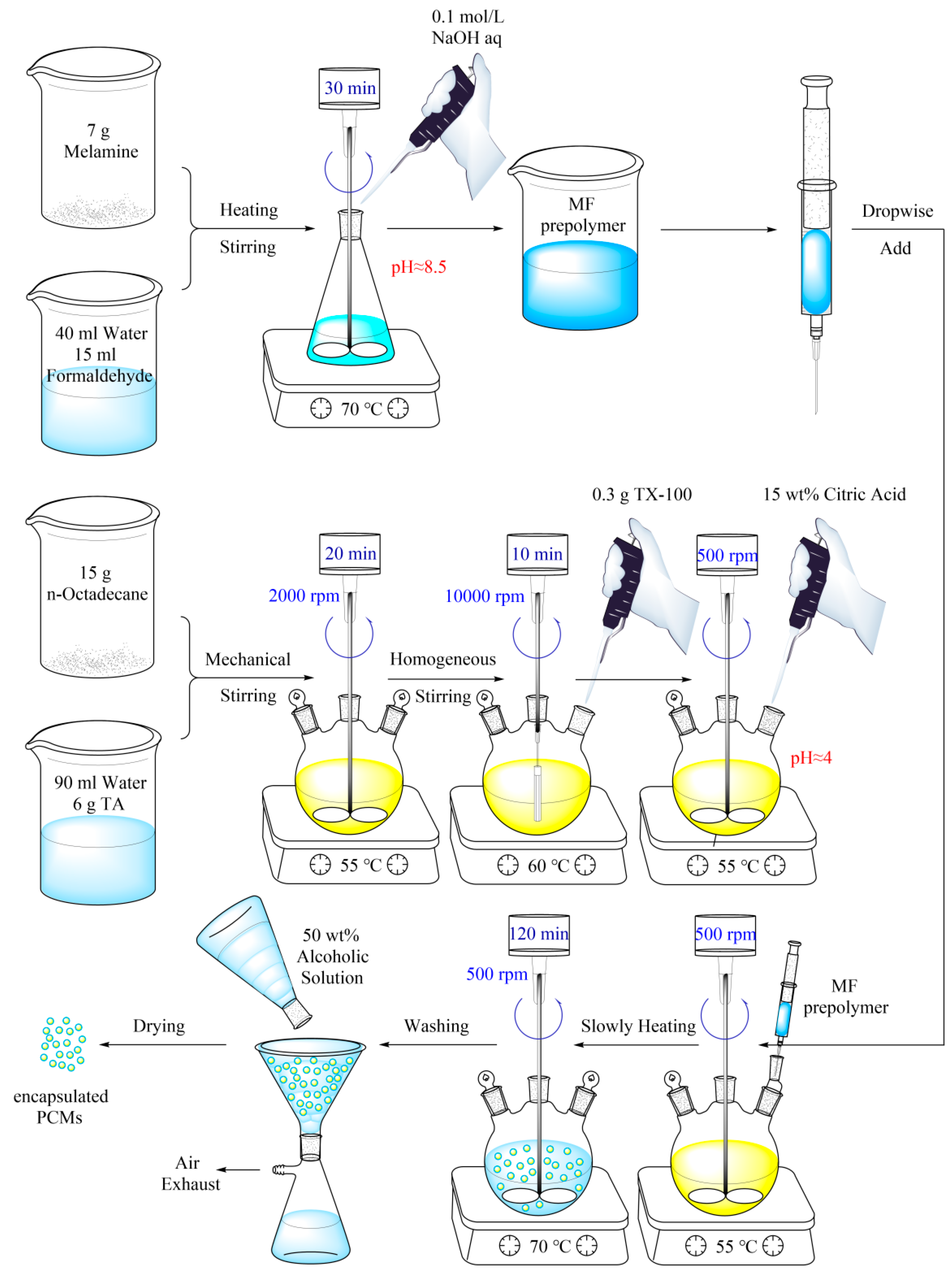
2.2. Preparation of Encapsulated n-Octadecane with MF Resin Shell
2.3. Chemical Characterization and Morphology of microPCMs
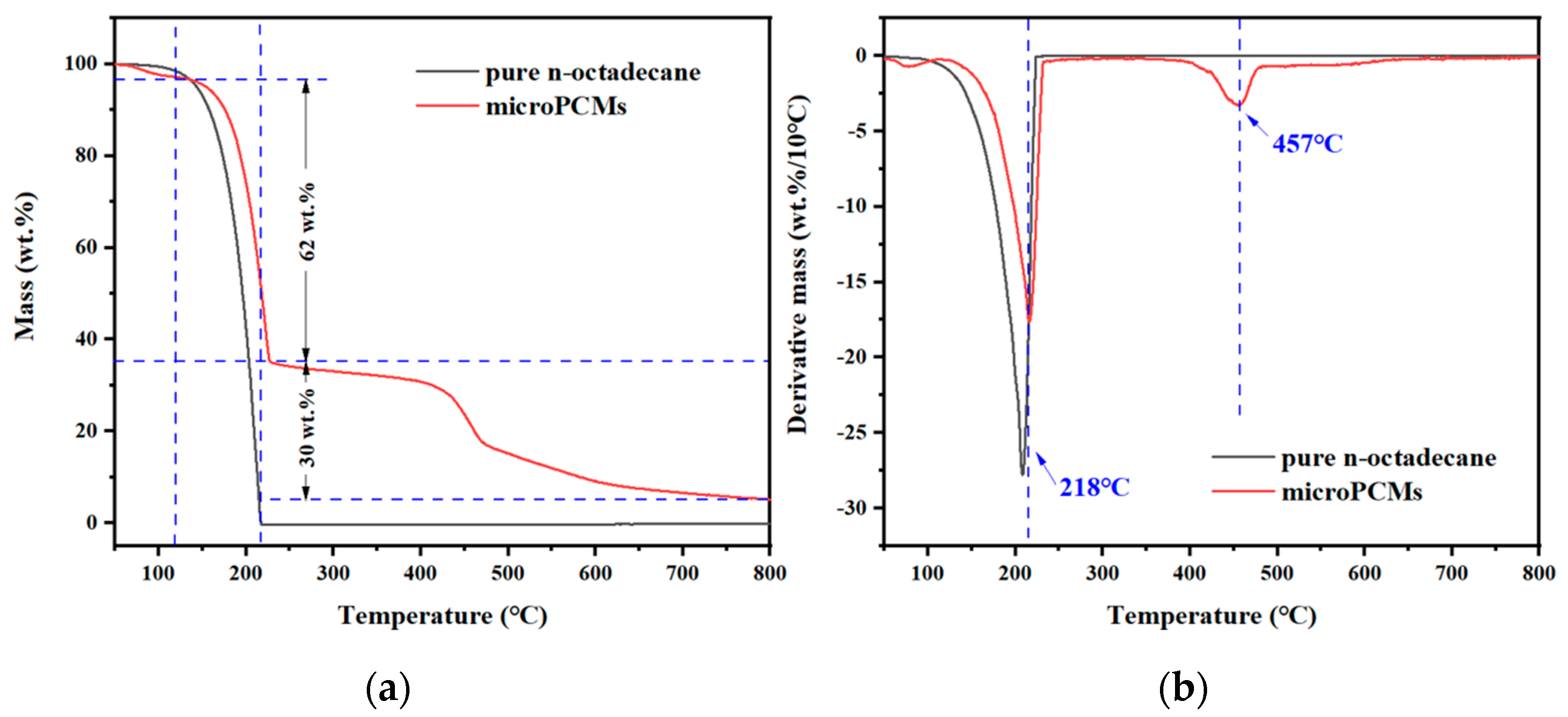
2.4. Thermal Properties and Thermal Stability of microPCMs
3. Temperature Control Experiments
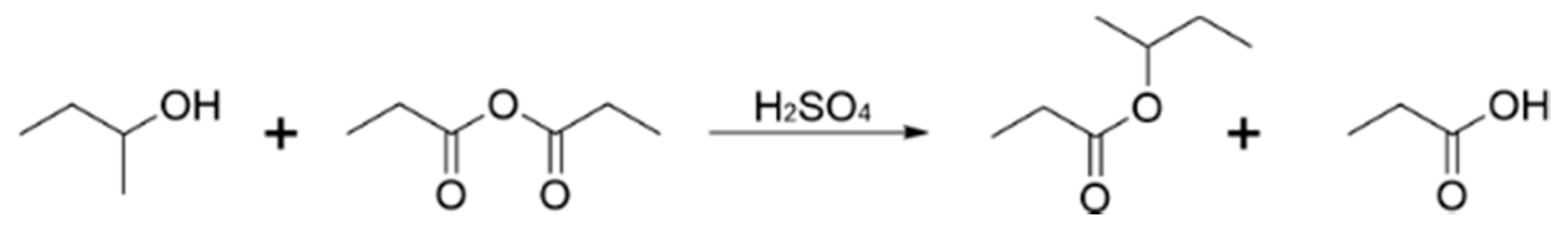
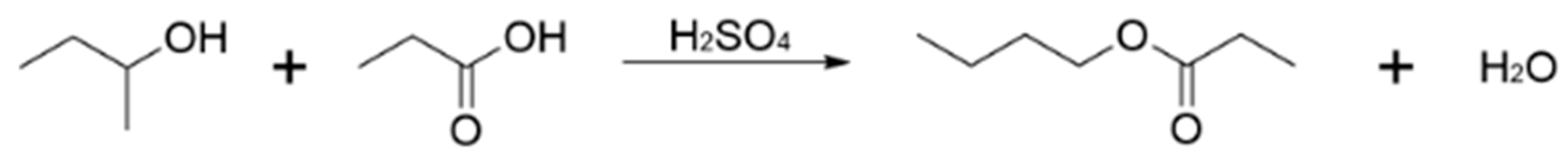
3.1. Reaction Scheme
3.2. Experimental Materials
3.3. Experimental Procedures
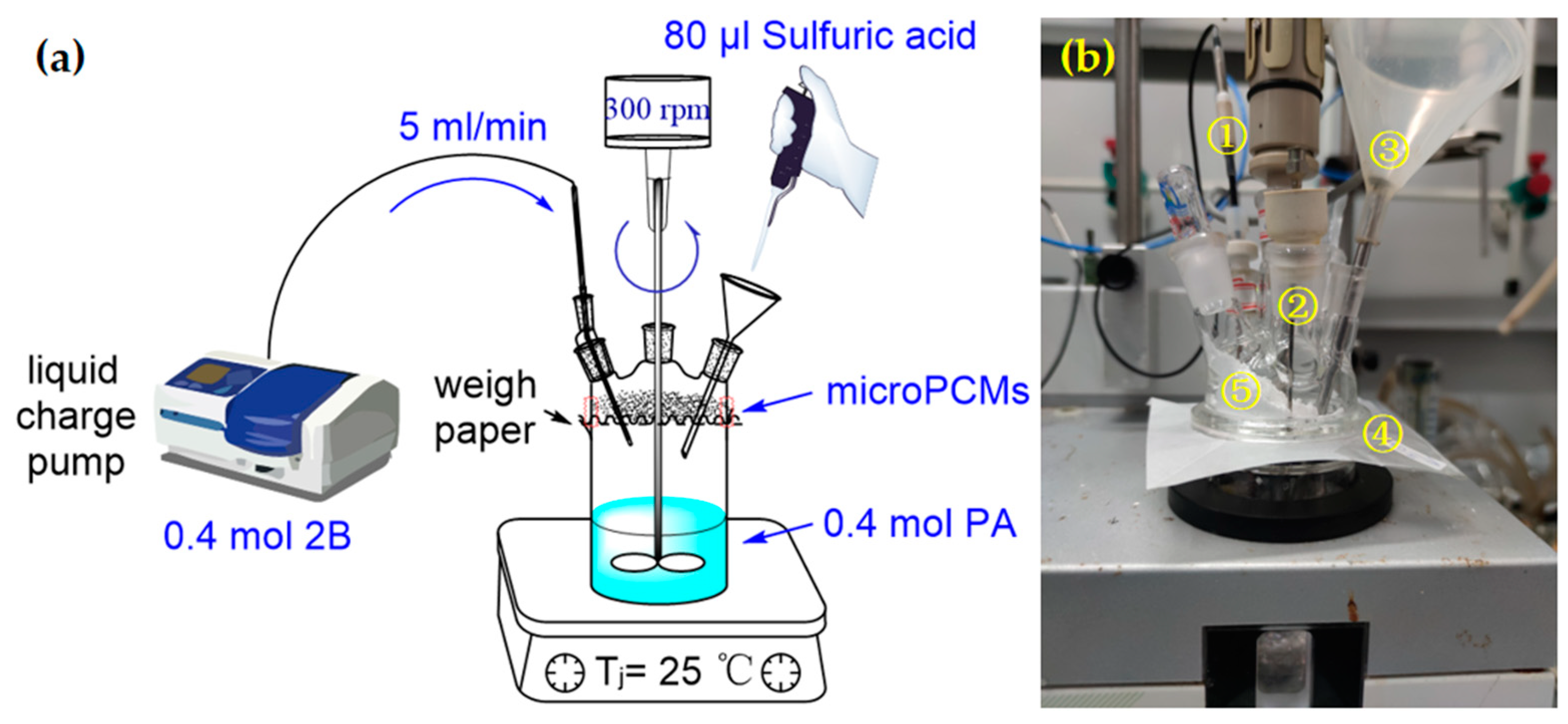
3.3.1. Experimental Equipment
3.3.2. Experimental Process
4. Result and Discussion
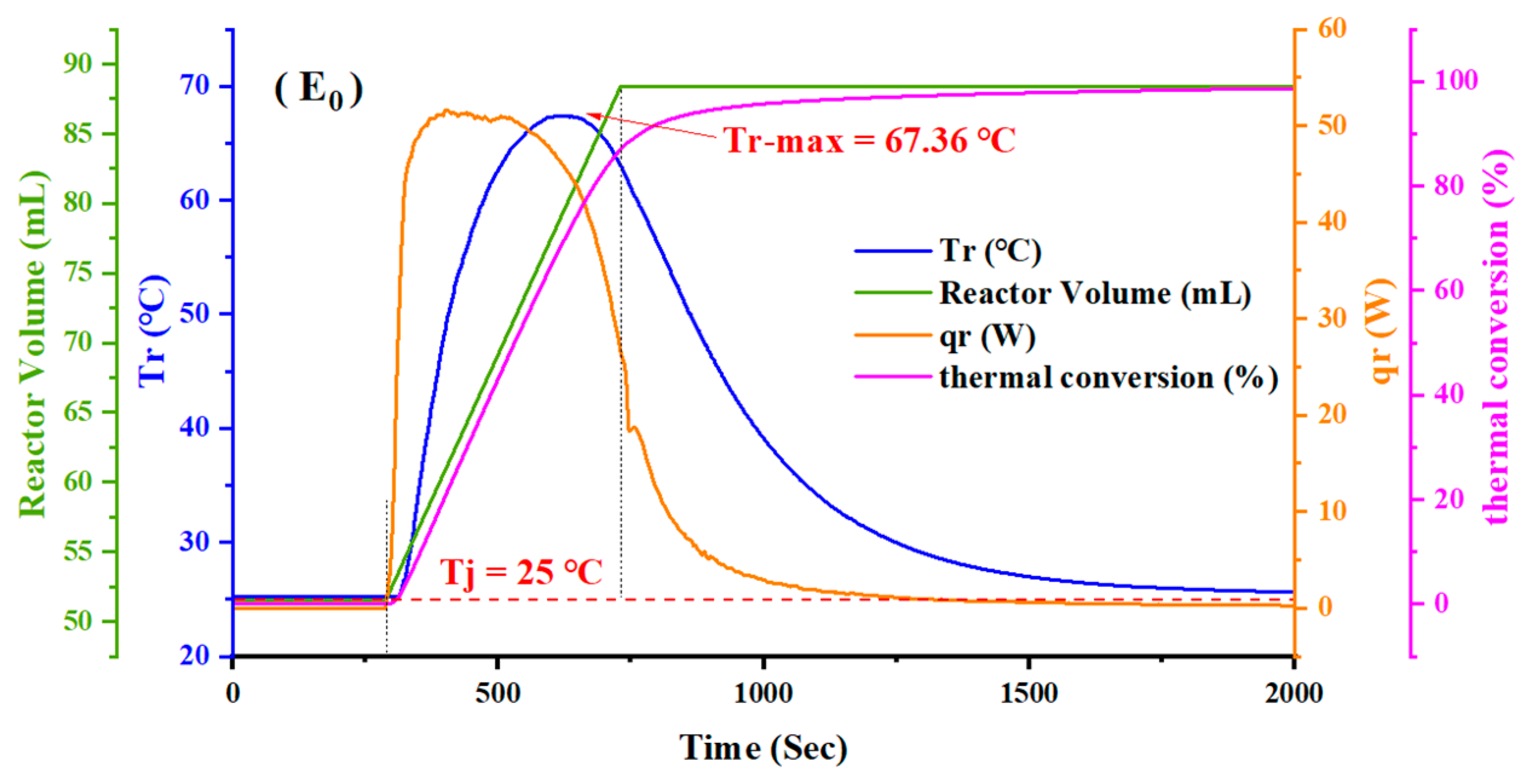
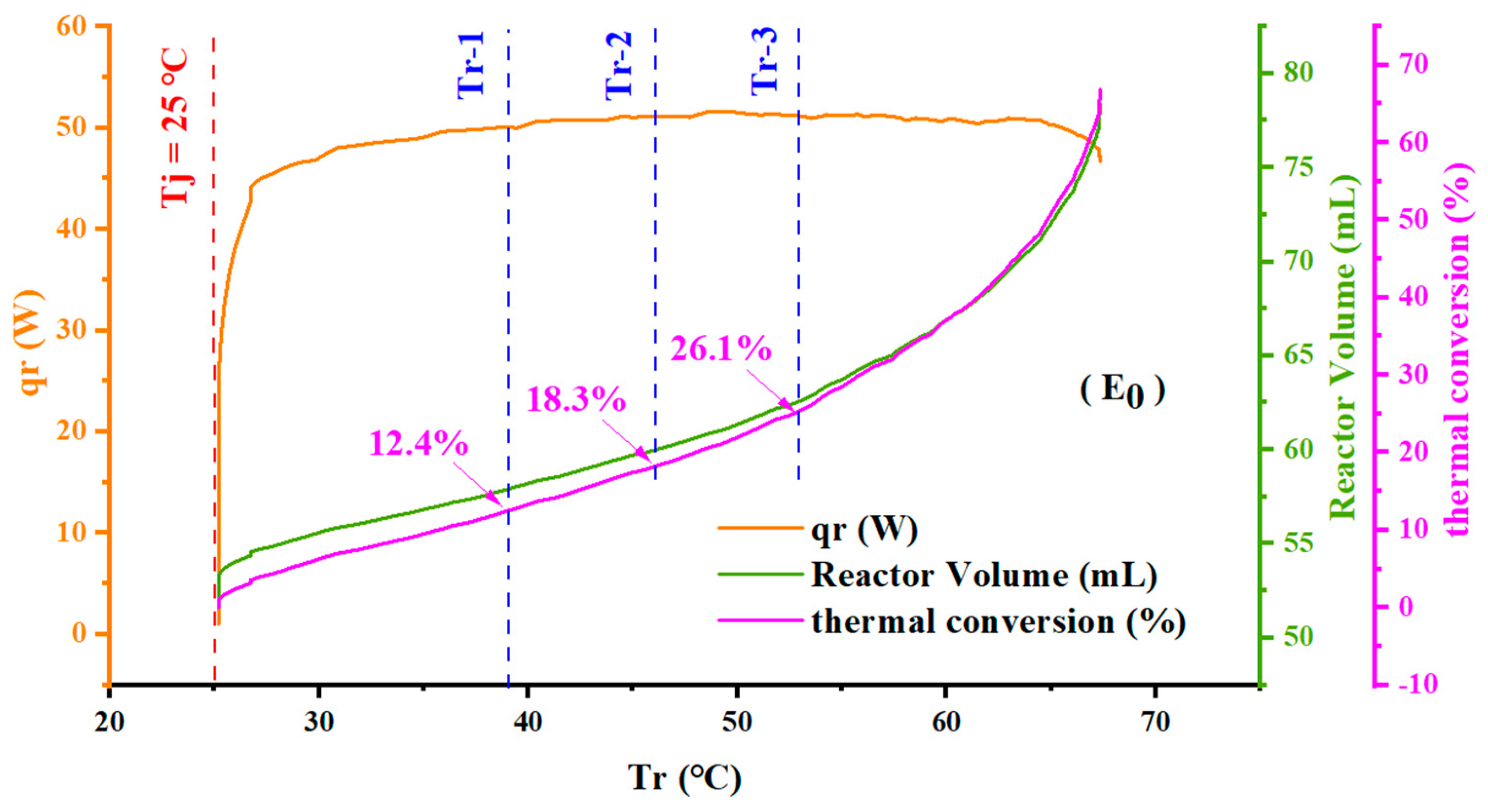
4.1. Experimental Results
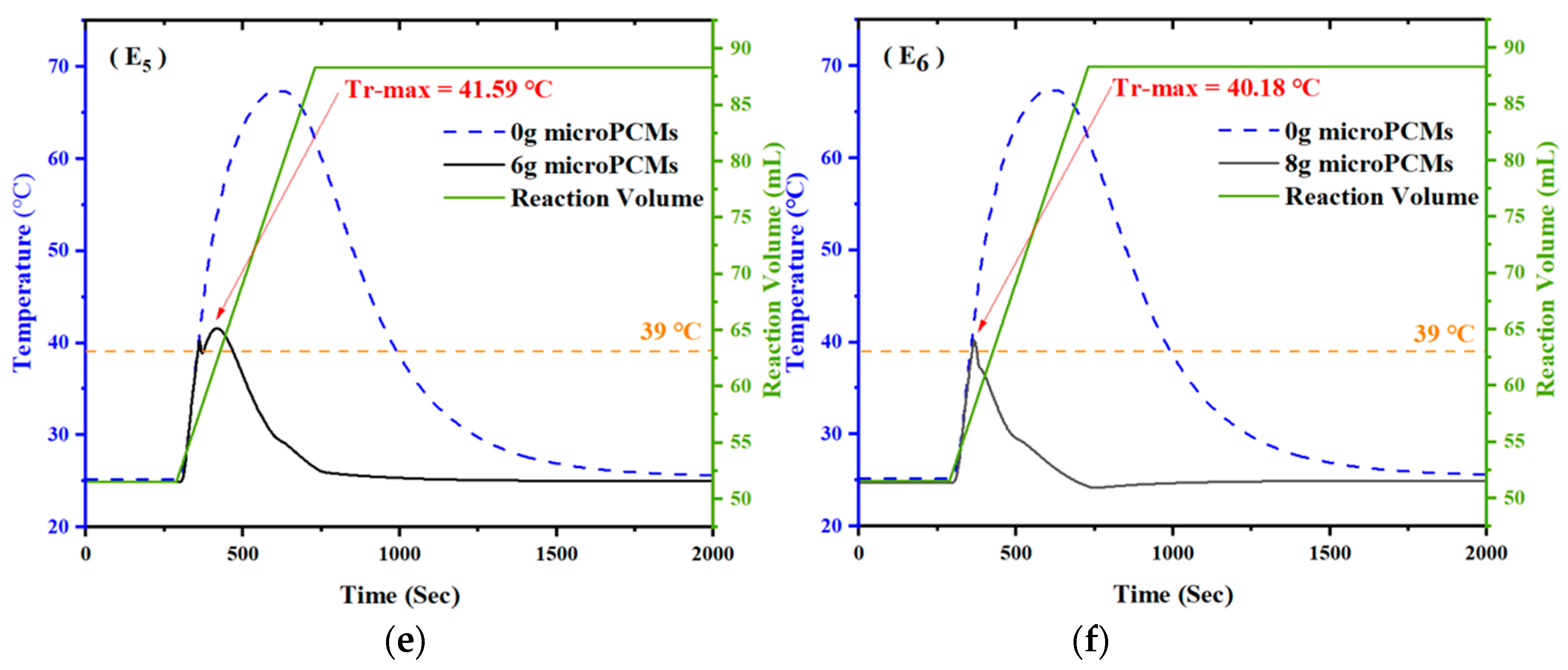
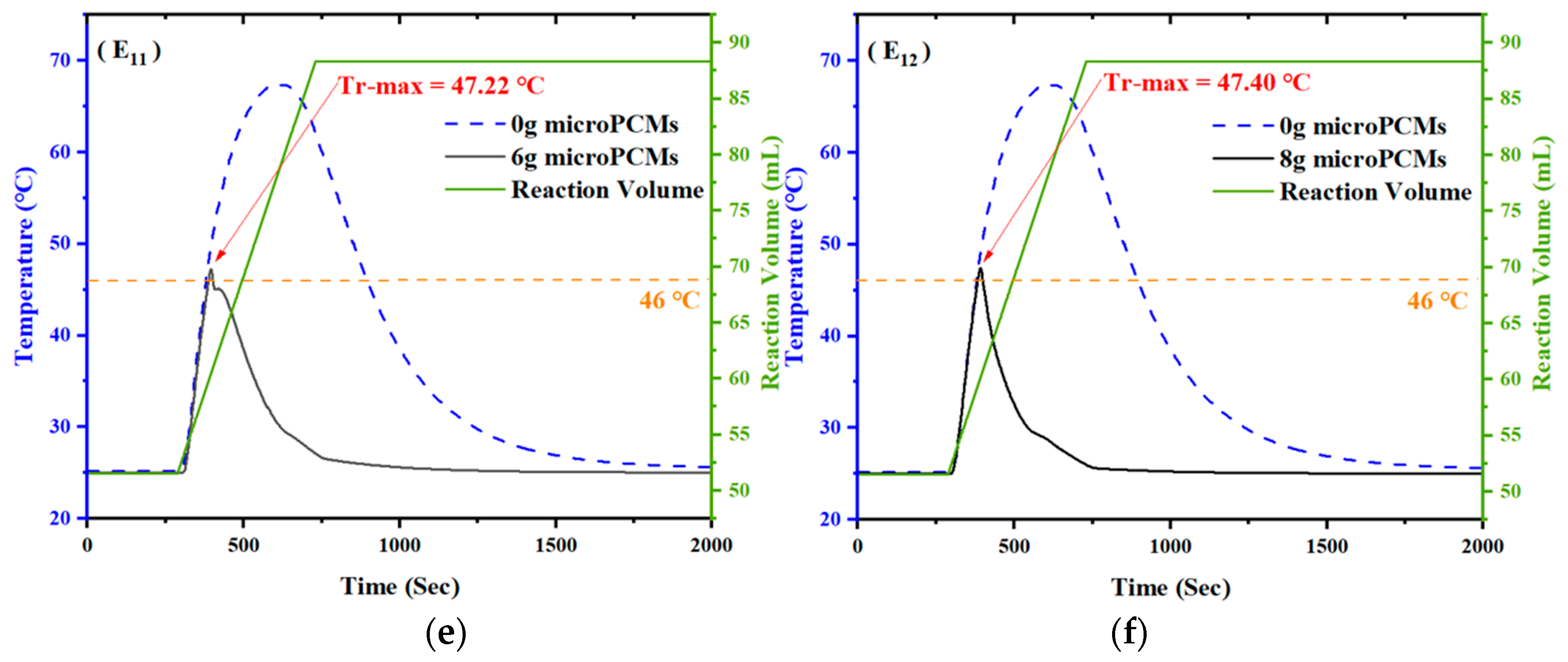
4.2. Effect of the Amount of microPCMs on Temperature Control
4.3. Effect of the Addition Temperature of microPCMs
5. Conclusions
- (1)
- The temperature control effect of microPCMs on exothermic reaction is related to the addition amount. Maximum temperature of the reaction system declined by 5.06 °C, 11.24 °C, 16.00 °C, and 22.01 °C when 2g, 4g, 5g, and 5.5g of microPCMs was added into the reactor at the temperature of 39 °C, respectively. Meanwhile, at the addition temperature of 46 °C, the maximum temperature declined by 6.40 °C, 10.52 °C, 13.70 °C, and 23.43 °C when the same amounts of microPCMs were added.
- (2)
- For an exothermic reaction, the temperature control effect of microPCMs becomes better with the increase of addition temperature. When 2 g, 4 g, 5 g, and 5.5 g of microPCMs was added at the temperature of 39 °C, the temperature of the reaction system continued to rise by 23.30 °C, 17.07 °C, 12.36 °C, and 6.35 °C, respectively, before it began to decline. In comparison, the temperature of the reaction system continued to rise by 14.96 °C, 10.84 °C, 7.66 °C, and 3.33 °C, respectively, while the addition temperature increased to 46 °C. At the temperature of 53 °C, even 2 g of microPCMs could effectively control the reaction temperature.
- (3)
- The effect mechanism of n-octadecane@MF resin microPCMs on the temperature control of a semi-batch esterification reaction system is the combination of both physical and chemical actions. The physical action is mainly reflected in the heat exchange between the microPCMs and the reaction system and the melting endothermic of the core material. The chemical reaction is caused by the anion exchange between the MF resin and anion in the reaction system, which could absorb the catalyst so as to suspend the reaction processes.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, C.C.; Wang, T.C.; Chen, L.Y.; Dai, J.H.; Shu, C.M. Loss prevention in the petrochemical and chemical-process high-tech industries in Taiwan. J. Loss Prev. Process Ind. 2010, 23, 531–538. [Google Scholar] [CrossRef]
- Dakkoune, A.; Hassimi, L.V.; Leveneur, S.; Lefebvre, D.; Estel, L. Risk Anysis of French Chemical Industry. Saf. Sci. 2018, 105, 77–85. [Google Scholar] [CrossRef]
- Jiang, J.C.; Wu, H.; Ni, L.; Zou, M.Y. CFD simulation to study batch reactor thermal runaway behaviour based on esterification reaction. Process Saf. Environ. Protect. 2018, 120, 87–96. [Google Scholar] [CrossRef]
- Strasser, W. CFD study of an evaporiative trickle bed reactor: Mal-distribution and thermal runaway included by feed disturbances. Chem. Eng. J. 2010, 161, 257–268. [Google Scholar] [CrossRef]
- Anastasov, A.I. A study of the influence of the operating parameters on the temperature of the hot spot in a fixed bed reactor. Chem. Eng. J. 2002, 86, 287–297. [Google Scholar] [CrossRef]
- Rudniak, L.; Machniewski, P.M.; Milewaka, A.; Molga, E. CFD modelling of stirred tank chemical reactors: Homogeneous and heterogeneous reaction systems. Chem. Eng. Sci. 2004, 59, 5233–5239. [Google Scholar] [CrossRef]
- Henda, R.; Machac, A.; Nilsson, B. Heat and mass transport of a in a nonlinear fixed-bed catalytic reactor: Hot spots and thermal runaway. Chem. Eng. J. 2008, 143, 195–200. [Google Scholar] [CrossRef]
- Anderson, R.C. Some Problems in Chemical Kinetics and Reactivity. J. Am. Chem. Soc. 1959, 81, 6344. [Google Scholar] [CrossRef]
- Thomas, P.H.; Bowes, P.C. Some aspects of the self-heating and ignition of solid cellulosic materials. Br. J. Appl. Phys. 1961, 12, 222. [Google Scholar] [CrossRef]
- Adler, J.; Enig, J.W. The critical conditions in the thermal explosion theory with reactant. Combust. Flame 1964, 8, 97–103. [Google Scholar] [CrossRef]
- Morbidelli, M.; Varma, A. Parametric sensitivity and runaway in chemical reactors. Sadhana 1987, 10, 133–148. [Google Scholar] [CrossRef]
- Morbidelli, M.; Varma, A. A generalized criterion for parametric sensitivity: Application to thermal explosion theory. Chem. Eng. Sci. 1988, 43, 91–102. [Google Scholar] [CrossRef]
- Vajda, S.; Rabitz, H. Parametric sensitivity and self-similarity in thermal explosion theory. Chem. Eng. Sci. 1992, 47, 1063–1078. [Google Scholar] [CrossRef]
- Strozzi, F.; Zaldivar, J. A general method for assessing the thermal stability of batch chemical reactors by sensitivity calculation based on Lyapunov exponents. Chem. Eng. Sci. 1994, 49, 2681–2688. [Google Scholar] [CrossRef]
- Strozzi, F.; Alo, S.M.A.; Zaldi’Var, J.M. A method for assessing thermal stability of batch reactors by sensitivity calculation based on Lyapunov exponents: Experimental verification. Chem. Eng. Sci. 1994, 49, 5549–5561. [Google Scholar] [CrossRef]
- Steensma, M.; Westerterp, K.R. Thermally safe operation of a cooled semi-batch reactor. Chem. Eng. Sci. 1994, 43, 2125–2132. [Google Scholar] [CrossRef] [Green Version]
- Steensma, M.; Westerterp, K.R. Thermally safe operation of a semibatch reactor for liquid-liquid reactions. Slow reactions. Ind. Eng. Chem. Res. 1990, 29, 1259–1270. [Google Scholar] [CrossRef]
- Steensma, M.; Westerterp, K.R. Thermally safe operation of a semibatch reactor for liquid-liquid reactions-fast reactions. Chem. Eng. Technol. 1991, 14, 367–375. [Google Scholar] [CrossRef] [Green Version]
- Kummer, A.; Varga, T. What do we know about reactor runaway?—A review. Process. Saf. Environ. Prot. 2021, 147, 460–476. [Google Scholar] [CrossRef]
- Liu, X.F.; Meng, T.Y.; Yu, J.L. Based on VSP2, safety reliability analysis and evaluation of thermal runaway reaction relief device were carried out. J. Chem. Eng. Uni. 2010, 24, 1046–1051. [Google Scholar]
- Watanabe, W.S.; Zhang, D.K. The effect of inherent and added inorganic matter on low-temperature oxidation reaction of coal. Fuel Process. Technol. 2001, 74, 145–160. [Google Scholar] [CrossRef]
- Wang, Y.W.; Duh, Y.S.; Chu, C.M. Thermal runaway hazards of tert-butyl hydroperoxide by calorimrtric studies. J. Therm. Anal. Calorim. 2009, 95, 553–557. [Google Scholar] [CrossRef]
- Wang, T.; Wang, S.; Luo, R.; Zhu, C.; Akiyama, T.; Zhang, Z. Microencapsulation of phase change materials with binary cores and calcium carbonate shell for thermal energy storge. Appl. Energy 2016, 171, 113–119. [Google Scholar] [CrossRef]
- Aftab, W.; Huang, X.; Wu, W.; Liang, Z.; Mahmood, A.; Zou, R. Nanoconfined phase change materials for thermal energy applications. Energy Environ. Sci. 2018, 11, 1392–1424. [Google Scholar] [CrossRef]
- Liu, C.Z.; Rao, Z.H.; Zhao, J.T.; Huo, Y.T.; Li, Y.M. Review on nanoencapsulated phase change materials: Preparation, characterization and heat transfer enhancement. Nano Energy 2015, 13, 814–826. [Google Scholar] [CrossRef]
- Luo, R.; Wang, S.; Wang, T.; Zhu, C.; Nomura, T.; Akiyama, T. Fabrication of paraffin@SiO2 shape-stabilized composite change material via chemical precipication method for building energy conservation. Energy Build. 2015, 108, 373–380. [Google Scholar] [CrossRef]
- Yuan, Y.C.; Rong, M.Z.; Zhang, M.Q. Preparation and characterization of microencapsulated polythiol. Polymer 2008, 49, 2531–2541. [Google Scholar] [CrossRef]
- Wei, K.; Wang, Y.; Ma, B. Effects of microencapsulated phase change materials on the performance of asphalt binders. Renew. Energy 2019, 132, 931–940. [Google Scholar] [CrossRef]
- Babapoor, A.; Azizi, M.; Karimi, G. Thermal management of a Li-ion battery using carbon fiber-PCM Composties. Appl. Therm. Eng. 2015, 82, 281–290. [Google Scholar] [CrossRef]
- Jamekhorshid, A.; Sadrameli, S.M.; Farid, M. A review of microencapsulation methods of phase change (PCMs) as a thermal energy storage (TES) medium. Renew. Sustain. Energy Rev. 2014, 31, 531–542. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Zhang, G.H. Review on microencapsulated phase change materials (MEPCMs): Fabrication, characterization and applications. Renew. Sust. Energy Rev. 2011, 15, 3813–3832. [Google Scholar] [CrossRef]
- Cheng, F.; Wei, Y.; Zhang, Y.; Wang, F.; Shen, T.; Zong, C. Preparation and characterization of phase-change material nanocapsules with amphiphilic polyurethane synthesized by 3-allyloxy-1, 2-propanediol. J. Appl. Polym. Sci. 2013, 130, 1879–1889. [Google Scholar] [CrossRef]
- Wang, L.Y.; Tsai, P.S.; Yang, Y.M. Preparation of silica microspheres encapsulateing phase-change material by sol-gel method in O/W emulsion. J. Microencapsule 2006, 23, 3–14. [Google Scholar] [CrossRef]
- Cao, F.; Yang, B. Supercooling suppression of microencapsulated phase change materials by optimizing shell composition and structure. Appl. Energy 2014, 113, 1512–1518. [Google Scholar] [CrossRef]
- Sharama, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Saeid, G.M.; Ali, M.; Omid, M.; Behshad, S.M.; Hassan, J.M.; Mohammad, Z.; Hossein, A.M. A review on the applicatioins of micro-/nano-encapsulated phase change material slurry in heat transfer and thermal storage systems. J. Therm. Anal. Calorim. 2021, 145, 245–268. [Google Scholar]
- Zhang, M.H.; Hong, Y.; Ding, S.J.; Hu, J.J.; Fan, Y.X.; Voevodin, A.A.; Su, M. Encapsulated Nano-Heat-Sinks for Thermal Management of Heterogeneous Chemical Reactions. Nanoscale 2010, 2, 2790. [Google Scholar] [CrossRef]
- Odunsi, A.O.; O’donovan, T.S.; Reay, D.A. Temperature Stabilisation in Fischer-Tropsch Reactors Using Phase Change Material (PCM). Appl. Therm. Eng. 2016, 93, 1377–1393. [Google Scholar] [CrossRef] [Green Version]
- Odunsi, A.O.; O’donovan, T.S.; Reay, D.A. Dynamic Modeling of Fixed-Bed Fischer-Tropsch Reactors with Phase Change Material Diluents. Chem. Eng. Technol. 2016, 39, 2066–2076. [Google Scholar] [CrossRef]
- Li, K.Z.; Cheng, X.M.; Li, N.N.; Zhu, X.; Wei, Y.G.; Zhai, K.; Wang, H. A Yolk/Shell Strategy for Designing Hybrid Phase Change Materials for Heat Management in Catalytic Reactions. J. Chem. Mater. Chem. A 2017, 5, 24232–24246. [Google Scholar] [CrossRef]
- Chen, Q.; Ni, L.; Jiang, J.C.; Parker, T.; Chen, Z.Q.; Cheng, Z.; Jiang, W.; Wang, Q.S. Inhibition of exothermic runaway of batch reactors for the homogeneous esterification using nano-encapsulated phase change materials. Appl. Therm. Eng. 2020, 178, 115531. [Google Scholar] [CrossRef]
- Moreno, P.; Miro, L.; Sole, A.; Barreneche, C.; Sole, C.; Martorell, L.; Cabeza, L.F. Corrosion of metal and metal alloy containers in contact with phase change materials (PCM) for potential heating and cooling applications. Appl. Energy 2014, 125, 238–245. [Google Scholar] [CrossRef]
- Salunkhe, P.B.; Shembekar, P.S. A review on effect of phase change material encapsulation on the thermal performance of a system. Renew. Sust. Energ. Rev. 2012, 16, 5603–5616. [Google Scholar] [CrossRef]
- Liang, S.; Li, Q.; Zhu, Y.; Chen, K.; Tian, C.; Wang, J.; Bai, R. Nanoencapsulation of n-octadecane phase change material with silica shell through interfacial hydrolysis and polycondensation in miniemulsion. Energy 2015, 93, 1684–1692. [Google Scholar] [CrossRef]
- Su, W.; Darkwa, J.; Kokogiannakis, G. Review of solid-liquid phase change materials and their encapsulation technologies. Renew. Sustain. Energy Rev. 2015, 48, 373–391. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Wu, D. Silica encapsulation of n-octadecane via sol–gel process: A novel microencapsulated phase-change material with enhanced thermal conductivity and performance. J. Colloid Interface Sci. 2010, 343, 246–255. [Google Scholar] [CrossRef]
- Fan, Z.G.; Han, P.J.; Zhang, Z.G.; Hu, Z.J. Optimized emulsifier combination based microencapsulated phase change materials (MicroPCMs): Preparation, characterization, and applications. Renew. Sustain. Energy Rev. 2020, 12, 014102.1–014102.7. [Google Scholar] [CrossRef]
- Cheng, Z.; Ni, L.; Wang, J.J.; Jiang, J.C.; Yao, H.; Chen, Q.; Cui, F.S.; Jiang, W.; Ye, S.L. Process hazard evaluation and exothermic mechanism for the synthesis of n-butylmagnesium bromide Grinard reagent in different solvents. Process Saf. Environ. Prot. 2021, 147, 654–673. [Google Scholar] [CrossRef]
- Dhanuka, V.R.; Malshe, V.C.; Chandalia, S.B. Kinetics of the liquid phase esterification of carb-Xylic acids with alcohols in the presence of acid catalysts: Re-interpretation of published data. Chem. Eng. Sci. 1977, 32, 551–556. [Google Scholar] [CrossRef]










| Experimental Materials | CAS No. | Molecular Formula | Weight (wt.%) | Source |
|---|---|---|---|---|
| n-octadecane | 593-45-3 | C18H38 | ≥99.0 wt.% | Aladdin Reagent (Shanghai, China) |
| Melamine | 1058-78-1 | C3H6N6 | ≥99.0 wt.% | Aladdin Reagent (Shanghai, China) |
| Ethanol | 64-47-5 | C2H6O | ≥99.7 wt.% | Ya Sheng Chemical (Wuxi, China) |
| Formaldehyde solution | 50-00-0 | CH2O | 36 wt.%~38wt.% | Xi Long Scientific (Shantou, China) |
| TA | --- | --- | ≈19 wt.% | Rui Gong Chemical Reagent (Shanghai, China) |
| Triton X-100 | 9002-93-1 | C34H62O11 | ≥95.0 wt.% | Sinopharm Chemical Reagent (Shanghai, China) |
| Sodium hydroxide | 1310-73-2 | NaOH | ≥99.0 wt.% | Sinopharm Chemical Reagent (Shanghai, China) |
| Anhydrous Citric acid | 77-92-9 | C6H8O7 | ≥99.5 wt.% | Sinopharm Chemical Reagent (Shanghai, China) |
| Distilled water | 7732-18-5 | H2O | --- | Wan Qing Glass & Instrument (Nanjing, China) |
| Sequence Number | ① | ② | ③ | ④ | ⑤ | ⑥ | ⑦ | ⑧ | ⑨ | ⑩ | ⑪ |
| Peak position (cm−1) | 717 | 1470 | 2849 | 2915 | 2960 | 810 | 1010 | 1160 | 1347 | 1568 | 3400 |
| Sample | Tm (°C) | ΔHm (J/g) | Encapsulation Ratio (%) | Char Yield at 800 °C |
|---|---|---|---|---|
| n-octadecane | 27.28 | 230.2 | --- | 0.19% |
| microPCMs | 27.47 | 159.3 | 69.2 | 4.88% |
| Experimental Materials | CAS No. | Molecular Formula | Weight (wt.%) | Source |
|---|---|---|---|---|
| Propionic anhydride | 123-62-6 | C6H10O3 | ≥97.0 wt.% | Aladdin Reagent (Shanghai, China) |
| 2-Butanol | 78-92-2 | C4H10O | ≥95.0 wt.% | Sinopharm Chemical Reagent (Shanghai, China) |
| Sulfuric acid | 7664-93-9 | H2SO4 | ≥98.0 wt.% | Ling Feng Chemical Reagent (Shanghai, China) |
| microPCMs | --- | --- | --- | Laboratory made |
| Test | Addition Temperature (°C) | Addition Amounts (g) | Maximum Temperature of Reactor (°C) |
|---|---|---|---|
| E0 | --- | 0 | 67.36 |
| E1 | 39 | 2 | 62.30 |
| E2 | 39 | 4 | 56.07 |
| E3 | 39 | 5 | 51.36 |
| E4 | 39 | 5.5 | 45.35 |
| E5 | 39 | 6 | 41.59 |
| E6 | 39 | 8 | 40.18 |
| E7 | 46 | 2 | 60.96 |
| E8 | 46 | 4 | 56.84 |
| E9 | 46 | 5 | 53.66 |
| E10 | 46 | 5.5 | 49.33 |
| E11 | 46 | 6 | 47.22 |
| E12 | 46 | 8 | 47.40 |
| E13 | 53 | 2 | 53.51 |
| E14 | 53 | 4 | 53.95 |
| E15 | 53 | 6 | 54.03 |
| E16 | 53 | 8 | 54.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Ni, L.; Chen, Q.; Jiang, J.; Zhou, K. Temperature Control of Exothermic Reactions Using n-Octadecane@MF Resin microPCMs Based on Esterification Reactions. Processes 2022, 10, 239. https://doi.org/10.3390/pr10020239
Li C, Ni L, Chen Q, Jiang J, Zhou K. Temperature Control of Exothermic Reactions Using n-Octadecane@MF Resin microPCMs Based on Esterification Reactions. Processes. 2022; 10(2):239. https://doi.org/10.3390/pr10020239
Chicago/Turabian StyleLi, Chenghao, Lei Ni, Qiang Chen, Juncheng Jiang, and Kuibin Zhou. 2022. "Temperature Control of Exothermic Reactions Using n-Octadecane@MF Resin microPCMs Based on Esterification Reactions" Processes 10, no. 2: 239. https://doi.org/10.3390/pr10020239
APA StyleLi, C., Ni, L., Chen, Q., Jiang, J., & Zhou, K. (2022). Temperature Control of Exothermic Reactions Using n-Octadecane@MF Resin microPCMs Based on Esterification Reactions. Processes, 10(2), 239. https://doi.org/10.3390/pr10020239








