Supercritical Technology-Based Date Sugar Powder Production: Process Modeling and Simulation
Abstract
:1. Introduction
2. Materials and Methods
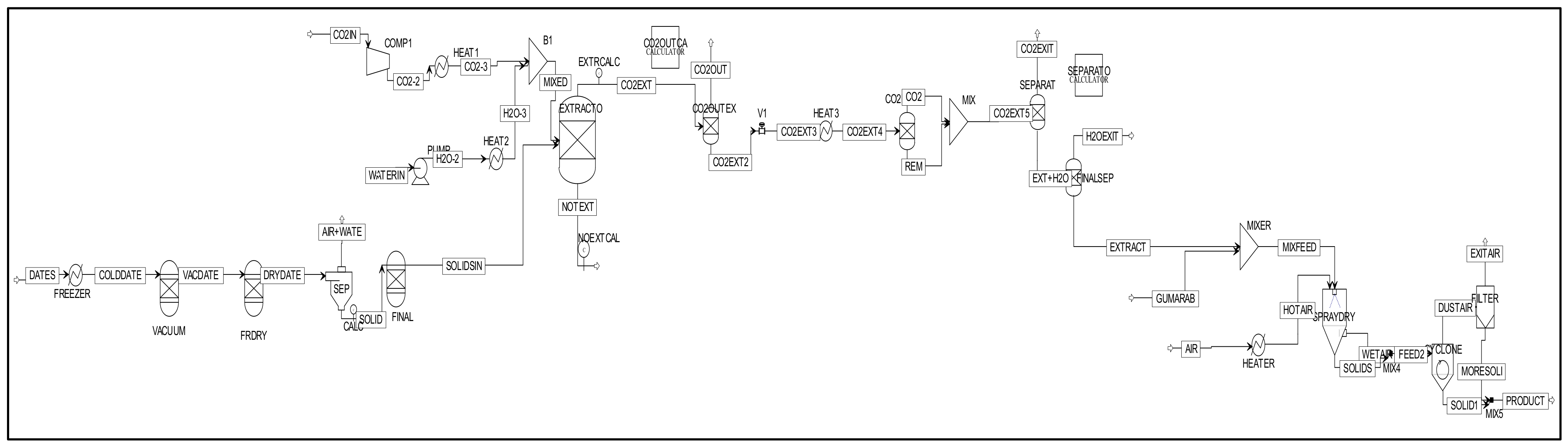
2.1. IDSPP Flowsheet
2.2. Materials
2.3. Data
2.4. Method
3. Results and Discussions
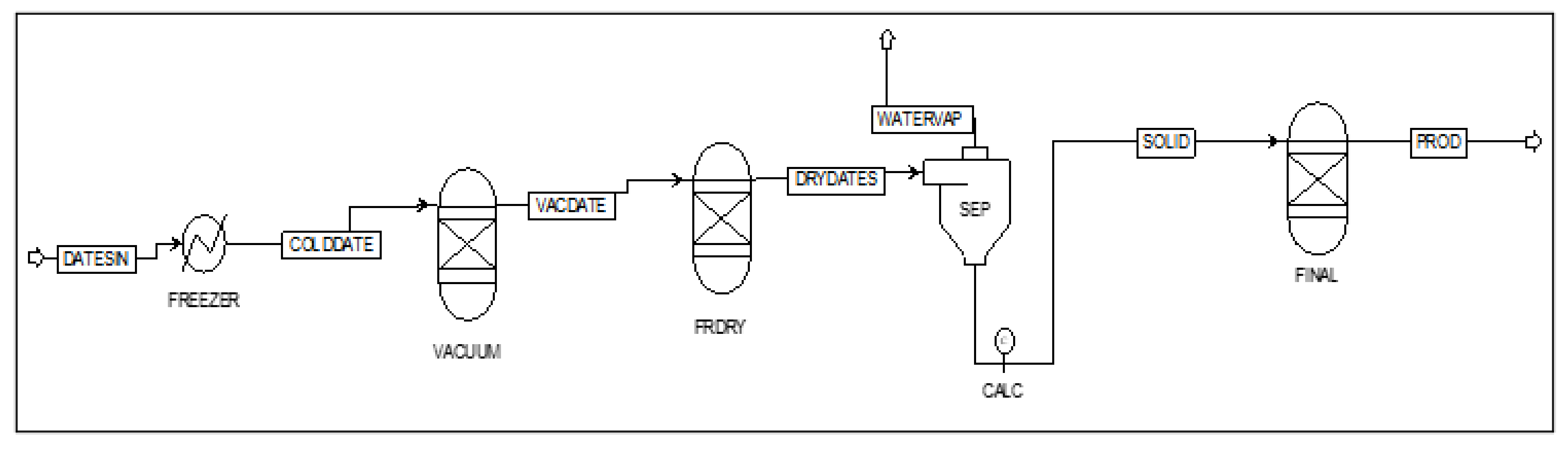
3.1. Freeze-Drying
3.2. Supercritical Extraction
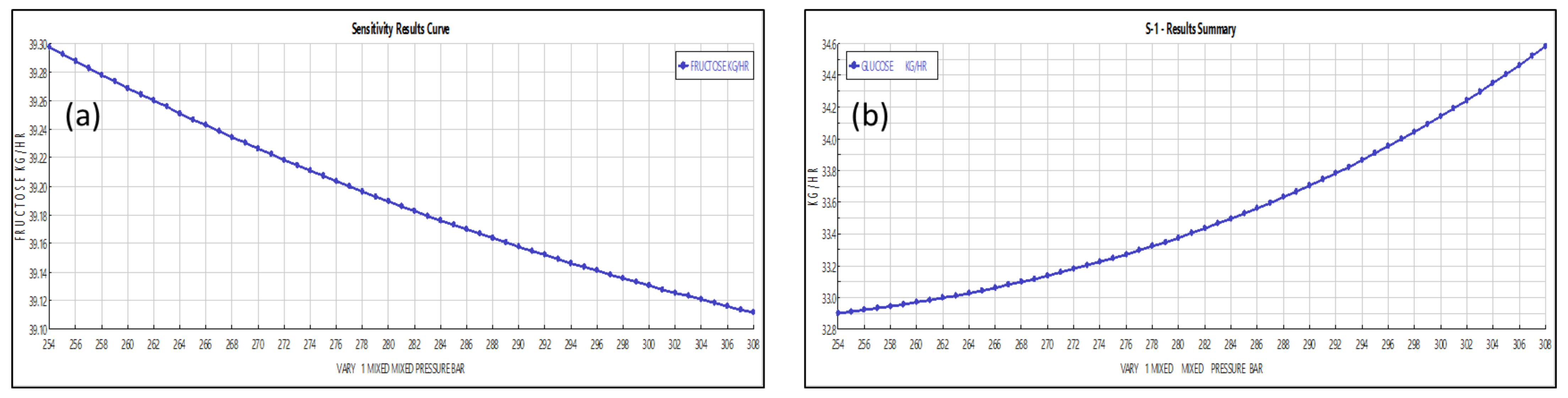
3.2.1. Operating Pressure
3.2.2. Extraction Temperature
3.2.3. Solvent Flow Rate and Composition
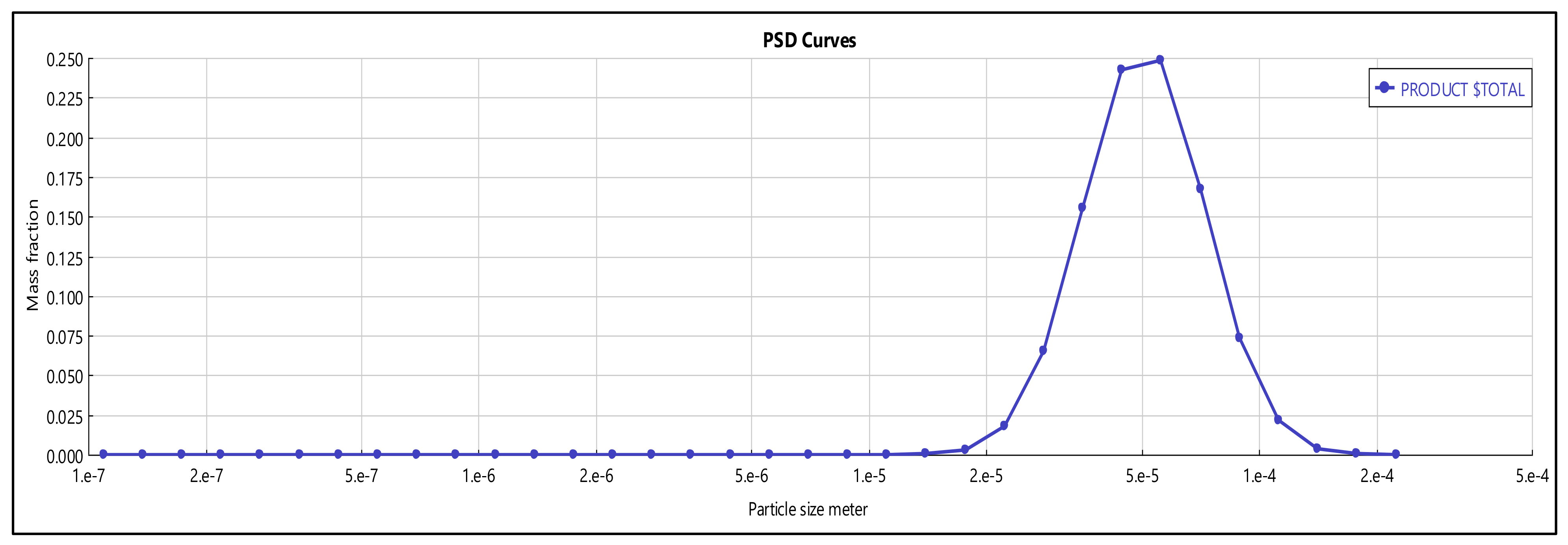
3.3. Spray-Drying
3.4. Product Solubility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| a | Empirical constant |
| A | Empirical constant |
| aFD | Freeze Drying experimental coefficient |
| B | Empirical constant |
| b | Empirical constant |
| bFD | Freeze Drying experimental coefficient |
| C | Empirical constant |
| D5 | Mass Median Diameter, mm |
| d50 | Median droplet size (μm) |
| DR | Diameter of the atomizer (m) |
| FD | Freeze Drying |
| GA | Gum Arabic |
| h | Height of the vanes (m) |
| IDSPP | Integrated Date Sugar Production Process |
| k | Empirical constant; k0–Drying constant (min−1); |
| k0 | Drying constant (min−1) |
| k1 | Drying constant (min−1) |
| M0 | Initial moisture content (g water/g dry solid) |
| mliq | Mass flow rate of the feed (kg/h) |
| MR | Moisture ratio |
| Ms | Mass flow ratio/vane (kg/h) |
| Mt | Moisture at a specific time (g water/g dry solid) |
| n | Number of blades |
| N | Rotational speed (rpm) |
| P | Total pressure of the system (bar) |
| p | Vane liquid loading correlation |
| PSD | Particle Size Distribution |
| q | Vane liquid loading correlation |
| r | Vane liquid loading correlation |
| S | Solubility of the solute in the supercritical solvent (g/L) |
| s | Vane liquid loading correlation |
| Sc | CO2–Supercritical Carbon dioxide |
| SCE | Supercritical Extraction |
| SD | Spray Drying |
| SRK | Soave-Redlich-Kwong |
| Sw | Solubility of the water in CO2 (kg H2O/kg CO2) |
| T | Temperature (°C) |
| U | Speed of the circumference of the disc (m/s) |
| UDF | User Defined Function |
| ygf | Solubility of the glucose/fructose in the supercritical carbon dioxide (mol solute/mol CO2) |
| ρs | Density of the pure solvent (g/L) |
References
- Voora, V.; Bermúdez, S.; Larrea, C. Global Market Report: Sugar; International Institute for Sustainable Development: Geneva, Switzerland, 2020. [Google Scholar]
- Rambabu, K.; Bharath, G.; Hai, A.; Banat, F.; Hasan, S.W.; Taher, H.; Zaid, H.F.M. Nutritional Quality and Physico-Chemical Characteristics of Selected Date Fruit Varieties of the United Arab Emirates. Processes 2020, 8, 256. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, I.A.; Ahmed, A.W.K.; Robinson, R.K. Chemical composition of date varieties as influenced by the stage of ripening. Food Chem. 1995, 54, 305–309. [Google Scholar] [CrossRef]
- Alharbi, K.L.; Raman, J.; Shin, H.-J. Date Fruit and Seed in Nutricosmetics. Cosmetics 2021, 8, 59. [Google Scholar] [CrossRef]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rambabu, K.; Edathil, A.A.; Nirmala, G.S.; Hasan, S.W.; Yousef, A.F.; Show, P.L.; Banat, F. Date-fruit syrup waste extract as a natural additive for soap production with enhanced antioxidant and antibacterial activity. Environ. Technol. Innov. 2020, 20, 101153. [Google Scholar] [CrossRef]
- Vallejo-Domínguez, D.; Rubio-Rosas, E.; Aguila-Almanza, E.; Hernández-Cocoletzi, H.; Ramos-Cassellis, M.E.; Luna-Guevara, M.L.; Rambabu, K.; Manickam, S.; Munawaroh, H.S.H.; Show, P.L. Ultrasound in the deproteinization process for chitin and chitosan production. Ultrason. Sonochem. 2021, 72, 105417. [Google Scholar] [CrossRef] [PubMed]
- Arumugham, T.; Rambabu, K.; Hasan, S.W.; Show, P.L.; Rinklebe, J.; Banat, F. Supercritical carbon dioxide extraction of plant phytochemicals for biological and environmental applications—A review. Chemosphere 2021, 271, 129525. [Google Scholar] [CrossRef] [PubMed]
- Al-Suod, H.; Ratiu, I.A.; Krakowska-Sieprawska, A.; Lahuta, L.; Górecki, R.; Buszewski, B. Supercritical fluid extraction in isolation of cyclitols and sugars from chamomile flowers. J. Sep. Sci. 2019, 42, 3243–3252. [Google Scholar] [CrossRef] [PubMed]
- Ratiu, I.-A.; Al-Suod, H.; Ligor, M.; Ligor, T.; Railean-Plugaru, V.; Buszewski, B. Complex investigation of extraction techniques applied for cyclitols and sugars isolation from different species of Solidago genus. Electrophoresis 2018, 39, 1966–1974. [Google Scholar] [CrossRef]
- Cavalcanti, R.N.; Albuquerque, C.L.C.; Meireles, M.A.A. Supercritical CO2 extraction of cupuassu butter from defatted seed residue: Experimental data, mathematical modeling and cost of manufacturing. Food Bioprod. Process. 2016, 97, 48–62. [Google Scholar] [CrossRef]
- Mirofci, S. A supercritical fluid extraction process to obtain valuable compounds from Eruca sativa leaves. Masters Thesis, University of Padova, Padua, Italy, 2014. [Google Scholar]
- Dohrn, R.; Buenz, A.P. Solubility enhancement of carbohydrates in carbon dioxide (Part 1 and 2). Int. Z. Lebensm. 1995, 46, 30–31. [Google Scholar]
- İZLİ, G. Total phenolics, antioxidant capacity, colour and drying characteristics of date fruit dried with different methods. Food Sci. Technol. 2016, 37, 139–147. [Google Scholar] [CrossRef] [Green Version]
- Seerangurayar, T.; Manickavasagan, A.; Al-Ismaili, A.M.; Al-Mulla, Y.A. Effect of carrier agents on flowability and microstructural properties of foam-mat freeze dried date powder. J. Food Eng. 2017, 215, 33–43. [Google Scholar] [CrossRef]
- Franceschinis, L.; Salvatori, D.M.; Sosa, N.; Schebor, C. Physical and functional properties of blackberry freeze-and spray-dried powders. Dry. Technol. 2014, 32, 197–207. [Google Scholar] [CrossRef]
- Shishehgarha, F.; Makhlouf, J.; Ratti, C. Freeze-drying characteristics of strawberries. Dry. Technol. 2002, 20, 131–145. [Google Scholar] [CrossRef]
- Manickavasagan, A.; Thangavel, K.; Dev, S.R.S.; Delfiya, D.S.A.; Nambi, E.; Orsat, V.; Raghavan, G.S.V. Physicochemical characteristics of date powder produced in a pilot-scale spray dryer. Dry. Technol. 2015, 33, 1114–1123. [Google Scholar] [CrossRef]
- Minh, N.P.; Vo, T.T.; Tu, T.T.C.; Vi, M.N.T.; Diem, N.T.N.; Thanh, H.T.T. Spray Drying Parameters Affecting to Dried Powder from Palmyra Palm (Borassus flabellifer) Juice. J. Pharm. Sci. Res. 2019, 11, 1382–1387. [Google Scholar]
- Anderson, D.M.W. Evidence for the safety of gum arabic (Acacia senegal (L.) Willd.) as a food additive—A brief review. Food Addit. Contam. 1986, 3, 225–230. [Google Scholar] [CrossRef]
- Georgetti, S.R.; Casagrande, R.; Souza, C.R.F.; Oliveira, W.P.; Fonseca, M.J.V. Spray drying of the soybean extract: Effects on chemical properties and antioxidant activity. LWT-Food Sci. Technol. 2008, 41, 1521–1527. [Google Scholar] [CrossRef]
- Glicksman, M. Gum arabic (Gum acacia). In Food Hydrocolloids; CRC Press: Boca Raton, FL, USA, 2019; pp. 7–29. [Google Scholar]
- Rockstraw, D.A. ASPEN Plus in the Chemical Engineering Curriculum: Suitable Course Content and Teaching Methodology. Chem. Eng. Educ. 2005, 39, 68–75. [Google Scholar]
- Rostamian, H.; Lotfollahi, M.N. New functionality for energy parameter of Redlich-Kwong equation of state for density calculation of pure carbon dioxide and ethane in liquid, vapor and supercritical phases. Period. Polytech. Chem. Eng. 2016, 60, 93–97. [Google Scholar] [CrossRef] [Green Version]
- Stahl, E.; Schilz, W.; Schütz, E.; Willing, E. A quick method for the microanalytical evaluation of the dissolving power of supercritical gases. Angew. Chem. Int. Ed. Engl. 1978, 17, 731–738. [Google Scholar] [CrossRef]
- Saldaña, M.D.A.; Alvarez, V.H.; Haldar, A. Solubility and physical properties of sugars in pressurized water. J. Chem. Thermodyn. 2012, 55, 115–123. [Google Scholar] [CrossRef]
- Chrastil, J. Solubility of solids and liquids in supercritical gases. J. Phys. Chem. 1982, 86, 3016–3021. [Google Scholar] [CrossRef]
- King, A.D., Jr.; Coan, C.R. Solubility of water in compressed carbon dioxide, nitrous oxide, and ethane. Evidence for hydration of carbon dioxide and nitrous oxide in the gas phase. J. Am. Chem. Soc. 1971, 93, 1857–1862. [Google Scholar] [CrossRef]
- Petersen, L.N.; Poulsen, N.K.; Niemann, H.H.; Utzen, C.; Jørgensen, J.B. An experimentally validated simulation model for a four-stage spray dryer. J. Process Control 2017, 57, 50–65. [Google Scholar] [CrossRef] [Green Version]
- Masters, K. Spray Drying Handbook; Halstead Press: New York, NY, USA, 1985. [Google Scholar]
- Nakagawa, K.; Ochiai, T. A mathematical model of multi-dimensional freeze-drying for food products. J. Food Eng. 2015, 161, 55–67. [Google Scholar] [CrossRef]
- Malekjani, N.; Jafari, S.M. Simulation of food drying processes by Computational Fluid Dynamics (CFD); recent advances and approaches. Trends Food Sci. Technol. 2018, 78, 206–223. [Google Scholar] [CrossRef]
- Li, S.; Stawczyk, J.; Zbicinski, I. CFD model of apple atmospheric freeze drying at low temperature. Dry. Technol. 2007, 25, 1331–1339. [Google Scholar] [CrossRef]
- Malaman, F.S.; Moraes, L.A.B.; West, C.; Ferreira, N.J.; Oliveira, A.L. Supercritical fluid extracts from the Brazilian cherry (Eugenia uniflora L.): Relationship between the extracted compounds and the characteristic flavour intensity of the fruit. Food Chem. 2011, 124, 85–92. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, M.; Xu, Q.; Ren, H.; Yin, J. Enhanced enzymatic hydrolysis of sorghum stalk by supercritical carbon dioxide and ultrasonic pretreatment. Appl. Biochem. Biotechnol. 2019, 188, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Oladipupo Kareem, M.; Edathil, A.A.; Rambabu, K.; Bharath, G.; Banat, F.; Nirmala, G.S.S.; Sathiyanarayanan, K. Extraction, characterization and optimization of high quality bio-oil derived from waste date seeds. Chem. Eng. Commun. 2021, 208, 801–811. [Google Scholar] [CrossRef]
- Islam, M.N.; Jo, Y.-T.; Jung, S.-K.; Park, J.-H. Thermodynamic and kinetic study for subcritical water extraction of PAHs. J. Ind. Eng. Chem. 2013, 19, 129–136. [Google Scholar] [CrossRef]
- Khajenoori, M.; Asl, A.H.; Hormozi, F. Proposed models for subcritical water extraction of essential oils. Chin. J. Chem. Eng. 2009, 17, 359–365. [Google Scholar] [CrossRef]
- Fazaeli, M.; Emam-Djomeh, Z.; Ashtari, A.K.; Omid, M. Effect of spray drying conditions and feed composition on the physical properties of black mulberry juice powder. Food Bioprod. Process. 2012, 90, 667–675. [Google Scholar] [CrossRef]
- Rambabu, K.; Muruganandam, L.; Velu, S. CFD simulation for separation of carbon dioxide-methane mixture by pressure swing adsorption. Int. J. Chem. Eng. 2014, 2014, 402756. [Google Scholar] [CrossRef]
- Cortés-Rojas, D.F.; Souza, C.R.F.; Oliveira, W.P. Optimization of spray drying conditions for production of Bidens pilosa L. dried extract. Chem. Eng. Res. Des. 2015, 93, 366–376. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Microencapsulation of pineapple peel extract by spray drying using maltodextrin, inulin, and arabic gum as wall matrices. Foods 2020, 9, 718. [Google Scholar] [CrossRef]
- Zhao, H.; Li, J.; Wang, L.; Li, C.; Li, P. Thermodynamic investigation of 1,3,5-trioxane, methyl acrylate, methyl acetate, and water mixtures, in terms of NRTL and UNIQUAC models. Ind. Eng. Chem. Res. 2019, 58, 18378–18386. [Google Scholar] [CrossRef]
- Ramu, A.G.; Telmenbayar, L.; Theerthagiri, J.; Yang, D.; Song, M.; Choi, D. Synthesis of a hierarchically structured Fe3O4—PEI nanocomposite for the highly sensitive electrochemical determination of bisphenol A in real samples. New J. Chem. 2020, 44, 18633–18645. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Lee, S.J.; Murthy, A.P.; Madhavan, J.; Choi, M.Y. Fundamental aspects and recent advances in transition metal nitrides as electrocatalysts for hydrogen evolution reaction: A review. Curr. Opin. Solid State Mater. Sci. 2020, 24, 100805. [Google Scholar] [CrossRef]
- Madhavan, J.; Theerthagiri, J.; Balaji, D.; Sunitha, S.; Choi, M.Y.; Ashokkumar, M. Hybrid advanced oxidation processes involving ultrasound: An overview. Molecules 2019, 24, 3341. [Google Scholar] [CrossRef] [Green Version]
- Senthil, R.A.; Priya, A.; Theerthagiri, J.; Selvi, A.; Nithyadharseni, P.; Madhavan, J. Facile synthesis of α-Fe2O3/WO3 composite with an enhanced photocatalytic and photo-electrochemical performance. Ionics 2018, 24, 3673–3684. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Senthil, R.A.; Priya, A.; Madhavan, J.; Michael, R.J.V.; Ashokkumar, M. Photocatalytic and photoelectrochemical studies of visible-light active α-Fe2O3—gC3N4 nanocomposites. Rsc Adv. 2014, 4, 38222–38229. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Senthil, R.A.; Senthilkumar, B.; Polu, A.R.; Madhavan, J.; Ashokkumar, M. Recent advances in MoS2 nanostructured materials for energy and environmental applications—A review. J. Solid State Chem. 2017, 252, 43–71. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Salla, S.; Senthil, R.A.; Nithyadharseni, P.; Madankumar, A.; Arunachalam, P.; Maiyalagan, T.; Kim, H.-S. A review on ZnO nanostructured materials: Energy, environmental and biological applications. Nanotechnology 2019, 30, 392001. [Google Scholar] [CrossRef]





| Unit | Temperature (°C) | Pressure (bar) |
|---|---|---|
| FREEZER | −42.0 | 1.01325 |
| VACUUM | −42.0 | 0.00048 |
| FRDRY | 20.0 | 0.0001 |
| SEP | 20.0 | 0.0001 |
| FINAL | 20.0 | 1.01325 |
| COMP1 | 783.9 | 308 |
| HEAT1 | 90.0 | 308 |
| PUMP | 40.5 | 308 |
| HEAT2 | 82.0 | 308 |
| MIX1 | 65.0 | 308 |
| EXTRACTO | 65.0 | 308 |
| CO2OUTEX | 65.0 | 308 |
| V1 VALVE | 31.3 | 72 |
| HEAT3 | 32.0 | 1 |
| CO2DENS | 32.0 | 1 |
| MIX2 | 18.2 | 1 |
| SEPARAT | 18.2 | 1 |
| FINALSEP | 18.2 | 1 |
| MIX | 20.0 | 1 |
| AIRHEAT | 150.0 | 1 |
| DRYER | 150.0 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bushnaq, H.; Krishnamoorthy, R.; Abu-Zahra, M.; Hasan, S.W.; Taher, H.; Alomar, S.Y.; Ahmad, N.; Banat, F. Supercritical Technology-Based Date Sugar Powder Production: Process Modeling and Simulation. Processes 2022, 10, 257. https://doi.org/10.3390/pr10020257
Bushnaq H, Krishnamoorthy R, Abu-Zahra M, Hasan SW, Taher H, Alomar SY, Ahmad N, Banat F. Supercritical Technology-Based Date Sugar Powder Production: Process Modeling and Simulation. Processes. 2022; 10(2):257. https://doi.org/10.3390/pr10020257
Chicago/Turabian StyleBushnaq, Hooralain, Rambabu Krishnamoorthy, Mohammad Abu-Zahra, Shadi W. Hasan, Hanifa Taher, Suliman Yousef Alomar, Naushad Ahmad, and Fawzi Banat. 2022. "Supercritical Technology-Based Date Sugar Powder Production: Process Modeling and Simulation" Processes 10, no. 2: 257. https://doi.org/10.3390/pr10020257
APA StyleBushnaq, H., Krishnamoorthy, R., Abu-Zahra, M., Hasan, S. W., Taher, H., Alomar, S. Y., Ahmad, N., & Banat, F. (2022). Supercritical Technology-Based Date Sugar Powder Production: Process Modeling and Simulation. Processes, 10(2), 257. https://doi.org/10.3390/pr10020257










