Experimental and Numerical Investigation of the Dehydration of Ca(OH)2 at Low Steam Pressures
Abstract
:1. Introduction
2. Materials and Methods
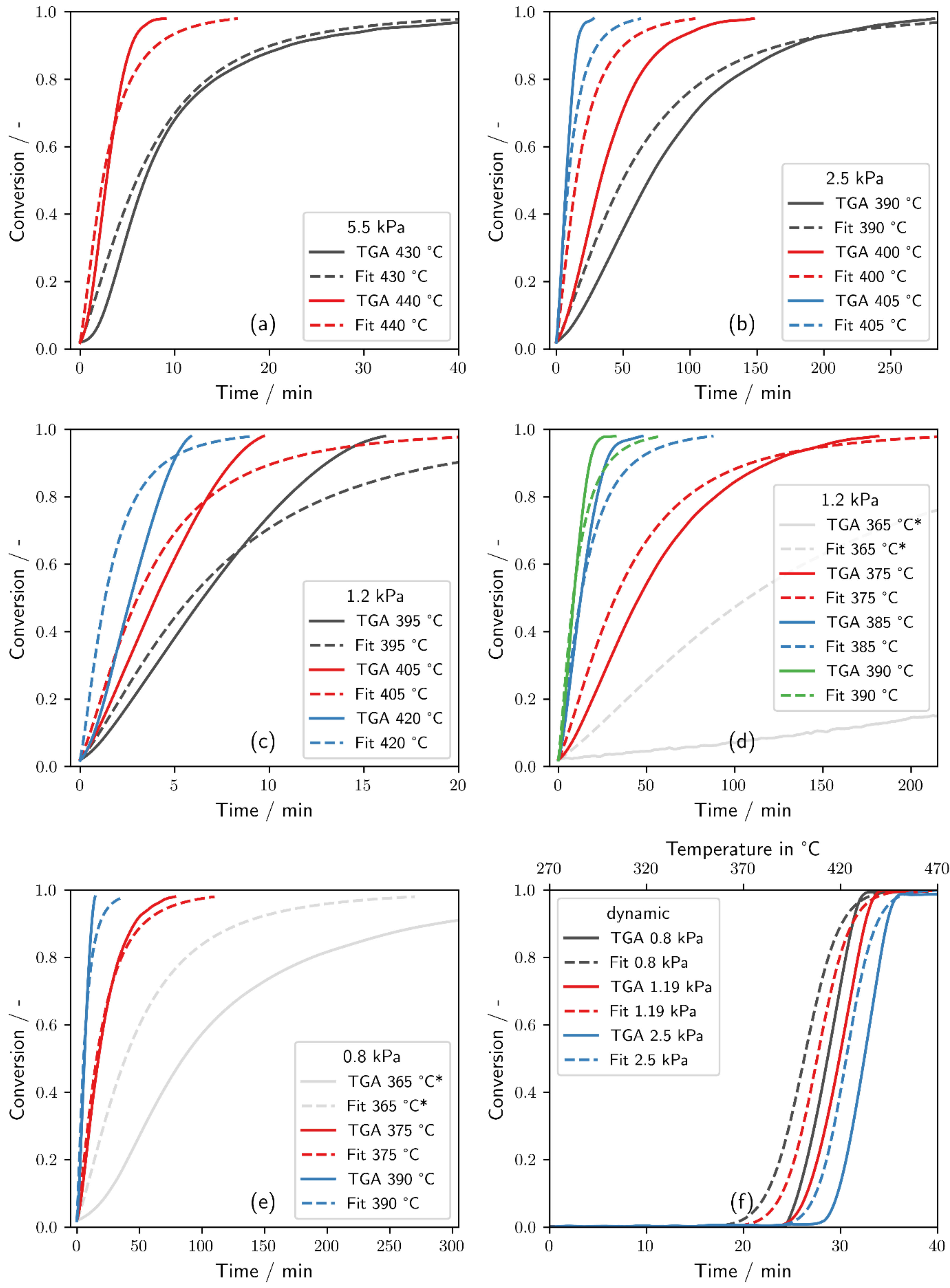
2.1. Thermogravimetric Dehydration Measurements and Fit of the Effective Reaction Rate
2.2. Lab-Scale Experiments
2.3. Simulation Model
2.3.1. Assumptions and Governing Equations
- No strong three-dimensional effects occur, and a two-dimensional rotation symmetric model is sufficient (based on [28]).
- The CaO/Ca(OH)2 bulk acts as a continuum with an equally distributed density over the whole filling level (i.e., no structural changes of the bulk such as agglomerates occur).
- The bulk outside the simulated area (Figure 1) has a negligible impact (since there are no further temperature measurements in this area, the temperature field can hardly be validated there).
- Local thermal equilibrium is assumed.
- Radiative heat transfer is negligible (based on [26]).
- The reaction rate is independent from the particle diameter.
2.3.2. Initial and Boundary Conditions
3. Results and Discussion
3.1. Thermogravimetric Dehydration Measurements and Fit of the Effective Reaction Rate
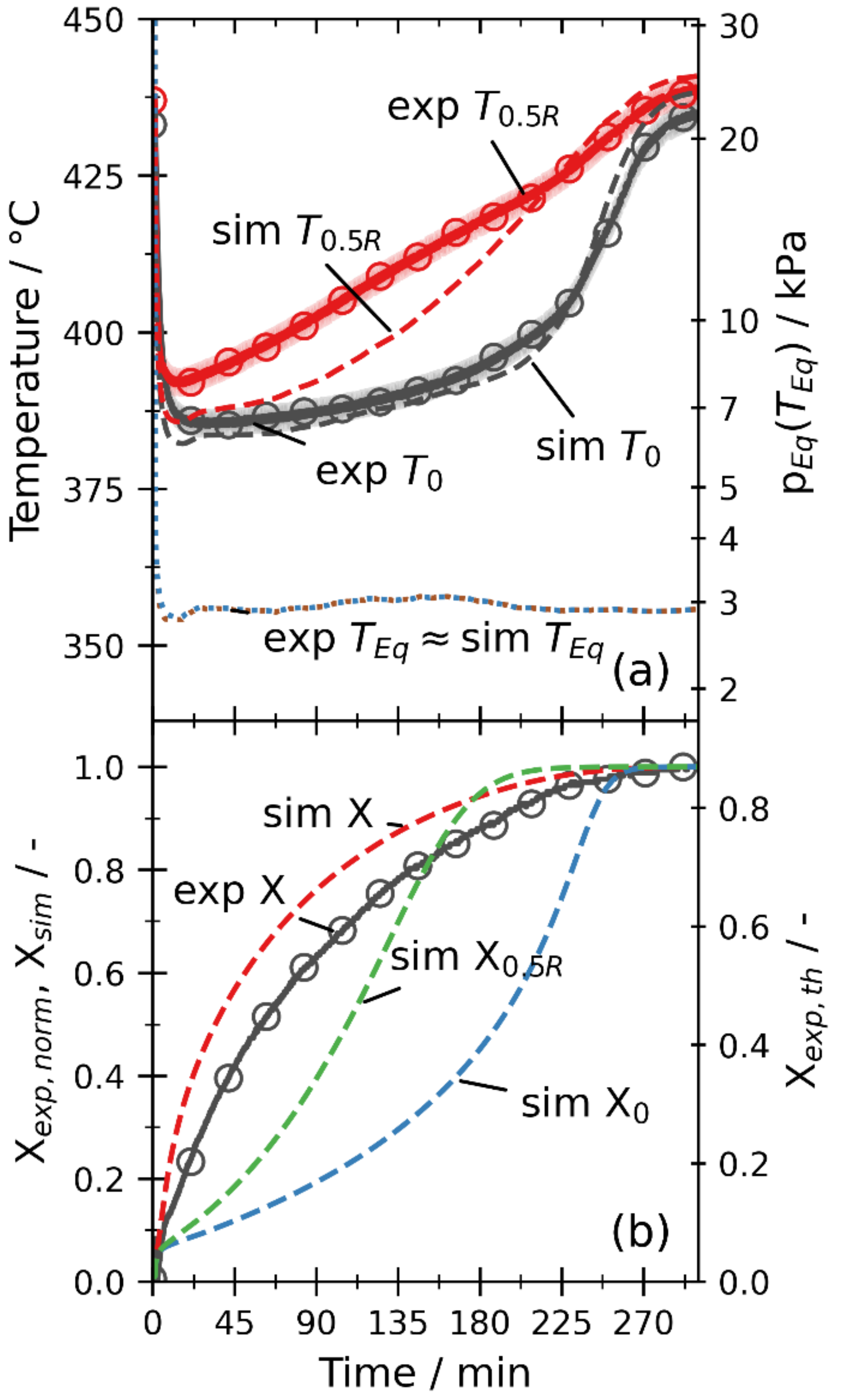
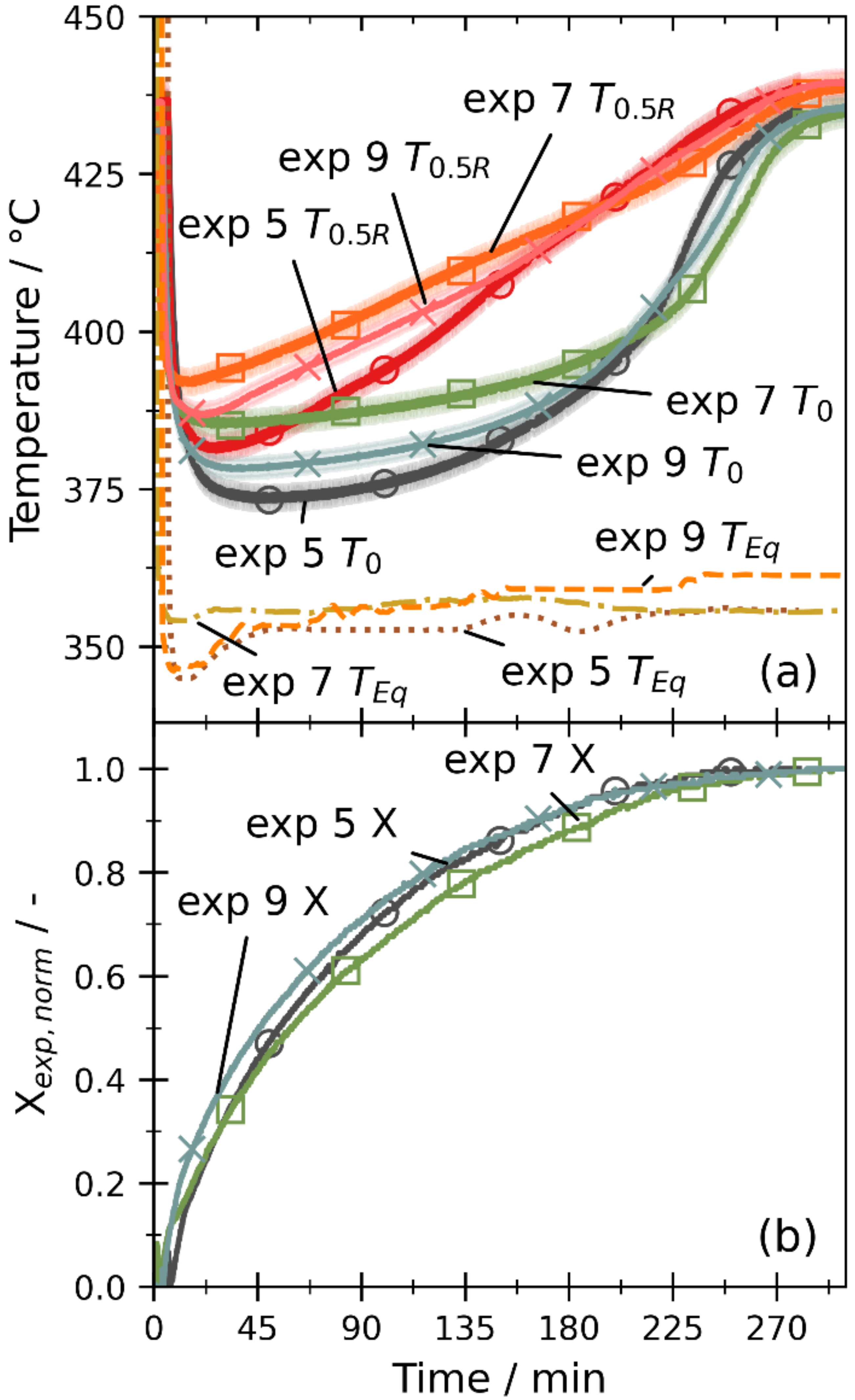
3.2. Comparison of Experiments and Simulations
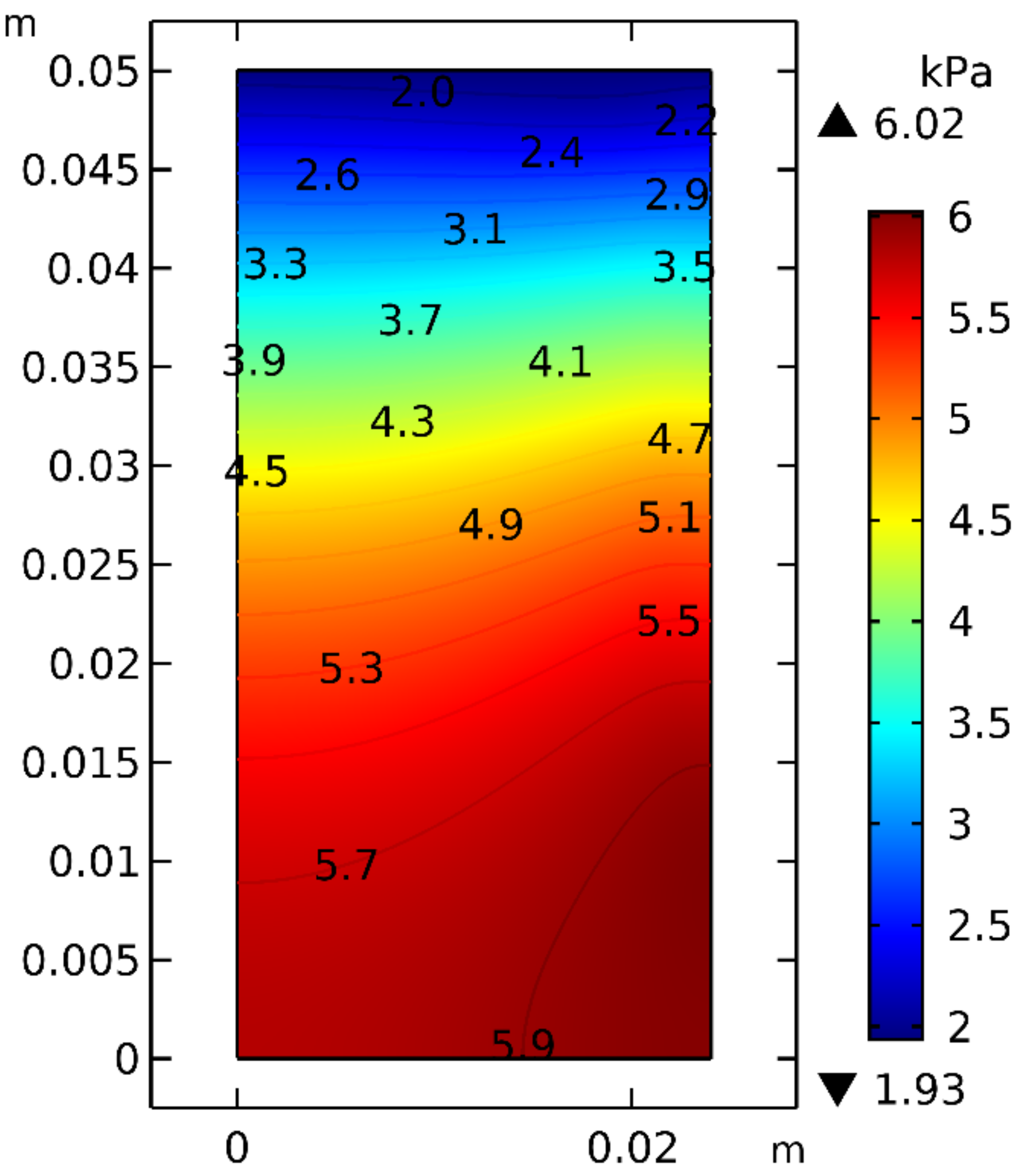
3.2.1. Thermal Conductivity of the Bulk
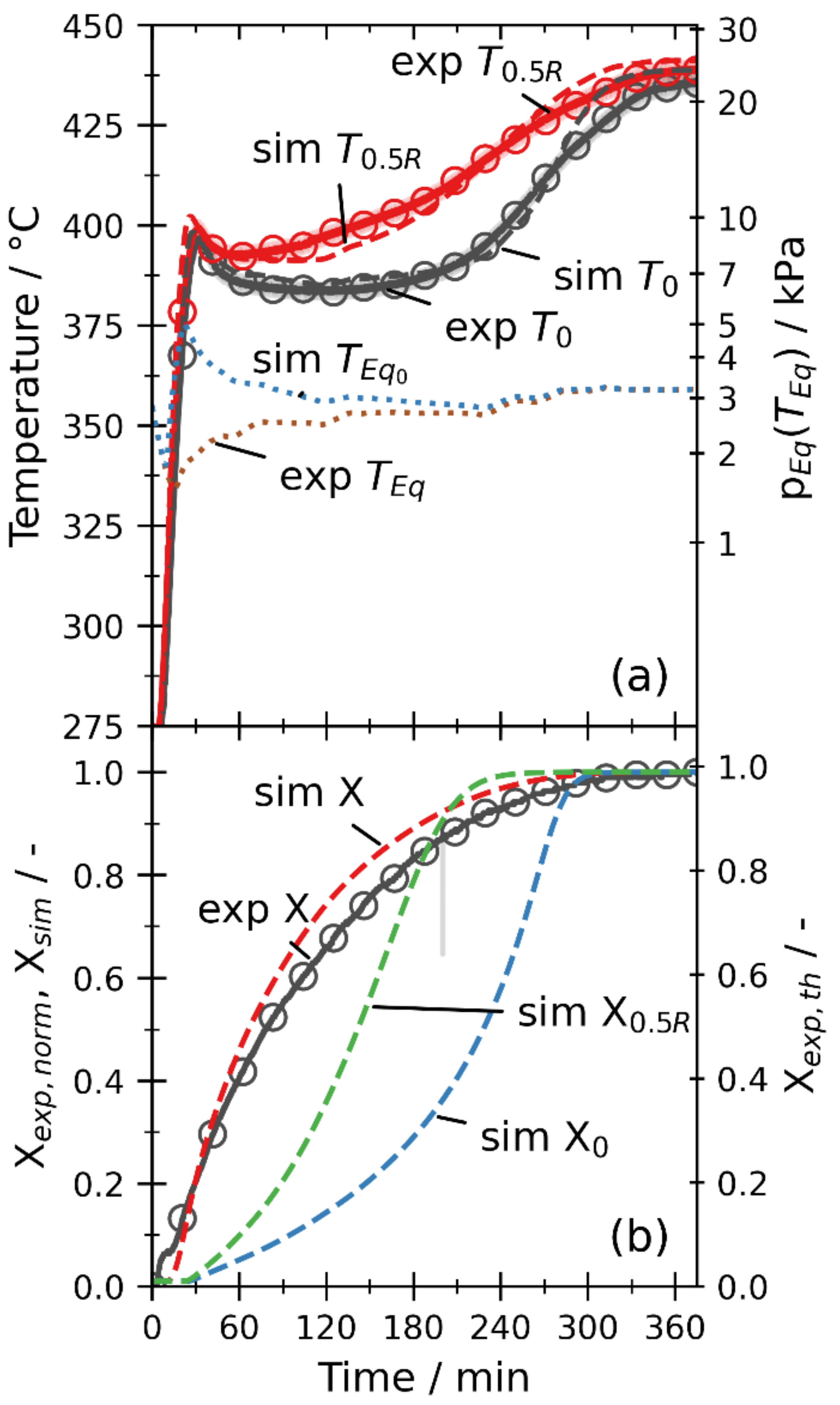
3.2.2. Dehydration by Temperature Increase
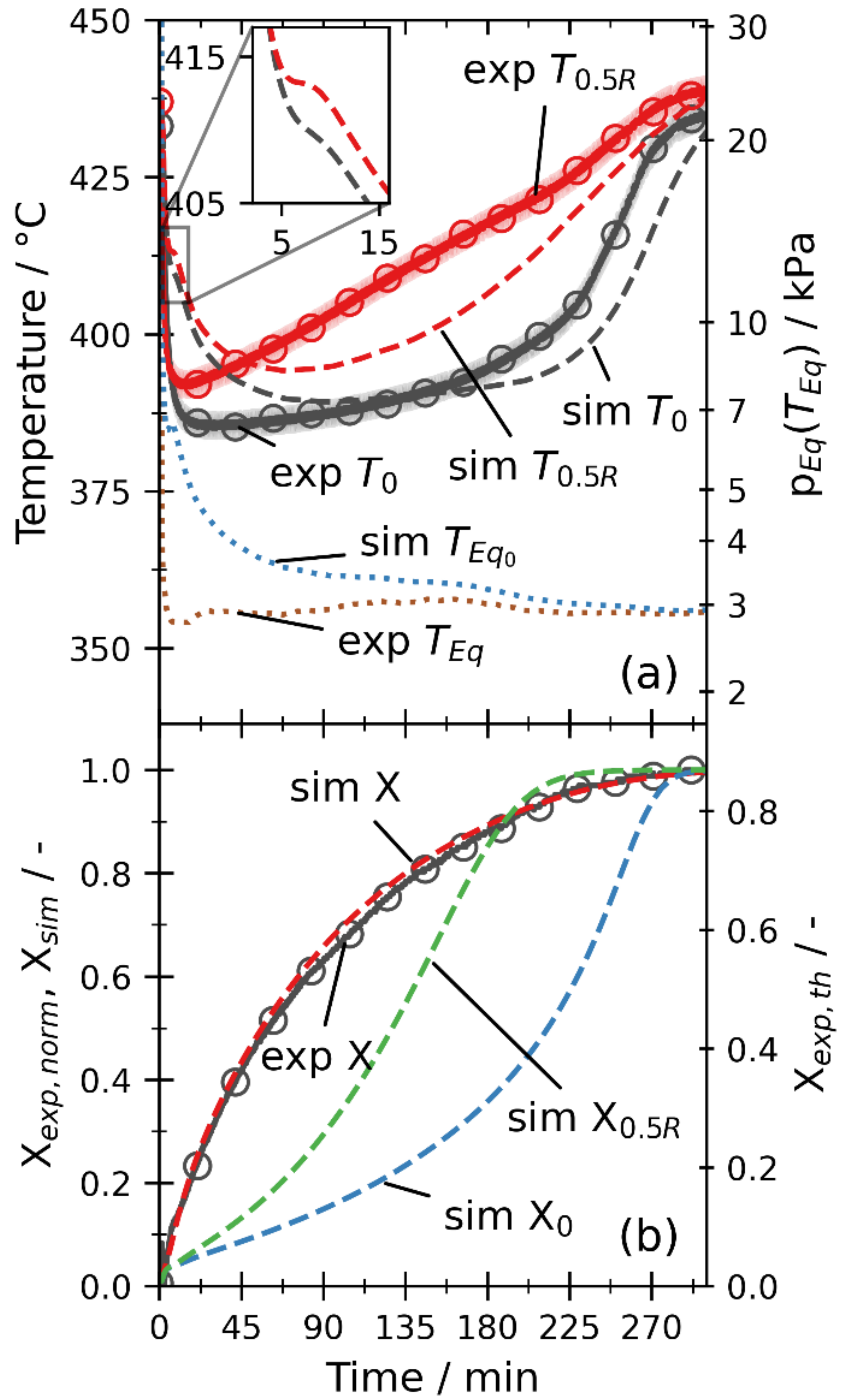
3.2.3. Dehydration by Pressure Reduction
3.2.4. Variation of Thermal Input
3.2.5. Overall Discussion
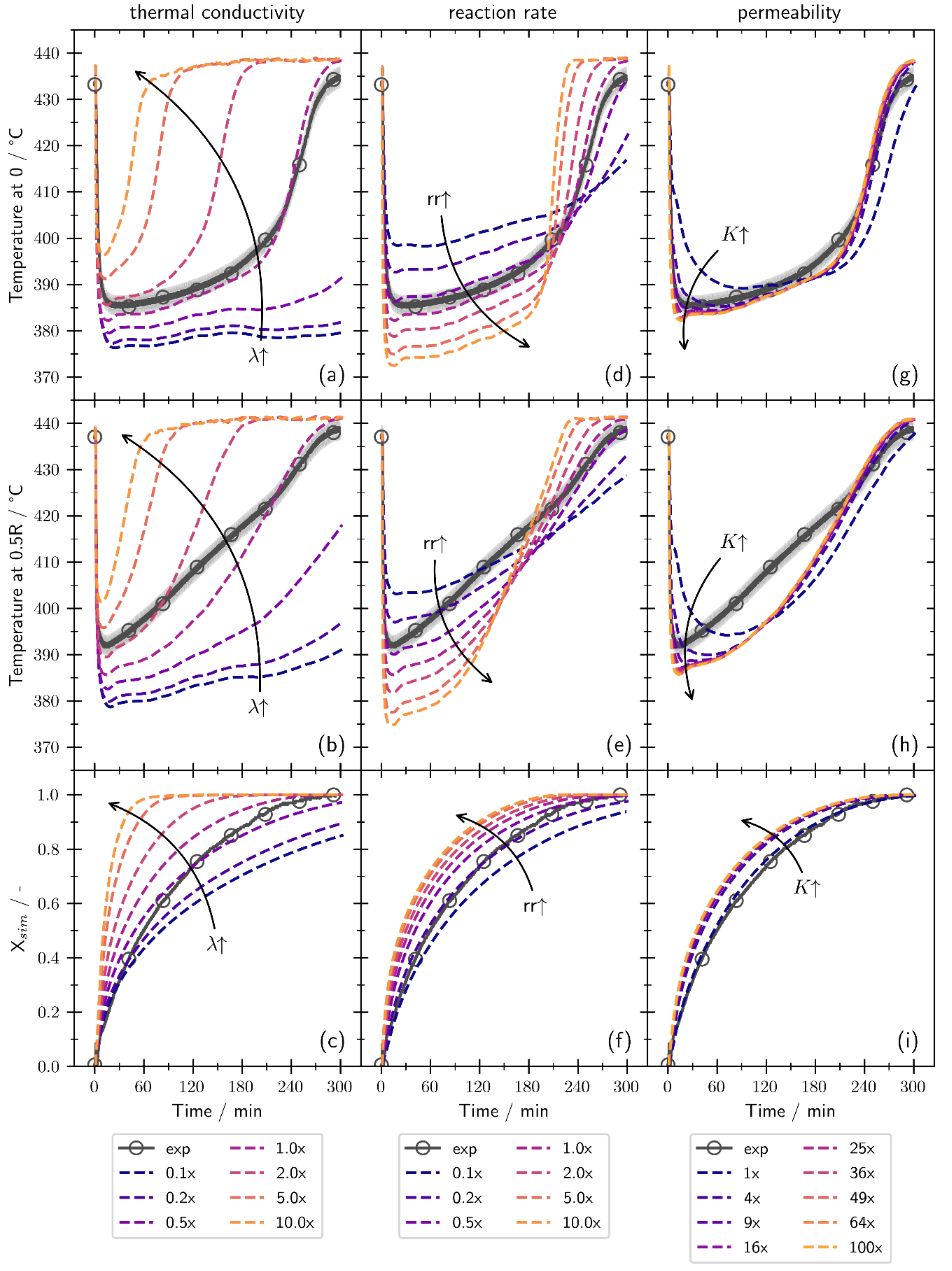
3.3. Sensitivity Study
4. Conclusions
- The reaction kinetics becomes comparatively slow (i.e., 200 min or longer until full conversion is reached) in the analyzed pressure range for temperatures approximately closer than 40–50 K to the equilibrium temperature by Samms and Evans.
- Assuming a one-step reaction, common models for the reaction kinetics cannot adequately describe the slow kinetics “close” to the equilibrium and fast kinetics further away from the equilibrium in one equation.
- A new equation for the reaction rate has been derived for a pressure and temperature range between 0.8 and 5.5 kPa and 375 and 440 °C.
- The gas transport is adequately described by the Carman–Kozeny equation for dehydrations initiated by a temperature increase. However, when the dehydration is triggered by a pressure reduction, structural changes of the bulk occur (e.g., channeling), and the permeability is enhanced. These structural changes have been incorporated by increasing the permeability by a factor of 100.
- When the channeling effects are considered by the increased permeability, the simulation yields mean absolute errors below 10 K for the temperatures and below 0.08 for the global conversion. Thus, the simulation model is validated within the measurement uncertainties of the setup for different operation modes, temperatures, and pressures.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Symbol | Description | Unit |
| Reaction enthalpy | J/mol | |
| Density | kg/m3 | |
| Thermal conductivity | W/m/K | |
| Dynamic viscosity | Pa s | |
| a | Fitting coefficient | - |
| b | Fitting coefficient | - |
| Isobaric heat capacity | J/kg/K | |
| Particle size | m | |
| K | Permeability | m² |
| m | Fitting coefficient | - |
| Initial molar mass of Ca(OH)2 | mol | |
| p | Pressure | Pa |
| p’ | Fitting coefficient | - |
| T | Temperature | K |
| t | Time | s |
| u | Velocity | m/s |
| X | Conversion | - |
| Indices: | ||
| Eq | Equilibrium | |
| exp | Experiment | |
| i | Measurement number | |
| j | Point in time |
References
- Fujimoto, S.; Bilgen, E.; Ogura, H. Dynamic simulation of CaO/Ca(OH)2 chemical heat pump systems. Exergy Int. J. 2002, 2, 6–14. [Google Scholar] [CrossRef]
- Stengler, J.; Linder, M. Thermal energy storage combined with a temperature boost: An underestimated feature of thermochemical systems. Appl. Energy 2020, 262, 114530. [Google Scholar] [CrossRef]
- Rosemary, J.K.; Bauerle, G.L.; Springer, T.H. Solar Energy Storage Using Reversible Hydration-Dehydration of CaO-Ca(OH)2. J. Energy 1979, 3, 321–322. [Google Scholar] [CrossRef]
- Schaube, F.; Koch, L.; Wörner, A.; Müller-Steinhagen, H. A thermodynamic and kinetic study of the de- and rehydration of Ca(OH)2 at high H2O partial pressures for thermo-chemical heat storage. Thermochim. Acta 2012, 538, 9–20. [Google Scholar] [CrossRef]
- Halstead, P.E.; Moore, A.E. The thermal dissociation of calcium hydroxide. J. Chem. Soc. 1957, 0, 3873–3875. [Google Scholar] [CrossRef]
- Samms, J.A.C.; Evans, B.E. Thermal dissociation of Ca(OH)2 at elevated pressures. J. Appl. Chem. 1968, 18, 5–8. [Google Scholar] [CrossRef]
- Schaube, F.; Antje, W.Ă.; Tamme, R. High Temperature Thermochemical Heat Storage for Concentrated Solar Power Using Gas-Solid Reactions. J. Sol. Energy Eng. 2011, 133, 31006. [Google Scholar] [CrossRef]
- Papapetrou, M.; Kosmadakis, G.; Cipollina, A.; Commare, U.L.; Micale, G. Industrial waste heat: Estimation of the technically available resource in the EU per industrial sector, temperature level and country. Appl. Therm. Eng. 2018, 138, 207–216. [Google Scholar] [CrossRef]
- Yan, J.; Zhao, C.Y. Thermodynamic and kinetic study of the dehydration process of CaO/Ca(OH)2 thermochemical heat storage system with Li doping. Chem. Eng. Sci. 2015, 138, 86–92. [Google Scholar] [CrossRef]
- Gupta, A.; Armatis, P.D.; Sabharwall, P.; Fronk, B.M.; Utgikar, V. Kinetics of Ca(OH)2 decomposition in pure Ca(OH)2 and Ca(OH)2-CaTiO3 composite pellets for application in thermochemical energy storage system. Chem. Eng. Sci. 2021, 246, 116986. [Google Scholar] [CrossRef]
- Khachani, M.; el Hamidi, A.; Halim, M.; Arsalane, S. Non-isothermal kinetic and thermodynamic studies of the dehydroxylation process of synthetic calcium hydroxide Ca(OH)2. J. Mater. Environ. Sci. 2014, 5, 615–624. [Google Scholar]
- Koga, N.; Favergeon, L.; Kodani, S. Impact of atmospheric water vapor on the thermal decomposition of calcium hydroxide: A universal kinetic approach to a physico-geometrical consecutive reaction in solid–gas systems under different partial pressures of product gas. Phys. Chem. Chem. Phys. 2019, 21, 11615–11632. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H. Kinetic Study of Ca(OH)2/CaO Reversible Thermochemical Reaction for Thermal Energy Storage by Means of Chemical Reaction. Kagaku Kogaku Ronbunshu 1985, 11, 542–548. [Google Scholar] [CrossRef] [Green Version]
- Criado, Y.A.; Alonso, M.; Abanades, J.C. Kinetics of the CaO/Ca(OH)2Hydration/Dehydration Reaction for Thermochemical Energy Storage Applications. Ind. Eng. Chem. Res. 2014, 53, 12594–12601. [Google Scholar] [CrossRef] [Green Version]
- Angerer, M.; Becker, M.; Härzschel, S.; Kröper, K.; Gleis, S.; Vandersickel, A.; Spliethoff, H. Design of a MW-scale thermo-chemical energy storage reactor. Energy Rep. 2018, 4, 507–519. [Google Scholar] [CrossRef]
- Criado, Y.A.; Huille, A.; Rougé, S.; Abanades, J.C. Experimental investigation and model validation of a CaO/Ca(OH)2 fluidized bed reactor for thermochemical energy storage applications. Chem. Eng. J. 2017, 313, 1194–1205. [Google Scholar] [CrossRef] [Green Version]
- Rougé, S.; Criado, Y.A.; Soriano, O.; Abanades, J.C. Continuous CaO/Ca(OH)2 Fluidized Bed Reactor for Energy Storage: First Experimental Results and Reactor Model Validation. ACS Publ. 2017, 56, 844–852. [Google Scholar] [CrossRef] [Green Version]
- Bian, Z.; Li, Y.; Zhang, C.; Zhao, J.; Wang, T.; Lei, W. Heat release performance and evolution of CaO particles under fluidization for CaO/Ca(OH)2 thermochemical heat storage. Process Saf. Environ. Prot. 2021, 155, 166–176. [Google Scholar] [CrossRef]
- Schaube, F.; Kohzer, A.; Schütz, J.; Wörner, A.; Müller-Steinhagen, H. De- and rehydration of Ca(OH)2 in a reactor with direct heat transfer for thermo-chemical heat storage. Part A: Experimental results. Chem. Eng. Res. Des. 2013, 91, 856–864. [Google Scholar] [CrossRef]
- Schmidt, M.; Szczukowski, C.; Roßkopf, C.; Linder, M.; Wörner, A. Experimental results of a 10 kW high temperature thermochemical storage reactor based on calcium hydroxide. Appl. Therm. Eng. 2014, 62, 553–559. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Zhao, C.Y. Experimental study of CaO/Ca(OH)2 in a fixed-bed reactor for thermochemical heat storage. Appl. Energy 2016, 175, 277–284. [Google Scholar] [CrossRef]
- Dai, L.; Long, X.-F.; Lou, B.; Wu, J. Thermal cycling stability of thermochemical energy storage system Ca(OH)2/CaO. Appl. Therm. Eng. 2018, 133, 261–268. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, Y.; Zhao, J. Development on Thermochemical Energy Storage Based on CaO-Based Materials: A Review. Sustainability 2018, 10, 2660. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M.; Gutierrez, A.; Linder, M. Thermochemical energy storage with CaO/Ca(OH)2—Experimental investigation of the thermal capability at low vapor pressures in a lab scale reactor. Appl. Energy 2017, 188, 672–681. [Google Scholar] [CrossRef]
- Nagel, T.; Shao, H.; Singh, A.K.; Watanabe, N.; Roßkopf, C.; Linder, M.; Wörner, A.; Kolditz, O. Non-equilibrium thermochemical heat storage in porous media: Part 1–Conceptual model. Energy 2013, 60, 254–270. [Google Scholar] [CrossRef]
- Schaube, F.; Utz, I.; Wörner, A.; Müller-Steinhagen, H. De- and rehydration of Ca(OH)2 in a reactor with direct heat transfer for thermo-chemical heat storage. Part B: Validation of model. Chem. Eng. Res. Des. 2013, 91, 865–873. [Google Scholar] [CrossRef]
- Xiao, S.; Praditia, T.; Oladyshkin, S.; Nowak, W. Global sensitivity analysis of a CaO/Ca(OH)2 thermochemical energy storage model for parametric effect analysis. Appl. Energy 2021, 285, 116456. [Google Scholar] [CrossRef]
- Ranjha, Q.; Oztekin, A. Numerical analyses of three-dimensional fixed reaction bed for thermochemical energy storage. Renew. Energy 2017, 111, 825–835. [Google Scholar] [CrossRef]
- Seitz, G.; Mohammadi, F.; Class, H. Thermochemical Heat Storage in a Lab-Scale Indirectly Operated CaO/Ca(OH)2 Reactor—Numerical Modeling and Model Validation through Inverse Parameter Estimation. Appl. Sci. 2021, 11, 682. [Google Scholar] [CrossRef]
- Wang, M.; Chen, L.; Zhou, Y.; Tao, W.-Q. Numerical Simulation of the Physical–Chemical–Thermal Processes During Hydration Reaction of the Calcium Oxide/Calcium Hydroxide System in an Indirect Reactor. Transp. Porous Media 2020, 140, 667–696. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Šesták, J.; Berggren, G. Study of the kinetics of the mechanism of solid-state reactions at increasing temperatures. Thermochim. Acta 1971, 3, 1–12. [Google Scholar] [CrossRef]
- Newville, M.; Stensitzki, T.; Allen, D.B.; Ingargiola, A. LMFIT: Non-Linear Least-Square Minimization and Curve-Fitting for Python. Astrophys. Source Code Libr. 2014, ascl-1606. [Google Scholar] [CrossRef]
- Funayama, S.; Takasu, H.; Zamengo, M.; Kariya, J.; Kim, S.T.; Kato, Y. Performance of thermochemical energy storage of a packed bed of calcium hydroxide pellets. Energy Storage 2019, 1, e40. [Google Scholar] [CrossRef] [Green Version]
- Funayama, S.; Takasu, H.; Zamengo, M.; Kariya, J.; Kim, S.T.; Kato, Y. Composite material for high-temperature thermochemical energy storage using calcium hydroxide and ceramic foam. Energy Storage 2019, 1, e53. [Google Scholar] [CrossRef] [Green Version]
- Risthaus, K.; Bürger, I.; Linder, M.; Schmidt, M. Numerical analysis of the hydration of calcium oxide in a fixed bed reactor based on lab-scale experiments. Appl. Energy 2020, 261, 114351. [Google Scholar] [CrossRef]
- Barin, I. Thermochemical Data of Pure Substances; Wiley: Weinheim, Germany; New York, NY, USA, 1995. [Google Scholar] [CrossRef]
- Roßkopf, C.; Haas, M.; Faik, A.; Linder, M.; Wörner, A. Improving powder bed properties for thermochemical storage by adding nanoparticles. Energy Convers. Manag. 2014, 86, 93–98. [Google Scholar] [CrossRef]
- Gollsch, M.; Afflerbach, S.; Drexler, M.; Linder, M. Structural integrity of calcium hydroxide granule bulks for thermochemical energy storage. Sol. Energy 2020, 208, 873–883. [Google Scholar] [CrossRef]
- Schmidt, M.; Linder, M. Power generation based on the Ca(OH)2/CaO thermochemical storage system—Experimental investigation of discharge operation modes in lab scale and corresponding conceptual process design. Appl. Energy 2017, 203, 594–607. [Google Scholar] [CrossRef]
- Mahmoudi, A.; Donkers, P.A.J.; Walayat, K.; Peters, B.; Shahi, M. A thorough investigation of thermochemical heat storage system from particle to bed scale. Chem. Eng. Sci. 2021, 246, 116877. [Google Scholar] [CrossRef]



| Pressure/kPa | Isothermal: Temperature/°C | Dynamic (5 K/min): Temperature/°C | ||||||
|---|---|---|---|---|---|---|---|---|
| 0.8 | (365) | 375 | 390 | 270–460 | ||||
| 1.2 | (365 1) | 375 | 385 | 390 | 395 | 405 | 420 | 270–475 |
| 2.5 | 390 | 400 | 405 | 270–490 | ||||
| 5.5 | 430 | 440 | ||||||
| Name | Type | Average Steam Pressure/kPa | Start Temperature/°C | Heater Temperature/°C |
|---|---|---|---|---|
| E3 1 | p− | 4.8 | 450 | 450 |
| E5 | p− | 2.9 | 450 | 450 |
| E7 | p− | 3 | 450 | 450 |
| E9 | p− | 3.3 | 450 | 450 |
| E11 | T+ | 3.6 | 350 | 490 |
| E13 | T+ | 2.8 | 280 | 450 |
| E15 | p− | 3.4 | 490 | 490 |
| Parameter | Unit | Symbol | Value | Reference | |
|---|---|---|---|---|---|
| Thermal conductivity, solid matrix | W/(m K) | 0.6 0.7 | Fitted (see Section 3.2.1) Fitted (see Section 3.2.1) | ||
| Particle diameter | µm | 5 | |||
| Gas constant | J/(mol K) | R | 8.314 | ||
| Reaction enthalpy | kJ/mol | 101 | at 450 °C | [37] | |
| Isobaric heat capacity CaO Ca(OH)2 | J/(kg K) | 923 1504 | at 450 °C at 450 °C | [37] |
| Experiment | Type | Theoretical Final Conversion | Permeability Factor | MAEs | ||
|---|---|---|---|---|---|---|
| T0/K | T0.5R/K | X/- | ||||
| E3 | p− | 79% * | 100 | 0.93 | 3.98 | 0.03 |
| E5 | p− | 86% | 100 | 4.27 | 2.86 | 0.06 |
| E7 | p− | 87% | 100 | 2.75 | 6.23 | 0.08 |
| E9 | p− | 92% | 100 | 3.48 | 3.1 | 0.08 |
| E11 | T+ | 94% | 1 | 6.9 | 9.9 | 0.02 |
| E13 | T+ | 98% | 1 | 3 | 3 | 0.04 |
| E15 | p− | 83% | 100 | 6.02 | 8.17 | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Risthaus, K.; Bürger, I.; Lutz, M.; Funayama, S.; Kato, Y.; Linder, M.; Schmidt, M. Experimental and Numerical Investigation of the Dehydration of Ca(OH)2 at Low Steam Pressures. Processes 2022, 10, 325. https://doi.org/10.3390/pr10020325
Risthaus K, Bürger I, Lutz M, Funayama S, Kato Y, Linder M, Schmidt M. Experimental and Numerical Investigation of the Dehydration of Ca(OH)2 at Low Steam Pressures. Processes. 2022; 10(2):325. https://doi.org/10.3390/pr10020325
Chicago/Turabian StyleRisthaus, Kai, Inga Bürger, Michael Lutz, Shigehiko Funayama, Yukitaka Kato, Marc Linder, and Matthias Schmidt. 2022. "Experimental and Numerical Investigation of the Dehydration of Ca(OH)2 at Low Steam Pressures" Processes 10, no. 2: 325. https://doi.org/10.3390/pr10020325






