Experimental and Numerical Validation of the One-Process Modeling Approach for the Hydration of K2CO3 Particles
Abstract
:1. Introduction
2. Theoretical Background
2.1. Elementary Steps
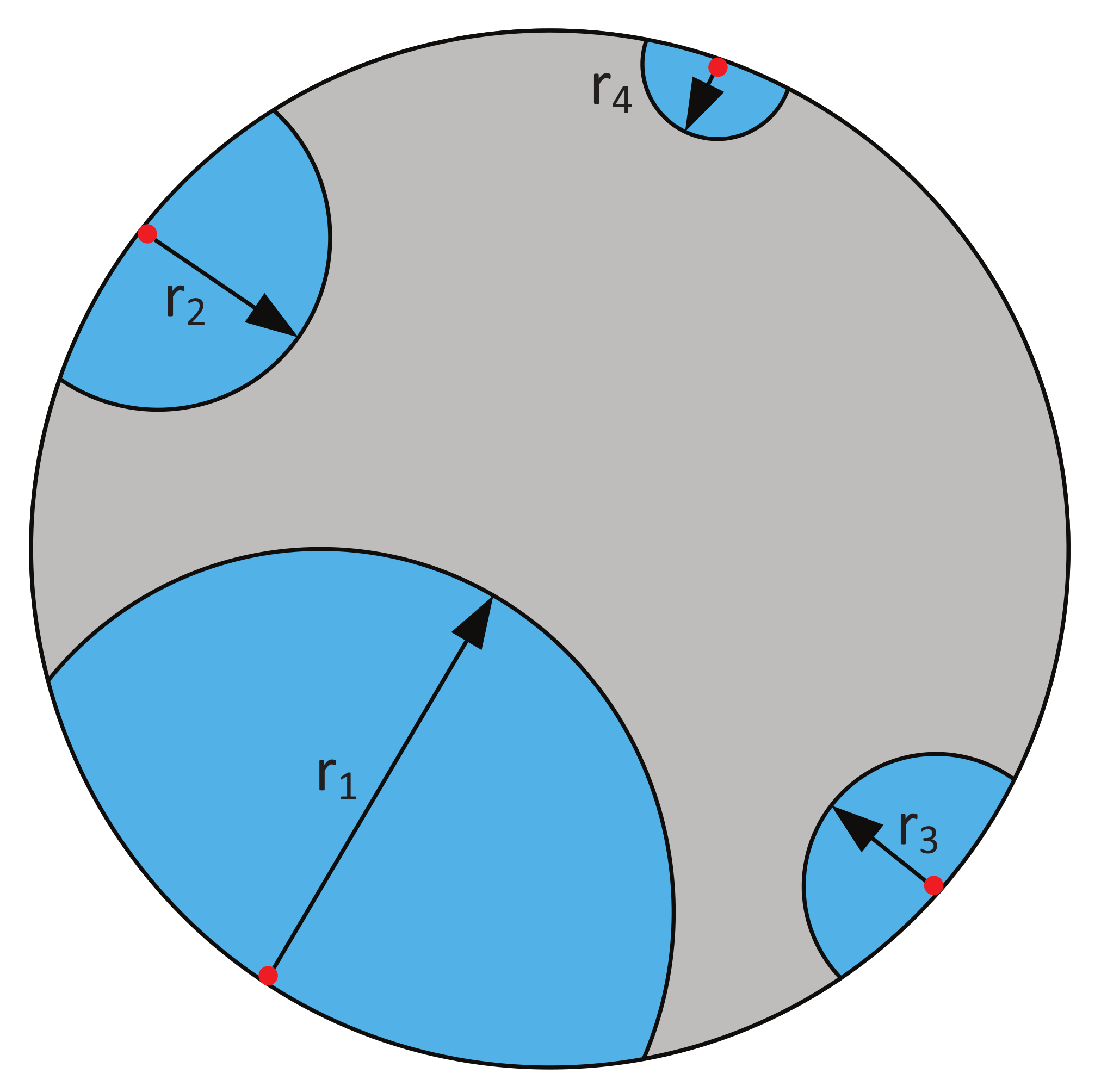
2.2. Nucleation and Nuclei Growth
2.3. Solid–Gas Reaction Models
- (1)
- There should be no accumulation of intermediate species; the reaction is in a pseudo-steady state [17].
- (2)
- (3)
2.4. One-Process and Two-Process Models
3. Materials and Experimental Methods
3.1. Sample Preparation
3.2. STA Experiments
4. Results and Discussion
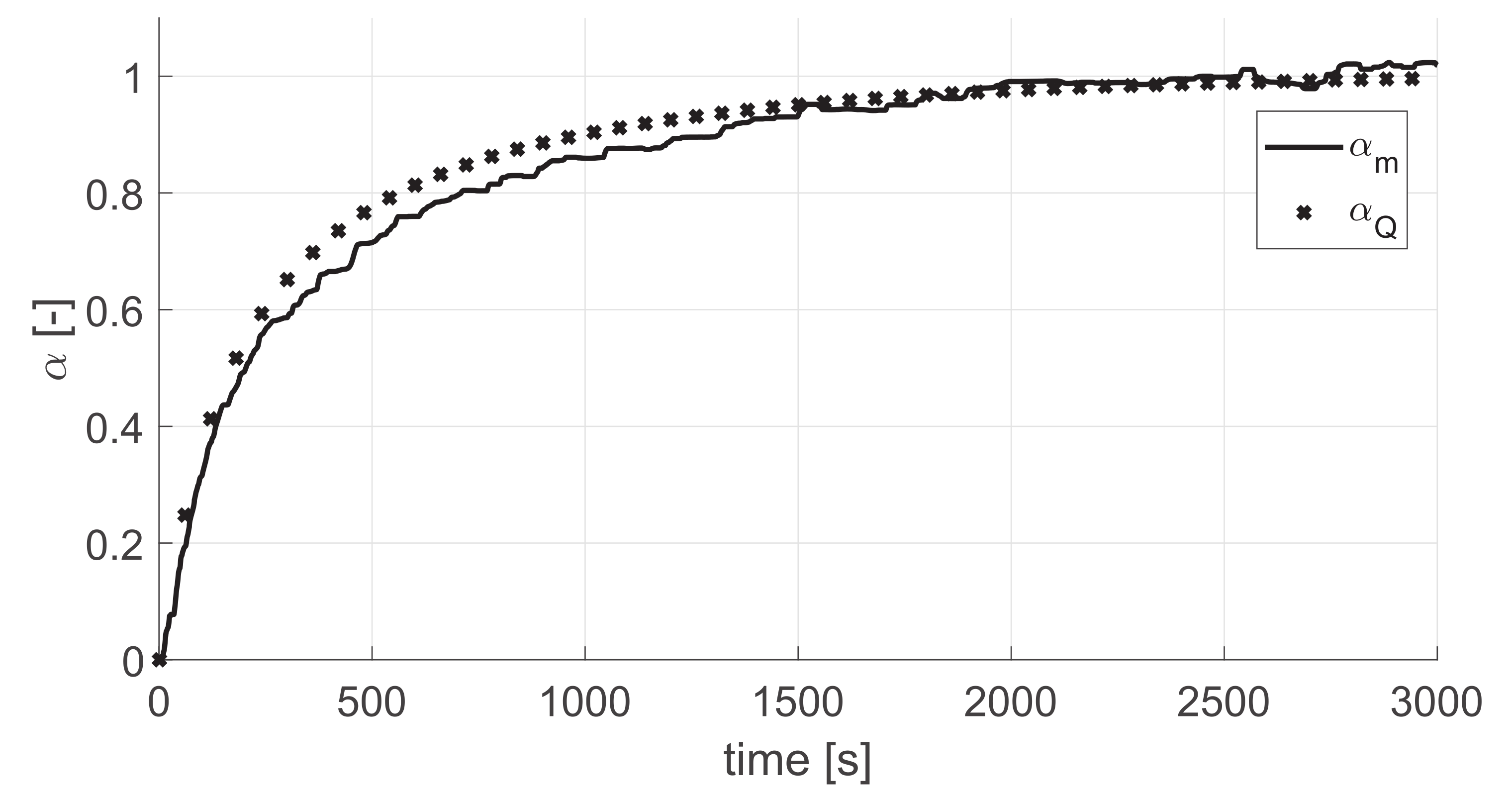
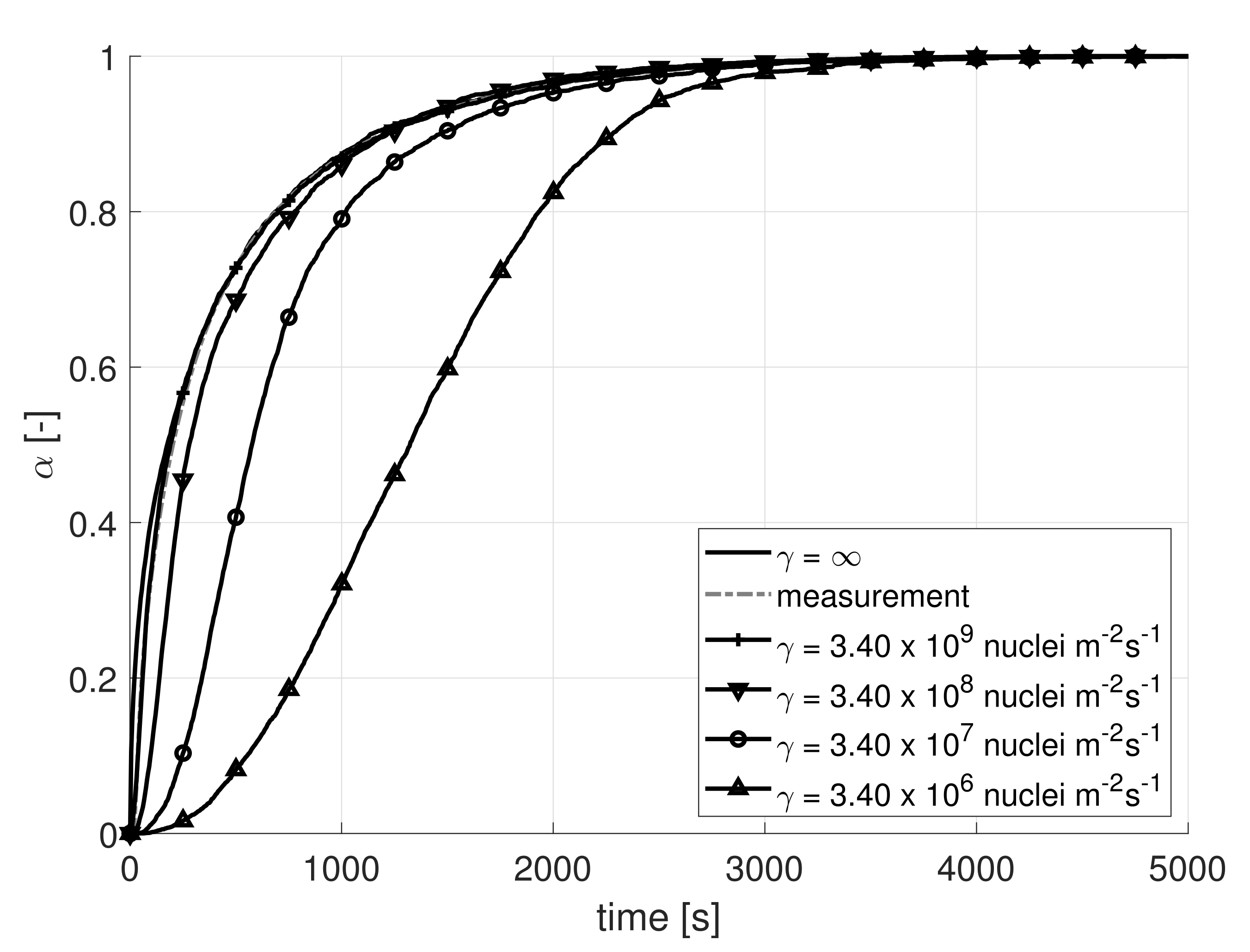
4.1. Flow Rate Dependency
4.2. Pseudo-Steady State Assumption
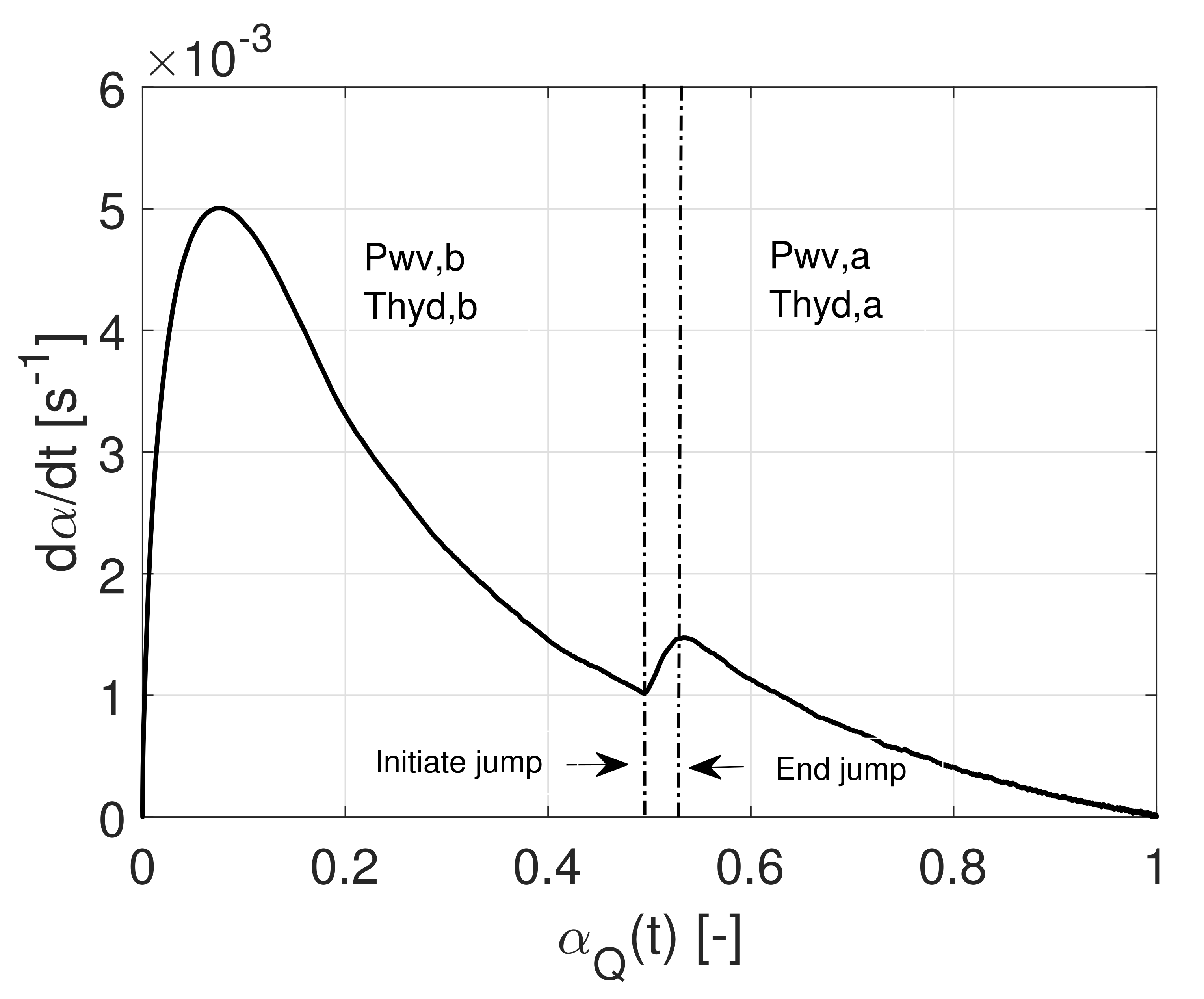
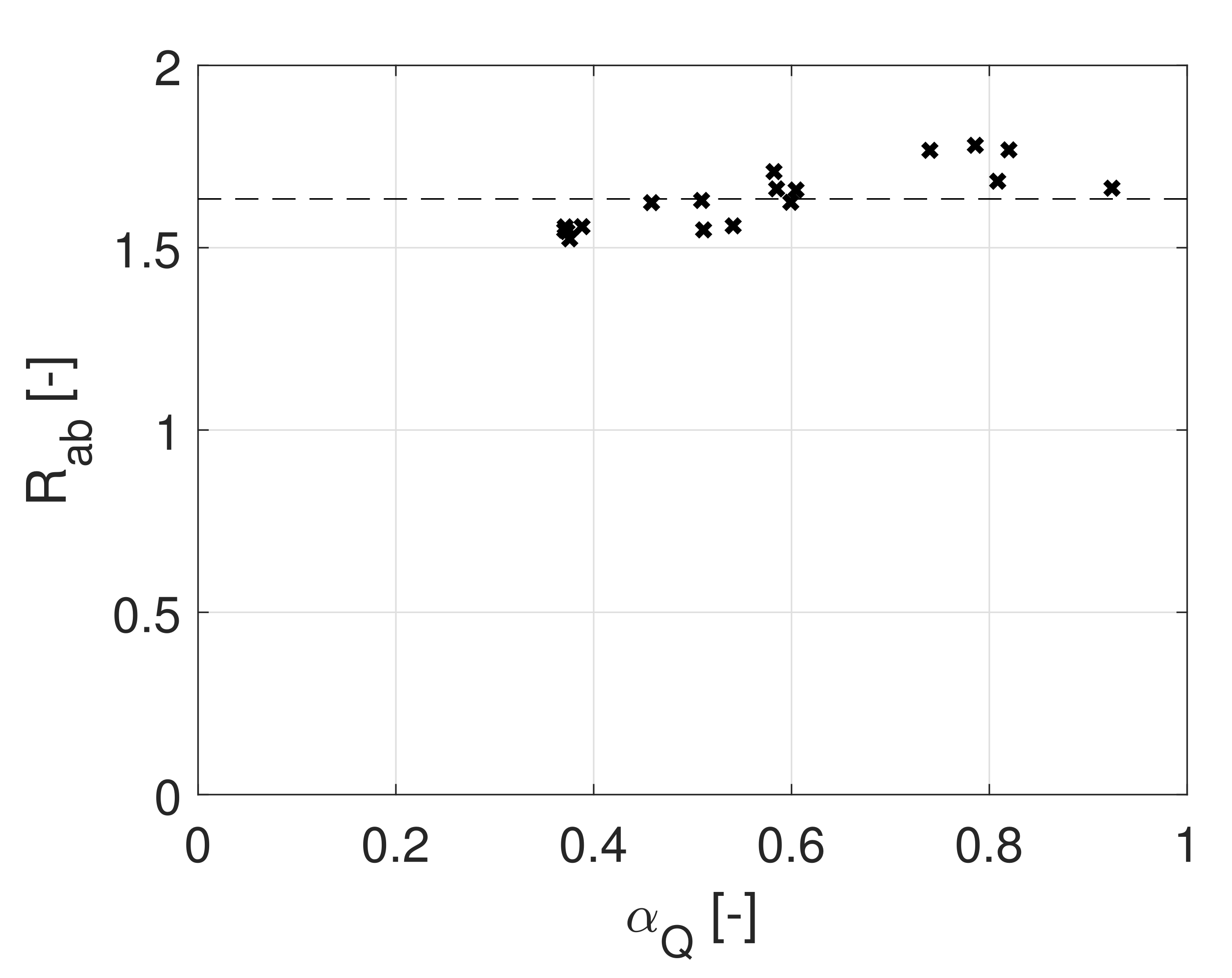
4.3. RDS Assumption—Jump Experiments
4.3.1. The ‘-Test’
4.3.2. The ‘-Test’
4.4. Modeling
4.4.1. One-Process Model—Instantaneous Nucleation Limited by Diffusion
4.4.2. Two-Process Models—Simultaneous Nucleation and Growth
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations Climate Change. The Paris Agreement: Essential Elements. Available online: https://unfccc.int/process-and-meetings/the-paris-agreement/the-paris-agreement (accessed on 20 December 2019).
- Quadrelli, R.; Silva, M.; Park, J.; Garcia, V. Energy Efficiency Indicators—Highlights; IEA Publications: Paris, France, 2019. [Google Scholar]
- Rindt, C.; Lan, S.; Gaeini, M.; Zhang, H.; Nedea, S.; Smeulders, D.M. Phase change materials and thermochemical materials for large-scale energy storage. In Continuous Media with Microstructure 2; Springer: Eindhoven, The Netherlands, 2016; pp. 187–197. [Google Scholar]
- Jänchen, J.; Ackermann, D.; Stach, H.; Brösicke, W. Studies of the water adsorption on zeolites and modified mesoporous materials for seasonal storage of solar heat. Sol. Energy 2004, 76, 339–344. [Google Scholar] [CrossRef]
- N’Tsoukpoe, K.E.; Schmidt, T.; Rammelberg, H.U.; Watts, B.A.; Ruck, W.K. A systematic multi-step screening of numerous salt hydrates for low temperature thermochemical energy storage. Appl. Energy 2014, 124, 1–16. [Google Scholar] [CrossRef]
- Donkers, P.; Sögütoglu, L.; Huinink, H.; Fischer, H.; Adan, O. A review of salt hydrates for seasonal heat storage in domestic applications. Appl. Energy 2017, 199, 45–68. [Google Scholar] [CrossRef]
- N’tsoukpoe, K.E.; Liu, H.; Le Pierrès, N.; Luo, L. A review on long-term sorption solar energy storage. Renew. Sustain. Energy Rev. 2009, 13, 2385–2396. [Google Scholar] [CrossRef]
- Barreneche, C.; Fernández, A.I.; Cabeza, L.F.; Cuypers, R. Thermophysical characterization and thermal cycling stability of two TCM: CaCl2 and zeolite. Appl. Energy 2015, 137, 726–730. [Google Scholar] [CrossRef] [Green Version]
- Sögütoglu, L.; Donkers, P.; Fischer, H.; Huinink, H.; Adan, O. In-depth investigation of thermochemical performance in a heat battery: Cyclic analysis of K2COl3, MgCl2 and Na2S. Appl. Energy 2018, 215, 159–173. [Google Scholar] [CrossRef]
- Wagman, D.D.; Evans, W.; Parker, V.; Schumm, R.; Halow, I. The NBS Tables of Chemical Thermodynamic Properties: Selected Values for Inorganic and C1 and C2 Organic Substances in SI Units; Technical Report; American Chemical Society: Washington, DC, USA, 1982. [Google Scholar]
- Gaeini, M.; Shaik, S.; Rindt, C. Characterization of potassium carbonate salt hydrate for thermochemical energy storage in buildings. Energy Build. 2019, 196, 178–193. [Google Scholar] [CrossRef]
- Stanish, M.; Perlmutter, D. Rate processes in cycling a reversible gas-solid reaction. AIChE J. 1984, 30, 56–62. [Google Scholar] [CrossRef]
- Stanish, M.; Perlmutter, D. Kinetics and transport effects in the dehydration of crystalline potassium carbonate hydrate. AIChE J. 1983, 29, 806–812. [Google Scholar] [CrossRef]
- Favergeon, L.; Morandini, J.; Pijolat, M.; Soustelle, M. A General Approach for Kinetic Modeling of Solid-Gas Reactions at Reactor Scale: Application to Kaolinite Dehydroxylation. Oil Gas Sci. Technol. 2013, 68, 1039–1048. [Google Scholar] [CrossRef] [Green Version]
- Fopah Lele, A.; Kuznik, F.; Rammelberg, H.U.; Schmidt, T.; Ruck, W.K. Thermal decomposition kinetic of salt hydrates for heat storage systems. Appl. Energy 2015, 154, 447–458. [Google Scholar] [CrossRef]
- Sögütoglu, L.C.; Birkelbach, F.; Werner, A.; Fischer, H.; Huinink, H.; Adan, O. Hydration of salts as a two-step process: Water adsorption and hydrate formation. Thermochim. Acta 2021, 695, 178819. [Google Scholar] [CrossRef]
- Michèle, P.; Loic, F.; Michel, S. From the drawbacks of the Arrhenius-f (α) rate equation towards a more general formalism and new models for the kinetic analysis of solid–gas reactions. Thermochim. Acta 2011, 525, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Pijolat, M.; Valdivieso, F.; Soustelle, M. Experimental test to validate the rate equation “dα/dt = kf (α)” used in the kinetic analysis of solid state reactions. Thermochim. Acta 2005, 439, 86–93. [Google Scholar] [CrossRef]
- Pijolat, M.; Soustelle, M. Experimental tests to validate the rate-limiting step assumption used in the kinetic analysis of solid-state reactions. Thermochim. Acta 2008, 478, 34–40. [Google Scholar] [CrossRef]
- Brun, C.; Valdivieso, F.; Pijolat, M.; Soustelle, M. Reduction by hydrogen of Ul3Ol8 into UO2: Nucleation and growth, influence of hydration. Phys. Chem. Chem. Phys. 1999, 1, 471–477. [Google Scholar] [CrossRef]
- Favergeon, L.; Pijolat, M.; Valdivieso, F.; Helbert, C. Experimental study and Monte-Carlo simulation of the nucleation and growth processes during the dehydration of Li2SO4·H2O single crystals. Phys. Chem. Chem. Phys. 2005, 7, 3723–3727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansour, M.; Favergeon, L.; Pijolat, M. Kinetic modeling of low temperature oxidation of copper nanoparticles by O2. Thermochim. Acta 2013, 570, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Nahdi, K.; Perrin, S.; Pijolat, M.; Rouquerol, F.; Ariguib, N.; Ayadi, M. Nucleation and anisotropic growth model for isothermal kaolinite dehydroxylation under controlled water vapour pressure. Phys. Chem. Chem. Phys. 2002, 4, 1972–1977. [Google Scholar] [CrossRef]
- Pijolat, M.; Favergeon, L. Kinetics and mechanisms of solid-gas reactions. In Handbook of Thermal Analysis and Calorimetry; Elsevier: Saint-Étienne, France, 2018; Volume 6, pp. 173–212. [Google Scholar]
- Pijolat, M.; Brun, C.; Valdivieso, F.; Soustelle, M. Reduction of uranium oxide U3O8 to UO2 by hydrogen. Solid State Ionics 1997, 101, 931–935. [Google Scholar] [CrossRef]
- Rouchon, L.; Favergeon, L.; Pijolat, M. Analysis of the kinetic slowing down during carbonation of CaO by CO2. J. Therm. Anal. Calorim. 2013, 113, 1145–1155. [Google Scholar] [CrossRef] [Green Version]
- Sögütoglu, L.C.; Steiger, M.; Houben, J.; Biemans, D.; Fischer, H.R.; Donkers, P.; Huinink, H.; Adan, O.C. Understanding the hydration process of salts: The impact of a nucleation barrier. Cryst. Growth Des. 2019, 19, 2279–2288. [Google Scholar] [CrossRef]
- L’vov, B.V. Thermal Decomposition of Solids and Melts: New Thermochemical Approach to the Mechanism, Kinetics and Methodology; Springer Science & Business Media: Budapest, Hungary, 2007; Volume 7. [Google Scholar]
- Volmer, M.; Weber, A. Keimbildung in übersättigten Gebilden. Z. Phys. Chem. 1926, 119, 277–301. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Galwey, A.K.; Brown, M.E. Thermal Decomposition of Ionic Solids: Chemical Properties and Reactivities of Ionic Crystalline Phases; Elsevier: Grahamstown, South Africa, 1999. [Google Scholar]
- Khawam, A.; Flanagan, D.R. Solid-state kinetic models: Basics and mathematical fundamentals. J. Phys. Chem. B 2006, 110, 17315–17328. [Google Scholar] [CrossRef] [PubMed]
- Risthaus, K.; Bürger, I.; Linder, M.; Schmidt, M. Numerical analysis of the hydration of calcium oxide in a fixed bed reactor based on lab-scale experiments. Appl. Energy 2020, 261, 114351. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Schick, C.; Verevkin, S.P.; Heym, F.; Androsch, R.; Hunkel, M.; Steinbacher, M.; Pijolat, M.; Favergeon, L.; Koga, N.; et al. Handbook of Thermal Analysis and Calorimetry; Brown, M.E., Gallagher, P.K., Eds.; Elsevier: Birmingham, AL, USA, 2018; p. 503. [Google Scholar]
- Vyazovkin, S. Isoconversional Kinetics. In Handbook of Thermal Analysis and Calorimetry; Elsevier: Birmingham, AL, USA, 2008; Volume 5, pp. 503–538. [Google Scholar] [CrossRef]
- Lebrun, M.; Spinner, B. Models of heat and mass transfers in solid—Gas reactors used as chemical heat pumps. Chem. Eng. Sci. 1990, 45, 1743–1753. [Google Scholar] [CrossRef]
- Vyazovkin, S. Modern isoconversional kinetics: From misconceptions to advances. In Handbook of Thermal Analysis and Calorimetry; Elsevier: Birmingham, AL, USA, 2018; Volume 6, pp. 131–172. [Google Scholar]
- Stengler, J.; Bürger, I.; Linder, M. Thermodynamic and kinetic investigations of the SrBr2 hydration and dehydration reactions for thermochemical energy storage and heat transformation. Appl. Energy 2020, 277, 115432. [Google Scholar] [CrossRef]
- Neveux, L.; Chiche, D.; Perez-Pellitero, J.; Favergeon, L.; Gay, A.S.; Pijolat, M. New insight into the ZnO sulfidation reaction: Mechanism and kinetics modeling of the ZnS outward growth. Phys. Chem. Chem. Phys. 2013, 15, 1532–1545. [Google Scholar] [CrossRef] [Green Version]
- Perrin, S.; Pijolat, M.; Valdivieso, F.; Soustelle, M. Kinetic study of the effect of a sudden change in temperature during the reduction of U3O8 into UO2 by hydrogen. Solid State Ionics 2001, 141, 109–115. [Google Scholar] [CrossRef]
- Favergeon, L.; Pijolat, M.; Soustelle, M. Surface nucleation and anisotropic growth models for solid-state reactions. Thermochim. Acta 2017, 654, 18–27. [Google Scholar] [CrossRef]
- Beving, M.; Frijns, A.; Rindt, C.; Smeulders, D. Effect of cycle-induced crack formation on the hydration behaviour of K2CO3 particles: Experiments and modelling. Thermochim. Acta 2020, 692, 178752. [Google Scholar] [CrossRef]
- Koga, N.; Tanaka, H. Effect of sample mass on the kinetics of thermal decomposition of a solid: II. Isothermal dehydration of Li2SO4·H2O. J. Therm. Anal. Calorim. 1993, 40, 1173–1179. [Google Scholar] [CrossRef]
- Van Essen, V.; Zondag, H.; Gores, J.; Bleijendaal, L.; Bakker, M.; Schuitema, R.; Van Helden, W.; He, Z.; Rindt, C. Characterization of MgSO4 hydrate for thermochemical seasonal heat storage. J. Sol. Energy Eng. 2009, 131, 041014. [Google Scholar] [CrossRef]
- Fisher, R.; Ding, Y.; Sciacovelli, A. Hydration kinetics of K2CO3, MgCl2 and vermiculite-based composites in view of low-temperature thermochemical energy storage. J. Energy Storage 2021, 38, 102561. [Google Scholar] [CrossRef]
- Surla, K.; Valdivieso, F.; Pijolat, M.; Soustelle, M.; Prin, M. Kinetic study of the oxidation by oxygen of liquid Al–Mg 5% alloys. Solid State Ionics 2001, 143, 355–365. [Google Scholar] [CrossRef]
- Tupin, M.; Pijolat, M.; Valdivieso, F.; Soustelle, M. Oxidation kinetics of ZrNbO in steam: Differences between the pre-and post-transition stages. J. Nucl. Mater. 2005, 342, 108–118. [Google Scholar] [CrossRef] [Green Version]
- Tupin, M.; Pijolat, M.; Valdivieso, F.; Soustelle, M.; Frichet, A.; Barberis, P. Differences in reactivity of oxide growth during the oxidation of Zircaloy-4 in water vapour before and after the kinetic transition. J. Nucl. Mater. 2003, 317, 130–144. [Google Scholar] [CrossRef] [Green Version]
- Helbert, C.; Touboul, E.; Perrin, S.; Carraro, L.; Pijolat, M. Stochastic and deterministic models for nucleation and growth in non-isothermal and/or non-isobaric powder transformations. Chem. Eng. Sci. 2004, 59, 1393–1401. [Google Scholar] [CrossRef]
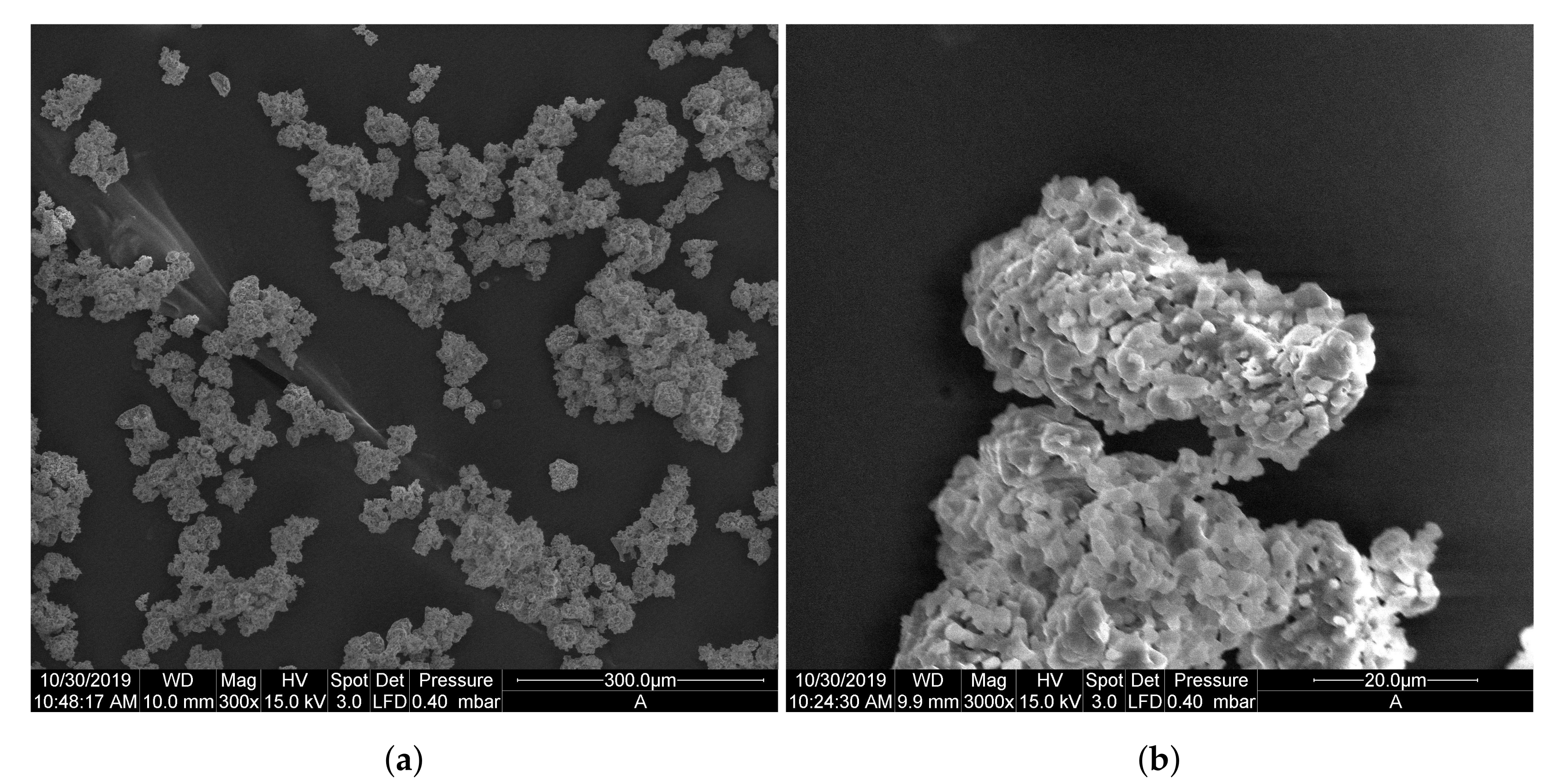
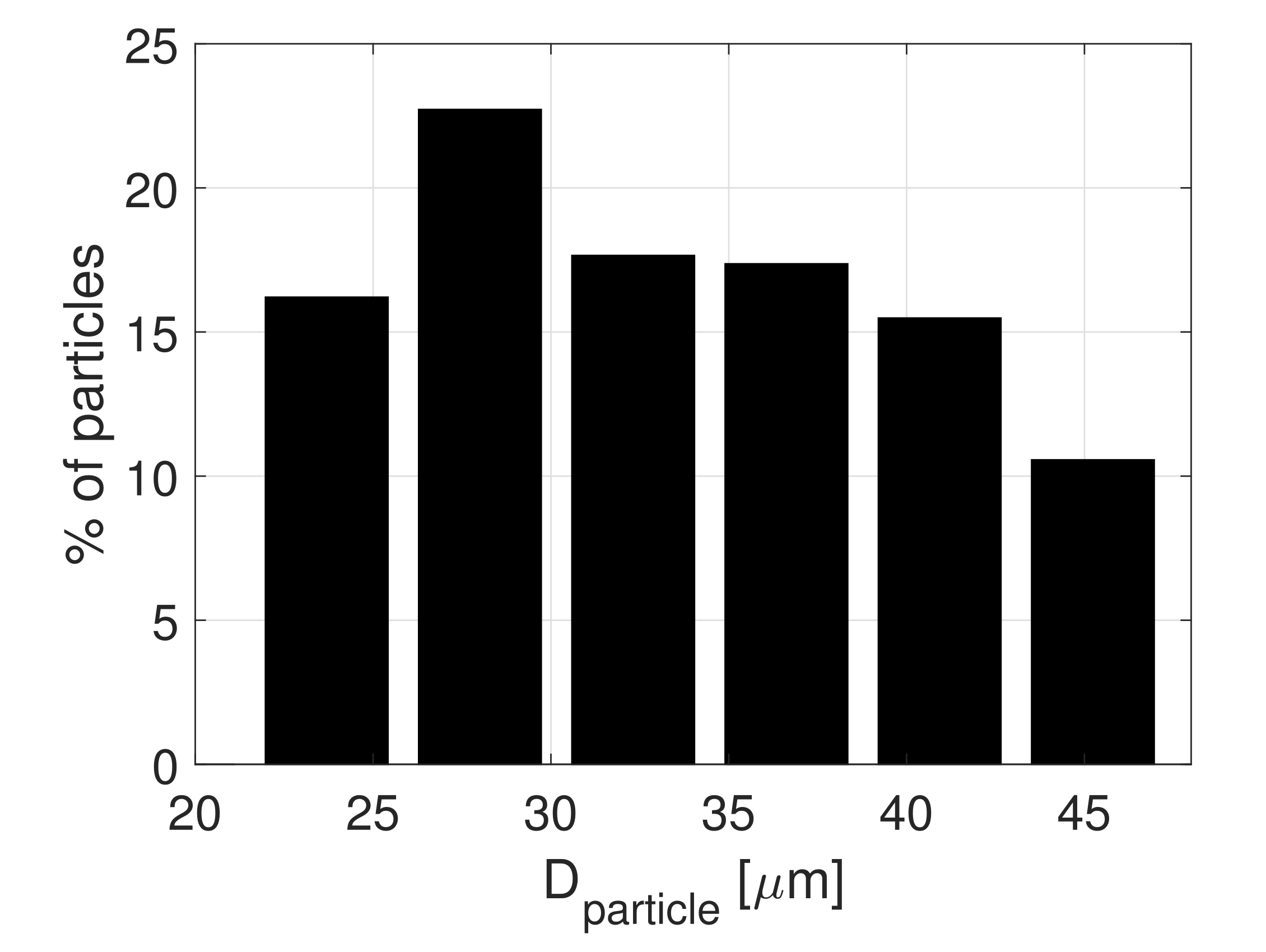
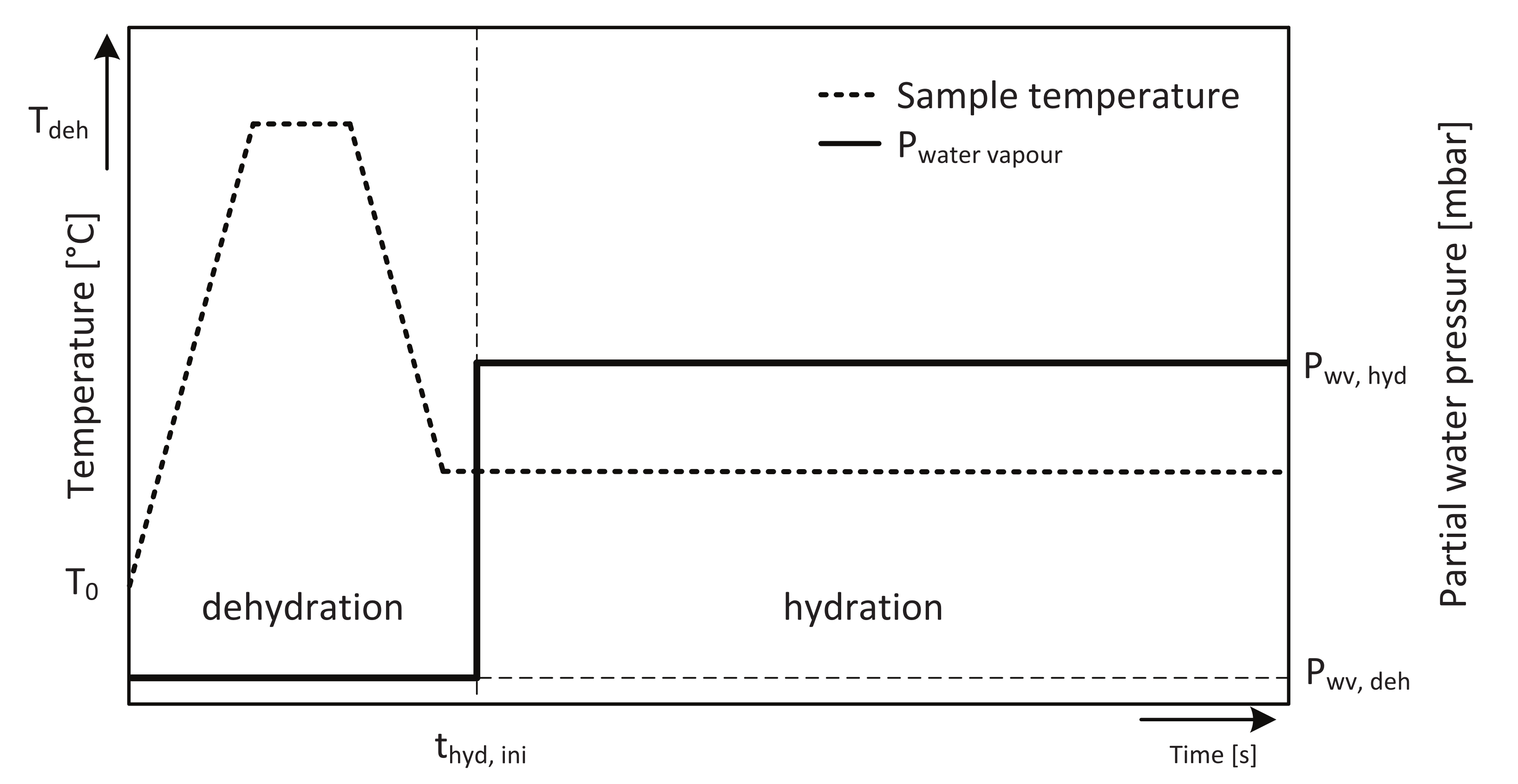
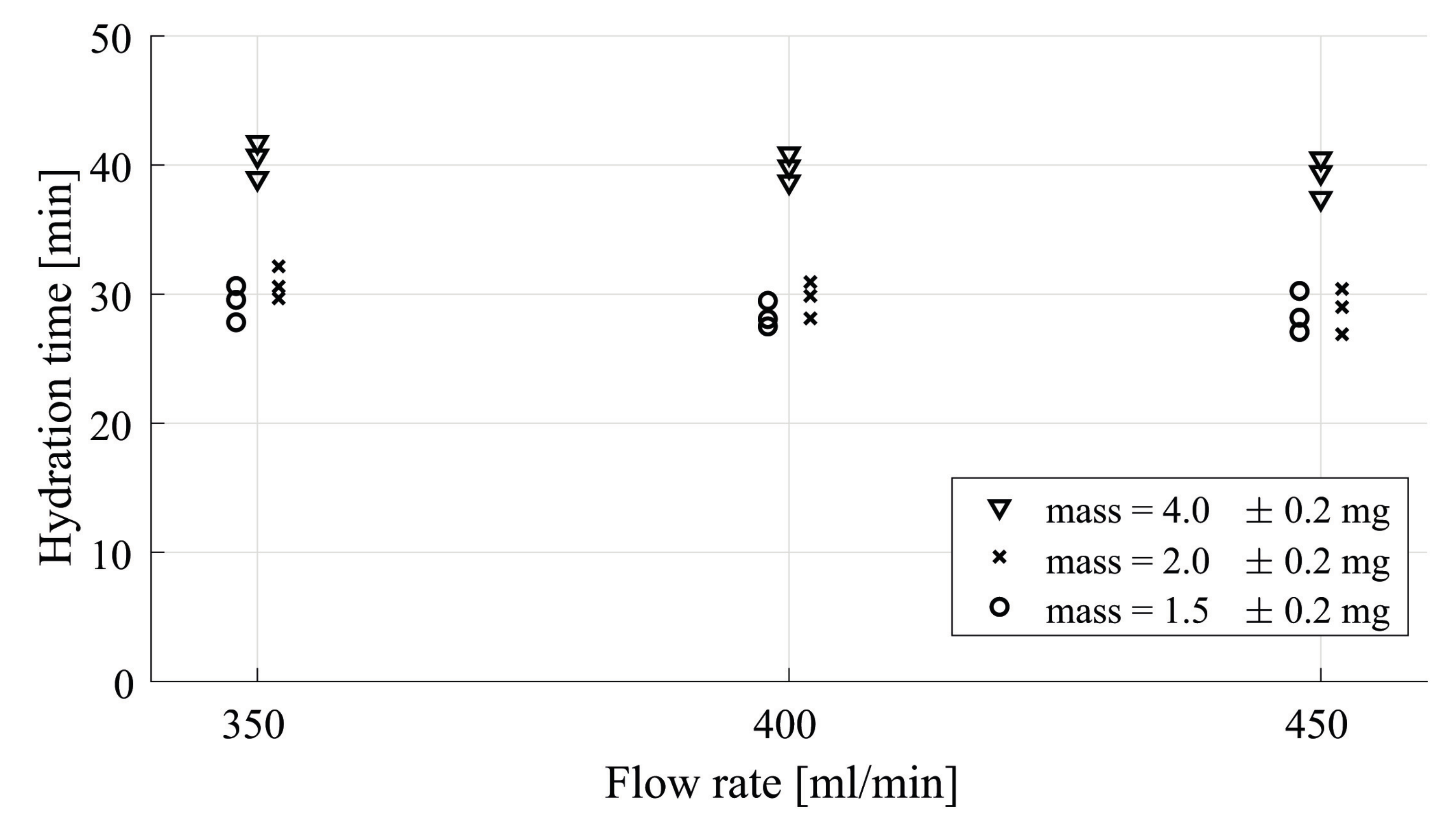
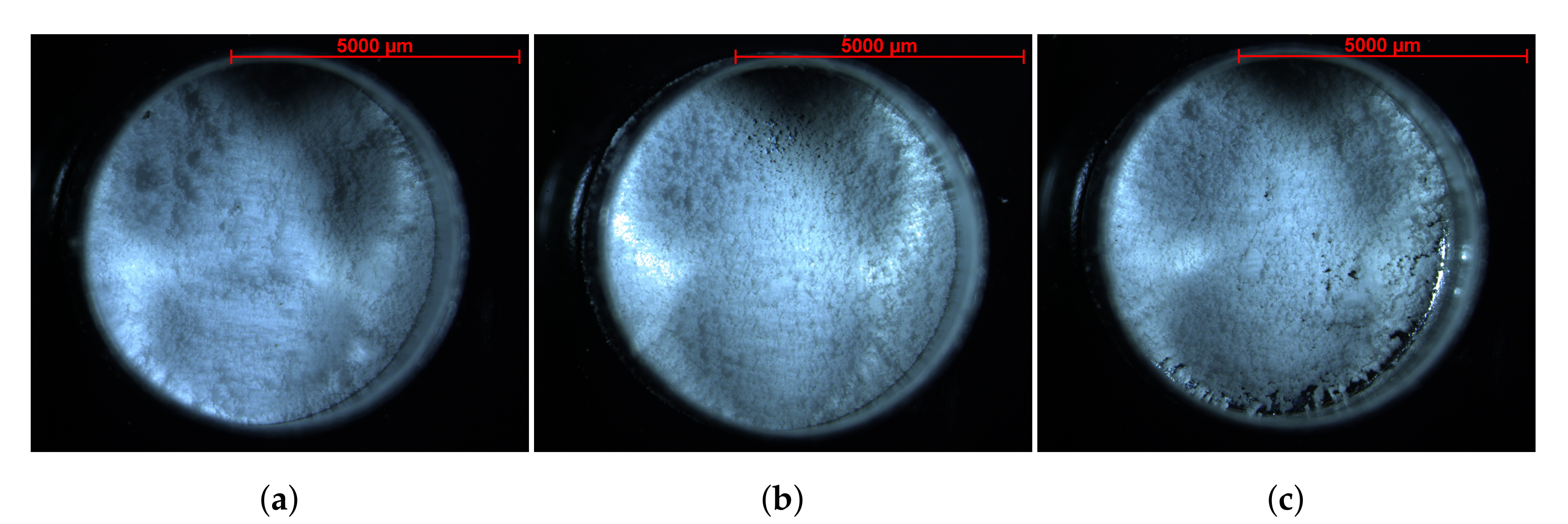



| Sample [mg] | # Particles [-] | # Layers [-] |
|---|---|---|
| 4.0 | ∼67,000 | 2.4 |
| 2.0 | ∼33,000 | 1.2 |
| 1.5 | ∼25,000 | 0.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beving, M.; Romme, J.; Donkers, P.; Frijns, A.; Rindt, C.; Smeulders, D. Experimental and Numerical Validation of the One-Process Modeling Approach for the Hydration of K2CO3 Particles. Processes 2022, 10, 547. https://doi.org/10.3390/pr10030547
Beving M, Romme J, Donkers P, Frijns A, Rindt C, Smeulders D. Experimental and Numerical Validation of the One-Process Modeling Approach for the Hydration of K2CO3 Particles. Processes. 2022; 10(3):547. https://doi.org/10.3390/pr10030547
Chicago/Turabian StyleBeving, Max, Joris Romme, Pim Donkers, Arjan Frijns, Camilo Rindt, and David Smeulders. 2022. "Experimental and Numerical Validation of the One-Process Modeling Approach for the Hydration of K2CO3 Particles" Processes 10, no. 3: 547. https://doi.org/10.3390/pr10030547
APA StyleBeving, M., Romme, J., Donkers, P., Frijns, A., Rindt, C., & Smeulders, D. (2022). Experimental and Numerical Validation of the One-Process Modeling Approach for the Hydration of K2CO3 Particles. Processes, 10(3), 547. https://doi.org/10.3390/pr10030547






