Catalytic Decomposition of n-C7 Asphaltenes Using Tungsten Oxides–Functionalized SiO2 Nanoparticles in Steam/Air Atmospheres
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
2.2.1. Asphaltene Isolation and Characterization
2.2.2. Nanoparticles Preparation
2.2.3. Surface Functionalization
2.2.4. Nanoparticles Characterization
2.2.5. Equilibrium Adsorption Isotherms
2.2.6. Thermogravimetric Analysis
3. Results and Discussion
3.1. Nanoparticle Characterization
3.2. Asphaltene Adsorption Isotherms
Effect of Synthesis Conditions
3.3. Catalytic Steam Decomposition of n-C7 Asphaltenes
3.3.1. Mass Loss Analysis
3.3.2. Estimation of the Effective Activation Energies
3.3.3. Analysis of the Gaseous Products Evolved during Catalytic Decomposition Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| API | American Petroleum Institute |
| C0 | Initial concentration of asphaltenes |
| CE | Equilibrium concentrations in solution after adsorption |
| DLS | Dynamic light scattering |
| EHO | Extra-heavy oil |
| HO | Heavy oil |
| H | Henry’s law constant |
| HTR | High-temperature reaction |
| K | Self-association degree |
| LTR | Low-temperature reaction |
| LHC | Light hydrocarbons |
| MSAP | Mean particle size of active phase |
| OFW | Ozawa–Flynn–Wall |
| PAH | Polyaromatic hydrocarbons |
| qm | Maximum adsorbed amount |
| TEOR | Thermal enhanced oil recovery |
| TEO | Transition element oxides |
| TGA | Thermogravimetric analysis |
| SLE | Solid–liquid equilibrium |
| SBET (m2) | BET surface area |
| SSS | Sodium silicate solution |
| WSN | Tungsten functionalized silica nanoparticles |
| XPS | X-ray photoelectron spectroscopy |
| Universal gas constant | |
| Absolute temperature | |
| Activation energy |
References
- Zaid, A.M.; Al-Dousari, M.M. Forces Driving Oil and Gas Demand Cycles. In Proceedings of the International Petroleum Technology Conference, Dubai, United Arab Emirates, 4–8 December 2007. [Google Scholar]
- Alboudwarej, H.; Felix, J.J.; Taylor, S.; Badry, R.; Bremner, C.; Brough, B.; Skeates, C.; Baker, A.; Palmer, D.; Pattison, K. La importancia del petróleo pesado. Oilfield Rev. 2006, 18, 38–58. [Google Scholar]
- Medina, O.E.; Gallego, J.; Rodríguez, E.; Franco, C.A.; Cortés, F.B. Effect of pressure on the oxidation kinetics of Asphaltenes. Energy Fuels 2019, 33, 10734–10744. [Google Scholar] [CrossRef]
- Medina, O.E.; Olmos, C.; Lopera, S.H.; Cortés, F.B.; Franco, C.A. Nanotechnology applied to thermal enhanced oil recovery processes: A review. Energies 2019, 12, 4671. [Google Scholar] [CrossRef] [Green Version]
- Mitra-Kirtley, S.; Mullins, O.C. Sulfur chemical moieties in carbonaceous materials. In Asphaltenes, Heavy Oils, and Petroleomics; Springer: New York, NY, USA, 2007; pp. 157–188. [Google Scholar]
- Tissot, B.P.; Welte, D.H. Petroleum Formation and Occurrence; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Groenzin, H.; Mullins, O.C. Asphaltene molecular size and structure. J. Phys. Chem. A 1999, 103, 11237–11245. [Google Scholar] [CrossRef]
- Mullins, O.C. The asphaltenes. Annu. Rev. Anal. Chem. 2011, 4, 393–418. [Google Scholar] [CrossRef]
- Strausz, O.P.; Lown, E.M. The Chemistry of Alberta Oil Sands, Bitumens and Heavy Oils; Alberta Energy Research Institute: Calgary, AB, Canada, 2003. [Google Scholar]
- Yudin, I.; Nikolaenko, G.; Gorodetskii, E.; Kosov, V.; Melikyan, V.; Markhashov, E.; Frot, D.; Briolant, Y. Mechanisms of asphaltene aggregation in toluene–heptane mixtures. J. Pet. Sci. Eng. 1998, 20, 297–301. [Google Scholar] [CrossRef]
- Murgich, J.; Abanero, J.A.; Strausz, O.P. Molecular recognition in aggregates formed by asphaltene and resin molecules from the Athabasca oil sand. Energy Fuels 1999, 13, 278–286. [Google Scholar] [CrossRef]
- Demirbas, A. Deposition and flocculation of asphaltenes from crude oils. Pet. Sci. Technol. 2016, 34, 6–11. [Google Scholar] [CrossRef]
- Medina, O.E.; Gallego, J.; Cespedes, S.; Nassar, N.N.; Montoya, T.; Corteś, F.B.; Franco, C.A. Effect of pressure on thermo-oxidative reactions of saturates, aromatics, and resins (S-Ar-R) from extra-heavy crude oil. Fuel 2021, 311, 122596. [Google Scholar] [CrossRef]
- Adams, J.J. Asphaltene adsorption, a literature review. Energy Fuels 2014, 28, 2831–2856. [Google Scholar] [CrossRef]
- Zahedi, G.; Fazlali, A.; Hosseini, S.; Pazuki, G.; Sheikhattar, L. Prediction of asphaltene precipitation in crude oil. J. Pet. Sci. Eng. 2009, 68, 218–222. [Google Scholar] [CrossRef]
- Nassar, N.N.; Hassan, A.; Pereira-Almao, P. Application of nanotechnology for heavy oil upgrading: Catalytic steam gasification/cracking of asphaltenes. Energy Fuels 2011, 25, 1566–1570. [Google Scholar] [CrossRef]
- Medina, O.E.; Gallego, J.; Redondo, J.D.; Corteś, F.B.; Franco, C.A. Effect of pressure on the thermo-oxidative behavior of saturates, aromatics, and resins (S-Ar-R) mixtures. Fuel 2021, 122787. [Google Scholar] [CrossRef]
- Allenson, S.J.; Walsh, M.A. A novel way to treat asphaltene deposition problems found in oil production. In Proceedings of the International Symposium on Oilfield Chemistry, Houston, TX, USA, 18 February 1997. [Google Scholar]
- Chang, J.; Ivory, J. Steam-Air Injection Process. In Proceedings of the SPE Canada Heavy Oil Technical Conference, Calgary, AB, Canada, 8 June 2016. [Google Scholar]
- Kokal, S.L.; Sayegh, S.G. Asphaltenes: The cholesterol of petroleum. In Proceedings of the Middle East Oil Show, Bahrain, Saudi Arabia, 15 March 1997. [Google Scholar]
- Saniere, A.; Hénaut, I.; Argillier, J. Pipeline transportation of heavy oils, a strategic, economic and technological challenge. Oil Gas Sci. Technol. 2004, 59, 455–466. [Google Scholar] [CrossRef]
- Medina, O.E.; Gallego, J.; Nassar, N.N.; Acevedo, S.A.; Cortés, F.B.; Franco, C.A. Thermo-Oxidative Decomposition Behaviors of Different Sources of n-C7 Asphaltenes at High-Pressure Conditions. Energy Fuels 2020, 34, 8740. [Google Scholar] [CrossRef]
- Bagci, A.S. Wet forward combustion for heavy oil recovery. Energy Sources Part A 2006, 28, 221–232. [Google Scholar] [CrossRef]
- Lapene, A.; Castanier, L.M.; Debenest, G.; Quintard, M.Y.; Kamp, A.M.; Corre, B. Effects of Water on Kinetics of Wet In-Situ Combustion. In Proceedings of the SPE Western Regional Meeting, San Jose, CA, USA, 1 January 2009; p. 12. [Google Scholar]
- Ward, G.D.; Ward, C.E. Insitu Wet Combustion Process for Recovery of Heavy Oils. Google Patents US4691773A, 8 September 1987. [Google Scholar]
- Sarathi, P.S. In-Situ Combustion Handbook—Principles and Practices; National Petroleum Technology Office: Sawfa, Saudi Arabia, 1999.
- Greaves, M.; Young, T.; El-Usta, S.; Rathbone, R.; Ren, S.; Xia, T. Air injection into light and medium heavy oil reservoirs: Combustion tube studies on West of Shetlands Clair oil and light Australian oil. Chem. Eng. Res. Des. 2000, 78, 721–730. [Google Scholar] [CrossRef]
- Franco, C.A.; Montoya, T.; Nassar, N.N.; Pereira-Almao, P.; Cortés, F.B. Adsorption and Subsequent Oxidation of Colombian Asphaltenes onto Nickel and/or Palladium Oxide Supported on Fumed Silica Nanoparticles. Energy Fuels 2013, 27, 7336–7347. [Google Scholar] [CrossRef]
- Medina, O.E.; Gallego, J.; Acevedo, S.; Riazi, M.; Ocampo-Pérez, R.; Cortés, F.B.; Franco, C.A. Catalytic Conversion of n-C7 Asphaltenes and Resins II into Hydrogen Using CeO2-Based Nanocatalysts. Nanomaterials 2021, 11, 1301. [Google Scholar] [CrossRef]
- Cardona, L.; Medina, O.E.; Céspedes, S.; Lopera, S.H.; Cortés, F.B.; Franco, C.A. Effect of Steam Quality on Extra-Heavy Crude Oil Upgrading and Oil Recovery Assisted with PdO and NiO-Functionalized Al2O3 Nanoparticles. Processes 2021, 9, 1009. [Google Scholar] [CrossRef]
- Medina, O.E.; Gallego, J.; Pérez-Cadenas, A.F.; Carrasco-Marín, F.; Cortés, F.B.; Franco, C.A. Insights into the Morphology Effect of Ceria on the Catalytic Performance of NiO–PdO/CeO2 Nanoparticles for Thermo-oxidation of n-C7 Asphaltenes under Isothermal Heating at Different Pressures. Energy Fuels 2021, 35, 18170–18184. [Google Scholar] [CrossRef]
- Mateus, L.; Moreno-Castilla, C.; López-Ramón, M.V.; Cortés, F.B.; Álvarez, M.Á.; Medina, O.E.; Franco, C.A.; Yebra-Rodríguez, Á. Physicochemical characteristics of calcined MnFe2O4 solid nanospheres and their catalytic activity to oxidize para-nitrophenol with peroxymonosulfate and n-C7 asphaltenes with air. J. Environ. Manag. 2021, 281, 111871. [Google Scholar] [CrossRef]
- Arias-Madrid, D.; Medina, O.E.; Gallego, J.; Acevedo, S.; Correa-Espinal, A.A.; Cortés, F.B.; Franco, C.A. NiO, Fe2O3, and MoO3 Supported over SiO2 Nanocatalysts for Asphaltene Adsorption and Catalytic Decomposition: Optimization through a Simplex–Centroid Mixture Design of Experiments. Catalysts 2020, 10, 569. [Google Scholar] [CrossRef]
- Hashemi, R.; Nassar, N.N.; Almao, P.P. Nanoparticle technology for heavy oil in-situ upgrading and recovery enhancement: Opportunities and challenges. Appl. Energy 2014, 133, 374–387. [Google Scholar] [CrossRef]
- Hosseinpour, N.; Mortazavi, Y.; Bahramian, A.; Khodatars, L.; Khodadadi, A.A. Enhanced pyrolysis and oxidation of asphaltenes adsorbed onto transition metal oxides nanoparticles towards advanced in-situ combustion EOR processes by nanotechnology. Appl. Catal. A Gen. 2014, 477, 159–171. [Google Scholar] [CrossRef]
- Nassar, N.N.; Hassan, A.; Pereira-Almao, P. Metal Oxide Nanoparticles for Asphaltene Adsorption and Oxidation. Energy Fuels 2011, 25, 1017–1023. [Google Scholar] [CrossRef]
- Cortés, F.B.; Chejne, F.; Carrasco-Marín, F.; Pérez-Cadenas, A.F.; Moreno-Castilla, C. Water sorption on silica-and zeolite-supported hygroscopic salts for cooling system applications. Energy Convers. Manag. 2012, 53, 219–223. [Google Scholar] [CrossRef]
- Montoya, T.; Coral, D.; Franco, C.A.; Nassar, N.N.; Cortés, F.B. A novel solid–liquid equilibrium model for describing the adsorption of associating asphaltene molecules onto solid surfaces based on the “chemical theory”. Energy Fuels 2014, 28, 4963–4975. [Google Scholar] [CrossRef]
- Krishnamoorti, R. Extracting the benefits of nanotechnology for the oil industry. J. Pet. Technol. 2006, 58, 24–26. [Google Scholar] [CrossRef]
- Medina, O.E.; Galeano-Caro, D.; Ocampo-Pérez, R.; Perez-Cadenas, A.F.; Carrasco-Marín, F.; Franco, C.A.; Corteś, F.B. Development of a monolithic carbon xerogel-metal composite for crude oil removal from oil in-saltwater emulsions: Evaluation of reuse cycles. Microporous Mesoporous Mater. 2021, 327, 111424. [Google Scholar] [CrossRef]
- Medina, O.E.; Galeano-Caro, D.; Castelo-Quibén, J.; Ocampo-Pérez, R.; Perez-Cadenas, A.F.; Carrasco-Marín, F.; Franco, C.A.; Corteś, F.B. Monolithic carbon xerogels-metal composites for crude oil removal from oil in-saltwater emulsions and subsequent regeneration through oxidation process: Composites synthesis, adsorption studies, and oil decomposition experiments. Microporous Mesoporous Mater. 2021, 319, 111039. [Google Scholar] [CrossRef]
- Franco, C.A.; Montoya, T.; Nassar, N.N.; Cortés, F.B. NiO nd PdO supported on fumed silica nanoparticles for adsorption and catalytic steam gasification of colombian c7asphaltenes. In Handbook on Oil Production Research; Nova Science Hauppauge: New York, NY, USA, 2014. [Google Scholar]
- Nassar, N.N.; Franco, C.A.; Montoya, T.; Cortés, F.B.; Hassan, A. Effect of oxide support on Ni–Pd bimetallic nanocatalysts for steam gasification of n-C7 asphaltenes. Fuel 2015, 156, 110–120. [Google Scholar] [CrossRef]
- Medina, O.E.; Gallego, J.; Restrepo, L.G.; Cortés, F.B.; Franco, C.A. Influence of the Ce4+/Ce3+ Redox-Couple on the Cyclic Regeneration for Adsorptive and Catalytic Performance of NiO-PdO/CeO2±δ Nanoparticles for n-C7 Asphaltene Steam Gasification. Nanomaterials 2019, 9, 734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medina, O.E.; Caro-Vélez, C.; Gallego, J.; Cortés, F.B.; Lopera, S.H.; Franco, C.A. Upgrading of Extra-Heavy Crude Oils by Dispersed Injection of NiO–PdO/CeO2±δ Nanocatalyst-Based Nanofluids in the Steam. Nanomaterials 2019, 9, 1755. [Google Scholar] [CrossRef] [Green Version]
- Franco-Ariza, C.A.; Guzmán-Calle, J.D.; Cortés, F.B. Adsorption and catalytic oxidation of asphaltenes in fumed silica nanoparticles: Effect of the surface acidity. Dyna 2016, 83, 171. [Google Scholar] [CrossRef]
- Nassar, N.N.; Hassan, A.; Carbognani, L.; Lopez-Linares, F.; Pereira-Almao, P. Iron oxide nanoparticles for rapid adsorption and enhanced catalytic oxidation of thermally cracked asphaltenes. Fuel 2012, 95, 257–262. [Google Scholar] [CrossRef]
- Nassar, N.N.; Hassan, A.; Pereira-Almao, P. Comparative oxidation of adsorbed asphaltenes onto transition metal oxide nanoparticles. Colloids Surf. A: Physicochem. Eng. Asp. 2011, 384, 145–149. [Google Scholar] [CrossRef]
- Nassar, N.N.; Hassan, A.; Pereira-Almao, P. Effect of surface acidity and basicity of aluminas on asphaltene adsorption and oxidation. J. Colloid Interface Sci. 2011, 360, 233–238. [Google Scholar] [CrossRef]
- Nassar, N.N.; Hassan, A.; Pereira-Almao, P. Effect of the particle size on asphaltene adsorption and catalytic oxidation onto alumina particles. Energy Fuels 2011, 25, 3961–3965. [Google Scholar] [CrossRef]
- Nassar, N.N.; Hassan, A.; Pereira-Almao, P. Thermogravimetric studies on catalytic effect of metal oxide nanoparticles on asphaltene pyrolysis under inert conditions. J. Therm. Anal. Calorim. 2012, 110, 1327–1332. [Google Scholar] [CrossRef]
- Barton, D.G.; Soled, S.L.; Iglesia, E. Solid acid catalysts based on supported tungsten oxides. Top. Catal. 1998, 6, 87–99. [Google Scholar] [CrossRef]
- Speight, J.G. Fouling in Refineries; Gulf Professional Publishing: Houston, TX, USA, 2015. [Google Scholar]
- J Spivey, J.; Dooley, K.M.; Han, Y.-F.; Meunier, F.; Tshentu, Z.R.; Dai, W.-L.; Jin, R.; Efstathiou, A.; Meunier, F.; Coppens, M.-O.; et al. Catalysis; Royal Society of Chemistry: Picadilly, UK, 2016; Volume 28. [Google Scholar]
- Kung, H.H. Transition Metal Oxides: Surface Chemistry and Catalysis; Elsevier: Amsterdam, The Netherlands, 1989; Volume 45. [Google Scholar]
- Wasmi, B.A.; Al-Amiery, A.A.; Kadhum, A.A.H.; Mohamad, A.B. Novel approach: Tungsten oxide nanoparticle as a catalyst for malonic acid ester synthesis via ozonolysis. J. Nanomater. 2014, 2014, 2. [Google Scholar] [CrossRef]
- Barre, L.; Espinat, D.; Rosenberg, E.; Scarsella, M. Colloidal structure of heavy crudes and asphaltene soltutions. Rev. De L’institut Français Du Pétrole 1997, 52, 161–175. [Google Scholar] [CrossRef] [Green Version]
- ASTM. D5236-13 Standard Test Method for Distillation of Heavy Hydrocarbon Mixtures (Vacuum Potstill Method). Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- ASTM. D2892 Standard Test Method for Distillation of Crude Petroleum (15-Theoretical Plate Column). Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- Franco, C.; Patiño, E.; Benjumea, P.; Ruiz, M.A.; Cortés, F.B. Kinetic and thermodynamic equilibrium of asphaltenes sorption onto nanoparticles of nickel oxide supported on nanoparticulated alumina. Fuel 2013, 105, 408–414. [Google Scholar] [CrossRef]
- Franco, C.A.; Nassar, N.N.; Montoya, T.; Ruíz, M.A.; Cortés, F.B. Influence of asphaltene aggregation on the adsorption and catalytic behavior of nanoparticles. Energy Fuels 2015, 29, 1610–1621. [Google Scholar] [CrossRef]
- Vert, M.; Hellwich, K.-H.; Hess, M.; Hodge, P.; Kubisa, P.; Rinaudo, M.; Schué, F. Terminology for biorelated polymers and applications (IUPAC Recommendations 2012). Pure Appl. Chem. 2012, 84, 377–410. [Google Scholar] [CrossRef]
- Medina, O.E.; Gallego, J.; Arias-Madrid, D.; Cortés, F.B.; Franco, C.A. Optimization of the load of transition metal oxides (Fe2O3, Co3O4, NiO and/or PdO) onto CeO2 nanoparticles in catalytic steam decomposition of n-C7 asphaltenes at low temperatures. Nanomaterials 2019, 9, 401. [Google Scholar] [CrossRef] [Green Version]
- Diao, W.; Tengco, J.M.M.; Regalbuto, J.R.; Monnier, J.R. Preparation and characterization of Pt–Ru bimetallic catalysts synthesized by electroless deposition methods. ACS Catal. 2015, 5, 5123–5134. [Google Scholar] [CrossRef]
- Geyer, R.; Hunold, J.; Keck, M.; Kraak, P.; Pachulski, A.; Schödel, R. Methods for determining the metal crystallite size of Ni supported catalysts. Chem. Ing. Tech. 2012, 84, 160–164. [Google Scholar] [CrossRef]
- Shastri, A.G.; Schwank, J. Metal dispersion of bimetallic catalysts via stepwise chemisorption and surface titration: I. Ru_AuSiO2. J. Catal. 1985, 95, 271–283. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Yao, H.; Dai, Q.; You, Z. Fourier Transform Infrared Spectroscopy characterization of aging-related properties of original and nano-modified asphalt binders. Constr. Build. Mater. 2015, 101, 1078–1087. [Google Scholar] [CrossRef]
- Leofanti, G.; Padovan, M.; Tozzola, G.; Venturelli, B. Surface area and pore texture of catalysts. Catal. Today 1998, 41, 207–219. [Google Scholar] [CrossRef]
- Guzmán, J.D.; Betancur, S.; Carrasco-Marín, F.; Franco, C.A.; Nassar, N.N.; Cortés, F.B. Importance of the Adsorption Method Used for Obtaining the Nanoparticle Dosage for Asphaltene-Related Treatments. Energy Fuels 2016, 30, 2052–2059. [Google Scholar] [CrossRef]
- Giraldo, J.; Nassar, N.N.; Benjumea, P.; Pereira-Almao, P.; Cortés, F.B. Modeling and prediction of asphaltene adsorption isotherms using Polanyi’s modified theory. Energy Fuels 2013, 27, 2908–2914. [Google Scholar] [CrossRef]
- Cortés, F.B.; Chejne, F.; Carrasco-Marín, F.; Moreno-Castilla, C.; Pérez-Cadenas, A.F. Water adsorption on zeolite 13X: Comparison of the two methods based on mass spectrometry and thermogravimetry. Adsorption 2010, 16, 141–146. [Google Scholar] [CrossRef]
- Montoya, T.; Argel, B.L.; Nassar, N.N.; Franco, C.A.; Cortés, F.B. Kinetics and mechanisms of the catalytic thermal cracking of asphaltenes adsorbed on supported nanoparticles. Pet. Sci. 2016, 13, 561–571. [Google Scholar] [CrossRef] [Green Version]
- Franco Ariza, C.A. Synthesis and Application of Supported Metallic and Multi-Metallic Oxides Nanoparticles for In-Situ Upgrading and Inhibition of Formation Damage; Universidad Nacional de Colombia: Bogota, Columbia, 2015. [Google Scholar]
- López, D.; Giraldo, L.J.; Salazar, J.P.; Zapata, D.M.; Ortega, D.C.; Franco, C.A.; Cortés, F.B. Metal Oxide Nanoparticles Supported on Macro-Mesoporous Aluminosilicates for Catalytic Steam Gasification of Heavy Oil Fractions for On-Site Upgrading. Catalysts 2017, 7, 319. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-F.; Lee, C.-Y.; Yeng, M.-Y.; Chiu, H.-T. The effect of calcination temperature on the crystallinity of TiO2 nanopowders. J. Cryst. Growth 2003, 247, 363–370. [Google Scholar] [CrossRef]
- Colque, S.; Payen, E.; Grange, P. Novel preparation of highly dispersed tungsten oxide on silica. J. Mater. Chem. 1994, 4, 1343–1348. [Google Scholar] [CrossRef]
- Elkins, T.W.; Hagelin-Weaver, H.E. Characterization of Mn–Na2WO4/SiO2 and Mn–Na2WO4/MgO catalysts for the oxidative coupling of methane. Appl. Catal. A: Gen. 2015, 497, 96–106. [Google Scholar] [CrossRef]
- Salmaoui, S.; Sediri, F.; Gharbi, N.; Perruchot, C.; Jouini, M. Hexagonal hydrated tungsten oxide nanomaterials: Hydrothermal synthesis and electrochemical properties. Electrochim. Acta 2013, 108, 634–643. [Google Scholar] [CrossRef]
- Terohid, S.A.A.; Heidari, S.; Jafari, A.; Asgary, S. Effect of growth time on structural, morphological and electrical properties of tungsten oxide nanowire. Appl. Phys. A 2018, 124, 567. [Google Scholar] [CrossRef] [Green Version]
- Biesinger, M.C. X-ray Photoelectron Spectroscopy (XPS) Reference Pages. Available online: http://www.xpsfitting.com/ (accessed on 1 May 2020).
- Agilent Scientific Instruments, Thermo Scientific XPS Simplified. Available online: https://xpssimplified.com/ (accessed on 1 May 2020).
- Susanti, D.; Diputra, A.A.G.P.; Tananta, L.; Purwaningsih, H.; Kusuma, G.E.; Wang, C.; Shih, S.; Huang, Y. WO3 nanomaterials synthesized via a sol-gel method and calcination for use as a CO gas sensor. Front. Chem. Sci. Eng. 2014, 8, 179–187. [Google Scholar] [CrossRef]
- Bursill, L.A. Structure of small defects in nonstoichiometric WO3−x. J. Solid State Chem. 1983, 48, 256–271. [Google Scholar] [CrossRef]
- Fukushi, D.; Sasaki, A.; Hirabayashi, H.; Kitano, M. Effect of oxygen vacancy in tungsten oxide on the photocatalytic activity for decomposition of organic materials in the gas phase. Microelectron. Reliab. 2017, 79, 1–4. [Google Scholar] [CrossRef]
- Tanaka, D.; Oaki, Y.; Imai, H. Enhanced photocatalytic activity of quantum-confined tungsten trioxide nanoparticles in mesoporous silica. Chem. Commun. 2010, 46, 5286–5288. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Feng, M.; Liu, F.; Liu, L.; Chen, H.; Gao, H.; Li, J. Structures and defects of WO3−x nanorods grown by in-situ heating tungsten filament. Chem. Phys. Lett. 2004, 389, 337–341. [Google Scholar] [CrossRef]
- Gayapan, K.; Sripinun, S.; Panpranot, J.; Praserthdam, P.; Assabumrungrat, S. Effect of pretreatment atmosphere of WOX/SiO2 catalysts on metathesis of ethylene and 2-butene to propylene. RSC Adv. 2018, 8, 11693–11704. [Google Scholar] [CrossRef] [Green Version]
- Medina, O.E.; Gallego, J.; Olmos, C.M.; Chen, X.; Cortés, F.B.; Franco, C.A. Effect of multifunctional nanocatalysts on n-C7 asphaltene adsorption and subsequent oxidation under high-pressure conditions. Energy Fuels 2020, 34, 6261–6278. [Google Scholar] [CrossRef]
- Ramirez-Corredores, M.M. The Science and Technology of Unconventional Oils: Finding Refining Opportunities; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Zimmer, A.K.; Becker, C.; Chambliss, C.K. Exploiting metal oxide nanoparticle selectivity in asphaltenes for identification of pyridyl-containing molecules. Energy Fuels 2013, 27, 4574–4580. [Google Scholar] [CrossRef]
- Hosseinpour, N.; Khodadadi, A.A.; Bahramian, A.; Mortazavi, Y. Asphaltene adsorption onto acidic/basic metal oxide nanoparticles toward in situ upgrading of reservoir oils by nanotechnology. Langmuir 2013, 29, 14135–14146. [Google Scholar] [CrossRef]
- Fritschy, G.; Papirer, E. Interactions between a bitumen, its components and model fillers. Fuel 1978, 57, 701–704. [Google Scholar] [CrossRef]
- Andreini, A.; Mol, J.C. Activity of supported tungsten oxide catalysts for the metathesis of propene. J. Chem. Soc. Faraday Trans. 1: Phys. Chem. Condens. Phases 1985, 81, 1705–1714. [Google Scholar] [CrossRef]
- Dudášová, D.; Simon, S.; Hemmingsen, P.V.; Sjöblom, J. Study of asphaltenes adsorption onto different minerals and clays: Part 1. Experimental adsorption with UV depletion detection. Colloids Surf. A: Physicochem. Eng. Asp. 2008, 317, 1–9. [Google Scholar] [CrossRef]
- Contreras, J.L.; Fuentes, G.A. Sintering of Supported Metal Catalysts. In Sintering-Methods and Products; InTech: London, UK, 2012. [Google Scholar]
- Siddiqui, M.N. Catalytic pyrolysis of Arab Heavy residue and effects on the chemistry of asphaltene. J. Anal. Appl. Pyrolysis 2010, 89, 278–285. [Google Scholar] [CrossRef]
- Hamedi Shokrlu, Y.; Babadagli, T. In-situ upgrading of heavy oil/bitumen during steam injection by use of metal nanoparticles: A study on in-situ catalysis and catalyst transportation. SPE Reserv. Eval. Eng. 2013, 16, 333–344. [Google Scholar] [CrossRef]
- Breysse, M.; Furimsky, E.; Kasztelan, S.; Lacroix, M.; Perot, G. Hydrogen activation by transition metal sulfides. Catal. Rev. 2002, 44, 651–735. [Google Scholar] [CrossRef]
- Kawai, T.; Goto, H.; Yamazaki, Y.; Ishikawa, T. Metathesis of n-alkenes over a CsNO3-Re2O7-Al2O3 catalyst. J. Mol. Catal. 1988, 46, 157–172. [Google Scholar] [CrossRef]
- Spamer, A.; Dube, T.; Moodley, D.; Van Schalkwyk, C.; Botha, J. The reduction of isomerisation activity on a WO3/SiO2 metathesis catalyst. Appl. Catal. A: Gen. 2003, 255, 153–167. [Google Scholar] [CrossRef]
- Van Roosmalen, A.; Mol, J. Active centers for the metathesis and isomerization of alkenes on tungsten-oxide/silica catalysts. J. Catal. 1982, 78, 17–23. [Google Scholar] [CrossRef]
- Nassar, N.N.; Hassan, A.; Vitale, G. Comparing kinetics and mechanism of adsorption and thermo-oxidative decomposition of Athabasca asphaltenes onto TiO2, ZrO2, and CeO2 nanoparticles. Appl. Catal. A: Gen. 2014, 484, 161–171. [Google Scholar] [CrossRef]
- Gradisher, L.; Dutcher, B.; Fan, M. Catalytic hydrogen production from fossil fuels via the water gas shift reaction. Applied Energy 2015, 139, 335–349. [Google Scholar] [CrossRef]
- Kök, M.V.; Pamir, M.R. Pyrolysis and combustion studies of fossil fuels by thermal analysis methods. J. Anal. Appl. Pyrolysis 1995, 35, 145–156. [Google Scholar] [CrossRef]
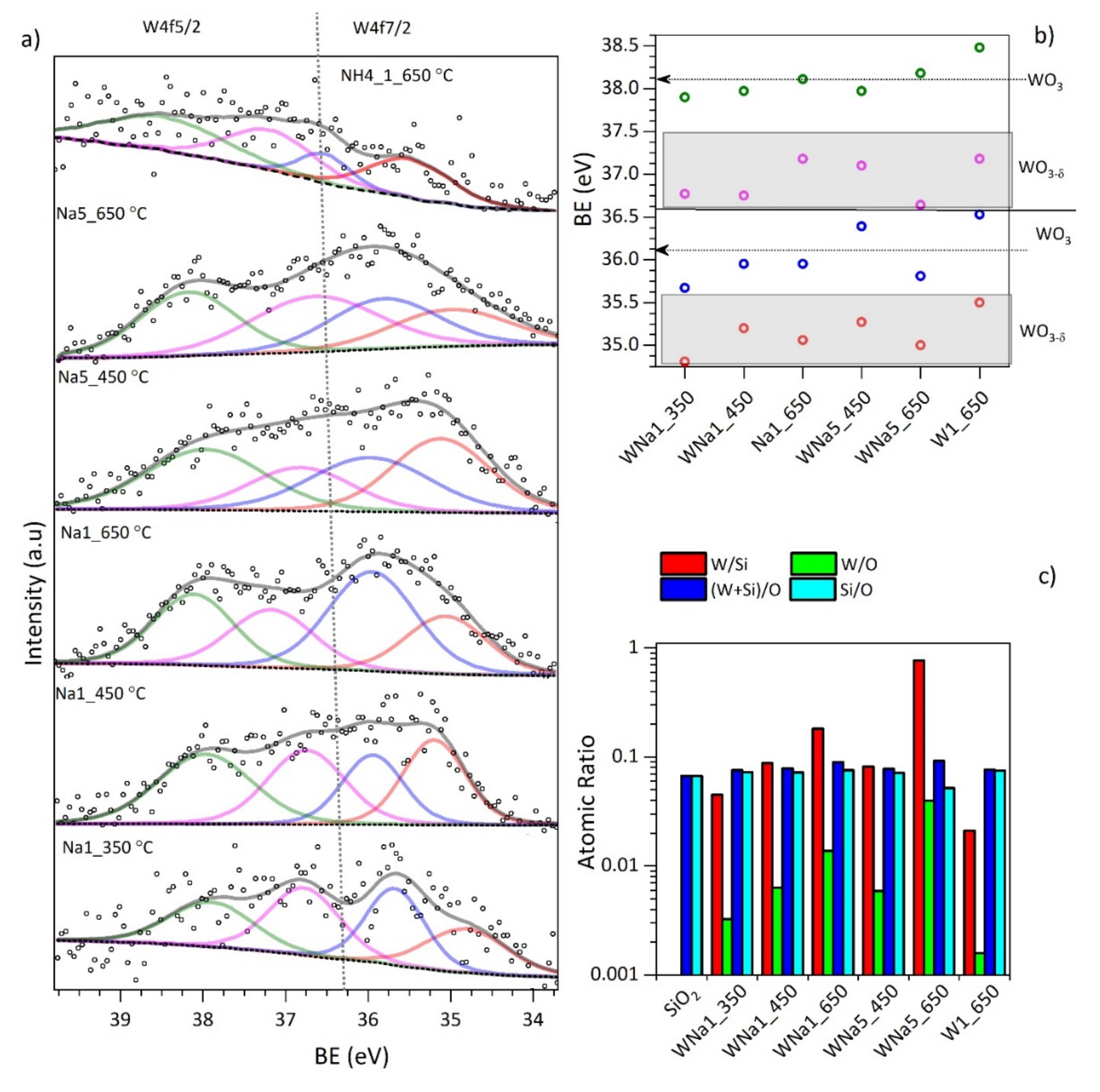
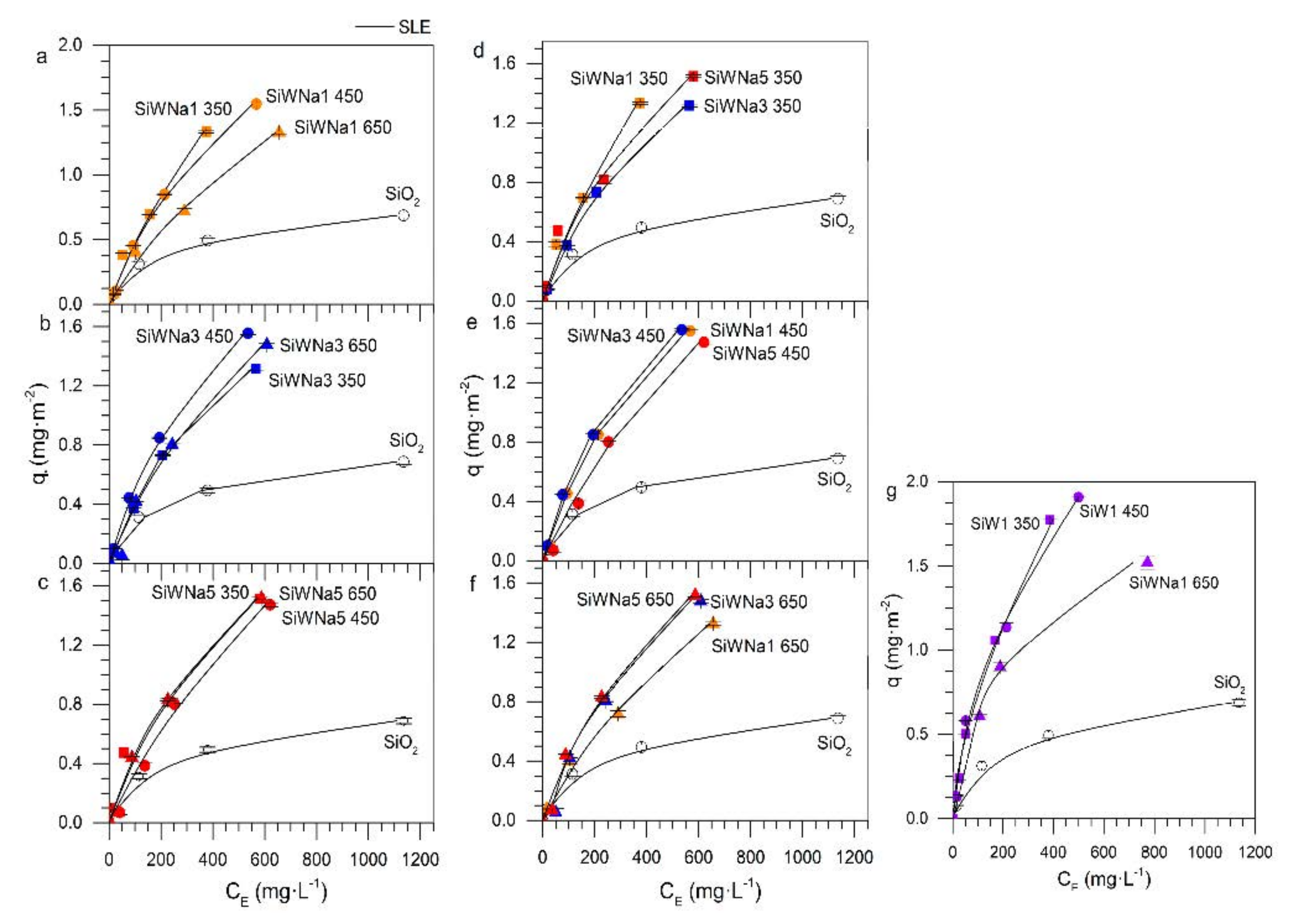
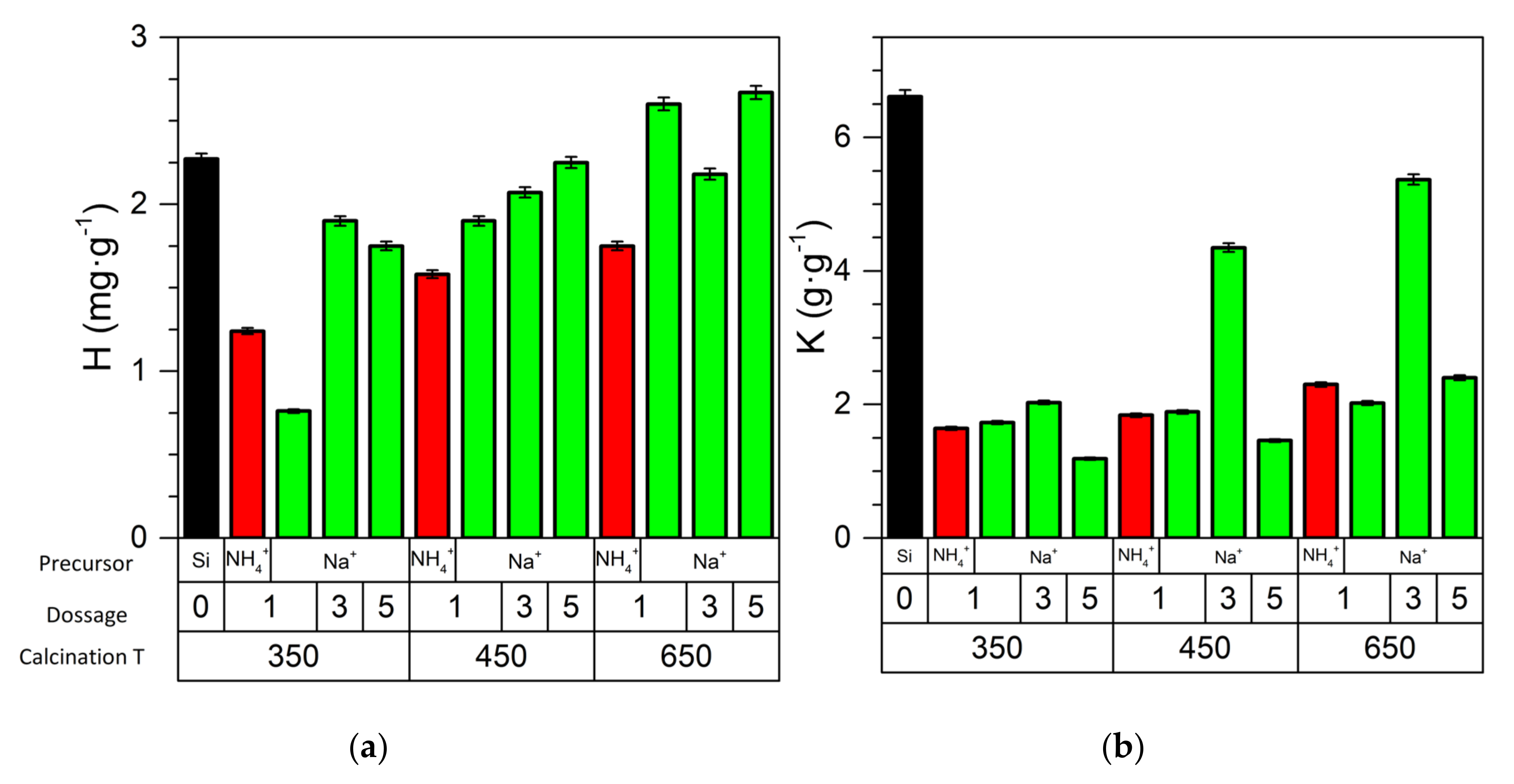


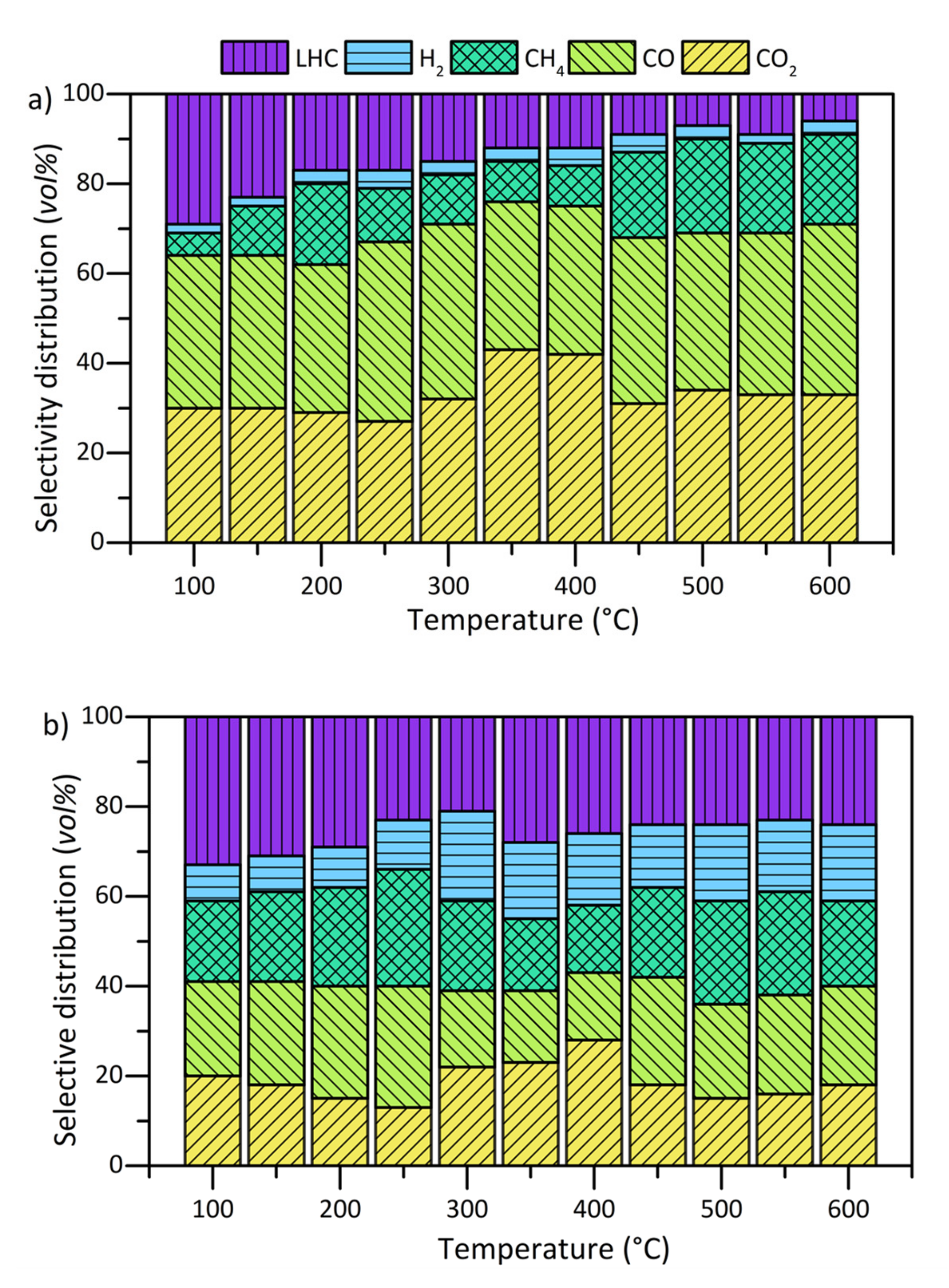
| Material | * SBET ± 0.1 m2/g | * MSAP ± 0.1 nm | Tungsten Dispersion (%) |
|---|---|---|---|
| SiO2 | 126.1 | - | - |
| SiWNa1 350 | 122.3 | 1.6 | 26.1 |
| SiWNa1 450 | 92.9 | 1.9 | 19.5 |
| SiWNa1 650 | 101.1 | 2.6 | 16.5 |
| SiWNa3 350 | 108.5 | 7.6 | 13.6 |
| SiWNa3 450 | 93.5 | 8.6 | 10.8 |
| SiWNa3 650 | 94.3 | 10.2 | 5.6 |
| SiWNa5 350 | 94.7 | 9.5 | 5.6 |
| SiWNa5 450 | 94.2 | 13.7 | 3.1 |
| SiWNa5 650 | 93.7 | 15.4 | 2.9 |
| SiW1 350 | 81.1 | 1.8 | 15.2 |
| SiW1 450 | 79.6 | 3.4 | 12.6 |
| SiW1 650 | 66.2 | 4.7 | 9.1 |
| Material | H (mg·g−1) | K × 10−4 (g/g) | qm (g·g−1) | R2 | χ2 |
|---|---|---|---|---|---|
| Si | 2.27 | 6.61 | 1.29 | 0.99 | 0.49 |
| SiWNa1 350 | 0.76 | 1.73 | 3.21 | 0.99 | 0.19 |
| SiWNa1 450 | 1.90 | 1.89 | 4.41 | 0.99 | 0.62 |
| SiWNa1 650 | 2.60 | 2.02 | 4.80 | 0.99 | 0.48 |
| SiWNa3 350 | 1.90 | 2.03 | 4.37 | 0.99 | 0.19 |
| SiWNa3 450 | 2.07 | 4.35 | 4.95 | 0.99 | 0.62 |
| SiWNa3 650 | 2.18 | 5.37 | 4.66 | 0.99 | 0.48 |
| SiWNa5 350 | 1.75 | 1.19 | 4.20 | 0.99 | 1.00 |
| SiWNa5 450 | 2.25 | 1.46 | 4.78 | 0.99 | 1.25 |
| SiWNa5 650 | 2.67 | 2.40 | 5.51 | 0.99 | 0.18 |
| SiW1 350 | 1.24 | 1.64 | 6.02 | 0.99 | 0.46 |
| SiW1 450 | 1.58 | 1.84 | 5.95 | 0.99 | 0.50 |
| SiW1 650 | 1.75 | 2.30 | 3.14 | 0.99 | 0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerón, K.M.; Arias-Madrid, D.; Gallego, J.; Medina, O.E.; Chinchilla, L.E.; Cortés, F.B.; Franco, C.A. Catalytic Decomposition of n-C7 Asphaltenes Using Tungsten Oxides–Functionalized SiO2 Nanoparticles in Steam/Air Atmospheres. Processes 2022, 10, 349. https://doi.org/10.3390/pr10020349
Cerón KM, Arias-Madrid D, Gallego J, Medina OE, Chinchilla LE, Cortés FB, Franco CA. Catalytic Decomposition of n-C7 Asphaltenes Using Tungsten Oxides–Functionalized SiO2 Nanoparticles in Steam/Air Atmospheres. Processes. 2022; 10(2):349. https://doi.org/10.3390/pr10020349
Chicago/Turabian StyleCerón, Karen M., Daniela Arias-Madrid, Jaime Gallego, Oscar E. Medina, Lidia E. Chinchilla, Farid B. Cortés, and Camilo A. Franco. 2022. "Catalytic Decomposition of n-C7 Asphaltenes Using Tungsten Oxides–Functionalized SiO2 Nanoparticles in Steam/Air Atmospheres" Processes 10, no. 2: 349. https://doi.org/10.3390/pr10020349
APA StyleCerón, K. M., Arias-Madrid, D., Gallego, J., Medina, O. E., Chinchilla, L. E., Cortés, F. B., & Franco, C. A. (2022). Catalytic Decomposition of n-C7 Asphaltenes Using Tungsten Oxides–Functionalized SiO2 Nanoparticles in Steam/Air Atmospheres. Processes, 10(2), 349. https://doi.org/10.3390/pr10020349










