Human Cytochrome P450 2C9 and Its Polymorphic Modifications: Electroanalysis, Catalytic Properties, and Approaches to the Regulation of Enzymatic Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrochemical Procedures
2.3. Preparation of Electrochemical Sensors
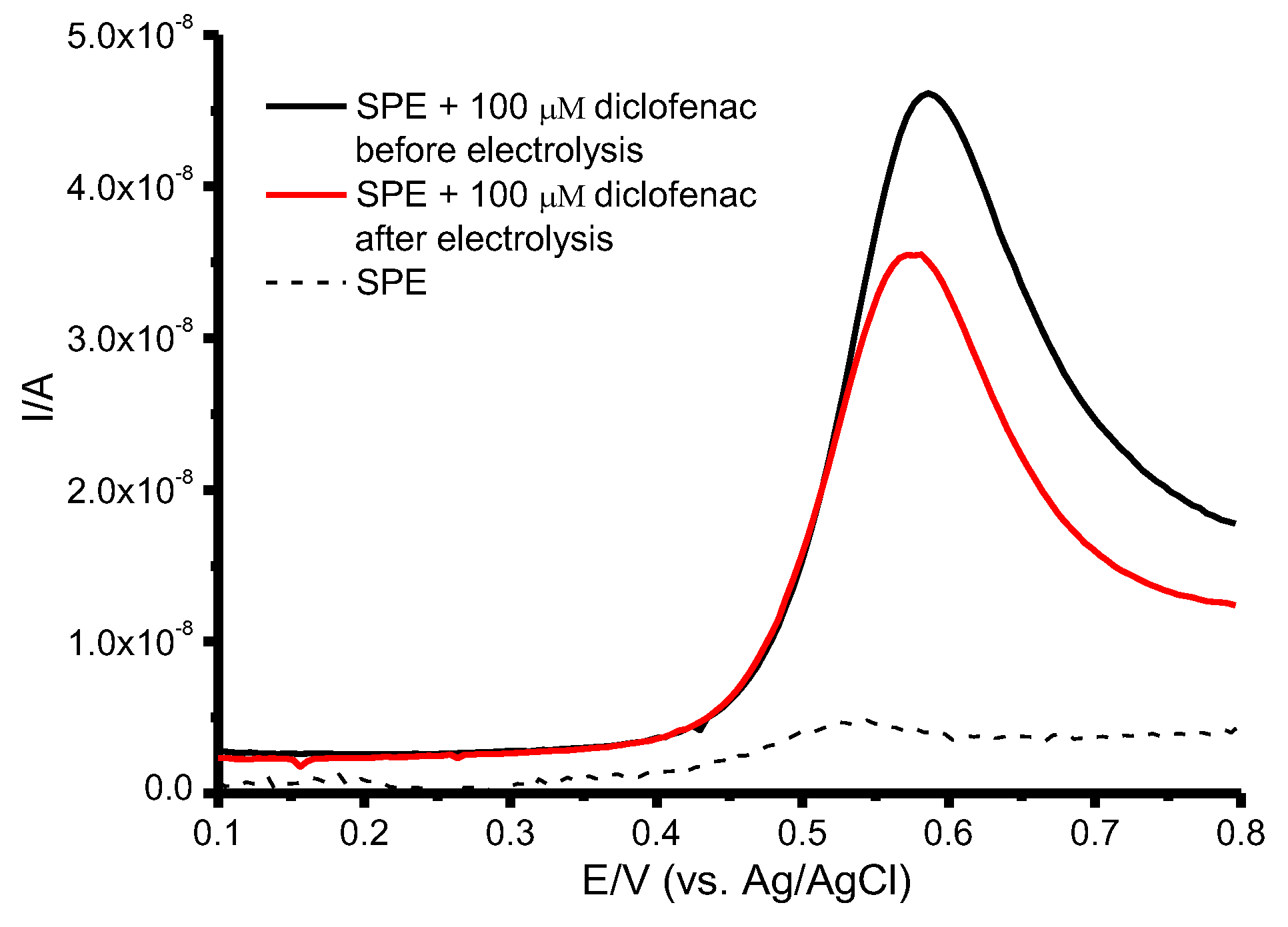
2.4. Electrocatalysis of P450 2C9, 2C9*2, and 2C9* in the Presence of Diclofenac
3. Results and Discussion
3.1. Electrochemical Characteristics and Electrocatalytic Properties of Cytochrome P450 2C9 and Polymorphic Modifications P450 2C9*2 and P450 2C9*3
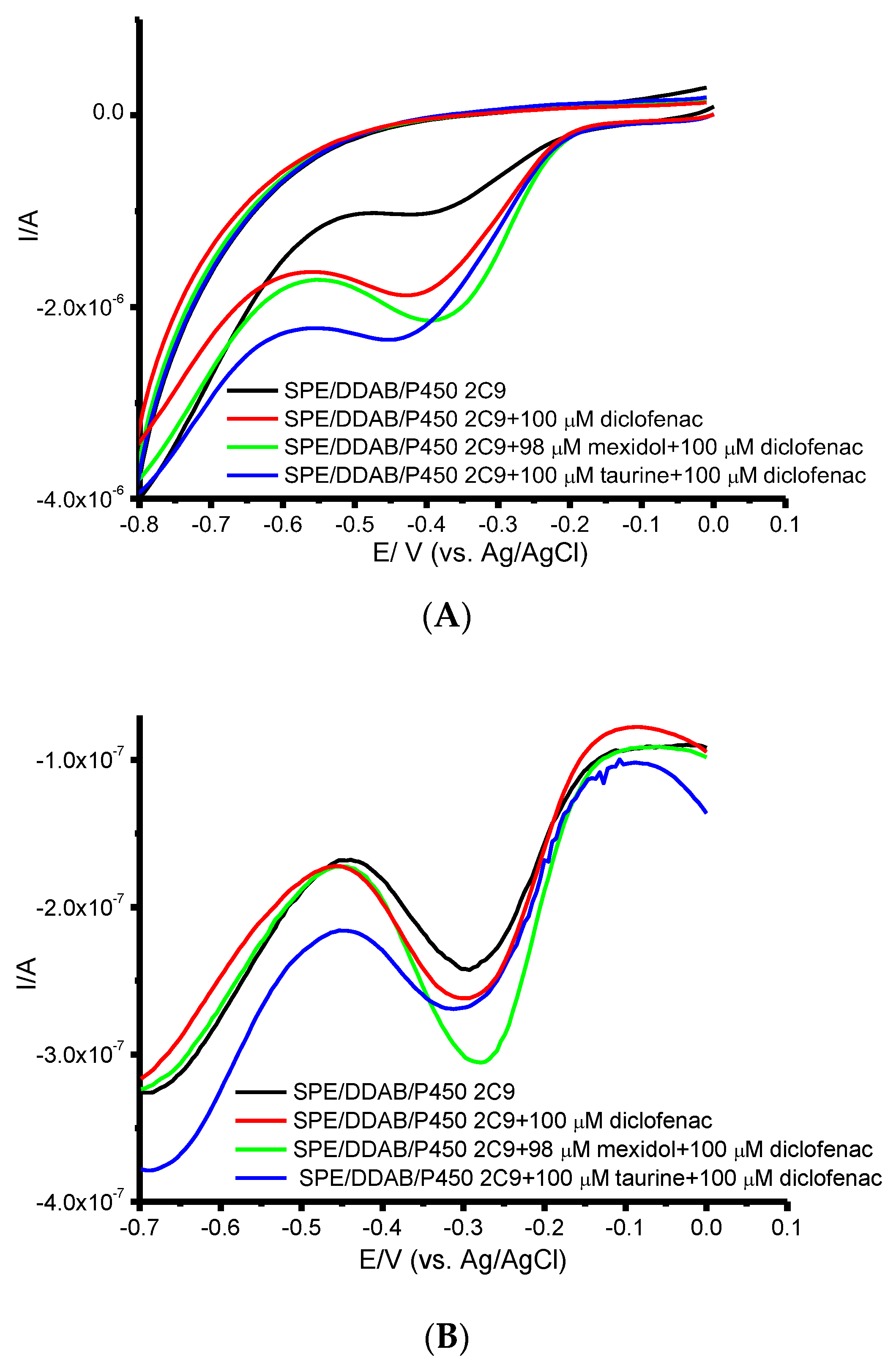
3.2. Influence of the Antioxidants on the Catalytic Activity of Cytochrome P450 2C9 and Polymorphic Modifications P450 2C9*2 and P450 2C9*3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef] [PubMed]
- Isvoran, A.; Louet, M.; Vladoiu, D.L.; Craciun, D.; Loriot, M.-A.; Villoutreix, B.O.; Miteva, M.A. Pharmacogenomics of the cytochrome P450 2C family: Impacts of amino acid variations on drug metabolism. Drug. Discov. Today. 2017, 22, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Manish, M.; Andrew, M.L.; Smriti, M. Cytochrome P450 2C9 polymorphism: Effect of amino acid substitutions on protein flexibility in the presence of tamoxifen. Comput. Biol. Chem. 2020, 84, 107166. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.-F.; Zhou, Z.-W.; Huang, M. Polymorphisms of human cytochrome P450 2C9 and the functional relevance. Toxicology 2010, 278, 165–188. [Google Scholar] [CrossRef]
- Patton, A.L.; Seely, K.A.; Yarbrough, A.L.; Fantegrossi, W.; James, L.P.; McCain, K.R.; Fujiwara, R.; Prather, P.L.; Moran, J.H.; Radominska-Pandya, A. Altered metabolism of synthetic cannabinoid JWH-018 by human cytochrome P450 2C9 and variants. Biochem. Biophys. Res. Commun. 2018, 498, 597–602. [Google Scholar] [CrossRef]
- Jarrar, Y.B.; Lee, S.J. Molecular functionality of CYP2C9 polymorphisms and their influence on drug therapy. Drug Metabol. Drug Interact. 2014, 29, 211–220. [Google Scholar] [CrossRef]
- Yamazaki, H.; Inoue, K.; Chiba, K.; Ozawa, N.; Kawai, T.; Suzuki, Y.; Goldstein, J.A.; Guengerich, F.P.; Shimada, T. Comparative Studies on the Catalytic Roles of Cytochrome P450 2C9 and Its Cys- and Leu-Variants in the Oxidation of Warfarin, Flurbiprofen, and Diclofenac by Human Liver Microsomes. Biochem. Pharmacol. 1998, 56, 243–251. [Google Scholar] [CrossRef]
- McBride, G.M.; Soo, J.Y.; Varcoe, T.; Morrison, J.L.; Wiese, M.D. Development of a method to determine cytochrome P450 1A2, 2C9, 2D6 and 3A4 activity sheep hepatic microsomes. J. Pharmacol. Toxicol. Methods. 2020, 106, 106934. [Google Scholar] [CrossRef]
- Yamazaki, H.; Inoue, K.; Shimada, T. Roles of two allelic 642 variants (Arg144Cys and Ile359Leu) of cytochrome P450 2C9 in the oxidation of tolbutamide and warfarin by human liver microsomes. Xenobiotica 1998, 28, 103–115. [Google Scholar] [CrossRef]
- Melet, A.; Assrir, N.; Jean, P.; Lopez-Garcia, M.P.; Marques-Soares, C.; Jaouen, M.; Dansette, P.M.; Sari, M.-A.; Mansuy, D. Substrate selectivity of human cytochrome P450 2C9: Importance of residues 476, 365, and 114 in recognition of diclofenac and sulfaphenazole and in mechanism-based inactivation by tienilic acid. Arch. Biochem. Biophys. 2003, 409, 80–91. [Google Scholar] [CrossRef]
- Maekawa, K.; Adachi, M.; Matsuzawa, Y.; Zhan, Q.; Kuroki, R.; Saito, Y.; Shah, M.B. Structural Basis of Single Nucleotide Polymorphisms in Cytochrome P450 2C9. Biochemistry 2017, 56, 5476–5480. [Google Scholar] [CrossRef] [PubMed]
- Mie, Y.; Tateyama, E.; Komatsu, Y. P-Aminothiophenol modification on gold surface improves stability for electrochemically driven cytochrome P450 microsome activity. Electrochim. Acta. 2014, 115, 364–369. [Google Scholar] [CrossRef]
- Panicco, P.; Castrignanò, S.; Sadeghi, S.J.; Di Nardo, G.; Gilardi, G. Engineered human CYP2C9 and its main polymorphic variants for bioelectrochemical measurements of catalytic response. Bioelectrochemistry 2021, 138, 107729. [Google Scholar] [CrossRef] [PubMed]
- Shumyantseva, V.V.; Makhova, A.A.; Bulko, T.V.; Kuzikov, A.V.; Masamrekh, R.A.; Shkel, T.; Usanov, S.; Gilep, A.; Archakov, A.I. Bioelectrochemical systems as technologies for studying drug interactions related to cytochrome P450. BioNanoScience 2018, 9, 79–86. [Google Scholar] [CrossRef]
- Im, S.C.; Waskell, L. The interaction of microsomal cytochrome P450 2B4 with its redox partners, cytochrome P450 reductase and cytochrome b5. Arch. Biochem. Biophys. 2011, 507, 144–153. [Google Scholar] [CrossRef] [Green Version]
- Shumyantseva, V.V.; Makhova, A.A.; Bulko, T.V.; Kuzikov, A.V.; Shich, E.V.; Suprun, E.V.; Kukes, V.; Usanov, S.; Archakov, A.I. The dose-dependent influence of antioxidant vitamins on electrochemically-driven cytochrome P450 3A4 catalysis. Oxid. Antioxid. Med. Sci. 2013, 2, 113–117. [Google Scholar] [CrossRef]
- Shumyantseva, V.V.; Makhova, A.A.; Bulko, T.V.; Kuzikov, A.V.; Shich, E.V.; Kukes, V.; Archakov, A.I. Electrocatalytic cycle of P450 cytochromes: The protective and stimulating roles of antioxidants. RSC Adv. 2015, 5, 71306. [Google Scholar] [CrossRef]
- Makhova, A.A.; Shikh, E.V.; Bulko, T.V.; Sizova, Z.M.; Shumyantseva, V.V. The influence of taurine and L-carnitine on 6 β-hydroxycortisol/cortisol ratio in human urine of healthy volunteers. Drug Metabol. Personal. Ther. 2019, 20190013. [Google Scholar] [CrossRef]
- Makhova, A.A.; Shumyantseva, V.V.; Shich, E.V.; Bulko, T.V.; Kukes, V.G.; Sizova, O.S.; Ramenskaya, G.V.; Usanov, S.A.; Archakov, A.I. Electroanalysis of cytochrome P450 3A4 catalytic properties with nanostructured electrodes: The influence of vitamin B group on diclofenac metabolism. BioNanoScience 2011, 1, 46–52. [Google Scholar] [CrossRef]
- Shumyantseva, V.V.; Makhova, A.A.; Bulko, T.V.; Bernhardt, R.; Kuzikov, A.V.; Shich, E.V.; Kukes, V.G.; Archakov, A.I. Taurine modulates catalytic activity of cytochrome P450 3A4. Biochemistry 2015, 80, 366–373. [Google Scholar] [CrossRef]
- Shumyantseva, V.V.; Shich, E.V.; Makhova, A.A.; Bulko, T.V.; Kukes, V.G.; Sizova, O.S.; Ramenskaya, G.V.; Usanov, S.A.; Archakov, A.I. The Influence of B-Group Vitamins on Monooxygenase Activity of Cytochrome P450 3A4: Pharmacokinetics and Electro Analysis of the Catalytic Properties. Biochem. Suppl. Ser. B Biomed. Chem. 2012, 6, 87–93. [Google Scholar] [CrossRef]
- Pechurskaya, T.A.; Lukashevich, O.P.; Gilep, A.A.; Usanov, S.A. Engineering, expression, and purification of “soluble” human cytochrome P45017alpha and its functional characterization. Biochemistry 2008, 73, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Omura, T.; Sato, R. The carbon monoxide-binding pigment of liver microsomes. II. Solubilization, purification, and properties. J. Biol. Chem. 1964, 239, 2379–2385. [Google Scholar] [CrossRef]
- Shumyantseva, V.V.; Bulko, T.V.; Archakov, A.I. Electrochemical reduction of cytochrome P450 as an approach to the construction of biosensors and bioreactors. J. Inorg. Biochem. 2005, 99, 1051–1063. [Google Scholar] [CrossRef]
- Schneider, E.; Clark, D.S. Cytochrome P450 (CYP) enzymes and the development of CYP biosensors. Biosens. Bioelectron. 2013, 39, 1–13. [Google Scholar] [CrossRef]
- Johnson, D.L.; Lewis, B.C.; Elliot, D.J.; Miners, J.O.; Martin, L.L. Electrochemical characterization of the human cytochrome P450 CYP2C9. Biochem. Pharmacol. 2005, 69, 1533–1541. [Google Scholar] [CrossRef]
- Lu, J.; Cui, D.; Li, H.; Zhang, Y.; Liu, S. Cytochrome P450 bienzymes assembled on Au/chitosan/reduced grapheme oxide nanosheets for electrochemically-driven drug cascade metabolism. Electrochim. Acta 2015, 165, 36–44. [Google Scholar] [CrossRef]
- Shumyantseva, V.V.; Kuzikov, A.V.; Masamrekh, R.A.; Bulko, T.V.; Archakov, A.I. From electrochemistry to enzyme kinetics of cytochrome P450. Biosens. Bioelectron. 2018, 121, 192–204. [Google Scholar] [CrossRef]
- Mi, L.; He, F.; Jiang, L.; Shangguan, L.; Zhang, X.; Ding, T.; Liu, A.; Zhang, Y.; Liu, S. Electrochemically-driven benzo [a] pyrene metabolism via human cytochrome P450 1A1 with reductase coated nitrogen-doped graphene nano- composites. J. Electroanal. Chem. 2017, 804, 23–28. [Google Scholar] [CrossRef]
- Murray, R.W.; Bard, A.J. Electroanalytical Chemistry; Marcel Dekker: New York, NY, USA, 2001; p. 191. [Google Scholar]
- Rusling, J.F.; Wang, B.; Yun, S. Electrochemistry of redox enzymes. In Bioelectrochemistry: Fundametals, Experimental Techniques and Applications; Bartlett, P.N., Ed.; John Wiley & Sons, Ltd.: New Jersey, NJ, USA, 2008; pp. 39–85. [Google Scholar] [CrossRef]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Sadeghi, S.J.; Ferrero, S.; Di Nardo, G.; Gilardi, G. Drug-drug interactions and cooperative effects detected in electrochemically driven human cytochrome P450 3A4. Bioelectrochem. 2012, 86, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Wu, Y. An amperometric biosensor based on human cytochrome P450 2C9 in polyacrylamide hydrogel films for bisphenol A determination. Sens. Actuators B. Chem. 2013, 178, 113–118. [Google Scholar] [CrossRef]
- Pandey, S.K.; Yadav, S.; Goel, Y.; Temre, M.K.; Singh, V.K.; Singh, S.M. Molecular docking of anti-inflammatory drug diclofenac with metabolic targets: Potential applications in cancer therapeutics. J. Theor. Biol. 2019, 465, 117–125. [Google Scholar] [CrossRef]
- Arisan, E.D.; Akar, R.O.; Rencuzogullari, O.; Yerlikaya, P.O.; Gurkan, A.C.; Akın, B.; Dener, E.; Kayhan, E.; Unsal, N.P. The molecular targets of diclofenac differs from ibuprofen to induce apoptosis and epithelial mesenchymal transition due to alternation on oxidative stress management p53 independently in PC3 prostate cancer cells. Prostate Int. 2019, 7, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Guan, X.; Dai, Z.; He, R.; Ding, X.; Yang, L.; Ge, G. Molecular probes for human cytochrome P450 enzymes: Recent progress and future perspectives. Coord. Chem. Rev. 2021, 421, 213600. [Google Scholar] [CrossRef]
- Wang, J. Analytical Electrochemistry, 3rd ed.; Wiley-VCH: Weinheim, Germany, 2006; p. 32. [Google Scholar]
- Kaminsky, L.S.; Zhang, Z.Y. Human P450 metabolism of warfarin. Pharmacol. Ther. 1997, 73, 67–74. [Google Scholar] [CrossRef]
- Haining, R.L.; Hunter, A.P.; Veronese, M.E.; Trager, W.F.; Rettie, A.E. Allelic variants of human cytochrome P450 2C9: Baculovirus-mediated expression, purification, structural characterization, substrate stereoselectivity, and prochiral selectivity of the wild-type and I359L mutant forms. Arch. Biochem. Biophys. 1996, 333, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, Y.; Si, D.; Fawcett, P.J.; Zhong, D.; Zhou, H. Catalytic activities of human cytochrome P450 2C9*1, 2C9*3 and 2C9*13. Xenobiotica 2005, 35, 853–861. [Google Scholar] [CrossRef]
- Shumyantseva, V.V.; Bulko, T.V.; Kuzikov, A.V.; Masamrekh, R.A.; Konyakhina, A.Y.; Romanenko, I.; Max, J.B.; Köhlere, M.; Gilep, A.A.; Usanov, S.A.; et al. All-electrochemical nanocomposite two-electrode setup for quantification of drugs and study of their electrocatalytical conversion by cytochromes P450. Electrochim. Acta. 2020, 336, 135579. [Google Scholar] [CrossRef]
- Letelier, M.A.; Jara-Sandoval, J.; Molina-Berríos, A.; Faúndez, M.; Aracena-Parks, P.; Aguilera, F. Melatonin protects the cytochrome P450 system through a novel antioxidant mechanism. Chem. Biol. Interact. 2010, 185, 208–214. [Google Scholar] [CrossRef]
- Gade, S.K.; Bhattacharya, S.; Manoj, K.M. Redox active molecules cytochrome c and vitamin C enhance heme-enzyme peroxidations by serving as non-specific agents for redox relay. Biochem. Biophys. Res. Commun. 2012, 419, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Bian, C.; Xiong, H.; Zhang, X.; Ye, Y.; Gu, H.; Wang, S. Electrochemical detection of BSA damage induced by Fenton reagents in room temperature ionic liquid. Sens. Actuators 2012, 169, 368–373. [Google Scholar] [CrossRef]
- de Montellano, P.R.O. Cytochrome P450: Structure, Mechanism, and Biochemistry; Springer: New York, NY, USA, 2015; pp. 523–785. [Google Scholar] [CrossRef]
- Samadi, M.; Haghi-Aminjan, H.; Sattari, M.; Shayesteh, M.R.H.; Bameri, B.; Armandeh, M.; Naddafi, M.; Eghbal, M.A.; Abdollahi, M. The role of taurine on chemotherapy-induced cardiotoxicity: A systematic review of non-clinical study. Life Sci. 2021, 265, 118813. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, A.M.; Javadivala, Z. A systematic review of preclinical studies on the efficacy of taurine for the treatment of rheumatoid arthritis. Amino Acids. 2021, 53, 783–800. [Google Scholar] [CrossRef] [PubMed]
- Miyata, M.; Tanaka, T.; Takahashi, K.; Funaki, A.; Sugiura, Y. Cholesterol-lowering effects of taurine through the reduction of ileal FXR signaling due to the alteration of ileal bile acid composition. Amino Acids. 2021, 53, 1523–1532. [Google Scholar] [CrossRef]
- Atila, A.; Alay, H.; Yaman, M.E.; Akman, ·T.C.; Cadirci, E.; Bayrak, ·B.; Celik, ·S.; Atila, ·N.E.; Yaganoglu, A.M.; Kadioglu, Y.; et al. The serum amino acid profile in COVID-19. Amino Acids 2021, 53, 1569–1588. [Google Scholar] [CrossRef]
- Jensen, S.B.; Thodberg, S.; Parween, S.; Moses, M.E.; Hansen, C.C.; Thomsen, J.; Sletfjerding, M.B.; Knudsen, C.; Giudice, R.D.; Lund, P.M.; et al. Biased cytochrome P450-mediated metabolism via small-molecule ligands binding P450 oxidoreductase. Nat. Commun. 2021, 12, 2260. [Google Scholar] [CrossRef]

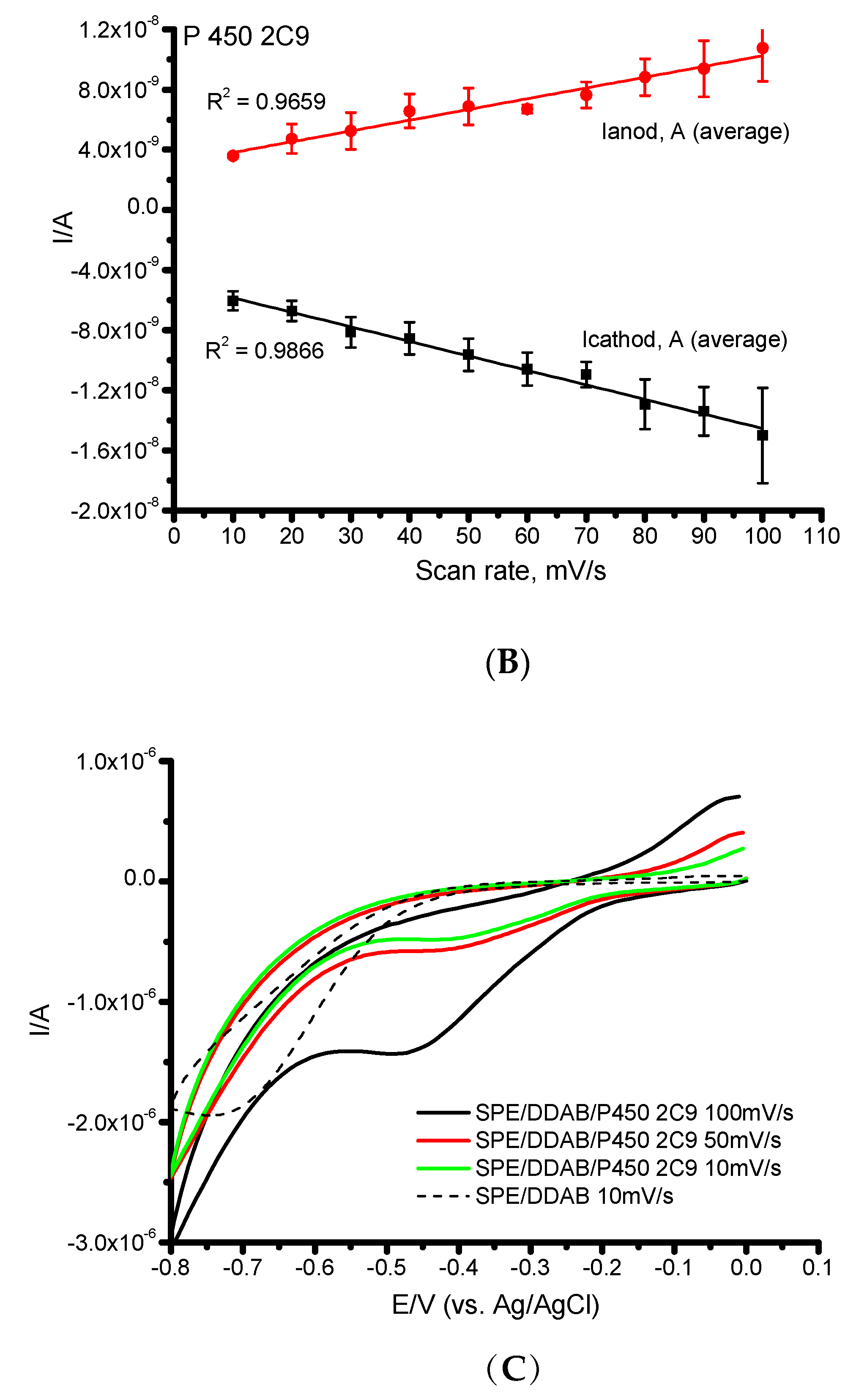
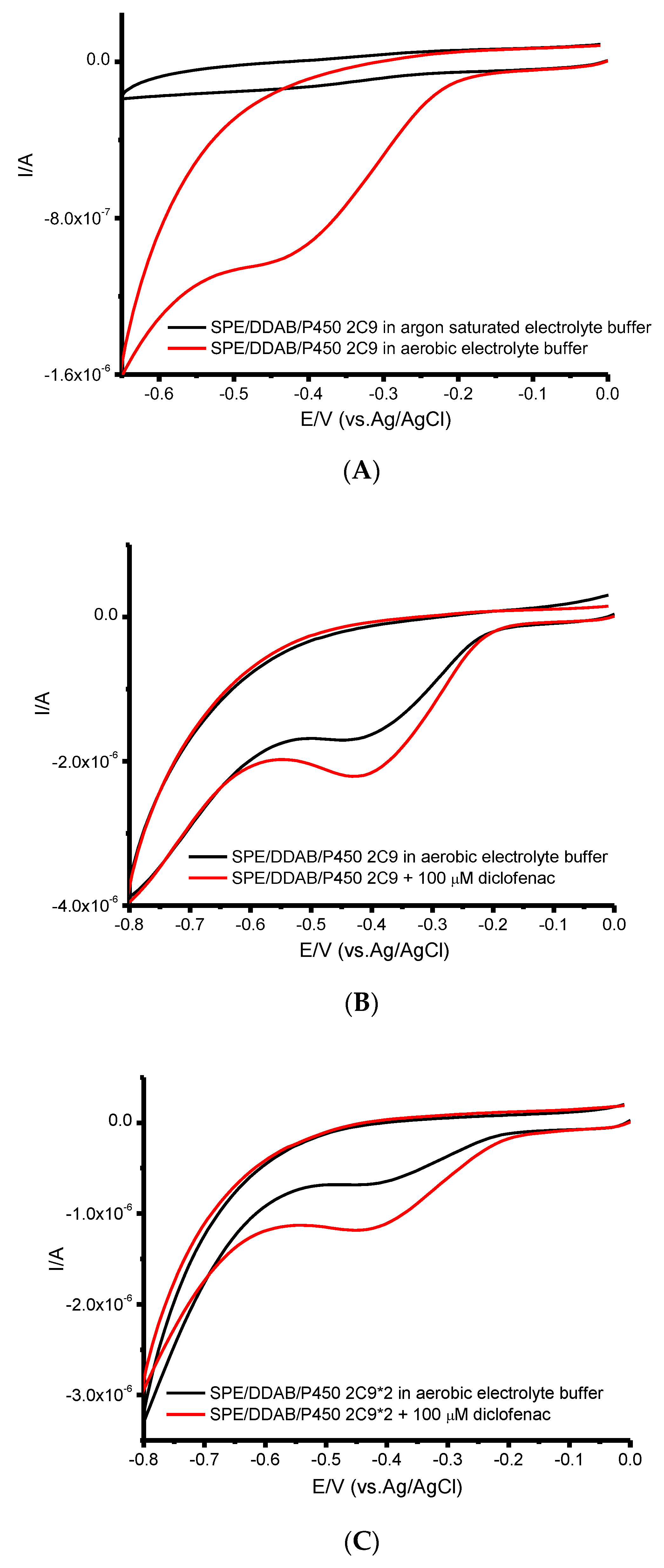
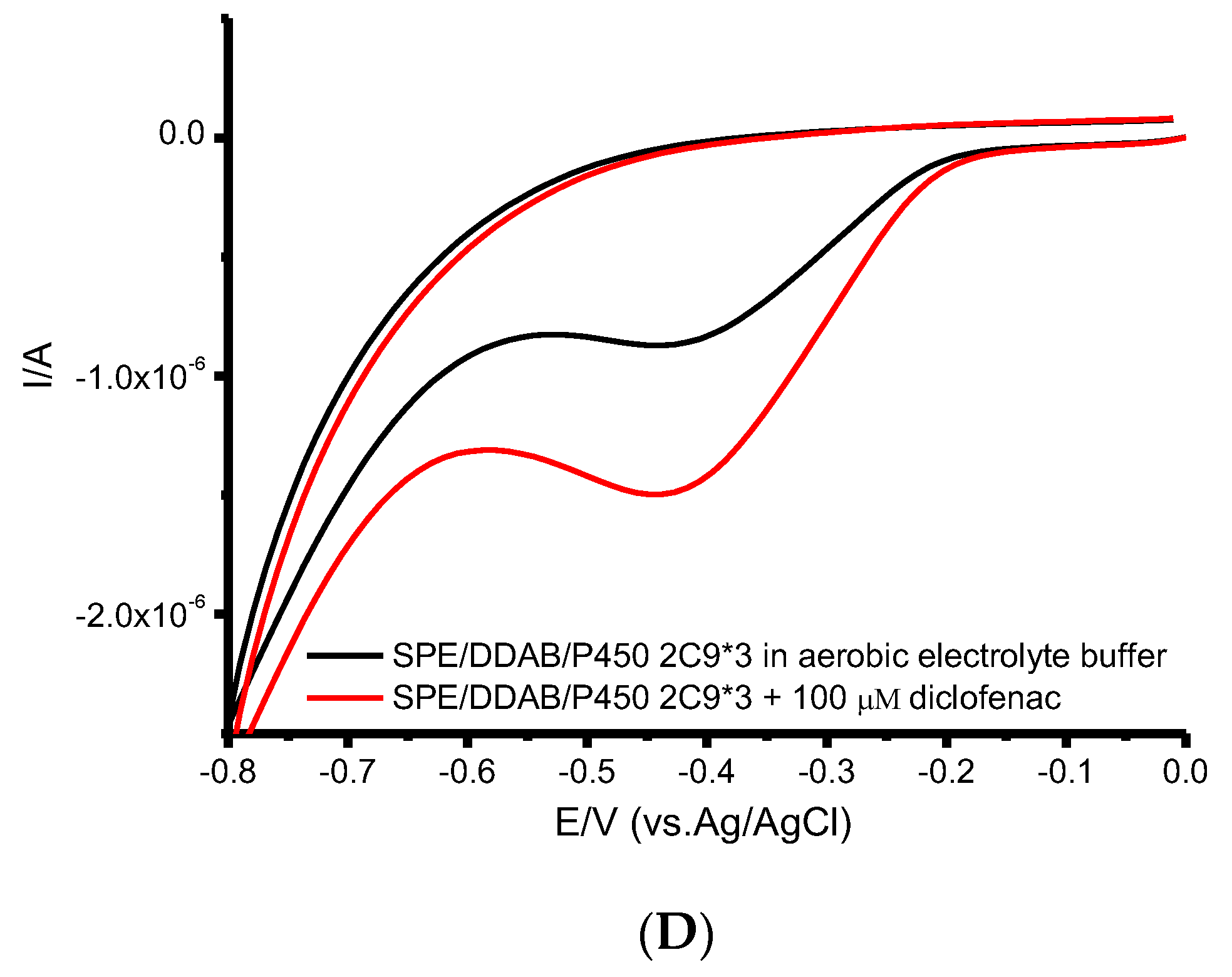
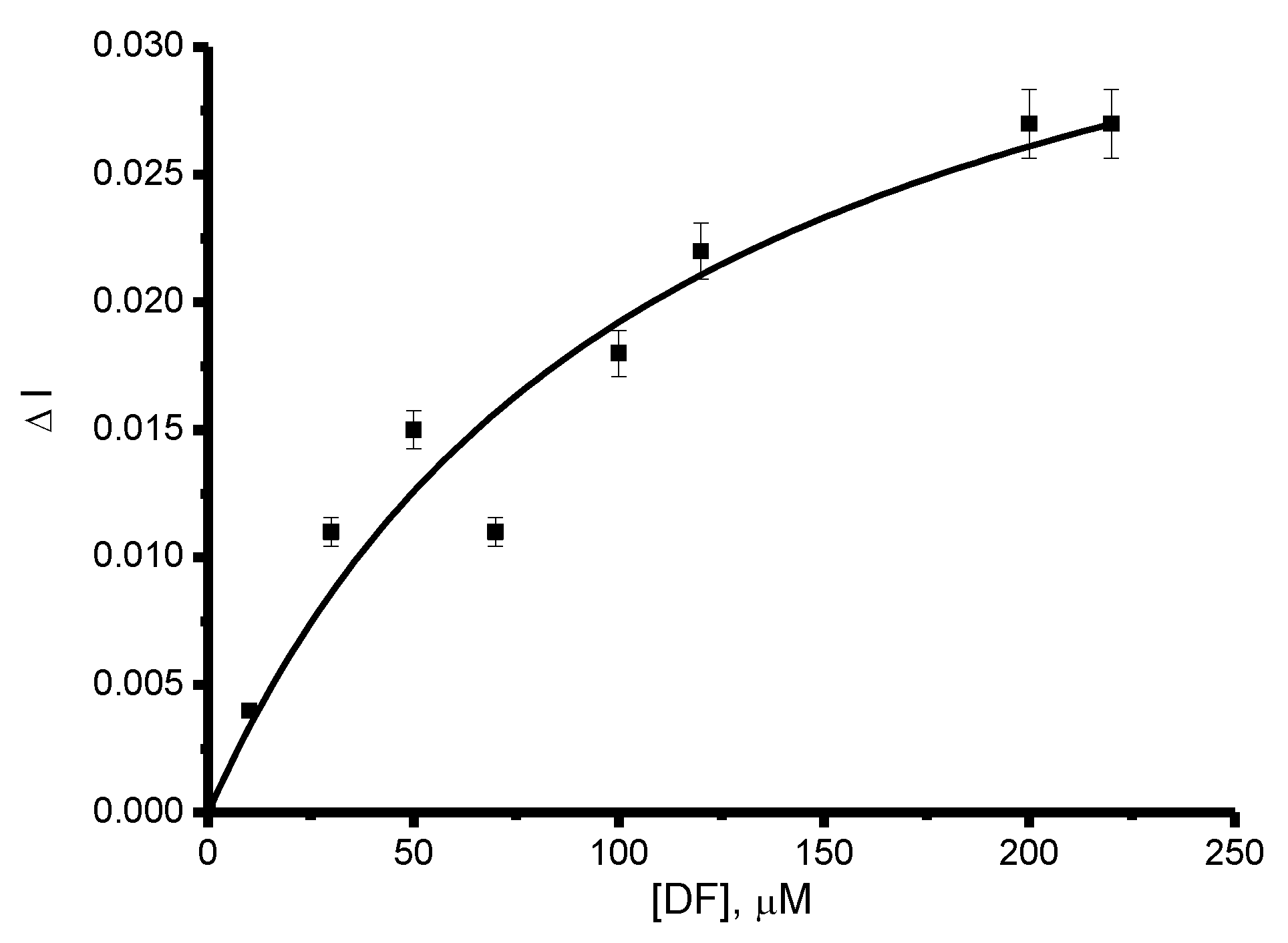
| CYP | Ec, V | Ea, V | Emid, V | ∆E, V | ks, s−1 | Γ0, mol/cm2 | Electroactive, % |
|---|---|---|---|---|---|---|---|
| CYP2C9 | −0.383 ± 0.01 | −0.252 ± 0.006 | −0.318 ± 0.01 | 0.131 | 0.54 ± 0.02 | (5.9 ± 1.1) 10−12 | 0.2 |
| CYP2C9*2 | −0.396 ± 0.01 | −0.252 ± 0.006 | −0.324 ± 0.01 | 0.144 | 0.48 ± 0.01 | (4.7 ± 3.5) 10−12 | 0.2 |
| CYP2C9*3 | −0.386 ± 0.02 | −0.249 ± 0.006 | −0.318 ± 0.03 | 0.138 | 0.50 ± 0.04 | (5.7 ± 2.2) 10−12 | 0.2 |
| CYP | Ered, V (in the Presence of Oxygen) | Ecat, V (in the Presence of Diclofenac) | Eonset, V | IDF/IO2 |
|---|---|---|---|---|
| CYP2C9 | −0.443 ± 0.020 | −0.393 ± 0.020 | −0.212 ± 0.002 | 1.80 ± 0.06 |
| CYP2C9*2 | −0.393 ± 0.030 | −0.361 ± 0.020 | −0.211 ± 0.002 | 1.33 ± 0.07 |
| CYP2C9*3 | −0.395 ± 0.020 | −0.406 ± 0.020 | −0.207 ± 0.001 | 2.18 ± 0.06 |
| CYP | Km (DF), μM | 100 μM DF Conversion | 100 μM DF + 98 μM Mexidol | 100 μM DF Conversion + 100 μM Taurine |
|---|---|---|---|---|
| CYP2C9 | 45 ± 5 | 19 ± 3% | 33 ± 5% | 29 ± 3% |
| CYP2C9*2 | 68 ± 2 | 11 ± 2% | 29 ± 3% | 16 ± 3% |
| CYP2C9*3 | 62 ± 2 | 17 ± 3% | 8 ± 3% | 19 ± 4% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shumyantseva, V.V.; Bulko, T.V.; Koroleva, P.I.; Shikh, E.V.; Makhova, A.A.; Kisel, M.S.; Haidukevich, I.V.; Gilep, A.A. Human Cytochrome P450 2C9 and Its Polymorphic Modifications: Electroanalysis, Catalytic Properties, and Approaches to the Regulation of Enzymatic Activity. Processes 2022, 10, 383. https://doi.org/10.3390/pr10020383
Shumyantseva VV, Bulko TV, Koroleva PI, Shikh EV, Makhova AA, Kisel MS, Haidukevich IV, Gilep AA. Human Cytochrome P450 2C9 and Its Polymorphic Modifications: Electroanalysis, Catalytic Properties, and Approaches to the Regulation of Enzymatic Activity. Processes. 2022; 10(2):383. https://doi.org/10.3390/pr10020383
Chicago/Turabian StyleShumyantseva, Victoria V., Tatiana V. Bulko, Polina I. Koroleva, Evgeniya V. Shikh, Anna A. Makhova, Maryia S. Kisel, Irina V. Haidukevich, and Andrei A. Gilep. 2022. "Human Cytochrome P450 2C9 and Its Polymorphic Modifications: Electroanalysis, Catalytic Properties, and Approaches to the Regulation of Enzymatic Activity" Processes 10, no. 2: 383. https://doi.org/10.3390/pr10020383







