Long-Term Behavior of Fuel Vapor Retaining Systems for Pure (E0) and Blended Fuels (E10) Part 2: Regeneration with Nitrogen of 70% Relative Humidity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Determination of the Evaporative Emissions (Type-IV Test)
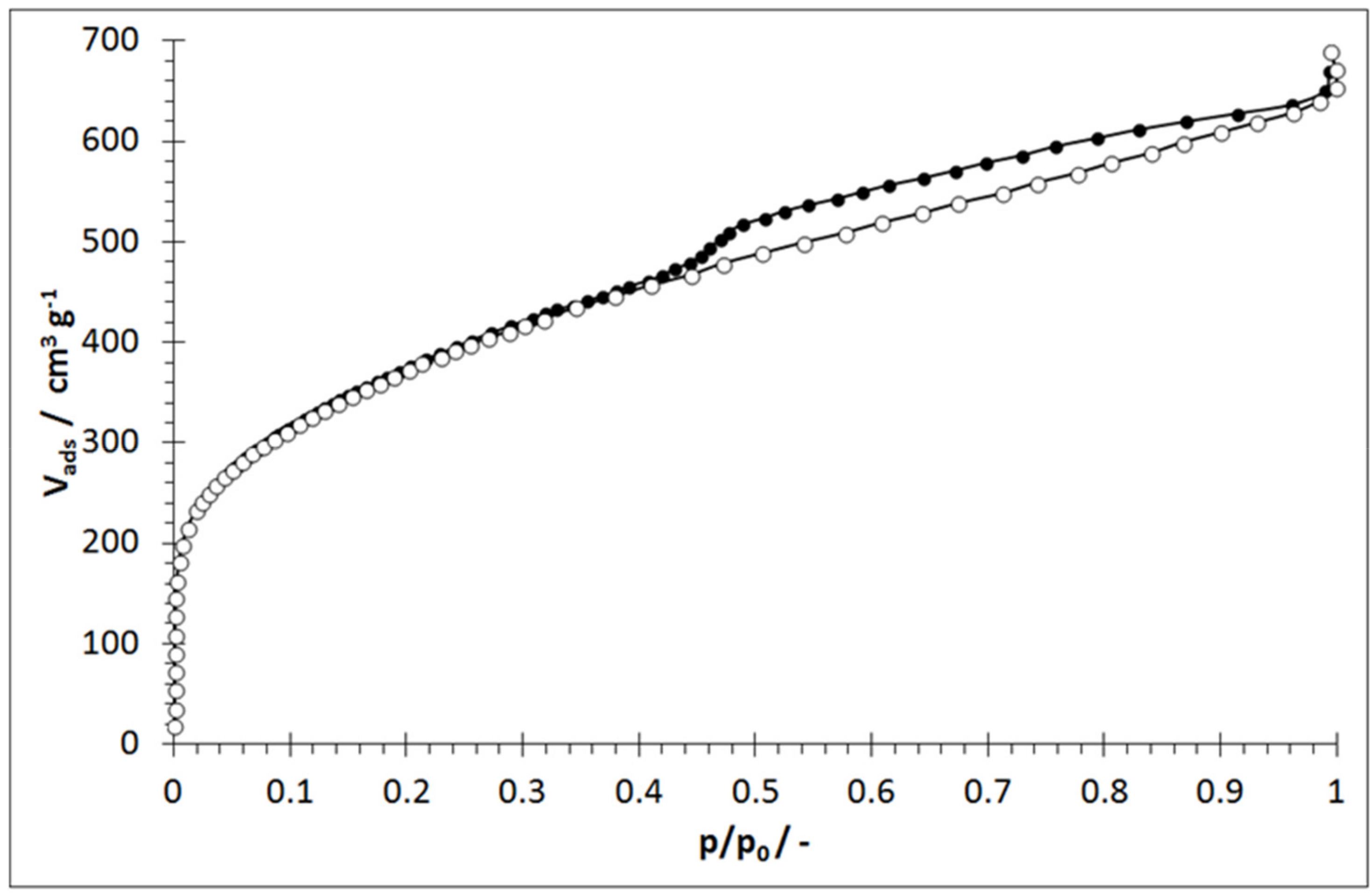
2.2. Determination of the Breakthrough Behavior
2.3. Determination of Water Content of the Activated Carbon Bed
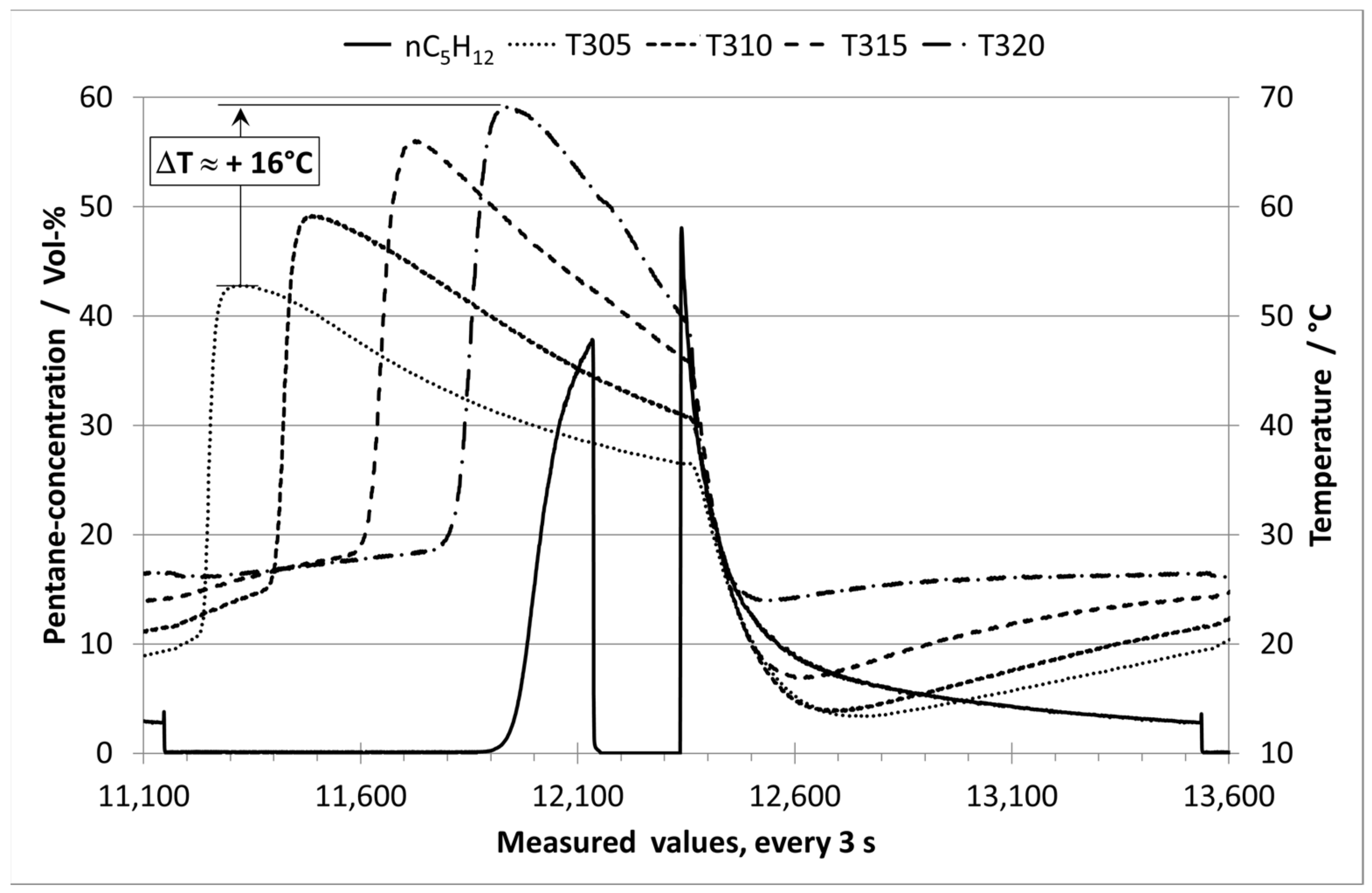
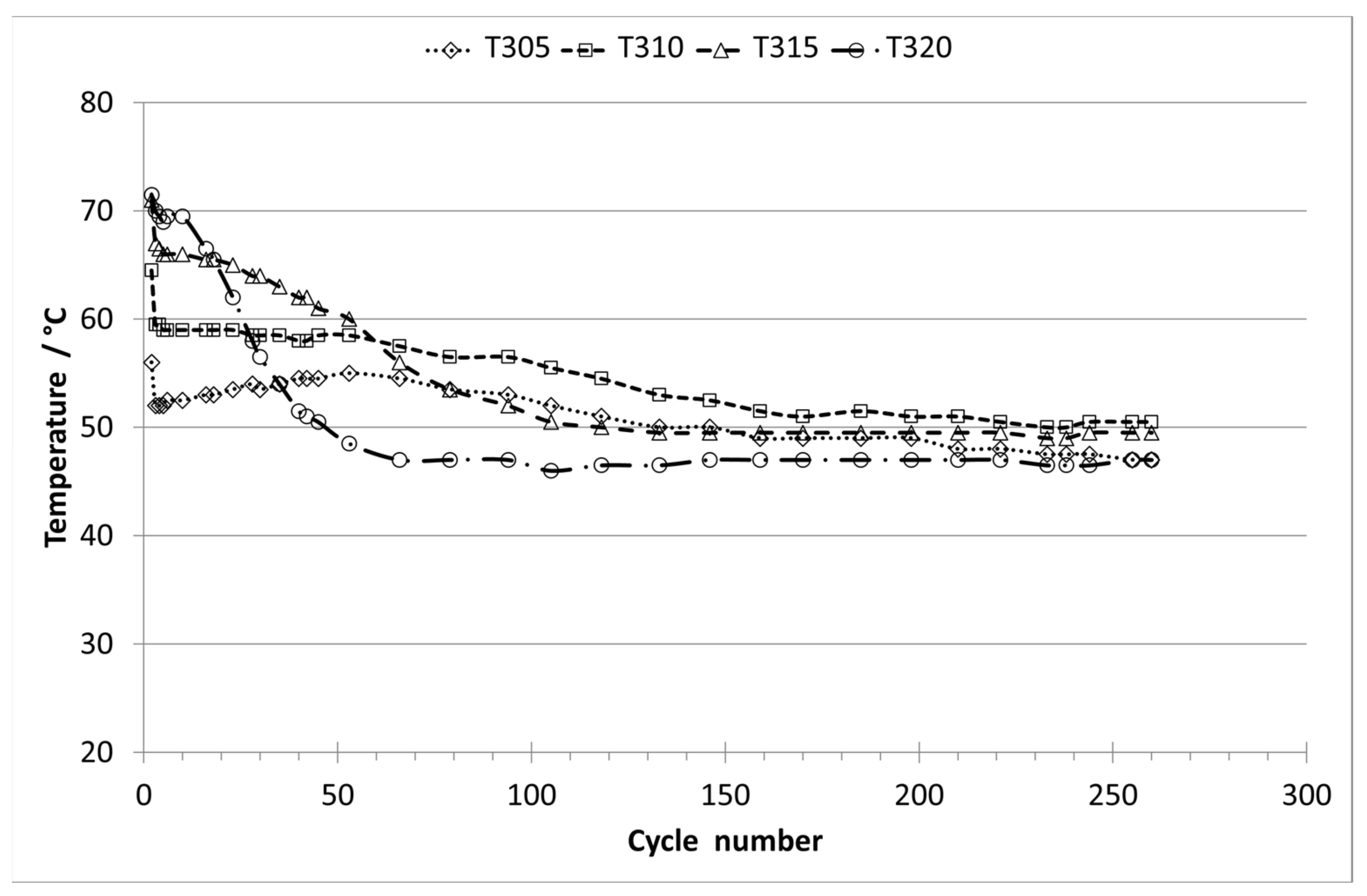
3. Results and Discussion
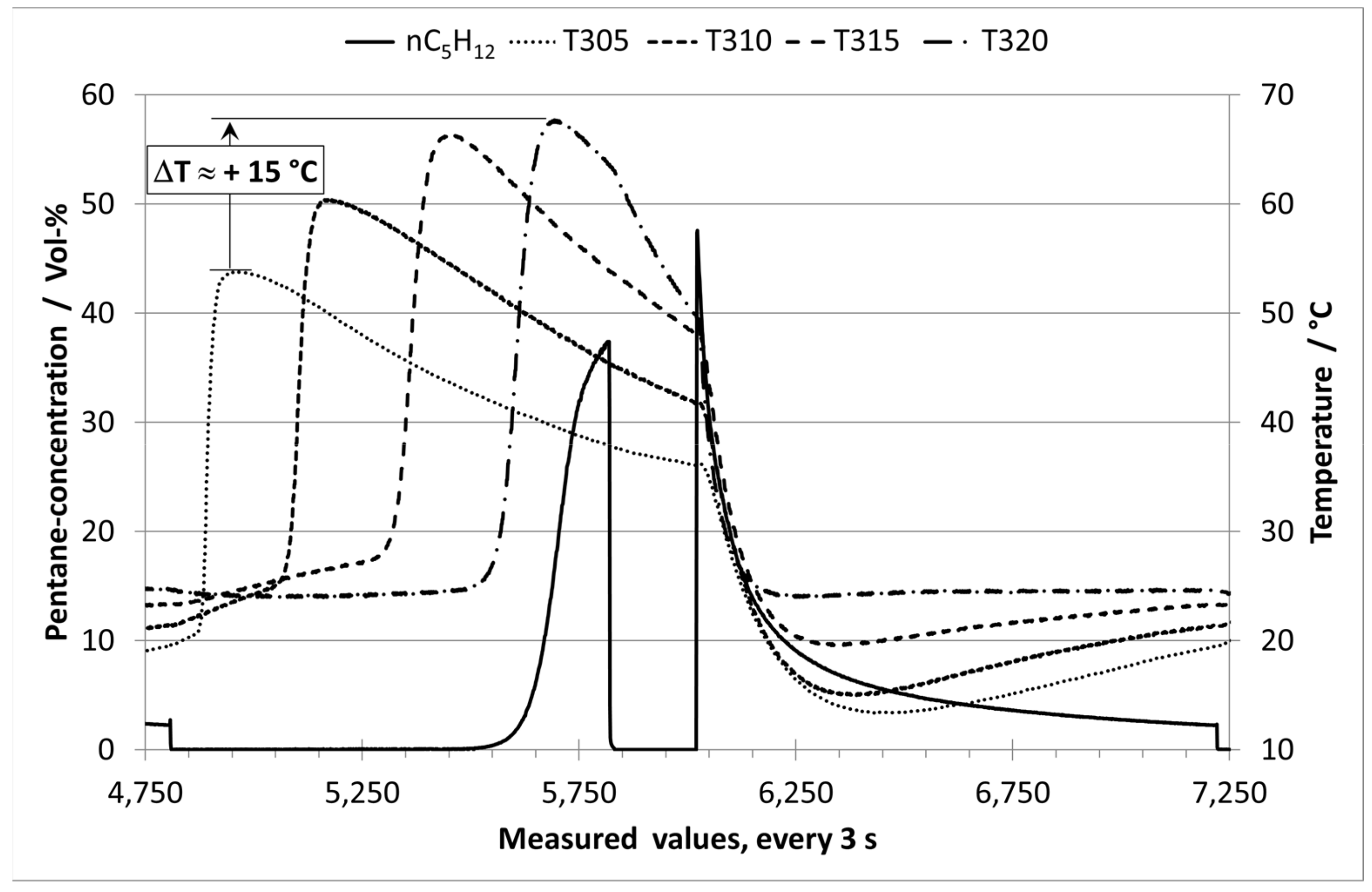
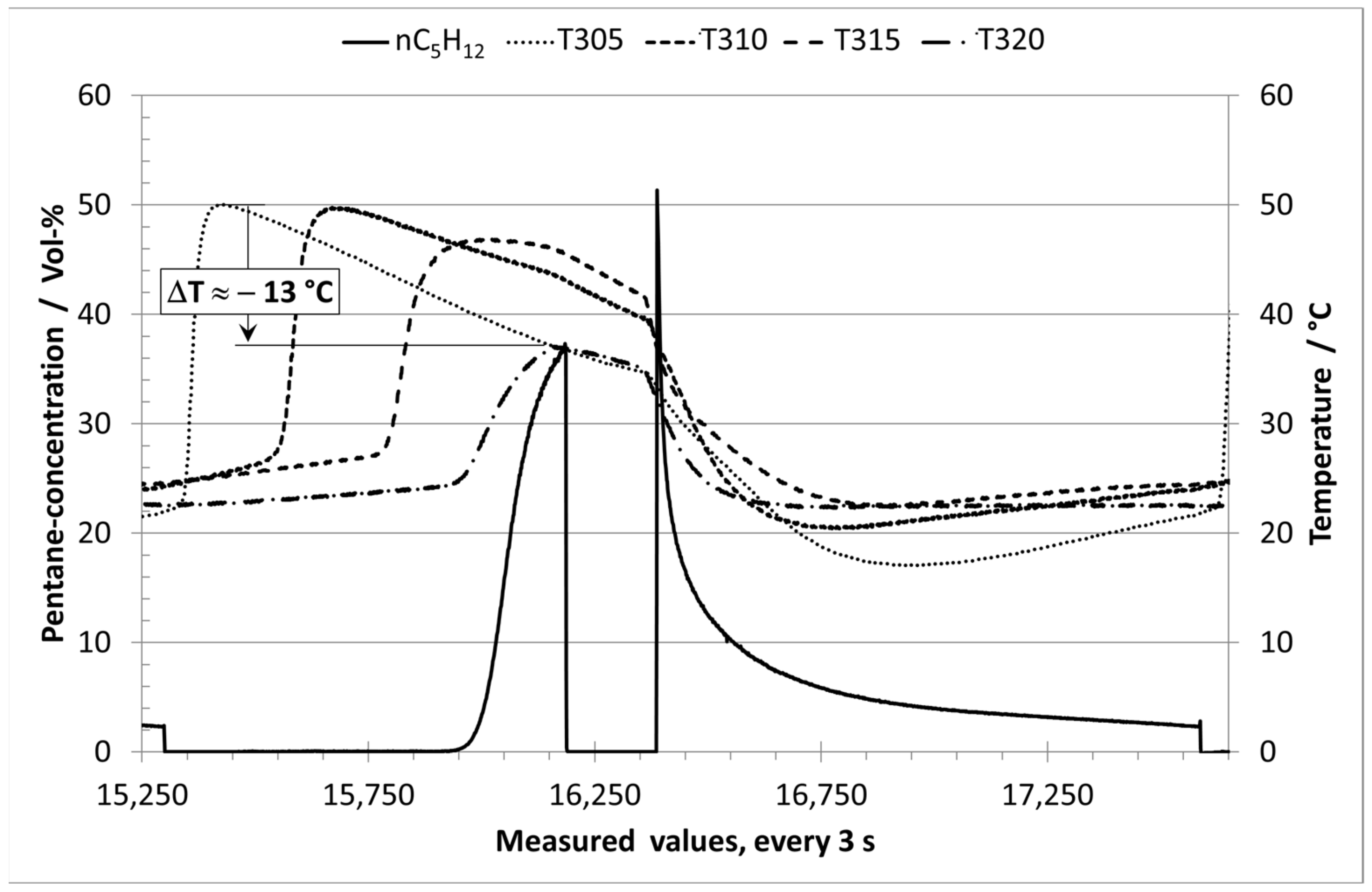
3.1. Analysis of Long-term Experiments with E0 and Humid Nitrogen (70% RH)
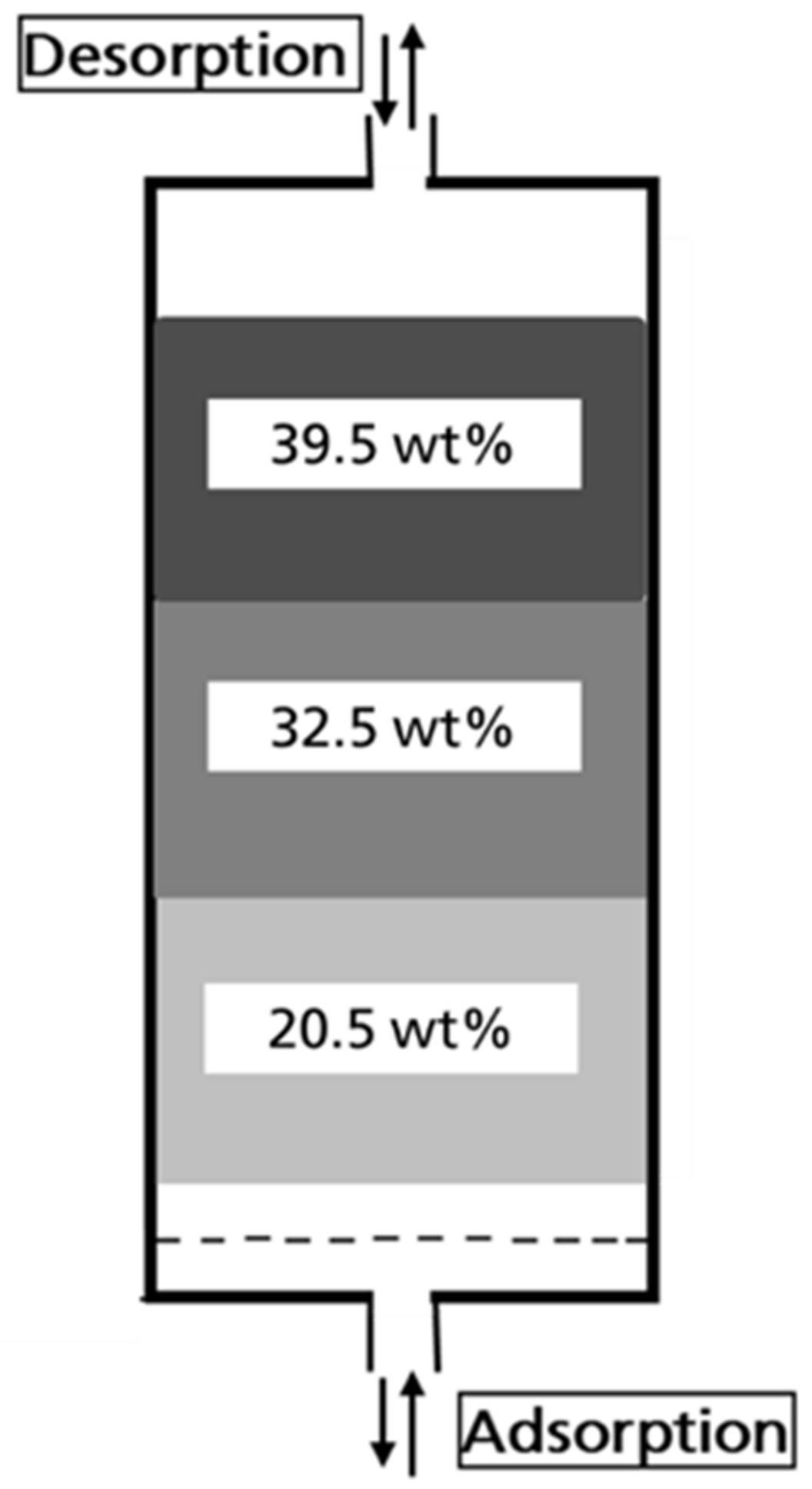
3.1.1. Water Content of the Activated Carbon for the system E0/humid N2
3.2. Analysis of Long-term Experiments with E10 Vapor Phase and Humid Nitrogen (70% RH)
3.2.1. Water Content of the Activated Carbon for the System E10/humid N2
3.3. Comparison of the Experiments with E10 and Purging with Dry or Humid Nitrogen
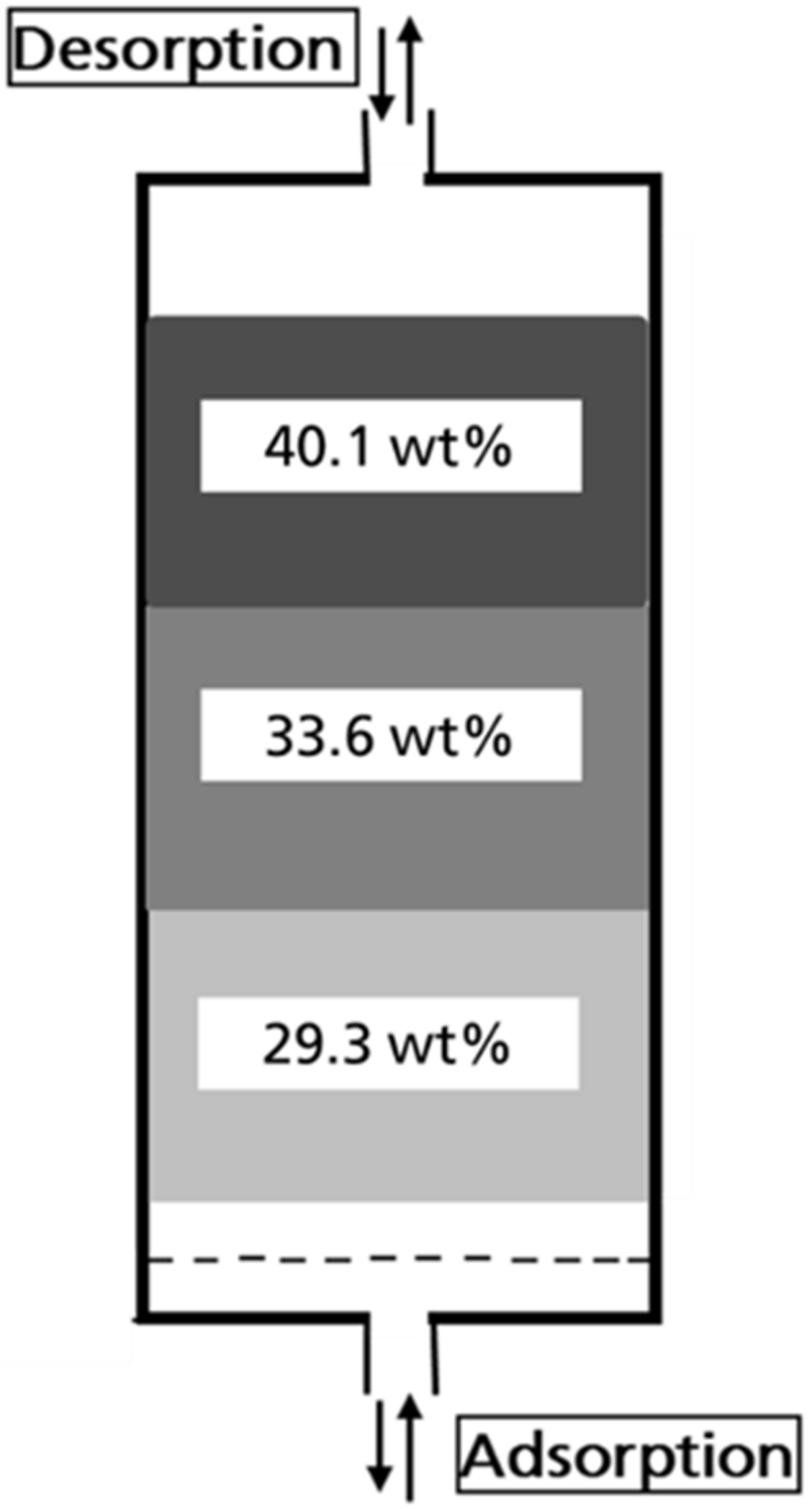
3.4. Determination of the Butane Working Capacity
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Directive 2009/28/EC of the European Parliament and of the Council. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32009L0028 (accessed on 6 September 2021).
- RICHTLINIE (EU) 2018/2001 DES EUROPÄISCHEN PARLAMENTS UND DES RATES vom 11. Dezember 2018 zur Förderung der Nutzung von Energie aus erneuerbaren Quellen. Available online: https://data.europa.eu/eli/dir/2018/2001/oj (accessed on 6 September 2021).
- Schmidt, H.; (TÜV Nord, Essen, Germany). Evaporative Emissions of in-use Vehicles from Germany and Sweden—Operational Conditions Affecting the Fuel System. Personal communication, 2009. [Google Scholar]
- Schmidt, H. Annual Report 2009: Swedish In-Service Testing Programme 2008 on Emissions from Passenger Cars and Light-Duty Trucks; TÜV Nord: Göteborg, Sweden, 2009. [Google Scholar]
- Martini, G.; Manfredi, U.; Rocha, M.; Marotta, A. Review of the European Test Procedure for Evaporative Emissions: Main Issues and Proposed Solutions; EUR 25640 EN; Publications Office of the European Union: Luxembourg, 2012; Available online: https://doi.org/10.2788/72123 (accessed on 27 November 2013). [CrossRef]
- Regelung Nr. 83 der Wirtschaftskommission der Vereinten Nationen für Europa (UN/ECE)—Einheitliche Bedingungen für die Genehmigung der Fahrzeuge Hinsichtlich der Emission von Schadstoffen aus dem Motor Entsprechend den Kraftstofferfordernissen des Motors. 2011. Available online: https://op.europa.eu/de/publication-detail/-/publication/2f8f0ce5-66fb-4a38-ae68-558ae1b04a5f/language-de (accessed on 22 February 2012).
- Part 86—Control of Emissions From New and In-Use Highway Vehicles and Engines. Available online: https://www.ecfr.gov/current/title-40/chapter-I/subchapter-C/part-86 (accessed on 8 October 2021).
- Das Klima in Deutschland. Available online: https://www.laenderdaten.info/Europa/Deutschland/Klima.php (accessed on 18 January 2022).
- Göbel, M.U.; Keller, J.U.; Meller, K.; Schmitz, I.; Seeger, T.; Schieferstein, E. Langzeitverhalten von Kraftstoffdampfrückhaltesystemen für Biokraftstoffe (E0, E10), Teil 1: Regeneration mit trockenem Spül-Stickstoff. Chem. Ing. Tech. 2021, 93, 1–10. [Google Scholar] [CrossRef]
- Amendment of Regulation (EC) No 692/2008: Regulation Proposal: Determination of Evaporative Emissions From Vehicles with Positive-Ignition Engines. Annex I, L199/18; Annex VI, L199/74. Available online: https://www.legislation.gov.uk/eur/2008/692/body (accessed on 13 August 2018).
- ASTM D5228: Standard Test Method for Determination of the Butane Working Capacity of Activated Carbon, 2000. Available online: https://www.beuth.de/de/regelwerke/astm (accessed on 29 September 2010).
- Keller, J.U.; Seeger, T.; Schieferstein, E. Koadsorptionsgleichgewichte von Kraftstoffdämpfen an feuchten Aktivkohlefiltern. Chem. Ing. Tech. 2014, 86, 58–66. [Google Scholar] [CrossRef]
- Kiefer, J.; Schorsch, S.; Seeger, T.; Leipertz, A. Online-Analyse der Zusammensetzung von Brenngasen mittels Raman-Spektroskopie. Chem. Ing. Tech. 2007, 79, 1327–1328. [Google Scholar] [CrossRef]
- Kiefer, J.; Seeger, T.; Steuer, S.; Schorsch, S.; Leipertz, A. Design and Characterization of a Raman-Scattering-Based Sensor System for Temporally Resolved Gas Analysis and its Application in a Gas Turbine Power Plant. Meas. Sci. Technol. 2008, 19, 085408. [Google Scholar] [CrossRef]
- Schlüter, S.; Krischke, F.; Popovska-Leipertz, N.; Seeger, T.; Breuer, G.; Jeleazcov, C.; Schüttler, J.; Leipertz, A. Demonstration of a signal enhanced fast Raman sensor for multi species gas analysis at a low pressure range for anesthesia monitoring. J. Raman Spectrosc. 2015, 46, 708–715. [Google Scholar] [CrossRef]
- Eichmann, S.C.; Kiefer, J.; Benz, J.; Kempf, T.; Leipertz, A.; Seeger, T. Determination of gas composition in a biogas plant using a Raman-based sensor system. Meas. Sci. Technol. 2014, 25, 075503. [Google Scholar] [CrossRef]
- Eichmann, S.C.; Weschta, M.; Kiefer, J.; Seeger, T.; Leipertz, A. Characterization of a fast gas analyzer based on Raman scattering for the analysis of synthesis gas. Rev. of Sci. Instrum. 2010, 81, 128104. [Google Scholar] [CrossRef] [PubMed]
- Eichmann, S.C.; Trost, J.; Seeger, T.; Zigan, L.; Leipertz, A. Application of linear Raman spectroscopy for the determination of acetone decomposition. Opt. Exp. 2011, 19, 11052–11058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schieferstein, E.; Seeger, T. Sensor system for long-term analysis of fuel vapour restraint systems. tm-Tech Mess. 2019, 87, 304–311. [Google Scholar] [CrossRef]






|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Göbel, M.U.; Keller, J.U.; Meller, K.; Schmitz, I.; Seeger, T.; Schieferstein, E. Long-Term Behavior of Fuel Vapor Retaining Systems for Pure (E0) and Blended Fuels (E10) Part 2: Regeneration with Nitrogen of 70% Relative Humidity. Processes 2022, 10, 397. https://doi.org/10.3390/pr10020397
Göbel MU, Keller JU, Meller K, Schmitz I, Seeger T, Schieferstein E. Long-Term Behavior of Fuel Vapor Retaining Systems for Pure (E0) and Blended Fuels (E10) Part 2: Regeneration with Nitrogen of 70% Relative Humidity. Processes. 2022; 10(2):397. https://doi.org/10.3390/pr10020397
Chicago/Turabian StyleGöbel, Manfred Ulrich, Jürgen U. Keller, Karl Meller, Ingo Schmitz, Thomas Seeger, and Eva Schieferstein. 2022. "Long-Term Behavior of Fuel Vapor Retaining Systems for Pure (E0) and Blended Fuels (E10) Part 2: Regeneration with Nitrogen of 70% Relative Humidity" Processes 10, no. 2: 397. https://doi.org/10.3390/pr10020397








