Data-Driven Machine Learning Intelligent Tools for Predicting Chromium Removal in an Adsorption System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Adsorbent Preparation and Characterization
2.3. Batch Experiment and Chromium Analysis
3. Network Development
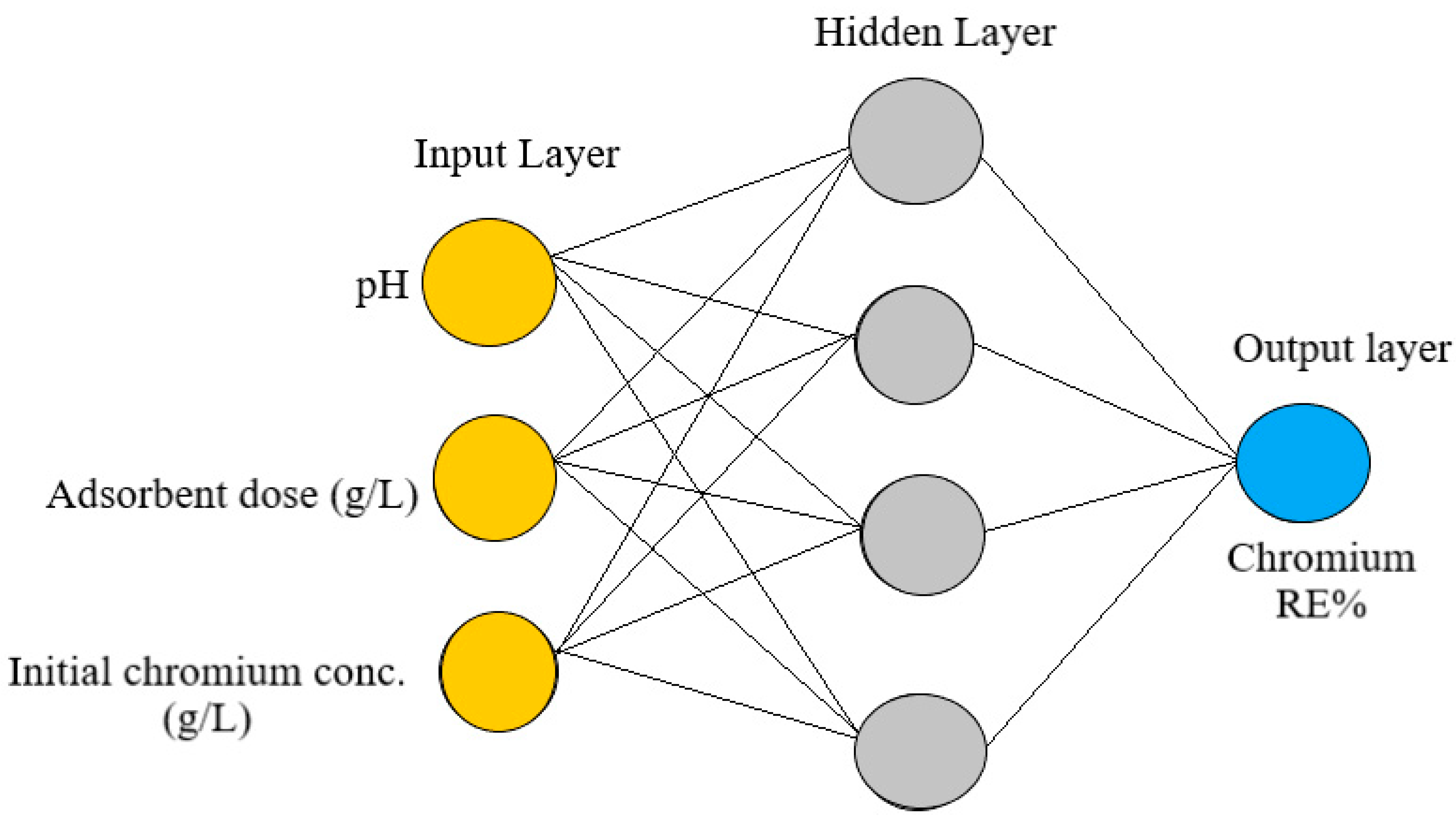
3.1. ANN Model
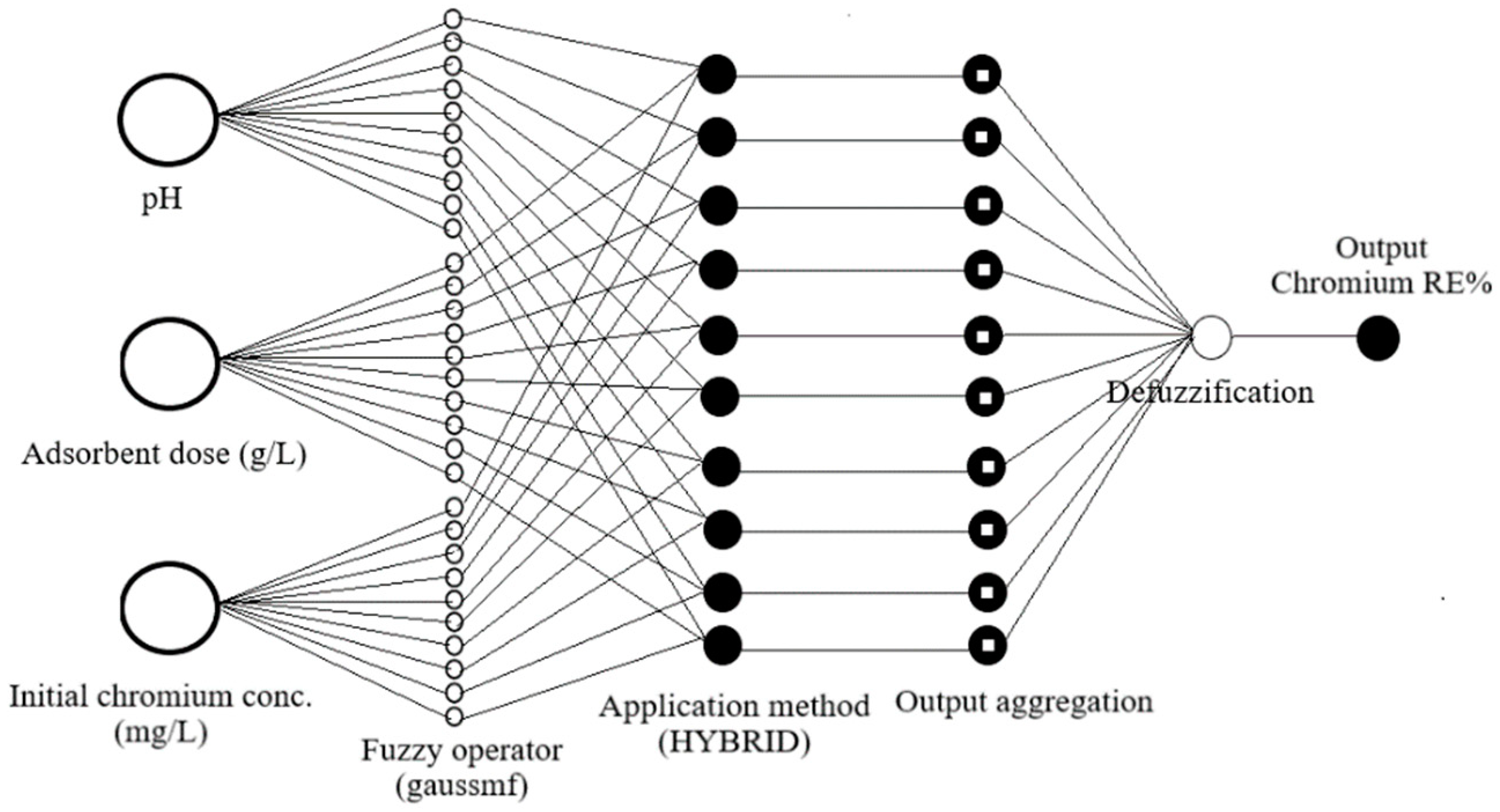
3.2. ANFIS Model
3.3. Data Processing in ANN and ANFIS
3.4. Statistical Evaluation
- Correlation coefficient (R)
- Sum of squares error (SSE)
- Sum of the absolute error (SAE)
- Average relative error (ARE)
- Absolute average deviation (AAD)
- Mean squared error (MSE)
- Root mean square error (RMSE)
- Hybrid fractional error function (HYBRID)
- Marquart’s percentage standard deviation (MPSD)
- Chi-square
3.5. Software Used
4. Results and Discussion
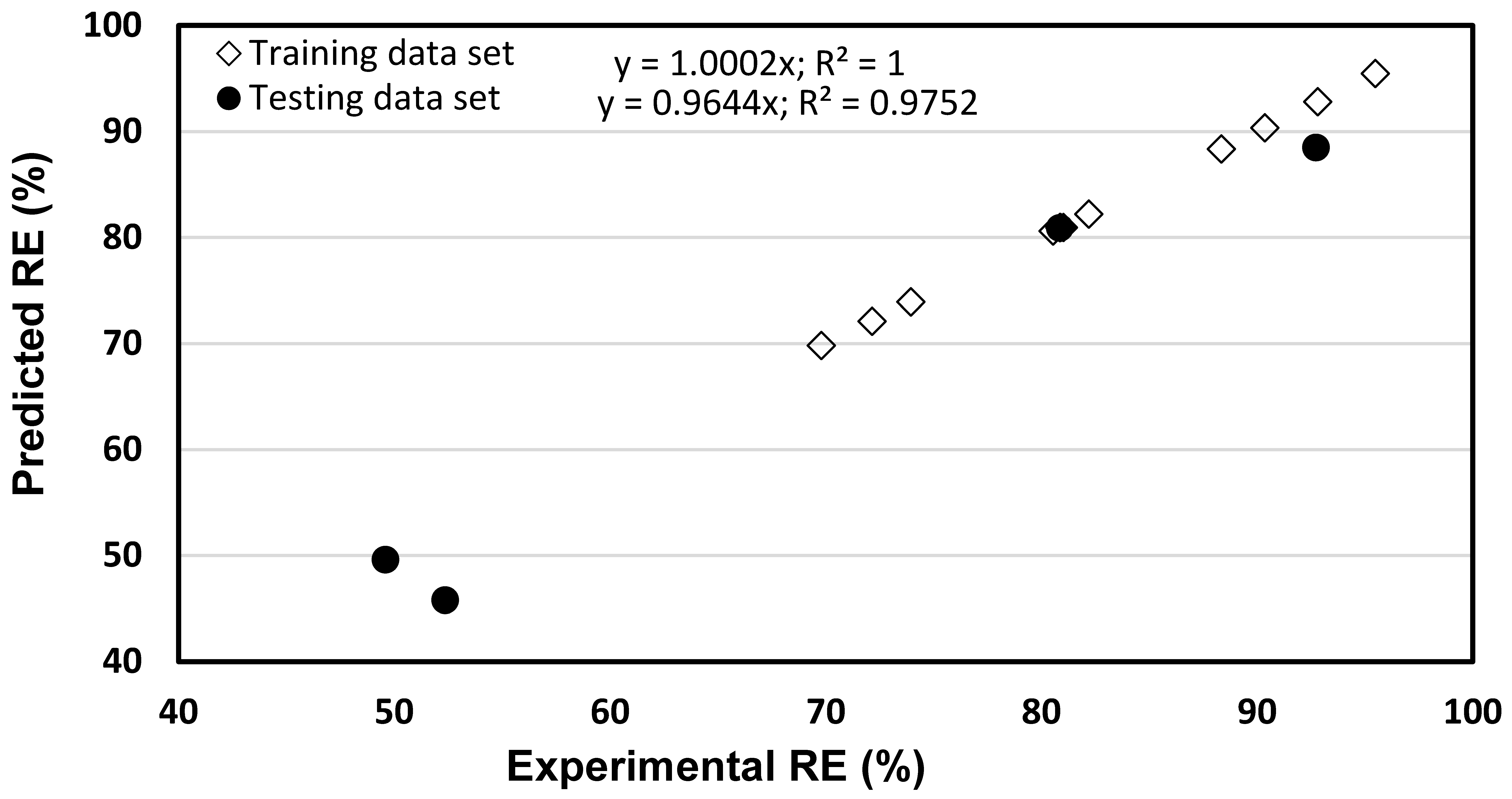
4.1. ANN Performance
4.2. ANFIS Performance
4.3. Comparison of ANN and ANFIS Models
4.4. Practical Implications of the Work and Future Research Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sridhar, A.; Ponnuchamy, M.; Kapoor, A.; Prabhakar, S. Valorization of food waste as adsorbents for toxic dye removal from contaminated waters: A review. J. Hazard. Mater. 2022, 424, 127432. [Google Scholar] [CrossRef]
- CPCB Remediation of Hazardous Waste Contaminated Dumpsites under National Clean Energy Fund (NCEF). Available online: https://cpcb.nic.in/displaypdf.php?id=aHdtZC9OQ0VGX1Byb2VqY3RfQmFja2dyb3VuZC5wZGY= (accessed on 18 January 2022).
- Central Pollution Control Board (CPCB) Industry Wise Emission Standards. Available online: https://cpcb.nic.in/effluent-emission/ (accessed on 18 January 2022).
- Vaiopoulou, E.; Gikas, P. Regulations for chromium emissions to the aquatic environment in Europe and elsewhere. Chemosphere 2020, 254, 126876. [Google Scholar] [CrossRef] [PubMed]
- WHO. Chromium in Drinking Water: Guidelines, WHO/SDE/WSH/03.04/04; WHO: Geneva, Switzerland, 2003. [Google Scholar]
- Pavesi, T.; Moreira, J.C. Mechanisms and individuality in chromium toxicity in humans. J. Appl. Toxicol. 2020, 40, 1183–1197. [Google Scholar] [CrossRef] [PubMed]
- Pakade, V.E.; Tavengwa, N.T.; Madikizela, L.M. Recent advances in hexavalent chromium removal from aqueous solutions by adsorptive methods. RSC Adv. 2019, 9, 26142–26164. [Google Scholar] [CrossRef] [Green Version]
- Fernández, P.M.; Viñarta, S.C.; Bernal, A.R.; Cruz, E.L.; Figueroa, L.I. Bioremediation strategies for chromium removal: Current research, scale-up approach and future perspectives. Chemosphere 2018, 208, 139–148. [Google Scholar] [CrossRef]
- Li, T.; Guan, Y.; Guo, C.; Yang, T.; Yu, Z.; Xu, G. Pilot scale experiment of an innovative magnetic bar magnetic separator for chromium removal from tannery wastewater. Process Saf. Environ. Prot. 2021, 149, 575–580. [Google Scholar] [CrossRef]
- Almeida, J.C.; Cardoso, C.E.; Tavares, D.S.; Freitas, R.; Trindade, T.; Vale, C.; Pereira, E. Chromium removal from contaminated waters using nanomaterials—A review. TrAC Trends Anal. Chem. 2019, 118, 277–291. [Google Scholar] [CrossRef]
- Dognani, G.; Hadi, P.; Ma, H.; Cabrera, F.C.; Job, A.; Agostini, D.L.; Hsiao, B.S. Effective chromium removal from water by polyaniline-coated electrospun adsorbent membrane. Chem. Eng. J. 2019, 372, 341–351. [Google Scholar] [CrossRef]
- Behera, S.K.; Sahni, S.; Tiwari, G.; Rai, A.; Mahanty, B.; Vinati, A.; Rene, E.R.; Pugazhendhi, A. Removal of chromium from synthetic wastewater using modified maghemite nanoparticles. Appl. Sci. 2020, 10, 3181. [Google Scholar] [CrossRef]
- Liu, W.; Yu, Y. Removal of recalcitrant trivalent chromium complexes from industrial wastewater under strict discharge standards. Environ. Technol. Innov. 2021, 23, 101644. [Google Scholar] [CrossRef]
- Rafique, M.I.; Usman, A.R.; Ahmad, M.; Al-Wabel, M.I. Immobilization and mitigation of chromium toxicity in aqueous solutions and tannery waste-contaminated soil using biochar and polymer-modified biochar. Chemosphere 2021, 266, 129198. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, Y.; Cao, Y.; Peng, F.; Cao, Y.; Shao, Q.; Liu, H.; Guo, Z. Hexavalent chromium removal over magnetic carbon nanoadsorbents: Synergistic effect of fluorine and nitrogen co-doping. J. Mater. Chem. A 2018, 6, 13062–13074. [Google Scholar] [CrossRef]
- Ben Khalifa, E.; Rzig, B.; Chakroun, R.; Nouagui, H.; Hamrouni, B. Application of response surface methodology for chromium removal by adsorption on low-cost biosorbent. Chemom. Intell. Lab. Syst. 2019, 189, 18–26. [Google Scholar] [CrossRef]
- Banerjee, A.; Ray, A. A survey of adsorption process parameter optimization related to degradation of environmental pollutants. In Intelligent Environmental Data Monitoring for Pollution Management; Elsevier: Amsterdam, The Netherlands, 2021; pp. 289–309. [Google Scholar]
- Jeon, J. The strengths and limitations of the statistical modeling of complex social phenomenon: Focusing on SEM, path analysis, or multiple regression models. Int. J. Econ. Manag. Eng. 2015, 9, 1634–1642. [Google Scholar] [CrossRef]
- Solgi, M.; Najib, T.; Ahmadnejad, S.; Nasernejad, B. Synthesis and characterization of novel activated carbon from Medlar seed for chromium removal: Experimental analysis and modeling with artificial neural network and support vector regression. Resour. Technol. 2017, 3, 236–248. [Google Scholar] [CrossRef]
- Du, J.; Shang, X.; Li, T.; Guan, Y. Recycling and modeling of chromium from sludge produced from magnetic flocculation treatment of chromium-containing wastewater. Process Saf. Environ. Prot. 2022, 157, 20–26. [Google Scholar] [CrossRef]
- Rodríguez-Romero, J.A.; Mendoza-Castillo, D.I.; Reynel-Ávila, H.E.; de Haro-Del Rio, D.A.; González-Rodríguez, L.M.; Bonilla-Petriciolet, A.; Duran-Valle, C.J.; Camacho-Aguilar, K.I. Preparation of a new adsorbent for the removal of arsenic and its simulation with artificial neural network-based adsorption models. J. Environ. Chem. Eng. 2020, 8, 103928. [Google Scholar] [CrossRef]
- Meher, S.K.; Behera, S.K.; Rene, E.R.; Park, H.-S. Comparative analysis on the application of neuro-fuzzy models for complex engineered systems: Case study from a landfill and a boiler. Expert Syst. 2017, 34, e12215. [Google Scholar] [CrossRef]
- Hong, E.; Yeneneh, A.M.; Sen, T.K.; Ang, H.M.; Kayaalp, A. ANFIS based modelling of dewatering performance and polymer dose optimization in a wastewater treatment plant. J. Environ. Chem. Eng. 2018, 6, 1957–1968. [Google Scholar] [CrossRef]
- Fu, Z.; Cheng, J.; Yang, M.; Batista, J.; Jiang, Y. Wastewater discharge quality prediction using stratified sampling and wavelet de-noising ANFIS model. Comput. Electr. Eng. 2020, 85, 106701. [Google Scholar] [CrossRef]
- Onu, C.E.; Nwabanne, J.T.; Ohale, P.E.; Asadu, C.O. Comparative analysis of RSM, ANN and ANFIS and the mechanistic modeling in eriochrome black-T dye adsorption using modified clay. S. Afr. J. Chem. Eng. 2021, 36, 24–42. [Google Scholar] [CrossRef]
- Foroutan, R.; Peighambardoust, S.J.; Mohammadi, R.; Omidvar, M.; Sorial, G.A.; Ramavandi, B. Influence of chitosan and magnetic iron nanoparticles on chromium adsorption behavior of natural clay: Adaptive neuro-fuzzy inference modeling. Int. J. Biol. Macromol. 2020, 151, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Rajabi Kuyakhi, H.; Tahmasebi Boldaji, R. Developing an adaptive neuro-fuzzy inference system based on particle swarm optimization model for forecasting Cr(VI) removal by NiO nanoparticles. Environ. Prog. Sustain. Energy 2021, 40, e13597. [Google Scholar] [CrossRef]
- Ghosal, P.S.; Kattil, K.V.; Yadav, M.K.; Gupta, A.K. Adsorptive removal of arsenic by novel iron/olivine composite: Insights into preparation and adsorption process by response surface methodology and artificial neural network. J. Environ. Manag. 2018, 209, 176–187. [Google Scholar] [CrossRef]
- Zafar, M.; Van Vinh, N.; Behera, S.K.; Park, H.-S. Ethanol mediated As(III) adsorption onto Zn-loaded pinecone biochar: Experimental investigation, modeling, and optimization using hybrid artificial neural network-genetic algorithm approach. J. Environ. Sci. 2017, 54, 114–125. [Google Scholar] [CrossRef]
- Saucedo-Delgado, B.G.; De Haro-Del Rio, D.A.; González-Rodríguez, L.M.; Reynel-Ávila, H.E.; Mendoza-Castillo, D.I.; Bonilla-Petriciolet, A.; Rivera de la Rosa, J. Fluoride adsorption from aqueous solution using a protonated clinoptilolite and its modeling with artificial neural network-based equations. J. Fluor. Chem. 2017, 204, 98–106. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Taghizadeh, M.; Taghizadeh, A. Mesoporous activated carbons of low-cost agricultural bio-wastes with high adsorption capacity: Preparation and artificial neural network modeling of dye removal from single and multicomponent (binary and ternary) systems. J. Mol. Liq. 2018, 269, 217–228. [Google Scholar] [CrossRef]
- Karri, R.R.; Sahu, J. Modeling and optimization by particle swarm embedded neural network for adsorption of zinc (II) by palm kernel shell based activated carbon from aqueous environment. J. Environ. Manag. 2018, 206, 178–191. [Google Scholar] [CrossRef]
- Karri, R.R.; Tanzifi, M.; Yaraki, M.T.; Sahu, J. Optimization and modeling of methyl orange adsorption onto polyaniline nano-adsorbent through response surface methodology and differential evolution embedded neural network. J. Environ. Manag. 2018, 223, 517–529. [Google Scholar] [CrossRef]
- Sharafi, K.; Pirsaheb, M.; Gupta, V.K.; Agarwal, S.; Moradi, M.; Vasseghian, Y.; Dragoi, E.-N. Phenol adsorption on scoria stone as adsorbent—Application of response surface method and artificial neural networks. J. Mol. Liq. 2019, 274, 699–714. [Google Scholar] [CrossRef]
- Pauletto, P.S.; Gonçalves, J.O.; Pinto, L.A.A.; Dotto, G.L.; Salau, N.P.G. Single and competitive dye adsorption onto chitosan–based hybrid hydrogels using artificial neural network modeling. J. Colloid Interface Sci. 2020, 560, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M.; Song, K.; Li, L. Fixed bed column and artificial neural network model to predict heavy metals adsorption dynamic on surfactant decorated graphene. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124076. [Google Scholar] [CrossRef]
- Moreno-Perez, J.; Bonilla-Petriciolet, A.; Castillo, D.I.M.; Reynel-Ávila, H.; Verde-Gómez, Y.; Trejo-Valencia, R. Artificial neural network-based surrogate modeling of multi-component dynamic adsorption of heavy metals with a biochar. J. Environ. Chem. Eng. 2018, 6, 5389–5400. [Google Scholar] [CrossRef]
- Li, W.; Wei, S.; Jiao, W.; Qi, G.; Liu, Y. Modelling of adsorption in rotating packed bed using artificial neural networks (ANN). Chem. Eng. Res. Des. 2016, 114, 89–95. [Google Scholar] [CrossRef]
- Behera, S.K.; Rene, E.R.; Park, H.-S. Neural network modeling of sorption of pharmaceuticals in engineered floodplain filtration system. Expert Syst. Appl. 2012, 39, 6052–6060. [Google Scholar] [CrossRef]
- Khatoon, N.; Khan, A.H.H.; Pathak, V.; Agnihotri, N.; Rehman, M. Removal of hexavalent chromium from synthetic waste water using synthetic nano zero valent iron (nZVI) as adsorbent. Int. J. Innov. Res. Sci. Eng. Technol. 2013, 2, 6140–6149. [Google Scholar]
- Bagheban Shahri, F.; Niazi, A. Synthesis of modified maghemite nanoparticles and its application for removal of acridine orange from aqueous solutions by using Box-Behnken design. J. Magn. Magn. Mater. 2015, 396, 318–326. [Google Scholar] [CrossRef]
- Han, M.J.; Behera, S.K.; Park, H.-S. Anaerobic co-digestion of food waste leachate and piggery wastewater for methane production: Statistical optimization of key process parameters. J. Chem. Technol. Biotechnol. 2012, 87, 1541–1550. [Google Scholar] [CrossRef]
- Lkhagvadulam, B.; Tsagaantsetseg, B.; Tergel, D.; Chuluunkhuyag, S. Removal of chromium from a tannery wastewater by using a maghemite nanoparticles. Int. J. Environ. Sci. Dev. 2017, 8, 696–702. [Google Scholar] [CrossRef] [Green Version]
- Predescu, A.; Nicolae, A.; Predescu, M.A.; Nicolae, A. Adsorption of Zn, Cu and Cd from waste waters by means of maghemite nanoparticles. UPB Sci. Bull. Ser. B Chem. Mater. Sci. 2012, 74, 255–264. [Google Scholar]
- Haykin, S.S. Neural Networks and Learning Machines; Prentice Hall: New York, NY, USA, 2009; ISBN 978-0-13-147139-9. [Google Scholar]
- Souza, P.R.; Dotto, G.L.; Salau, N.P.G. Artificial neural network (ANN) and adaptive neuro-fuzzy interference system (ANFIS) modelling for nickel adsorption onto agro-wastes and commercial activated carbon. J. Environ. Chem. Eng. 2018, 6, 7152–7160. [Google Scholar] [CrossRef]
- Al-Hmouz, A.; Shen, J.; Al-Hmouz, R.; Yan, J. Modeling and simulation of an adaptive neuro-fuzzy inference system (ANFIS) for mobile learning. IEEE Trans. Learn. Technol. 2012, 5, 226–237. [Google Scholar] [CrossRef]
- Niknam Shahrak, M.; Esfandyari, M.; Karimi, M. Efficient prediction of water vapor adsorption capacity in porous metal–organic framework materials: ANN and ANFIS modeling. J. Iran. Chem. Soc. 2019, 16, 11–20. [Google Scholar] [CrossRef]
- Zafar, M.; Kumar, S.; Kumar, S.; Dhiman, A. Artificial intelligence based modeling and optimization of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) production process by using Azohydromonas lata MTCC 2311 from cane molasses supplemented with volatile fatty acids: A genetic algorithm paradigm. Bioresour. Technol. 2012, 104, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Rego, A.S.C.; Valim, I.C.; Vieira, A.A.S.; Vilani, C.; Santos, B.F. Optimization of sugarcane bagasse pretreatment using alkaline hydrogen peroxide through ANN and ANFIS modelling. Bioresour. Technol. 2018, 267, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Houben, C.; Lapkin, A.A. Automatic discovery and optimization of chemical processes. Curr. Opin. Chem. Eng. 2015, 9, 1–7. [Google Scholar] [CrossRef] [Green Version]



| Pollutant (s) | Adsorbent | Batch/Continuous | Type of Algorithm | Input Parameters | Output Parameters | Data Points Used | Network Architecture | References |
|---|---|---|---|---|---|---|---|---|
| Arsenic | Iron/olivine composite | Batch | Levenberg-Marquardt (LM) BP algorithm | Cad, Ci, time, qag, and pH | REAs | 38 | 5:12:1 (As (III)T) 5:14:1 (As (V)T) | [28] |
| Ethanol mediated-Arsenic (III) | Zn-loaded pinecone (PC) biochar | Batch | BP algorithm and genetic algorithm | CAs, CEtOH, and pH | ACAs | 17 | 3:4:1 | [29] |
| Fluoride | Protonated clinoptilolite | Batch | Hybrid feedforward algorithm | pH, Ci, and temperature | ACF | 99 | 3:3:1 | [30] |
| Methylene blue (MB) | Biowaste AC 1 | Batch | BP algorithm | Ci, Cad, pH, temperature, and time | ACdye | 88 | 5:10:1 | [31] |
| Zinc (II) | Palm kernel shell AC 1 | Batch | PSO and LM-BP algorithm | Ci, pH, time, Cad, and temperature | REZn | 270 | 5:7:1 | [32] |
| Methyl orange dye | Polyaniline nano-adsorbent | Batch | LM-BP algorithm | pH, Ci, Cad, temperature, and time | REdye | 322 | 5:12:1 | [33] |
| Phenol | Scoria stone | Batch | CS-NN 3 | Cad, Ci, and time | REPh | 100 | 3:10:1 | [34] |
| Arsenic | Opuntia ficus indica biomass | Batch | Hybrid model | Ci, temperature, pH, and time | ACAs | 81 | 4:3:1 | [21] |
| AB and AR 4 | Chitosan hydrogels | Batch | LM-BP algorithm | Ci-AB, Ci-AR, time, ϕ, and w/w% | ACAB-AC | 315 | 5:10:10:10:1 | [35] |
| Copper and Manganese | GN-SDS 2 | Continuous | Quick prop algorithm | Ci, Cad, pH, and temperature | RECu,Mg | 40 | 4:10:1 | [36] |
| Cadmium, nickel, zinc, and copper | Bone Biochar | Continuous | LM-BP algorithm | Ci and time | Ct/C0 | 1420 | 2:6:1 | [37] |
| Coal-based pollutant | AC 1 | Continuous | LM–BP algorithm | β, ReL, t/tmax, and Ck/C0 | qt/qmax | 208 | 4:9:1 | [38] |
| Triclosan and ibuprofen | AC | Continuous | Gradient descent algorithm | Ci and q | C-t | 10 | 2:4:1 | [39] |
| Run | Actual Level of Factors | Chromium RE (%) | |||
|---|---|---|---|---|---|
| X1 | X2 (g/L) | X3 (mg/L) | Experimental | Predicted | |
| 1 | 4 | 2.5 | 10 | 80.54 | 84.38 |
| 2 | 8 | 2.5 | 10 | 69.79 | 69.87 |
| 3 | 4 | 7.5 | 10 | 92.72 | 94.54 |
| 4 | 8 | 7.5 | 10 | 90.36 | 89.56 |
| 5 | 8 | 2.5 | 30 | 52.35 | 49.72 |
| 6 | 8 | 7.5 | 30 | 82.20 | 77.54 |
| 7 | 2.64 | 5 | 20 | 95.48 | 93.94 |
| 8 | 9.36 | 5 | 20 | 73.95 | 74.85 |
| 9 | 6 | 0.795 | 20 | 49.58 | 49.69 |
| 10 | 6 | 9.204 | 20 | 88.34 | 87.69 |
| 11 | 6 | 5 | 3.182 | 92.81 | 91.28 |
| 12 | 6 | 5 | 36.82 | 72.15 | 72.98 |
| 13 | 6 | 5 | 20 | 80.85 | 80.99 |
| 14 | 6 | 5 | 20 | 80.89 | 80.99 |
| 15 | 6 | 5 | 20 | 80.84 | 80.99 |
| 16 | 6 | 5 | 20 | 80.87 | 80.99 |
| 17 | 6 | 5 | 20 | 83.49 | 80.99 |
| 18 | 6 | 5 | 20 | 81.02 | 80.99 |
| Characteristics | Features/Value |
|---|---|
| ANN model | |
| Network type | Feed-forward backpropagation |
| Training function | Trainlm (Levenberg Marquardt) |
| Number of hidden layers | 1 |
| Number of data used for network training | 14 |
| Number of data used for testing | 4 |
| Transfer function in hidden layer | tansig (Sigmoid) |
| Optimum no. of neurons in hidden layer | 3 |
| Epoch number | 164 |
| ANFIS Model | |
| Number of nodes | 58 |
| Number of linear parameters | 72 |
| Number of nonlinear parameters | 24 |
| Total number of parameters | 96 |
| Number of training data pairs | 14 |
| Number of validation data pairs | 04 |
| Number of fuzzy rules | 18 |
| Parameters | ANN Model | ANFIS Model | ||
|---|---|---|---|---|
| Training | Testing | Training | Testing | |
| R | 1.00 | 0.97 | 0.99 | 0.82 |
| R2 | 1.00 | 0.98 | 0.99 | 0.94 |
| SSE | 0.03 | 60.58 | 5.57 | 233.15 |
| SAE | 0.51 | 10.89 | 4.60 | 19.12 |
| ARE | 0.05 | 2.88 | 0.40 | 5.51 |
| AAD | 0.23 | 1009.72 | 39.75 | 3885.83 |
| MSE | <0.01 | 10.10 | 0.40 | 38.85 |
| RMSE | 0.05 | 3.18 | 0.63 | 6.23 |
| HYBRID | <0.01 | 33.66 | 0.61 | 144.72 |
| MPSD | 6.03 | 580.16 | 78.24 | 1203.03 |
| Chi-Square | <0.01 | 1.01 | 0.07 | 4.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zafar, M.; Aggarwal, A.; Rene, E.R.; Barbusiński, K.; Mahanty, B.; Behera, S.K. Data-Driven Machine Learning Intelligent Tools for Predicting Chromium Removal in an Adsorption System. Processes 2022, 10, 447. https://doi.org/10.3390/pr10030447
Zafar M, Aggarwal A, Rene ER, Barbusiński K, Mahanty B, Behera SK. Data-Driven Machine Learning Intelligent Tools for Predicting Chromium Removal in an Adsorption System. Processes. 2022; 10(3):447. https://doi.org/10.3390/pr10030447
Chicago/Turabian StyleZafar, Mohd, Ayushi Aggarwal, Eldon R. Rene, Krzysztof Barbusiński, Biswanath Mahanty, and Shishir Kumar Behera. 2022. "Data-Driven Machine Learning Intelligent Tools for Predicting Chromium Removal in an Adsorption System" Processes 10, no. 3: 447. https://doi.org/10.3390/pr10030447









