Grape Pomace Valorization by Extraction of Phenolic Polymeric Pigments: A Review
Abstract
:1. Introduction
2. Composition of Grape Pomace
3. The Chemistry of Polyphenols
3.1. Anthocyanins and Anthocyanin Derivatives
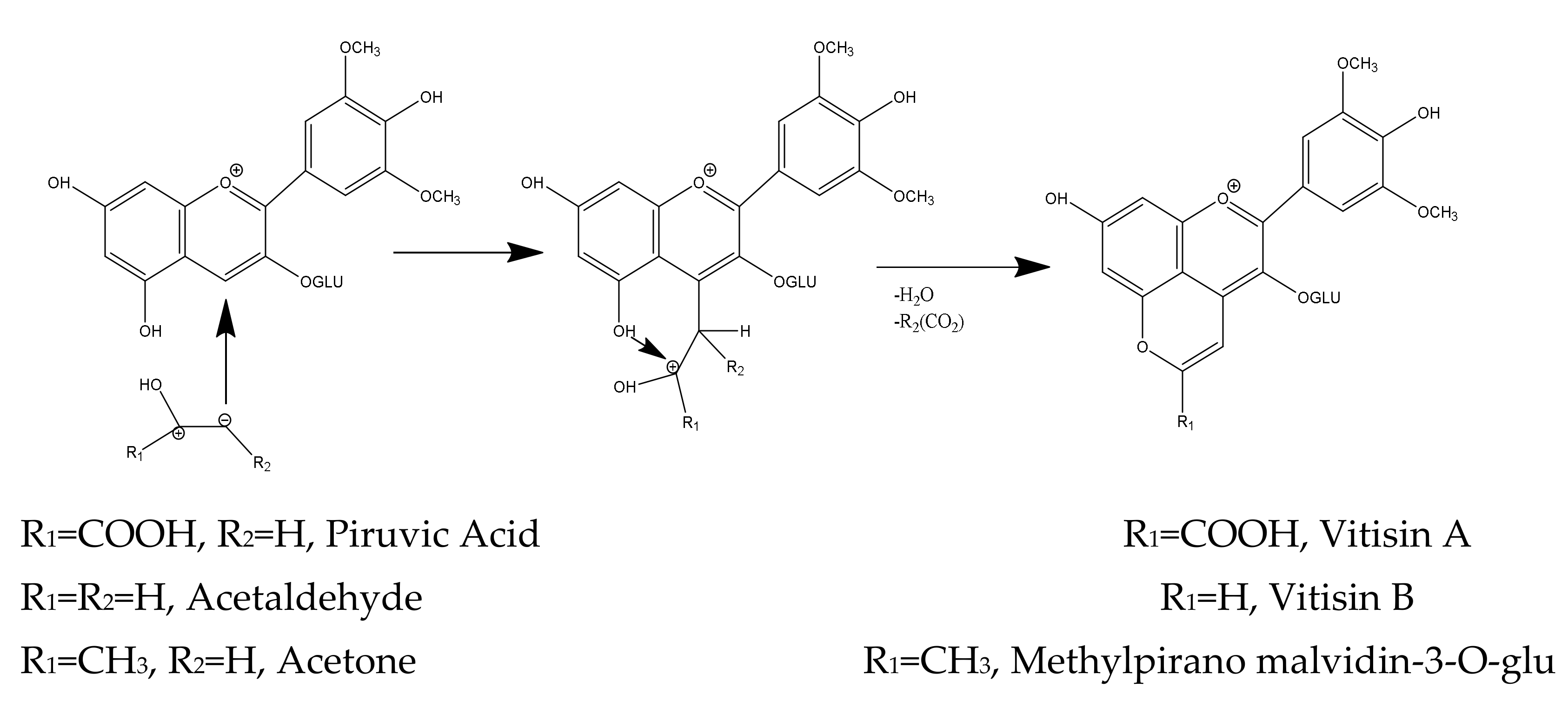
3.1.1. Pyranoanthocyanins
3.1.2. Vitisins
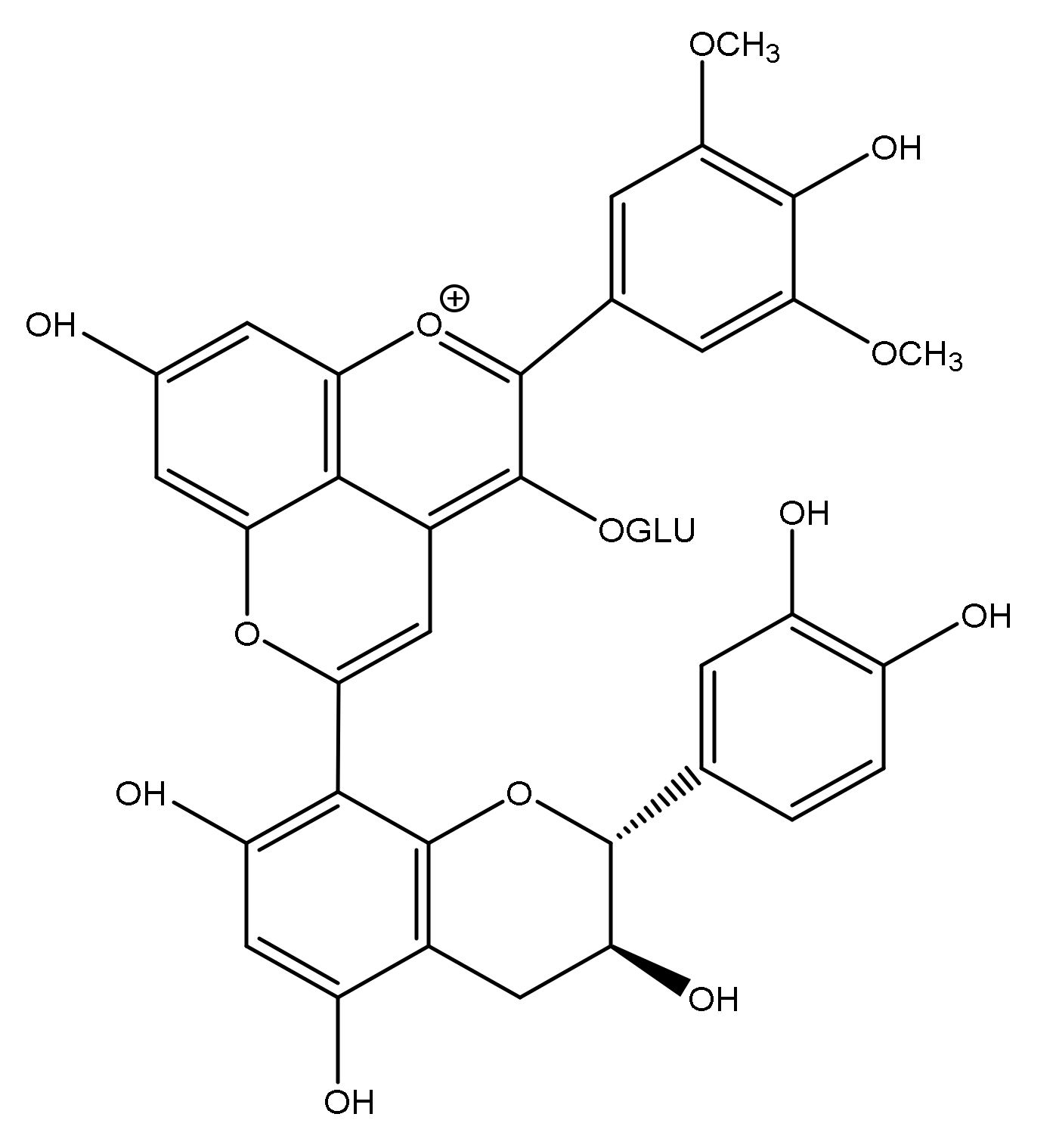
3.1.3. Flavanyl-Pyranoanthocyanins
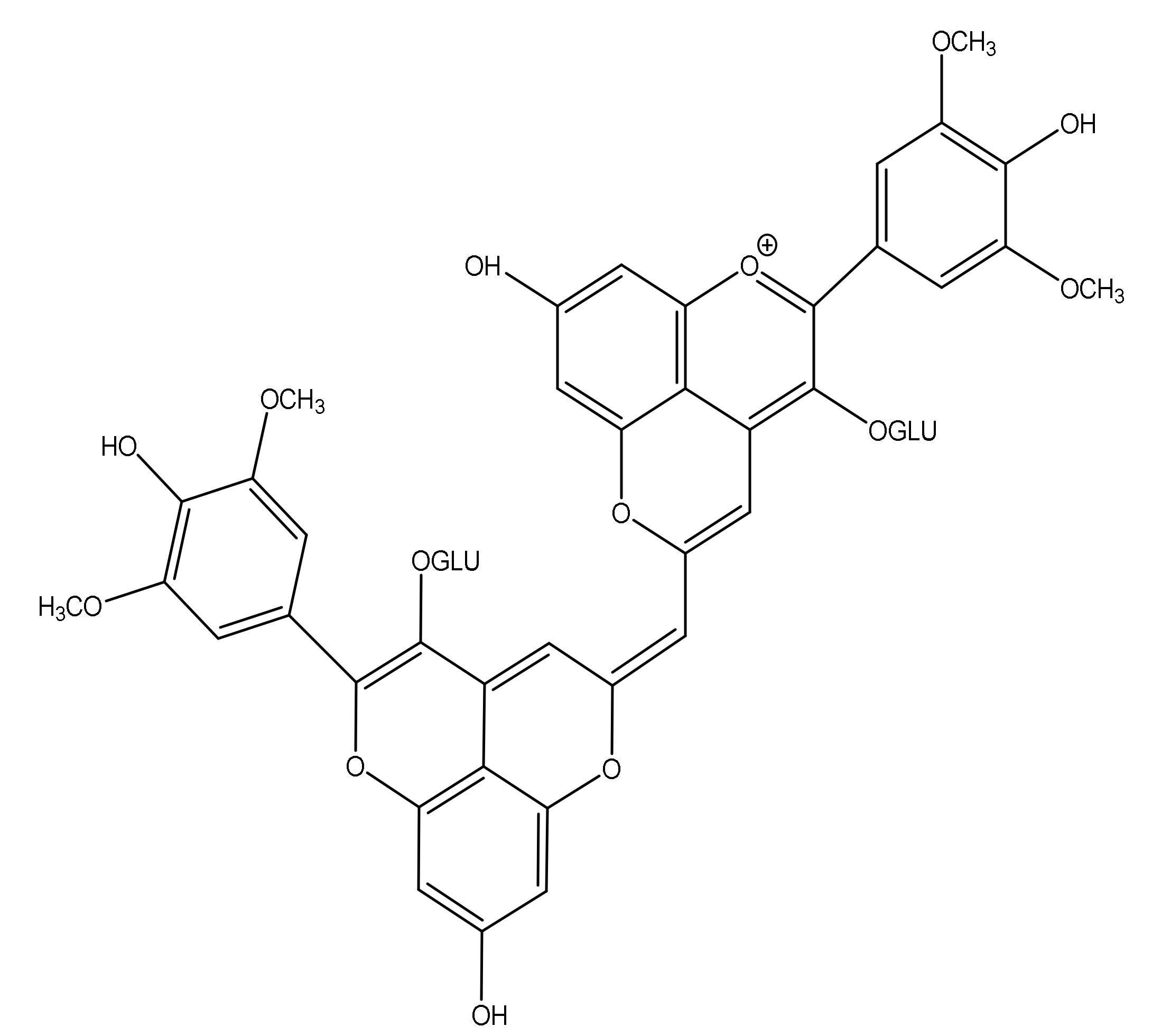
3.1.4. Pyranoanthocyanin Dimers
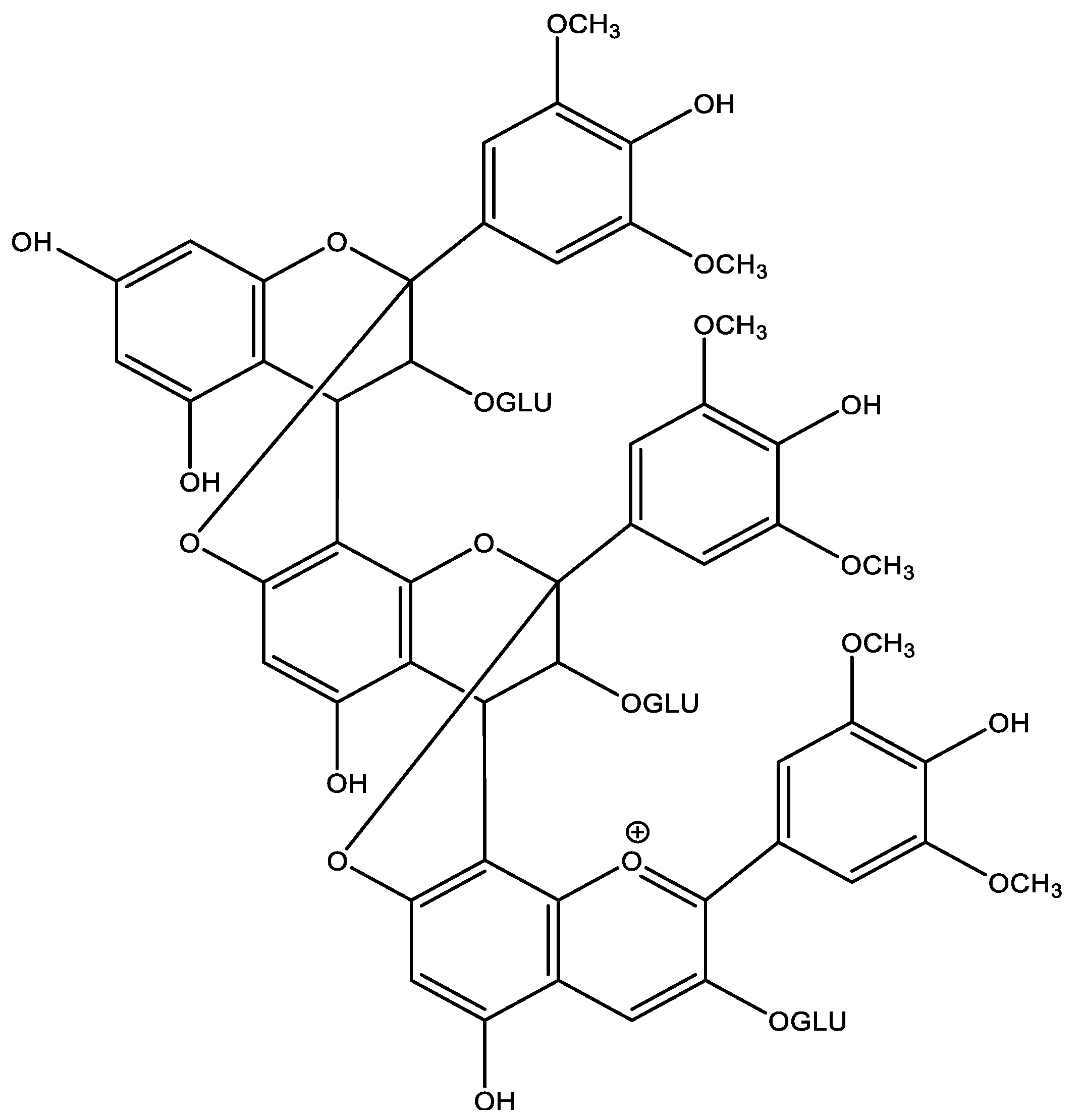
3.1.5. Polymeric Anthocyanins
4. Factors Influencing the Stability of Anthocyanins
4.1. Effect of pH
4.2. Effect of Concentration
4.3. Effect of Temperature
4.4. Light Effect
4.5. Effect of Oxygen
4.6. Effect of Co-Pigmentation
5. Bioactive Compound Extraction Methods
5.1. Solid-Liquid Extraction
5.2. Ultrasound Assisted Extraction (UAE)
5.3. Microwave Assisted Extraction (MAE)
5.4. Supercritical Fluid Extraction (SFE)
5.5. Natural Deep Eutectic Solvents Extraction (NADES)
5.6. Pressurized Liquid Extraction (PLE)
6. Beneficial Effects of Anthocyanins
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kammerer, D.; Claus, A.; Carle, R.; Schieber, A. Polyphenol screening of pomace from red and white grape varieties (Vitis vinifera L.) by HPLC-DAD-MS/MS. J. Agric. Food Chem. 2004, 52, 4360–4367. [Google Scholar] [CrossRef] [PubMed]
- Ghafoor, K.; Park, J.; Choi, Y. Optimization of supercritical fluid extraction of bioactive compounds from grape (Vitis labrusca B.) peel by using response surface methodology. Innov. Food Sci. Emerg. Technol. 2010, 11, 485–490. [Google Scholar] [CrossRef]
- Sirohi, R.; Tarafdar, A.; Singh, S.; Negi, T.; Gaur, V.K.; Gnansounou, E.; Bharathiraja, B. Green processing and biotechnological potential of grape pomace: Current trends and opportunities for sustainable biorefinery. Bioresour. Technol. 2010, 314, 123771. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.; Baenas, N.; Dominguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.A.; Garcia-Viguera, C. Natural bioactive compounds from winery by-products as health promoters: A review. Int. J. Mol. Sci. 2014, 15, 15638. [Google Scholar] [CrossRef] [Green Version]
- Lima, A.; Soares, C.M.F.; Paltram, R.; Halbwirth, H.; Bica, K. Extraction and consecutive purification of anthocyanins from grape pomace using ionic liquid solutions. Fluid Phase Equilibria 2017, 451, 68–78. [Google Scholar] [CrossRef]
- Bonfigli, M.; Godoy, E.; Reinheimer, M.A.; Scenna, N.J. Comparison between conventional and ultrasound-assisted techniques for extraction of anthocyanins from grape pomace. Experimental results and mathematical modeling. J. Food Eng. 2017, 207, 56–72. [Google Scholar] [CrossRef]
- Christ, K.L.; Burritt, R.L. Critical environmental concerns in wine production: An integrative review. J. Clean. Prod. 2013, 53, 232–242. [Google Scholar] [CrossRef]
- Boonchu, T.; Utama-Ang, N. Optimization of extraction and microencapsulation of bioactive compounds from red grape (Vitis vinifera L.) pomace. J. Food Sci. Technol. 2013, 52, 784–792. [Google Scholar] [CrossRef] [Green Version]
- Meini, M.R.; Cabezudo, I.; Boschetti, C.E.; Romanini, D. Recovery of phenolic antioxidants from Syrah grape pomace through the optimization of an enzymatic extraction process. Food Chem. 2019, 283, 257–264. [Google Scholar] [CrossRef]
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef]
- Cheynier, V. Phenolic compounds: From plants to foods. Phytochem. Rev. 2012, 11, 153–177. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Gonzaga, L.V.; Rizelio, V.M.; Gonçalves, A.E.D.S.S.; Genovese, M.I.; Fett, R. Phenolic compounds and antioxidant activity of seed and skin extracts of red grape (Vitis vinifera and Vitis labrusca) pomace from Brazilian winemaking. Food Res. Int. 2011, 44, 897–901. [Google Scholar] [CrossRef]
- Oliveira, J.; da Silva, M.A.; Teixeira, N.; de Freitas, V.; Salas, E. Screening of Anthocyanins and Anthocyanin-Derived Pigments in Red Wine Grape Pomace Using LC-DAD/MS and MALDI-TOF Techniques. J. Agric. Food Chem. 2015, 63, 7636–7644. [Google Scholar] [CrossRef] [PubMed]
- Sousa, E.C.; Uchôa-Thomaz, A.M.A.; Carioca, J.O.B.; De Morais, S.M.; De Lima, A.; Martins, C.G.; Alexandrino, C.D.; Ferreira, P.A.T.; Rodrigues, A.L.M.; Rodrigues, S.P.; et al. Chemical composition and bioactive compounds of grape pomace (Vitis vinifera L.), Benitaka variety, grown in the semiarid region of Northeast Brazil. Food Sci. Technol. 2014, 34, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Mendes, J.A.S.; Prozil, S.O.; Evtuguin, D.V.; Cruz, L.P. Towards comprehensive utilization of winemaking residues: Characterization of grape skins from red grape pomaces of variety Touriga Nacional. Ind. Crop. Prod. 2013, 43, 25–32. [Google Scholar] [CrossRef]
- Fontana, A.R.; Antoniolli, A.; Bottini, R. Grape pomace as a sustainable source of bioactive compounds: Extraction, characterization, and biotechnological applications of phenolics. J. Agric. Food Chem. 2013, 61, 8987–9003. [Google Scholar] [CrossRef]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A. Natural polyphenols: An overview Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef] [Green Version]
- Brezoiu, A.M.; Matei, C.; Deaconu, M.; Stanciuc, A.-M.; Trifan, A.; Gaspar, A.; Berger, D. Polyphenols extract from grape pomace. Characterization and valorisation through encapsulation into mesoporous silica-type matrices. Food Chem. Toxicol. 2019, 133, 110787. [Google Scholar] [CrossRef]
- Lu, Y.; Foo, L.Y. The polyphenol constituents of grape pomace. Food Chem. 1999, 65, 1–8. [Google Scholar] [CrossRef]
- Amico, V.; Napoli, E.M.; Renda, A.; Ruberto, G.; Spatafora, C.; Tringali, C. Constituents of grape pomace from the Sicilian cultivar ‘Nerello Mascalese’. Food Chem. 2004, 88, 599–607. [Google Scholar] [CrossRef]
- Boussetta, N.; Lanoisellé, J.; Bedel-cloutour, C.; Vorobiev, E. Extraction of soluble matter from grape pomace by high voltage electrical discharges for polyphenol recovery: Effect of sulphur dioxide and thermal treatments. J. Food Eng. 2009, 95, 192–198. [Google Scholar] [CrossRef]
- Vega, D.B. Formación y Evolución de Pigmentos de Tipo Pironoantociano en la Elaboración de Vinos Tintos y Rosados. PhD Thesis, Universidad de Castilla-La Mancha, Ciudad Real, Spain, 2013. [Google Scholar]
- Pina, F.; Oliveira, J.; de Freitas, V. Anthocyanins and derivatives are more than flavylium cations. Tetrahedron 2015, 71, 3107–3114. [Google Scholar] [CrossRef]
- Goula, A.M.; Thymiatis, K.; Kaderides, K. Valorization of grape pomace: Drying behavior and ultrasound extraction of phenolics. Food Bioprod. Process. 2016, 100, 132–144. [Google Scholar] [CrossRef]
- Bordiga, M.; Travaglia, F.; Locatelli, M. Valorisation of grape pomace: An approach that is increasingly reaching its maturity—A review. Int. J. Food Sci. Technol. 2019, 54, 933–942. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, S.; Zhang, X.; He, F.; Duan, C. An effective method for the semi-preparative isolation of high-purity anthocyanin monomers from grape pomace. Food Chem. 2020, 310, 125830. [Google Scholar] [CrossRef]
- Liazid, A.; Guerrero, R.F.; Cantos, E.; Palma, M.; Barroso, C.G. Microwave assisted extraction of anthocyanins from grape skins. Food Chem. 2011, 124, 1238–1243. [Google Scholar] [CrossRef]
- Caldas, T.W.; Mazza, K.E.L.; Teles, A.S.C.; Mattos, G.N.; Brígida, A.I.S.; Conte-Junior, C.A.; Borguini, R.G.; Godoy, R.L.O.; Cabral, L.M.C.; Tonon, R.V. Phenolic compounds recovery from grape skin using conventional and non-conventional extraction methods. Ind. Crops Prod. 2018, 111, 86–91. [Google Scholar] [CrossRef]
- Vidal, S.; Hayasaka, Y.; Meudec, E.; Cheynier, V.; Skouroumounis, G. Fractionation of Grape Anthocyanin Classes Using Multilayer Coil Countercurrent Chromatography with Step Gradient Elution. J. Agric. Food Chem. 2004, 52, 713–719. [Google Scholar] [CrossRef]
- Garrido, J.; Borges, F. Wine and grape polyphenols—A chemical perspective. Food Res. Int. 2013, 54, 1844–1858. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Ma, R.; Xu, Z.; Wang, J.; Chen, T.; Chen, F.; Wang, Z. Identification and quantification of anthocyanins in Kyoho grape juice-making pomace, Cabernet Sauvignon grape winemaking pomace and their fresh skin. J. Sci. Food Agric. 2013, 93, 1404–1411. [Google Scholar] [CrossRef]
- West, M.E.; Mauer, L.J. Color and Chemical Stability of a Variety of Anthocyanins and Ascorbic Acid in Solution and Powder Forms. J. Agric. Food Chem. 2013, 61, 4169–4179. [Google Scholar] [CrossRef] [PubMed]
- Shiraz, L.; Giacosa, S.; Marengo, F.; Guidoni, S.; Rolle, L.; Hunter, J.J. Anthocyanin yield and skin softening during maceration, as affected by vineyard row orientation and grape ripeness of Vitis vinifera. Food Chem. 2015, 174, 8–15. [Google Scholar] [CrossRef]
- Cortez, R.; Luna-vital, D.A.; Margulis, D.; de Mejia, E.G. Natural Pigments: Stabilization Methods of Anthocyanins for Food Applications. Compr. Rev. Food Sci. Food Saf. 2017, 16, 180–198. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1–16. [Google Scholar] [CrossRef] [Green Version]
- de Souza, V.B.; Thomazini, M.; César, J.; Balieiro, D.C.; Fávaro-trindade, C.S. Effect of spray drying on the physicochemical properties and color stability of the powdered pigment obtained from vinification byproducts of the Bordo grape (Vitis labrusca). Food Bioprod. Process. 2013, 93, 39–50. [Google Scholar] [CrossRef]
- Garzón, G.A. Anthocyanins as Natural Colorants and Bioactive Compounds. A Review. Acta Biol. Colomb. 2008, 13, 27–36. [Google Scholar]
- Fernandes, A.; Brás, N.F.; Mateus, N.; de Freitas, V. A study of anthocyanin self-association by NMR spectroscopy. New J. Chem. 2015, 39, 2602–2611. [Google Scholar] [CrossRef]
- Spigno, G.; de Faveri, D.M. Antioxidants from grape stalks and marc: Influence of extraction procedure on yield, purity and antioxidant power of the extracts. J. Food Eng. 2007, 78, 793–801. [Google Scholar] [CrossRef]
- Wang, W.; Jung, J.; Tomasino, E.; Zhao, Y. Optimization of solvent and ultrasound-assisted extraction for different anthocyanin rich fruit and their effects on anthocyanin compositions. LWT—Food Sci. Technol. 2016, 72, 229–238. [Google Scholar] [CrossRef] [Green Version]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef] [Green Version]
- Ongkowijoyo, P.; Luna-Vital, D.A.; de Mejia, E.G. Extraction techniques and analysis of anthocyanins from food sources by mass spectrometry: An update. Food Chem. 2018, 250, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Madureira, J.; Barros, L.; Verde, S.C.; Margaça, F.M.A.; Santos-Buelga, C.; Ferreira, I.C.F.R. Ionizing Radiation Technologies to Increase the Extraction of Bioactive Compounds from Agro-Industrial Residues: A Review. J. Agric. Food Chem. 2020, 68, 11054–11067. [Google Scholar] [CrossRef] [PubMed]
- Brahim, M.; Gambier, F.; Brosse, N. Optimization of polyphenols extraction from grape residues in water medium. Ind. Crops Prod. 2014, 52, 18–22. [Google Scholar] [CrossRef]
- Friedman, M. Antibacterial, antiviral, and antifungal properties of wines and winery byproducts in relation to their flavonoid content. J. Agric. Food Chem. 2014, 62, 6025–6042. [Google Scholar] [CrossRef]
- Guerrero, R.F.; Smith, P.; Bindon, K.A. Application of insoluble fibers in the fining of wine phenolics. J. Agric. Food Chem. 2013, 61, 4424–4432. [Google Scholar] [CrossRef] [PubMed]
- Kannampilly, N.J.; Devadas, C.T. Kinetic modelling of anthocyanin extraction from grape (Vitis vinifera) using response surface methodology. Int. J. Innov. Technol. Explor. Eng. 2019, 8, 3015–3019. [Google Scholar] [CrossRef]
- Kondratyuk, T.P.; Pezzuto, J.M. Natural product polyphenols of relevance to human health. Pharm. Biol. 2004, 42, 46–63. [Google Scholar] [CrossRef] [Green Version]
- El Gharras, H. Polyphenols: Food sources, properties and applications—A review. Int. J. Food Sci. Technol. 2009, 44, 2512–2518. [Google Scholar] [CrossRef]
- Quiñones, M.; Aleixandre, M.M.A. Los polifenoles, compuestos de origen natural con efectos saludables sobre el sistema cardiovascular. Nutr. Hosp. 2012, 27, 76–89. [Google Scholar] [CrossRef]
- Li, A.N.; Li, S.; Zhang, Y.J.; Xu, X.R.; Chen, Y.M.; Li, H.B. Resources and biological activities of natural polyphenols. Nutrients 2014, 6, 6020. [Google Scholar] [CrossRef]
- Valencia-Avilés, E.; Ignacio-Figueroa, I.; Sosa-Martínez, E.; Bartolomé-Camacho, M.C. Polyphenols: Antioxidant and toxicological properties. Rev. Fac. Cienc. Químicas 2017, 31, 15–29. [Google Scholar]
- Pervaiz, T.; Faghihi, F.; Salman, H.M.; Fang, J. Naturally occurring anthocyanin, structure, functions and biosynthetic pathway in fruit plants. J. Plant Biochem. 2017, 5, 1–9. [Google Scholar] [CrossRef]
- He, J.; Giusti, M.M. Anthocyanins: Natural colorants with health-promoting properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231. [Google Scholar] [CrossRef] [PubMed]
- Ribéreau-Gayon, P. Les composés phénoliques du raisin et du vin II. Les flavonosides et les anthocyanosides. Ann. Physiol. Végétale 1964, 3, 211–242. [Google Scholar]
- Hayasaka, Y.; Asenstorfer, R. Screening for potential pigments derived from anthocyanins in red wine using nanoelectrospray tandem mass spectrometry. J. Agric. Food Chem. 2002, 50, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Sigurdson, G.T.; Tang, P.; Giusti, M.M. Natural Colorants: Food colorants from natural sources. Annu. Rev. Food Sci. Technol. 2017, 8, 261–280. [Google Scholar] [CrossRef] [PubMed]
- Brouillard, R.; Delaporte, B. Chemistry of anthocyanin pigments. Kinetic and thermodynamic study of proton transfer, hydration, and tautomeric reactions of malvidin 3-glucoside. J. Am. Chem. Soc. 1977, 99, 8461–8468. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; Pacheco-Hernández, M.D.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Zheng, L.; Qiuhong, P.; Cui, X.; Duan, C. Optimization on anthocyanins extraction from wine grape skins using orthogonal test design. Food Sci. Biotechnol. 2010, 19, 1047–1053. [Google Scholar] [CrossRef]
- Giusti, M.; Wrolstad, R. Acylated anthocyanins from edible sources and their applications in food systems. Biochem. Eng. J. 2003, 14, 217–225. [Google Scholar] [CrossRef]
- Horbowicz, M.; Kosson, R.; Grzesiuk, A.; Dębski, H. Anthocyanins of fruits and vegetables-their occurrence, analysis and role in human nutrition. Vegetable Crops Research Bulletin. 2008, 68, 5–22. [Google Scholar] [CrossRef]
- González-Paramás, A.M.; da Silva, F.L.; Martín-López, P.; Macz-Pop, G.; Manzano, S.G.; Alcalde-Eon, C.; Pérez-Alonso, J.J.; Escribano-Bailon, M.T.; Rivas-Gonzalo, J.-C.; Santos-Buelga, C. Flavanol-anthocyanin condensed pigments in plant extracts. Food Chem. 2006, 94, 428–436. [Google Scholar] [CrossRef]
- Salas, E.; Atanasova, V.; Poncet-Legrand, C.; Meudec, E.; Mazauric, J.P.; Cheynier, V. Demonstration of the occurrence of flavanol-anthocyanin adducts in wine and in model solutions. Anal. Chim. Acta 2004, 513, 325–332. [Google Scholar] [CrossRef]
- Salas, E.; le Guernevé, C.; Fulcrand, H.; Poncet-Legrand, C.; Cheynier, V. Structure determination and colour properties of a new directly linked flavanol-anthocyanin dimer. Tetrahedron Lett. 2004, 45, 8725–8729. [Google Scholar] [CrossRef]
- Nave, F.; Teixeira, N.; Mateus, N.; de Freitas, V. The fate of flavanol-anthocyanin adducts in wines: Study of their putative reaction patterns in the presence of acetaldehyde. Food Chem. 2010, 121, 1129–1138. [Google Scholar] [CrossRef]
- Fulcrand, H.; Benabdeljalil, C.; Rigaud, J.; Cheynier, V.; Moutounet, M. A new class of wine pigments generated by reaction between pyruvic acid and grape anthocyanins. Phytochemistry 1998, 47, 1401–1407. [Google Scholar] [CrossRef]
- He, J.; Santos-Buelga, C.; Mateus, N.; de Freitas, V. Isolation and quantification of oligomeric pyranoanthocyanin-flavanol pigments from red wines by combination of column chromatographic techniques. J. Chromatogr. A 2006, 1134, 215–225. [Google Scholar] [CrossRef]
- Marquez, A.; Serratosa, M.P.; Merida, J. Pyranoanthocyanin derived pigments in wine: Structure and formation during winemaking. J. Chem. 2013, 2013, 1–15. [Google Scholar] [CrossRef]
- Revilla, I.; Perez-Magarino, S.; Gonzalez-SanJose, M.L. Identification of anthocyanin derivatives in grape skin extracts and red wines by liquid chromatography with diode array and mass spectrometric detection. J. Chromatogr. A 1999, 847, 83–90. [Google Scholar] [CrossRef]
- Salas, E.; Fulcrand, H.; Poncet-Legrand, C.; Meudec, E.; Kohler, N.; Winterhalter, P.; Cheynier, V. Isolation of flavanol-anthocyanin adducts by countercurrent chromatography. J. Chromatogr. Sci. 2005, 43, 488–493. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Ilárduya, M.; Sánchez-Fernández, C.; Viloria-Bernal, M.; López-Márquez, D.; Berrueta, L.; Gallo, B.; Vicente, F. Mass spectrometry fragmentation pattern of coloured flavanol-anthocyanin and anthocyanin-flavanol derivatives in aged red wines of Rioja. Aust. J. Grape Wine Res. 2012, 18, 203–214. [Google Scholar] [CrossRef]
- Oliveira, J.; de Freitas, V.; Silva, A.M.S.; Mateus, N. Reaction between hydroxycinnamic acids and anthocyanin-pyruvic acid adducts yielding new portisins. J. Agric. Food Chem. 2007, 55, 6349–6356. [Google Scholar] [CrossRef]
- Vivar-Quintana, A.M.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Anthocyanin-derived pigments and colour of red wines. Anal. Chim. Acta 2002, 458, 147–155. [Google Scholar] [CrossRef]
- Oliveira, J.; Mateus, N.; Silva, A.M.S.; de Freitas, V. Equilibrium forms of vitisin B pigments in an aqueous system studied by NMR and visible spectroscopy. J. Phys. Chem. B 2009, 113, 11352–11358. [Google Scholar] [CrossRef]
- Mateus, N.; de Freitas, V. Evolution and stability of anthocyanin-derived pigments during port wine aging. J. Agric. Food Chem. 2001, 49, 5217–5222. [Google Scholar] [CrossRef]
- He, F.; Liang, N.-N.; Mu, L.; Pan, Q.-H.; Wang, J.; Reeves, M.J.; Duan, C.-Q. Anthocyanins and their variation in red wines II. Anthocyanin derived pigments and their color evolution. Molecules 2012, 17, 1483. [Google Scholar] [CrossRef] [Green Version]
- Mateus, N.; Silva, A.M.S.; Vercauteren, J.; de Freitas, V. Occurrence of anthocyanin-derived pigments in red wines. J. Agric. Food Chem. 2001, 49, 4836–4840. [Google Scholar] [CrossRef]
- Oliveira, J.; da Silva, M.A.; Parola, A.J.; Mateus, N.; Brás, N.F.; Ramos, M.J.; de Freitas, V. Structural characterization of a A-type linked trimeric anthocyanin derived pigment occurring in a young Port wine. Food Chem. 2013, 141, 1987–1996. [Google Scholar] [CrossRef]
- Mateus, N.; Silva, A.M.S.; Rivas-Gonzalo, J.C.; Santos-Buelga, C.; de Freitas, V. A new class of blue anthocyanin-derived pigments isolated from red wines. J. Agric. Food Chem. 2003, 51, 1919–1923. [Google Scholar] [CrossRef]
- Alcalde-Eon, C.; Escribano-Bailón, M.T.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Separation of pyranoanthocyanins from red wine by column chromatography. Anal. Chim. Acta 2004, 513, 305–318. [Google Scholar] [CrossRef]
- Pissarra, J.; Lourenço, S.; González-Paramás, A.M.; Mateus, N.; Santos-Buelga, C.; de Freitas, V. Formation of new anthocyanin-alkyl/aryl-flavanol pigments in model solutions. Anal. Chim. Acta 2004, 513, 215–221. [Google Scholar] [CrossRef]
- Wang, H.; Race, E.J.; Shrikhande, A.J. Anthocyanin transformation in Cabernet Sauvignon wine during aging. ACS Symp. Ser. 2004, 886, 198–216. [Google Scholar] [CrossRef]
- Dipalmo, T.; Crupi, P.; Pati, S.; Clodoveo, M.L.; di Luccia, A. Studying the evolution of anthocyanin-derived pigments in a typical red wine of Southern Italy to assess its resistance to aging. LWT—Food Sci. Technol. 2016, 71, 1–9. [Google Scholar] [CrossRef]
- Zhang, X.K.; Lan, Y.B.; Huang, Y.; Zhao, X.; Duan, C.Q. Targeted metabolomics of anthocyanin derivatives during prolonged wine aging: Evolution, color contribution and aging prediction. Food Chem. 2021, 339, 127795. [Google Scholar] [CrossRef]
- Francia-Aricha, E.M.; Guerra, M.T.; Rivas-Gonzalo, J.C.; Santos-Buelga, C. New anthocyanin pigments formed after condensation with flavanols. J. Agric. Food Chem. 1997, 45, 2262–2266. [Google Scholar] [CrossRef]
- Cruz, L.; Teixeira, N.; Silva, A.M.S.; Mateus, N.; Borges, J.; de Freitas, V. Role of vinylcatechin in the formation of pyranomalvidin-3-glucoside-(+)- catechin. J. Agric. Food Chem. 2008, 56, 10980–10987. [Google Scholar] [CrossRef]
- de Freitas, V.; Mateus, N. Formation of pyranoanthocyanins in red wines: A new and diverse class of anthocyanin derivatives. Anal. Bioanal. Chem. 2011, 401, 1467–1477. [Google Scholar] [CrossRef]
- Oliveira, J.; Azevedo, J.; Silva, A.M.S.; Teixeira, N.; Cruz, L.; Mateus, N.; De Freitas, V. Pyranoanthocyanin dimers: A new family of turquoise blue anthocyanin-derived pigments found in port wine. J. Agric. Food Chem. 2010, 58, 5154–5159. [Google Scholar] [CrossRef]
- Jurd, L.; Somers, T.C. The formation of xanthylium salts from proanthocyanidins. Phytochemistry 1970, 9, 419–427. [Google Scholar] [CrossRef]
- Somers, T.C. The polymeric nature of wine pigments. Phytochemistry 1971, 10, 2175–2186. [Google Scholar] [CrossRef]
- Timberlake, C.F.; Bridle, P. Interactions Between Anthocyanins, Phenolic Compounds, and Acetaldehyde and Their Significance in Red Wines. Am. J. Enol. Vitic. 1976, 27, 97–105. [Google Scholar]
- Atanasova, V.; Fulcrand, H.; Cheynier, V.; Moutounet, M. Effect of oxygenation on polyphenol changes occurring in the course of wine-making. Anal. Chim. Acta 2002, 458, 15–27. [Google Scholar] [CrossRef]
- Es-Safi, N.E.; Fulcrand, H.; Cheynier, V.; Moutounet, M. Studies on the acetaldehyde-induced condensation of (-)-epicatechin and malvidin 3-O-glucoside in a model solution system. J. Agric. Food Chem. 1999, 47, 2096–2102. [Google Scholar] [CrossRef]
- Pissarra, J.; Mateus, N.; Rivas-Gonzalo, J.; Buelga, C.S.; de Freitas, V. Reaction between malvidin 3-glucoside and (+)-catechin in model solutions containing different aldehydes. J. Food Sci. 2003, 68, 476–481. [Google Scholar] [CrossRef]
- Salas, E.; Dueñas, M.; Schwarz, M.; Winterhalter, P.; Cheynier, V.; Fulcrand, H. Characterization of pigments from different high speed countercurrent chromatography wine fractions. J. Agric. Food Chem. 2005, 53, 4536–4546. [Google Scholar] [CrossRef]
- Remy, S.; Fulcrand, H.; Labarbe, B.; Cheynier, V.; Moutounet, M. First confirmation in red wine of products resulting from direct anthocyanin-tannin reactions. J. Sci. Food Agric. 2000, 80, 745–751. [Google Scholar] [CrossRef]
- Alcalde-Eon, C.; Escribano-Bailón, M.T.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Changes in the detailed pigment composition of red wine during maturity and ageing: A comprehensive study. Anal. Chim. Acta 2006, 563, 238–254. [Google Scholar] [CrossRef]
- Boido, E.; Alcalde-Eon, C.; Carrau, F.; Dellacassa, E.; Rivas-Gonzalo, J.C. Aging effect on the pigment composition and color of Vitis vinifera L. Cv. Tannat wines. Contribution of the main pigment families to wine color. J. Agric. Food Chem. 2006, 54, 6692–6704. [Google Scholar] [CrossRef]
- Berrueta, L.A.; Rasines-Perea, Z.; Prieto-Perea, N.; Asensio-Regalado, C.; Alonso-Salces, R.M.; Sánchez-Ilárduya, M.B.; Gallo, B. Formation and evolution profiles of anthocyanin derivatives and tannins during fermentations and aging of red wines. Eur. Food Res. Technol. 2020, 246, 149–165. [Google Scholar] [CrossRef]
- Pérez-Magariño, S.; González-San José, M.L. Evolution of Flavanols, Anthocyanins, and Their Derivatives during the Aging of Red Wines Elaborated from Grapes Harvested at Different Stages of Ripening. J. Agric. Food Chem. 2004, 52, 1181–1189. [Google Scholar] [CrossRef]
- Laitila, J.E.; Suvanto, J.; Salminen, J.P. Liquid chromatography–tandem mass spectrometry reveals detailed chromatographic fingerprints of anthocyanins and anthocyanin adducts in red wine. Food Chem. 2019, 294, 138–151. [Google Scholar] [CrossRef]
- Prat-García, S.; Oliveira, J.; Alamo-sanza, M.; de Freitas, V.; Nevares, I.; Mateus, N. Characterization of Anthocyanins and Anthocyanin-Derivatives in Red Wines during Ageing in Custom Oxygenation Oak Wood Barrels. Molecules 2021, 26, 64. [Google Scholar] [CrossRef]
- Fulcrand, H.; Atanasova, V.; Salas, E.; Cheynier, V. The fate of anthocyanins in wine: Are there determining factors? ACS Symp. Ser. 2004, 886, 68–88. [Google Scholar] [CrossRef]
- Jurd, L. Anthocyanidins and related compounds-XVI-The dimerization of flavylium salts in aqueus solutions. Tetrahedron 1972, 28, 493–504. [Google Scholar] [CrossRef]
- Cemeroglu, B.; Velioglu, S.; Isik, S. Degradation Kinetics of Anthocyanins in Sour Cherry Juice and Concentrate. J. Food Sci. 1994, 59, 1216–1218. [Google Scholar] [CrossRef]
- Patras, A.; Brunton, N.P.; O’Donnell, C.; Tiwari, B.K. Effect of thermal processing on anthocyanin stability in foods mechanisms and kinetics of degradation. Trends Food Sci. Technol. 2010, 21, 3–11. [Google Scholar] [CrossRef]
- Dangles, O.; Brouillard, R. Polyphenol interactions. The copigmentation case: Thermodynamic data from temperature variation and relaxation kinetics. Medium effect. Can. J. Chem. 1992, 70, 2174–2189. [Google Scholar] [CrossRef]
- Dangles, O.; Brouillard, R. A Spectroscopic method based on the anthocyanin copigmentation interaction and applied to the quantitative study of molecular complexes. J. Chem. Soc. Perkin Trans. 1992, 2, 247–257. [Google Scholar] [CrossRef]
- Dangles, O.; Saito, N.; Brouillard, R. Anthocyanin intramolecular copigment effect. Phytochemistry 1993, 34, 119–124. [Google Scholar] [CrossRef]
- Trouillas, P.; Sancho-García, J.C.; de Freitas, V.; Gierschner, J.; Otyepka, M.; Dangles, O. Stabilizing and modulating color by copigmentation: Insights from theory and experiment. Chem. Rev. 2016, 116, 4937–4982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, J.; Brás, N.F.; da Silva, M.A.; Mateus, N.; Parola, A.J.; de Freitas, V. Grape anthocyanin oligomerization: A putative mechanism for red color stabilization? Phytochemistry 2014, 105, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Týskiewicz, K.; Konkol, M.; Rój, E. The application of supercritical fluid extraction in phenolic compounds isolation from natural plant materials. Molecules 2018, 23, 2625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drevelegka, I.; Goula, A.M. Recovery of grape pomace phenolic compounds through optimized extraction and adsorption processes. Chem. Eng. Process. Process Intensif. 2020, 149, 107845. [Google Scholar] [CrossRef]
- Wang, M.; Wang, J.; Zhang, Y.; Xia, Q.; Bi, W.; Yang, X.; Chen, D.D.Y. Fast environment-friendly ball mill-assisted deep eutectic solvent-based extraction of natural products. J. Chromatogr. A 2016, 1443, 262–266. [Google Scholar] [CrossRef]
- Zainal-Abidin, M.H.; Hayyan, M.; Hayyan, A.; Jayakumar, N.S. New horizons in the extraction of bioactive compounds using deep eutectic solvents: A review. Anal. Chim. Acta 2017, 979, 1–23. [Google Scholar] [CrossRef]
- Bubalo, M.C.; Vidović, S.; Redovniković, I.R.; Jokić, S. New perspective in extraction of plant biologically active compounds by green solvents. Food Bioprod. Process. 2018, 109, 52–73. [Google Scholar] [CrossRef]
- Da Porto, C.; Natolino, A.; Decorti, D. Extraction of proanthocyanidins from grape marc by supercritical fluid extraction using CO2 as solvent and ethanol—Water mixture as co-solvent. J. Supercrit. Fluids 2014, 87, 59–64. [Google Scholar] [CrossRef]
- Barba, F.J.; Zhu, Z.; Koubaa, M.; Sant’Ana, A.S.; Orlien, V. Green alternative methods for the extraction of antioxidant bioactive compounds from winery wastes and by-products: A review. Trends Food Sci. Technol. 2016, 49, 96–109. [Google Scholar] [CrossRef]
- Pinelo, M.; Ruiz-Rodríguez, A.; Sineiro, J.; Señoráns, F.J.; Reglero, G.; Núñez, M.J. Supercritical fluid and solid-liquid extraction of phenolic antioxidants from grape pomace: A comparative study. Eur. Food Res. Technol. 2007, 226, 199–205. [Google Scholar] [CrossRef]
- Spigno, G.; Tramelli, L.; de Faveri, D.M. Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J. Food Eng. 2007, 81, 200–208. [Google Scholar] [CrossRef]
- Casazza, A.A.; Aliakbarian, B.; Mantegna, S.; Cravotto, G.; Perego, P. Extraction of phenolics from Vitis vinifera wastes using non-conventional techniques. J. Food Eng. 2010, 100, 50–55. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Comas-Serra, F.; Femenia, A.; Rosselló, C.; Simal, S. Effect of power ultrasound application on aqueous extraction of phenolic compounds and antioxidant capacity from grape pomace (Vitis vinifera L.): Experimental kinetics and modeling. Ultrason. Sonochem. 2015, 22, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, K.M.; Zhao, J.; Jin, Y.; Heo, S.R.; Han, S.Y.; Yoo, D.E.; Lee, J. Highly efficient extraction of anthocyanins from grape skin using deep eutectic solvents as green and tunable media. Arch. Pharm. Res. 2015, 38, 2143–2152. [Google Scholar] [CrossRef]
- Bubalo, M.C.; Ćurko, N.; Tomašević, M.; Ganić, K.K.; Redovnikovic, I.R. Green extraction of grape skin phenolics by using deep eutectic solvents. Food Chem. 2016, 200, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Da Porto, C.; Natolino, A.; Decorti, D. The combined extraction of polyphenols from grape marc: Ultrasound assisted extraction followed by supercritical CO2 extraction of ultrasound-raffinate. LWT 2015, 61, 98–104. [Google Scholar] [CrossRef] [Green Version]
- Drosou, C.; Kyriakopoulou, K.; Bimpilas, A.; Tsimogiannis, D.; Krokida, M. A comparative study on different extraction techniques to recover red grape pomace polyphenols from vinification byproducts. Ind. Crops Prod. 2015, 75, 141–149. [Google Scholar] [CrossRef]
- Fontana, A.; Antoniolli, A. Phenolics profiling of pomace extracts from different grape varieties cultivated in Argentina. RSC Adv. 2017, 7, 29446–29457. [Google Scholar] [CrossRef] [Green Version]
- Bosiljkov, T.; Dujmić, F.; Bubalo, M.C.; Hribar, J.; Vidrih, R.; Brnčić, M.; Zlatic, E.; Redovniković, I.R.; Jokić, S. Natural deep eutectic solvents and ultrasound-assisted extraction: Green approaches for extraction of wine lees anthocyanins. Food Bioprod. Process. 2017, 102, 195–203. [Google Scholar] [CrossRef]
- Pintać, D.; Majkić, T.; Torovic, L.; Orčić, D.; Beara, I.; Simin, N.; Mimica–Dukić, N.; Lesjak, M. Solvent selection for efficient extraction of bioactive compounds from grape pomace. Ind. Crops Prod. 2018, 111, 379–390. [Google Scholar] [CrossRef]
- Panić, M.; Radić, M.; Kraljić, K.; Škevin, D.; Radojčić, I. Ready-to-use green polyphenolic extracts from food by-products. Food Chem. 2019, 283, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Panić, M.; Gunjević, V.; Cravotto, G.; Redovniković, I.R. Enabling technologies for the extraction of grape-pomace anthocyanins using natural deep eutectic solvents in up-to-half-litre batches extraction of grape-pomace anthocyanins using NADES. Food Chem. 2019, 300, 125185. [Google Scholar] [CrossRef] [PubMed]
- Šuković, D.; Knežević, B.; Gašić, U.; Sredojević, M.; Ćirić, I.; Todić, S.; Mutić, J.; Tešić, Ž. Phenolic profiles of leaves, grapes and wine of grapevine variety vranac (Vitis vinifera L.) from Montenegro. Foods 2020, 9, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loarce, L.; Oliver-Simancas, R.; Marchante, L.; Díaz-Maroto, M.C.; Alañón, M.E. Modifiers based on natural deep eutectic mixtures to enhance anthocyanins isolation from grape pomace by pressurized hot water extraction. LWT 2021, 149, 111889. [Google Scholar] [CrossRef]
- Monrad, J.K.; Suárez, M.; Motilva, M.J.; King, J.W.; Srinivas, K.; Howard, L.R. Extraction of anthocyanins and flavan-3-ols from red grape pomace continuously by coupling hot water extraction with a modified expeller. Food Res. Int. 2014, 65, 77–87. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J.; Chen, G.; Ma, H. The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: A review. Food Biosci. 2020, 35, 100547. [Google Scholar] [CrossRef]
- Panja, P. Green extraction methods of food polyphenols from vegetable materials. Curr. Opin. Food Sci. 2018, 23, 173–182. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
- Eskilsson, C.S.; Bjorklund, E. Analytical-scale microwave-assisted extraction. J. Chromatogr. A 2000, 902, 227–250. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Zhang, H.F.; Yang, X.H.; Wang, Y. Microwave assisted extraction of secondary metabolites from plants: Current status and future directions. Trends Food Sci. Technol. 2011, 22, 672–688. [Google Scholar] [CrossRef]
- Kalli, E.; Lappa, I.; Bouchagier, P.; Tarantilis, P.A.; Skotti, E. Novel application and industrial exploitation of winery by-products. Bioresour. Bioprocess. 2018, 5, 46. [Google Scholar] [CrossRef]
- Brunner, G. Supercritical fluids: Technology and application to food processing. Food Eng. 2005, 67, 21–33. [Google Scholar] [CrossRef]
- Farías-Campomanes, A.M.; Rostagno, M.A.; Meireles, A.A. Production of polyphenol extracts from grape bagasse using supercritical fluids: Yield, extract composition and economic evaluation. J. Supercrit. Fluids 2013, 77, 70–78. [Google Scholar] [CrossRef]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef] [PubMed]
- Alonso, D.A.; Baeza, A.; Chinchilla, R.; Gómez Lucas, C.; Guillena, G.; Marset, X.; Pastor, I.M.; Ramón, D.J.; Ros Ñíguez, D.; Saavedra, B. Mezclas eutécticas como alternativa sostenible a los disolventes convencionales en Química Orgánica. An. Química 2018, 114, 79–87. [Google Scholar]
- Cunha, S.C.; Fernandes, J.O. Extraction techniques with deep eutectic solvents. Trends Anal. Chem. 2018, 105, 225–239. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Farajzadeh, M.A.; Mogaddam, M.R.A.; Feriduni, B. Simultaneous synthesis of a deep eutectic solvent and its application in liquid-liquid microextraction of polycyclic aromatic hydrocarbons from aqueous samples. RSC Adv. 2016, 6, 47990–47996. [Google Scholar] [CrossRef]
- Wei, Z.; Qi, X.; Li, T.; Luo, M.; Wang, W.; Zu, Y.; Fu, Y. Application of natural deep eutectic solvents for extraction and determination of phenolics in Cajanus cajan leaves by ultra performance liquid chromatography. Sep. Purif. Technol. 2015, 149, 237–244. [Google Scholar] [CrossRef]
- Zhao, B.Y.; Xu, P.; Yang, F.X.; Wu, H.; Zong, M.H.; Lou, W.Y. Biocompatible Deep Eutectic Solvents Based on Choline Chloride: Characterization and Application to the Extraction of Rutin from Sophora japonica. ACS Sustain. Chem. Eng. 2015, 3, 2746–2755. [Google Scholar] [CrossRef]
- Socas-Rodríguez, B.; Santana-Mayor, Á.; Herrera-Herrera, A.V.; Rodríguez-Delgado, M.Á. Deep eutectic solvents. In Green Sustainable Process for Chemical and Environmental Engineering and Science: Ionic Liquids as Green Solvents; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 123–177. [Google Scholar]
- Alvarez-Rivera, G.; Bueno, M.; Ballesteros-Vivas, D.; Mendiola, J.A.; Ibañez, E. Pressurized liquid extraction. In Liquid-Phase Extraction; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 375–398. [Google Scholar]
- Alañón, E.; Ivanovićd, M.; Pimentel-Morab, S.; Borrás-Linaresc, I.; Arráez-Román, D.; Segura-Carreterob, A. A novel sustainable approach for the extraction of value-added compounds from Hibiscus sabdariffa L. calyces by natural deep eutectic solvents. Food Res. Int. 2020, 137, 109646. [Google Scholar] [CrossRef] [PubMed]
- da Silva, D.T.; Pauletto, R.; Cavalheiro, S.D.S.; Bochi, V.C.; Rodrigues, E.; Weber, J.; Silva, C.D.B.D.; Morisso, F.D.P.; Barcia, M.T.; Emanuelli, T. Natural deep eutectic solvents as a biocompatible tool for the extraction of blueberry anthocyanins. J. Food Compos. Anal. 2020, 89, 103470. [Google Scholar] [CrossRef]
- Radošević, K.; Ćurko, N.; Srček, V.G.; Bubalo, M.C.; Tomašević, M.; Ganić, K.K.; Redovniković, I.R. Natural deep eutectic solvents as beneficial extractants for enhancement of plant extracts bioactivity. LWT-Food Sci. Technol. 2016, 73, 45–51. [Google Scholar] [CrossRef]
- Pereira, D.T.V.; Tarone, A.G.; Cazarin, C.B.B.; Barbero, G.F.; Martínez, J. Pressurized liquid extraction of bioactive compounds from grape marc. J. Food Eng. 2019, 240, 105–113. [Google Scholar] [CrossRef]
- De Oliveira, L.D.L.; de Carvalho, M.V.; Melo, L. Health promoting and sensory properties of phenolic compounds in food. Rev. Ceres 2014, 61, 764–779. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Ahmedna, M. Functional components of grape pomace: Their composition, biological properties and potential applications. Int. J. Food Sci. Technol. 2013, 48, 221–237. [Google Scholar] [CrossRef]



| Aglycone | Substitution | ||||||
|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | R4 | R5 | R6 | R7 | |
| Pelargonidin (Pd) | OH | OH | H | OH | H | OH | H |
| Cyanidin (Cy) | OH | OH | H | OH | OH | OH | H |
| Delphinidin (Dph) | OH | OH | H | OH | OH | OH | OH |
| Peonidin (Pn) | OH | OH | H | OH | OCH3 | OH | H |
| Petunidin(Pt) | OH | OH | H | OH | OCH3 | OH | OH |
| Malvidin (Mv) | OH | OH | H | OH | OCH3 | OH | OCH3 |
| Extraction Method | Extraction Conditions | Raw Material | Identified Compounds | References |
|---|---|---|---|---|
| SFE SLE | Supercritical CO2 Density range for CO2: 111.13–874 kg/m3 Modifier: ethanol Constant stirring rate:140 rpm Solvent: ethanol 96% and distilled water Extraction time: 30, 90 min Ratio s/l: 1, 5 v/w Temperature: 25, 50 °C | Garnacha grape pomace | Total phenolic content | [122] |
| SLE | First phase Temperature: 45 and 60 °C Maceration times: 1, 3, 5, 7, 9,15, 20, 24 h Solvent: absolute ethanol Ratio s/l: 4/1 v/w Second phase Temperature: 60 °C Maceration times: 5 h Solvent: ethanol-water (10–20–30–40–50–60%) Liquid extracts were freeze-dried | Barbera red grape pomace | Total phenolic content Anthocyanin content | [123] |
| UAE | Solvent: methanol acidifie with formic acid (95, 98, 100%) Time: 30, 60, 90 min Temperature: 25, 30, 35 °C | Cabernet Sauvignon grape skin | Total monomeric anthocyanins Total acylated anthocyanins Total polymeric anthocyanins | [61] |
| SFE | Supercritical CO2 Extraction temperatures: 37, 40, 43, 46 °C Pressures: 140, 150, 160, 170 kg cm−2 Modifier: ethanol 5, 6, 7, 8% | Vitis Labrusca B grape skin | Total phenolic content Total anthocyanins | [2] |
| MAE UAE | Solvent: methanol Temperature: 110 °C under nitrogen atmosphere Irradiation: 60 W Time: 60 min. Solvent: methanol Temperature: 25 °C Irradiation: 60 W Time: 60 min. | Skins and seeds of Pinot Noir cultivar from white vinification | Total phenolic content Total flavonoids o-diphenols | [124] |
| MAE | Temperature: 50, 100 °C Time: 5, 20 min Power: 100, 500 w Solvent: methanol-water 50, 80% | Tintilla de Rota grape skin | Total anthocyanins | [27] |
| UAE | Temperature: 20, 35, 50 °C Frequency: 50 kHz Acoustic power density: 435 W/L | Vitis vinifera L grape pomace | Total phenolic content | [125] |
| UAE DES | Solvent: water, methanol 80, 100%, ethanol 70, 100% Temperature: 25 °C Time: 45 min | Grape skin | Total anthocyanins | [126] |
| UAE, DES MAE | Temperature: 30, 90 °C Time: 15, 90 min Frequency: 35 kHz Temperature: 50, 90 °C Time: 15, 90 min Power: 100 W | Red grape skin | Total anthocyanins | [127] |
| UAE SFE | Solvent: ethanol-water (449.73 g/L) Temperature: 20, 50, 80 °C Frequency: 20 kHz Time: 4, 7, 10 min Supercritical CO2 Pressure: 8 MPa Temperature: 40 °C Solvent flow rate: 6 kg/h CO2 modified with 10% ethanol-water | Red grape pomace | Total phenolic content | [128] |
| UAE MAE | Solvent: ethanol-water (1:1) Frequency: 25 kHz Temperature: 20 °C Time: 60 min Solvent: ethanol-water (1:1) Temperature: 50 °C Power: 200 W Time: 60 min | Agiorgitiko grape pomace | Total phenolic content Total flavan-3-ol content | [129] |
| UAE | Frequency: 20 kHz Solvent: ethanol-water 50, 70%, methanol 70% Time: 4, 10, 20, 30, 40, 60 min Temperature: 20, 40 °C | Agiorgitiko grape pomace | Total phenolic content | [24] |
| SLE | Solvent: ethanol- water 50% v/v Solvent-to-sample ratio 5: 1 Time: 2 h Temperature: 60 °C | Malbec, Cabernet Sauvignon, Cabernet Franc and Merlot grape pomace | Total glycosylated Total acetylated Total coumaroylated | [130] |
| UAE SLE | Solvent: ethanol- water 50% v/v Frequency of 40 kHz Time: 5, 10, 15, 20, 25, 30 min Solid to solvent ratio: 1:40 Solvent: ethanol- water 50% v/v Time: 5, 10, 15, 20, 25, 30 min Solid to solvent ratio: 1:40 Agitation speed: 460 rpm | Red grape pomace | Total anthocyanins | [6] |
| UAE DES | Power: 190, 285, 380 W Frequency: 37 kHz Temperature: 35 °C Solid to solvent ratio: 0.1 g/mL Time: 15, 30, 45 min Water content in DES: 10, 30, 50% Time: 15, 30, 45 min Power: 190, 285, 380 W | Wine lees | Total anthocyanins | [131] |
| UAE MAE | Frequency: 20 kHz Power density: 1000 W/L Temperature: 28 °C Solvent: ethanol (8–92%) Frequency: 2458 MHz Power density: 1000 W/L Solvent: ethanol (8–92%) | Red grape pomace | Total phenolic content | [28] |
| SLE | Six extracting 80% methanol 80% ethanol Acetone Ethyl acetate Methanol: distilled water: formic acid | Cabernet Sauvignon, Italian Riesling varieties and Merlot variety grape pomace | Total phenolic content Total flavonoid content | [132] |
| DES Ultrasonic–microwave cooperative reactor | Microwave power: 300 W Ultrasound power: 50 W Time: 10 min Solvent: ethanol 70% Solvent: Choline chloride-citric acid, molar ratio 2:1 with 30% of water (v/v) | Red grape pomace | Total phenolic content | [133] |
| DES Ultrasonic–microwave cooperative reactor | Power 100 W Temperature: 65 °C Time: 50 min Solid-liquid ratios: of 0.03 g/mL of solvent Solvent: 70% of ethanol with 0.1% of HCl, v/v and NADES | Red grape pomace | Total anthocyanins | [134] |
| UAE | Solvent: methanol-water 70% solution containing 0.1% HCl Time: 60 min Temperature: 25 °C | Leaves, grapes and wine of Grapevine Variety Vranac | Phenolic Acids and their derivatives Anthocyanins and derivates Flavan-3-ols Flavanols Stilbenes | [135] |
| UAE MAE | Power: 130 W Frequency: 20 kHz Time: 2, 5, 10, 20, 30 min Temperature: between 20–60 °C Solvent: aqueous ethanol 0–100% Operating pressure: 75 bars Time: 1, 2, 3, 4, 5 min | Agiorgitiko grape pomace | Total phenolic content | [117] |
| DES PHWE | Number of cycles: 2 Time: 10 min Pressure: 1500 psi Temperature: 40–100 °C Solvent: NADES | Tempranillo grape pomace | Total anthocyanins Unpolymerized anthocyanins | [136] |
| NADES | Molar Ratio (mol/mol) | Total Anthocyanins | Water | NADESsynthesis Method | References |
|---|---|---|---|---|---|
| ChLa ChOx ChEth ChProp ChFruW ChMa ChGluW ChU | 1:2 1:1 1:2 1:2 2:1:1 3:1 2:1:1 1:2 | 3.10 3.44 2.94 3.15 2.66 2.52 1.92 2.76 | 25% | Heating and stirring Temperature 80 °C Time 2 h | [155] 1 |
| ChGlyCit ChGlyCit ChGlyCit ChGlyCit ChGly | 1:1:1 2:0.5:0.5 0.5:2:0.5 0.5:0.5:2 1.5:1.5 | 2.611 2.302 3.623 1.608 2.021 | 25% | Dried in an oven at 45 °C for 1 h before use. Heating and stirring Temperature 80 °C Time 30 min | [156] 2 |
| ChOx ChLa ChFruW ChEth ChProp ChU | 1:1 1:2 2:1:1 1:2 1:2 1:2 | 170.04 146.06 78.48 93.74 145.52 101.37 | - | Heating and stirring Temperature 80 °C Time 2 h | [136] 3 |
| ChCit ChMa ChProMa | 2:1 1:1 1:1:1 | 0.92 0.78 0.92 | 25% | Heating and stirring Temperature 80 °C Time 2 h | [134] 4 |
| ChMa ChCit ChGly ChGlu ChFru ChGal ChRib ChSuc ChMaltose ChMaltitol | 1:1 1:1 1:1 5:2 5:2 5:2 5:2 1:1 4:1 4:1 | 23 25 23.5 22 24.5 22 24 18 24 24.5 | 3:7 w/w | Freeze drying method | [126] 5 |
| ChXyl ChFru ChGlu ChGly ChMa ChCit ChOx | 2:1 1.9:1 2:1 1:2 1:1 1:1 1:1 | 4.4 4.2 3.9 4.5 5.3 4.9 5.0 | 50% 50% 50% 50% 75% 75% 75% | Heating and stirring Temperature 80 °C Time 2–6 h | [131] 6 |
| ChGlu ChFru ChXyl ChGly ChMa | 2:1 1.9:1 2:1 1:2 1:1 | 16 17 20 12 24 | 30% | Heating and stirring Temperature 80 °C Time 2–6 h | [157] 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castellanos-Gallo, L.; Ballinas-Casarrubias, L.; Espinoza-Hicks, J.C.; Hernández-Ochoa, L.R.; Muñoz-Castellanos, L.N.; Zermeño-Ortega, M.R.; Borrego-Loya, A.; Salas, E. Grape Pomace Valorization by Extraction of Phenolic Polymeric Pigments: A Review. Processes 2022, 10, 469. https://doi.org/10.3390/pr10030469
Castellanos-Gallo L, Ballinas-Casarrubias L, Espinoza-Hicks JC, Hernández-Ochoa LR, Muñoz-Castellanos LN, Zermeño-Ortega MR, Borrego-Loya A, Salas E. Grape Pomace Valorization by Extraction of Phenolic Polymeric Pigments: A Review. Processes. 2022; 10(3):469. https://doi.org/10.3390/pr10030469
Chicago/Turabian StyleCastellanos-Gallo, Lilisbet, Lourdes Ballinas-Casarrubias, José C. Espinoza-Hicks, León R. Hernández-Ochoa, Laila Nayzzel Muñoz-Castellanos, Miriam R. Zermeño-Ortega, Alejandra Borrego-Loya, and Erika Salas. 2022. "Grape Pomace Valorization by Extraction of Phenolic Polymeric Pigments: A Review" Processes 10, no. 3: 469. https://doi.org/10.3390/pr10030469








