Preparation of Polymer Composite Selective Permeable Membrane with Graphene Oxide and Application for Chemical Protective Clothing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membranes Preparation
2.3. Infrared Analysis
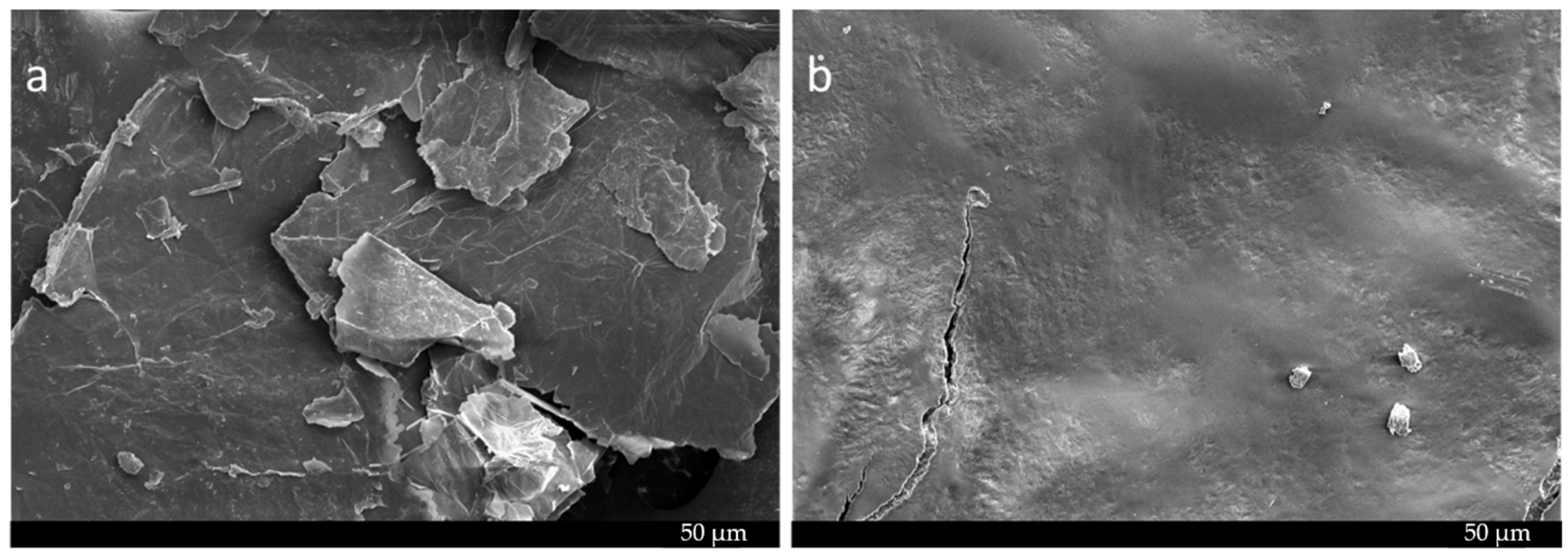
2.4. Morphology Analysis
2.5. Water Uptake (WU), Ion Exchange Capacity (IEC), and Linear Swelling Ratio (LSR)
2.6. Wettability
2.7. Mechanical Strength Test
2.8. Permeations and Selectivity Tests
3. Results
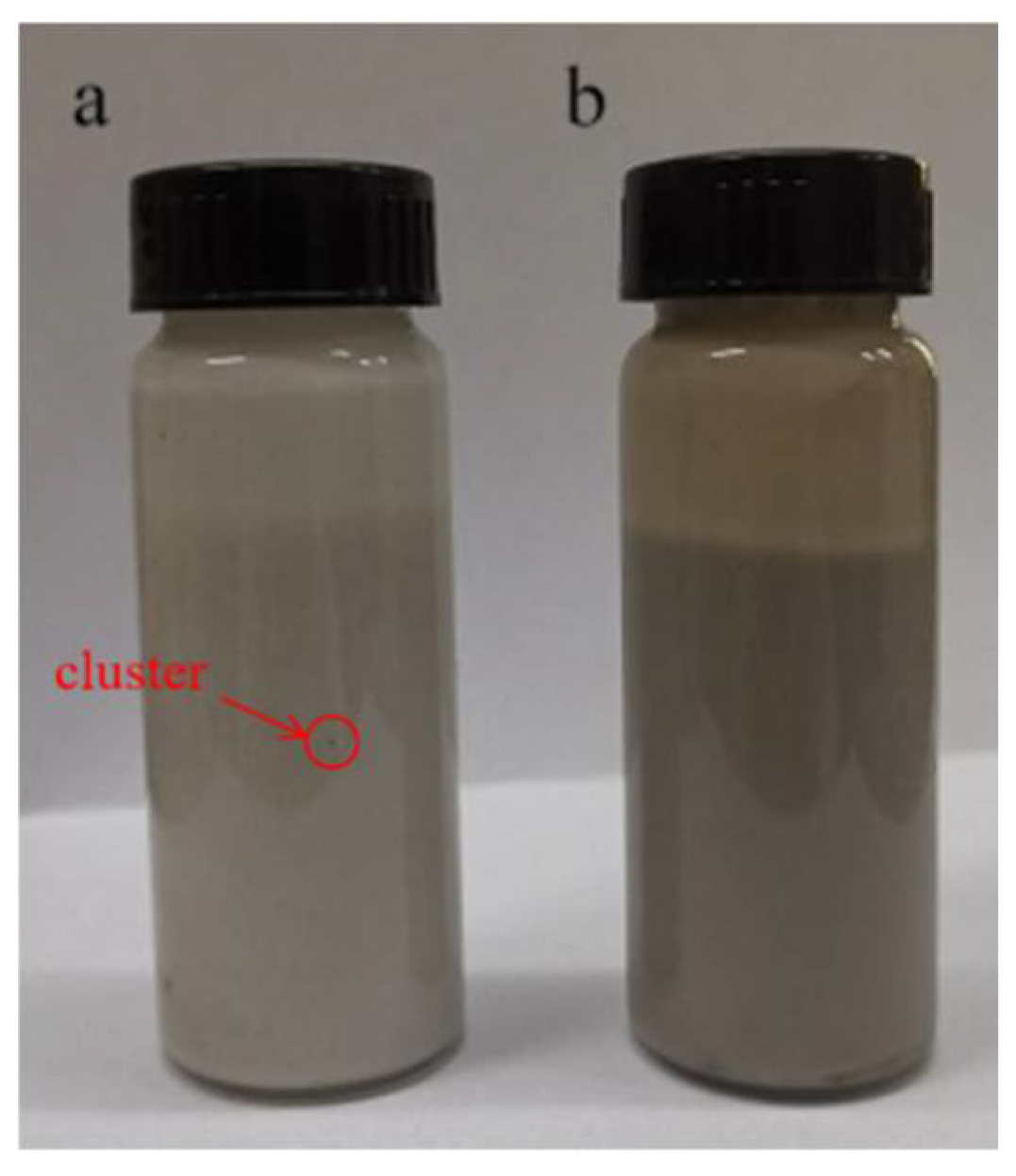
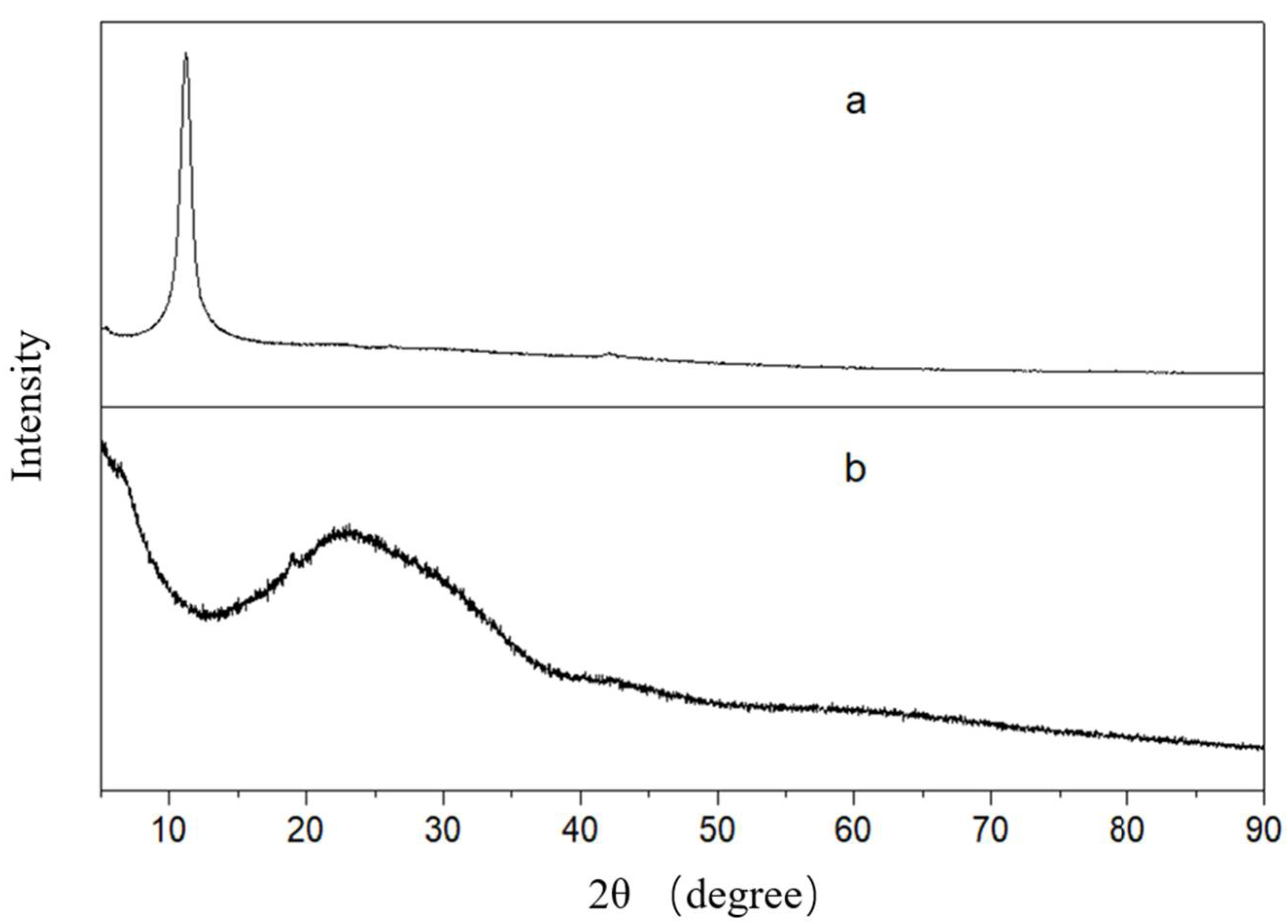
3.1. Synthesis and Characterizations of GO-SSS and PVDF-g-SSS Composite Membrane
3.2. IEC, WU, and LSR
3.3. Water Contact Angle (WCA)
3.4. Mechanical Properties
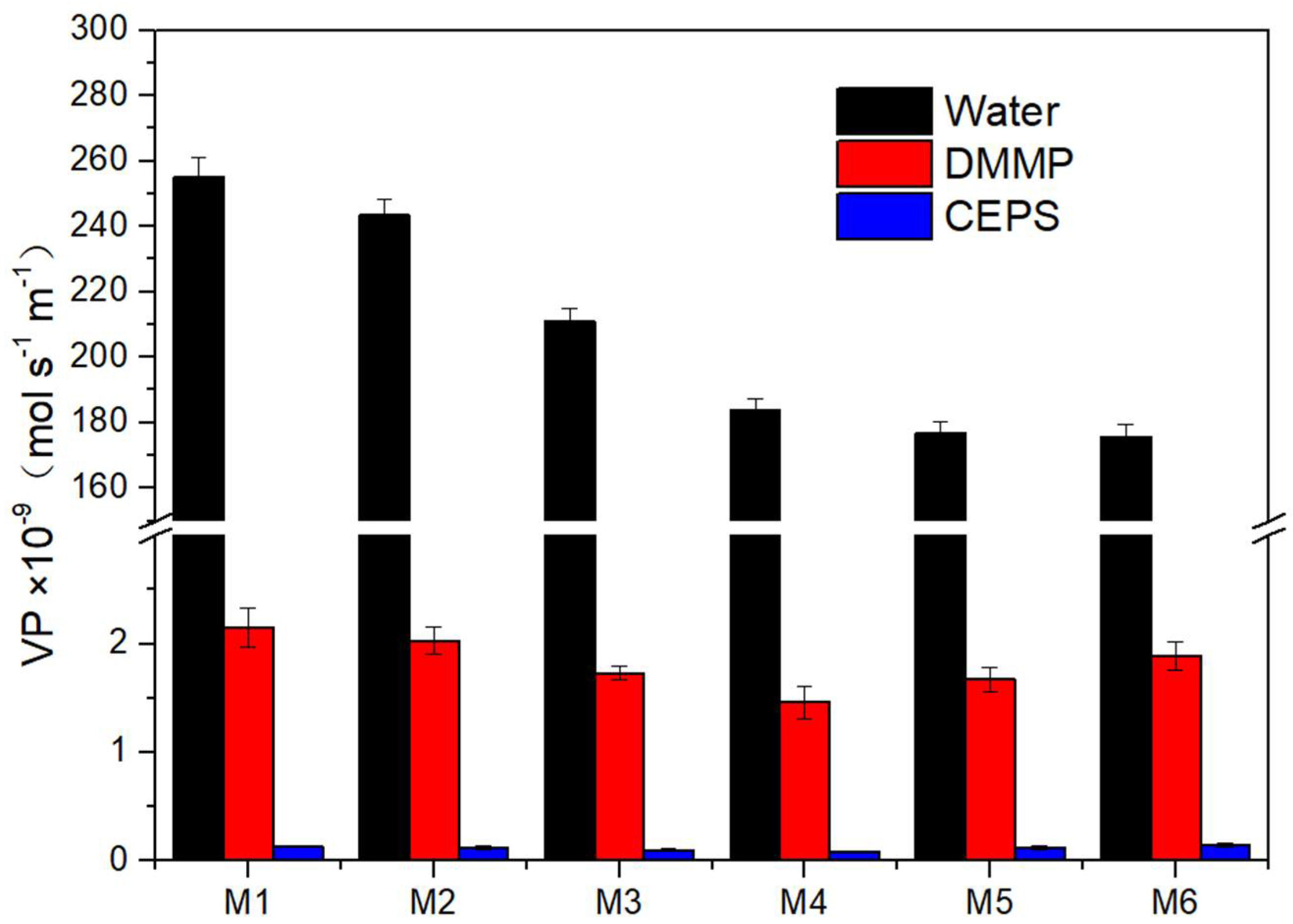
3.5. Permeations and Selectivity of Membranes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dubey, V.; Rao, N.; Maiti, S.N.; Gupta, A.K. Sorption of sulfur mustard and its oxygen analog in black and nonblack-filled butyl rubber membranes. J. Appl. Polym. Sci. 1998, 69, 503–511. [Google Scholar] [CrossRef]
- Almquist, C.B.; Hwang, S.T. The permeation of organophosphorus compounds in silicone rubber membranes. J. Membr. Sci. 1999, 153, 57–69. [Google Scholar] [CrossRef]
- Rivin, D.; Lindsay, R.S.; Shuely, W.J.; Rodriguez, A. Liquid permeation through nonporous barrier materials. J. Membr. Sci. 2005, 246, 39–47. [Google Scholar] [CrossRef]
- Lu, X.; Nguyen, V.; Zeng, X.; Elliott, B.J.; Gin, D.L. Selective rejection of a water-soluble nerve agent stimulant using a nanoporous lyotropic liquid crystal-butyl rubber vapor barrier material: Evidence for a molecular size-discrimination mechanism. J. Membr. Sci. 2008, 318, 397–404. [Google Scholar] [CrossRef]
- Schreuder-Gibson, H.L.; Truong, Q.; Walker, J.E.; Owens, J.R.; Wander, J.D.; Jones, W.E. Chemical and biological protection and detection in fabrics for protective clothing. MRS Bull. 2003, 28, 574–578. [Google Scholar] [CrossRef] [Green Version]
- Preparation and Properties of Spherical Activated Carbon-based Inner Materials for Chemical Protective Clothing. China Text. Lead. 2018, 64–67.
- Ramaseshan, R.; Sundarrajan, S.; Liu, Y.; Barhate, R.S.; Lala, N.L.; Ramakrishna, S. Functionalized polymer nanofibre membranes for protection from chemical warfare stimulants. Nanotechnology 2006, 17, 2947–2953. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Wang, D.; Li, H.; Li, L.; Zhang, S.; Zhou, C.; Zheng, X.; Men, Q.; Zhong, J.; et al. Preparation and Chemical Protective Clothing Application of PVDF Based Sodium Sulfonate Membrane. Membranes 2020, 10, 190. [Google Scholar] [CrossRef] [PubMed]
- Rivin, D.; Meermeier, G.; Schneider, N.S.; Vishnyakov, A.; Neimark, A.V. Simultaneous transport of water and organic molecules through polyelectrolyte membranes. J. Phys. Chem. B 2004, 108, 8900–8909. [Google Scholar] [CrossRef]
- Schneider, N.S.; Rivin, D. Interaction of dimethyl methylphosphonate with Nafion in acid and cation modifications. Polymer 2004, 45, 6309–6320. [Google Scholar] [CrossRef]
- Schneider, N.S.; Rivin, D. Solvent transport in hydrocarbon and perfluorocarbon ionomers. Polymer 2006, 47, 3119–3131. [Google Scholar] [CrossRef]
- Schneider, N.S.; Rivin, D. Steady state analysis of water vapor transport in ionomers. Polymer 2010, 51, 671–678. [Google Scholar] [CrossRef]
- Jung, K.H.; Pourdeyhimi, B.; Zhang, X.W. Chemical protection performance of polystyrene sulfonic acid-filled polypropylene nonwoven membranes. J. Membr. Sci. 2010, 362, 137–142. [Google Scholar] [CrossRef]
- Vishnyakov, A.; Neimark, A.V. Molecular Dynamics Simulation of Nanoscale Distribution and Mobility of Water and Dimethylmethylphosphonate in Sulfonated Polystyrene. J. Phys. Chem. B 2008, 112, 14905–14910. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Colon, E.; Perez-Perez, M.; Suleiman, D. Synthesis and characterization of novel phosphonated and sulfonated poly(styrene-isobutylene-styrene) for fuel cell and protective clothing applications. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 1424–1435. [Google Scholar] [CrossRef]
- Ruiz-Colon, E.; Perez-Perez, M.; Suleiman, D. Influence of carboxylated and phosphonated single-walled carbon nanotubes on the transport properties of sulfonated poly(styrene-isobutylene-styrene) membranes. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 2475–2495. [Google Scholar] [CrossRef]
- Ruiz-Colon, E.; Perez-Perez, M.; Suleiman, D. Transport properties of blended sulfonated poly(styrene-isobutylene-styrene) and isopropyl phosphate membranes. J. Appl. Polym. Sci. 2019, 136, 47009. [Google Scholar] [CrossRef]
- Suleiman, D.; Carreras, G.; Soto, Y. Effect of block composition, size and functionality of poly(styrene-isobutylene-styrene) copolymers. J. Appl. Polym. Sci. 2013, 128, 2297–2306. [Google Scholar] [CrossRef]
- Nafion 117. Available online: https://www.fuelcellstore.com/nafion-117?search=nafion%20117 (accessed on 18 December 2021).
- Scott, B.J.; Verbridge, S.S.; Alden, J.S.; van der Zande, A.M.; Parpia, J.M.; Craighead, H.G.; McEuen, P.L. Impermeable atomic membranes from graphene sheets. J. Nano Lett. 2008, 8, 2458–2462. [Google Scholar]
- Kan, L.; Xu, Z.; Gao, C. General Avenue to Individually Dispersed Graphene Oxide-Based Two-Dimensional Molecular Brushes by Free Radical Polymerization. Macromolecules 2011, 44, 444–452. [Google Scholar] [CrossRef]
- Ge, Q.; Ran, J.; Miao, J.; Yang, Z.; Xu, T. Click Chemistry Finds Its Way in Constructing an Ionic Highway in Anion-Exchange Membrane. ACS Appl. Mater. Interfaces 2015, 7, 28545–28553. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Lin, X.; Ge, L.; Wu, L.; Xu, T. A novel route for preparing highly proton conductive membrane materials with metal-organic frameworks. Chem. Commun. 2013, 49, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kim, J.; Jung, B. Morphology and transport properties of protons and methanol through partially sulfonated block copolymers. J. Membr. Sci. 2005, 250, 175–182. [Google Scholar] [CrossRef]
- Wijmans, J.G.; Baker, R.W. The solution-diffusion model—A review. J. Membr. Sci. 1995, 107, 1–21. [Google Scholar] [CrossRef]






| Sample | IEC (mmol/g) | WU (%) | LSR (%) | CA of Water (o) |
|---|---|---|---|---|
| M1 | 1.97 | 53.6 | 16. 8 | 40.1 ± 2.2 |
| M2 | 1.95 | 53.2 | 16.3 | 44.9 ± 2.6 |
| M3 | 1.90 | 53.1 | 16.1 | 50.2 ± 2.3 |
| M4 | 1.88 | 52.8 | 15.8 | 55.0 ± 2.4 |
| M5 | 1.83 | 52.4 | 15.6 | 58.3 ± 2.1 |
| M6 | 1.80 | 51.6 | 15.2 | 60.6 ± 1.8 |
| Sample | Thickness (μm) | Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|
| M1 | 120 | 17.5 ± 2.3 | 235.3 ± 18.8 |
| M2 | 117 | 13.2 ± 2.2 | 113.7 ± 14.2 |
| M3 | 113 | 15.3 ± 2.3 | 102.4 ± 19.5 |
| M4 | 109 | 13.7 ± 1.5 | 60.6 ± 15.4 |
| M5 | 106 | 14.1 ± 1.3 | 46.4 ± 8.4 |
| M6 | 103 | 12.9 ± 1.6 | 26.8 ± 5.3 |
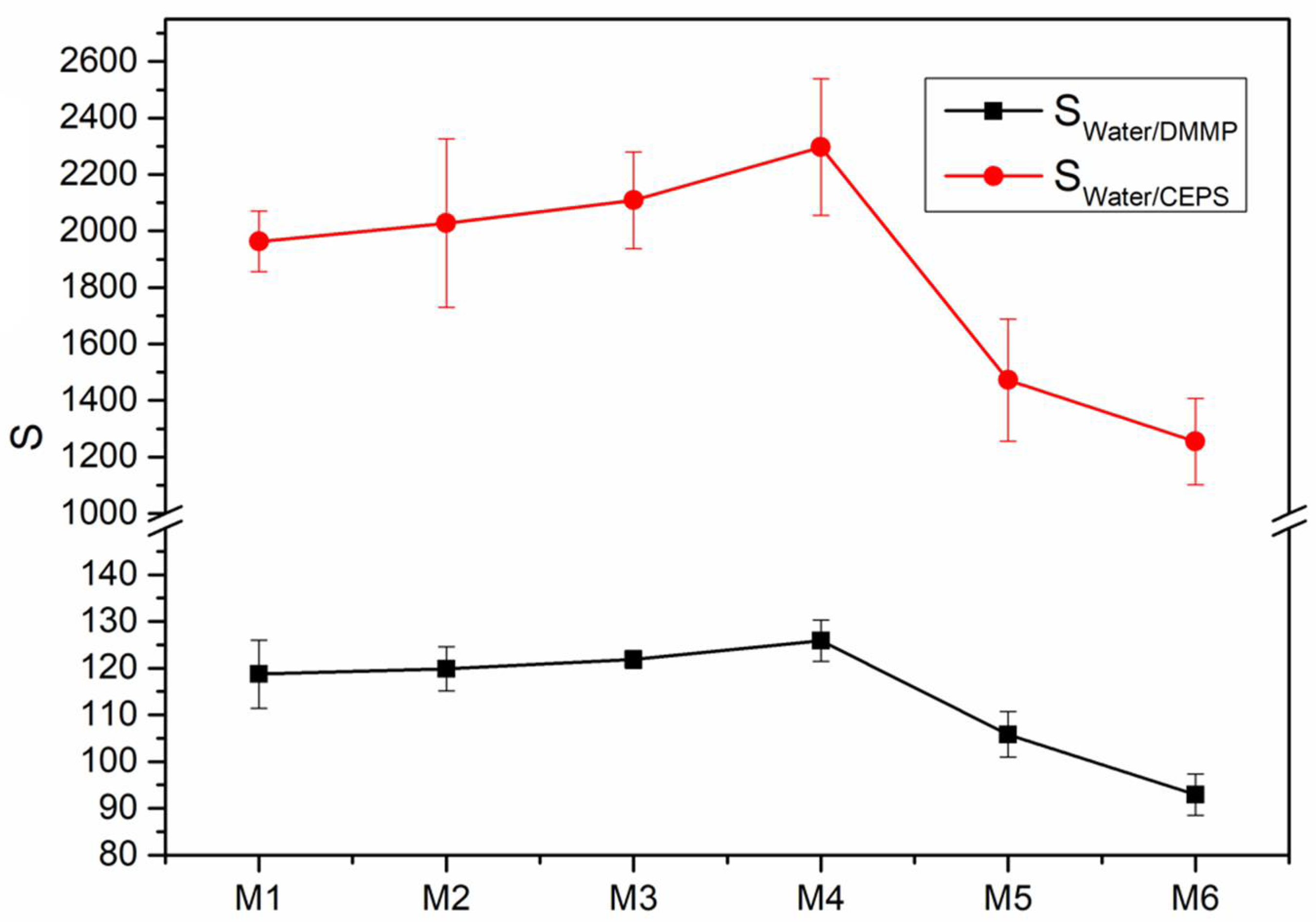
| Sample | VTR of Water (g m−2 24 h−1) | VP of Water × 10−9(mol s−1 m−1) | VP of DMMP × 10−9 (mol s−1 m−1) | VP of CEPS × 10−9 (mol s−1 m−1) | Selectivity (Water/DMMP) | Selectivity (Water/CEPS) |
|---|---|---|---|---|---|---|
| M1 | 3307 ± 75 | 255.23 ± 5.76 | 2.15 ± 0.18 | 0.13 ± 0.01 | 118.71 ± 7.26 | 1963.31 ± 106.72 |
| M2 | 3235 ± 64 | 243.36 ± 4.82 | 2.03 ± 0.12 | 0.12 ± 0.02 | 119.88 ± 4.71 | 2028.00 ± 297.83 |
| M3 | 2902 ± 55 | 210.89 ± 4.03 | 1.73 ± 0.06 | 0.10 ± 0.01 | 121.90 ± 1.89 | 2108.90 ± 170.59 |
| M4 | 2622 ± 52 | 183.78 ± 3.63 | 1.46 ± 0.08 | 0.08 ± 0.01 | 125.88 ± 4.41 | 2297.25 ± 241.78 |
| M5 | 2593 ± 51 | 176.74 ± 3.51 | 1.67 ± 0.11 | 0.12 ± 0.02 | 105.83 ± 4.87 | 1472.83 ± 216.22 |
| M6 | 2652 ± 56 | 175.66 ± 3.74 | 1.89 ± 0.13 | 0.14 ± 0.02 | 92.94 ± 4.41 | 1254.71 ± 152.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, H.; Li, Z.; Li, H.; Zhao, Y.; Li, X.; Liu, J.; Ji, Z. Preparation of Polymer Composite Selective Permeable Membrane with Graphene Oxide and Application for Chemical Protective Clothing. Processes 2022, 10, 471. https://doi.org/10.3390/pr10030471
Du H, Li Z, Li H, Zhao Y, Li X, Liu J, Ji Z. Preparation of Polymer Composite Selective Permeable Membrane with Graphene Oxide and Application for Chemical Protective Clothing. Processes. 2022; 10(3):471. https://doi.org/10.3390/pr10030471
Chicago/Turabian StyleDu, Haolin, Zenghe Li, Heguo Li, Yue Zhao, Xiaopeng Li, Jing Liu, and Zuohui Ji. 2022. "Preparation of Polymer Composite Selective Permeable Membrane with Graphene Oxide and Application for Chemical Protective Clothing" Processes 10, no. 3: 471. https://doi.org/10.3390/pr10030471
APA StyleDu, H., Li, Z., Li, H., Zhao, Y., Li, X., Liu, J., & Ji, Z. (2022). Preparation of Polymer Composite Selective Permeable Membrane with Graphene Oxide and Application for Chemical Protective Clothing. Processes, 10(3), 471. https://doi.org/10.3390/pr10030471







