Supercritical Foaming and Impregnation of Polycaprolactone and Polycaprolactone-Hydroxyapatite Composites with Carvacrol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of PCL-HA Composites by Solvent Casting
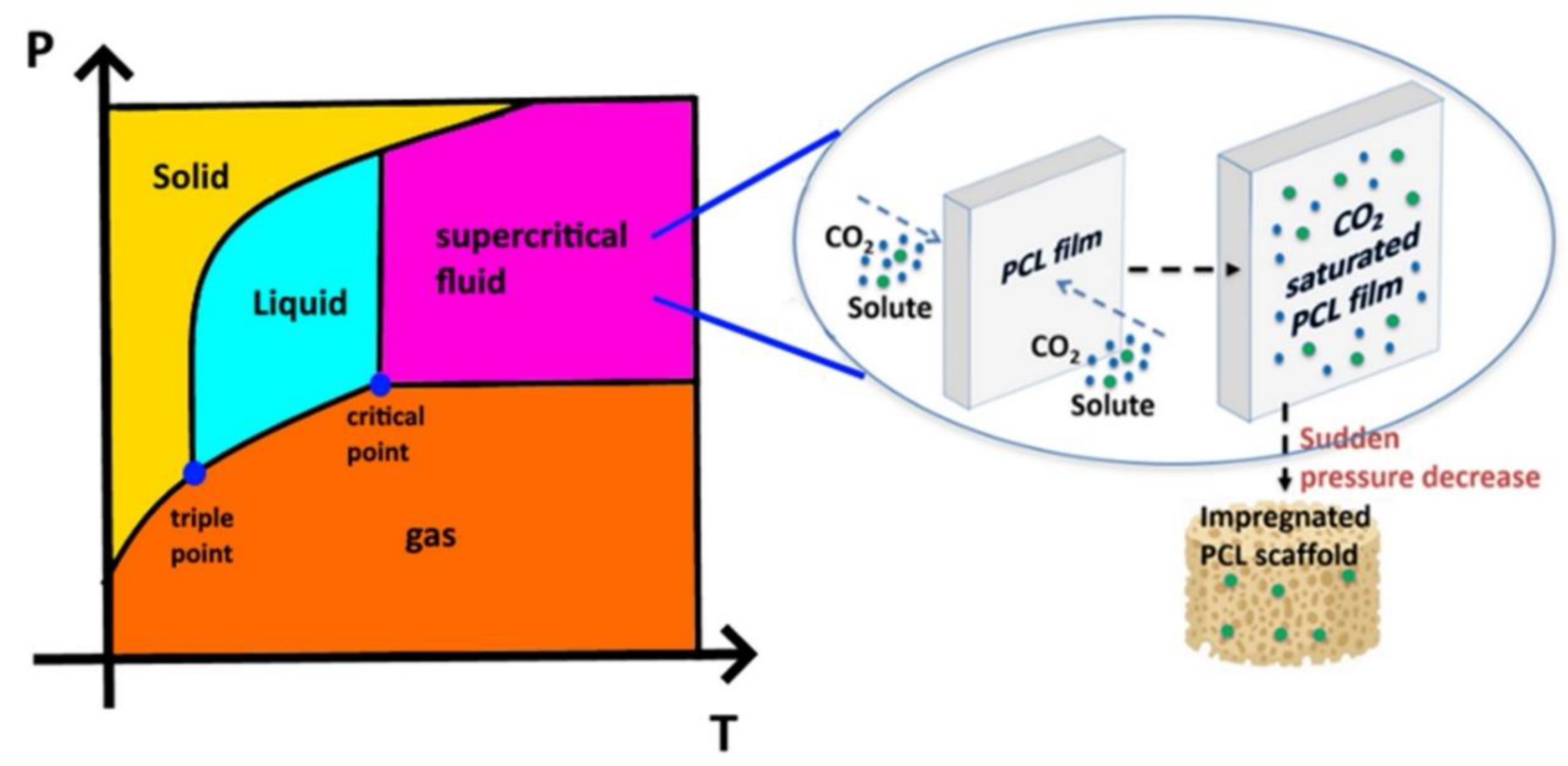
2.3. scCO2-Assisted Foaming and Impregnation Assays
2.4. Foam Characterization
3. Results and Discussion
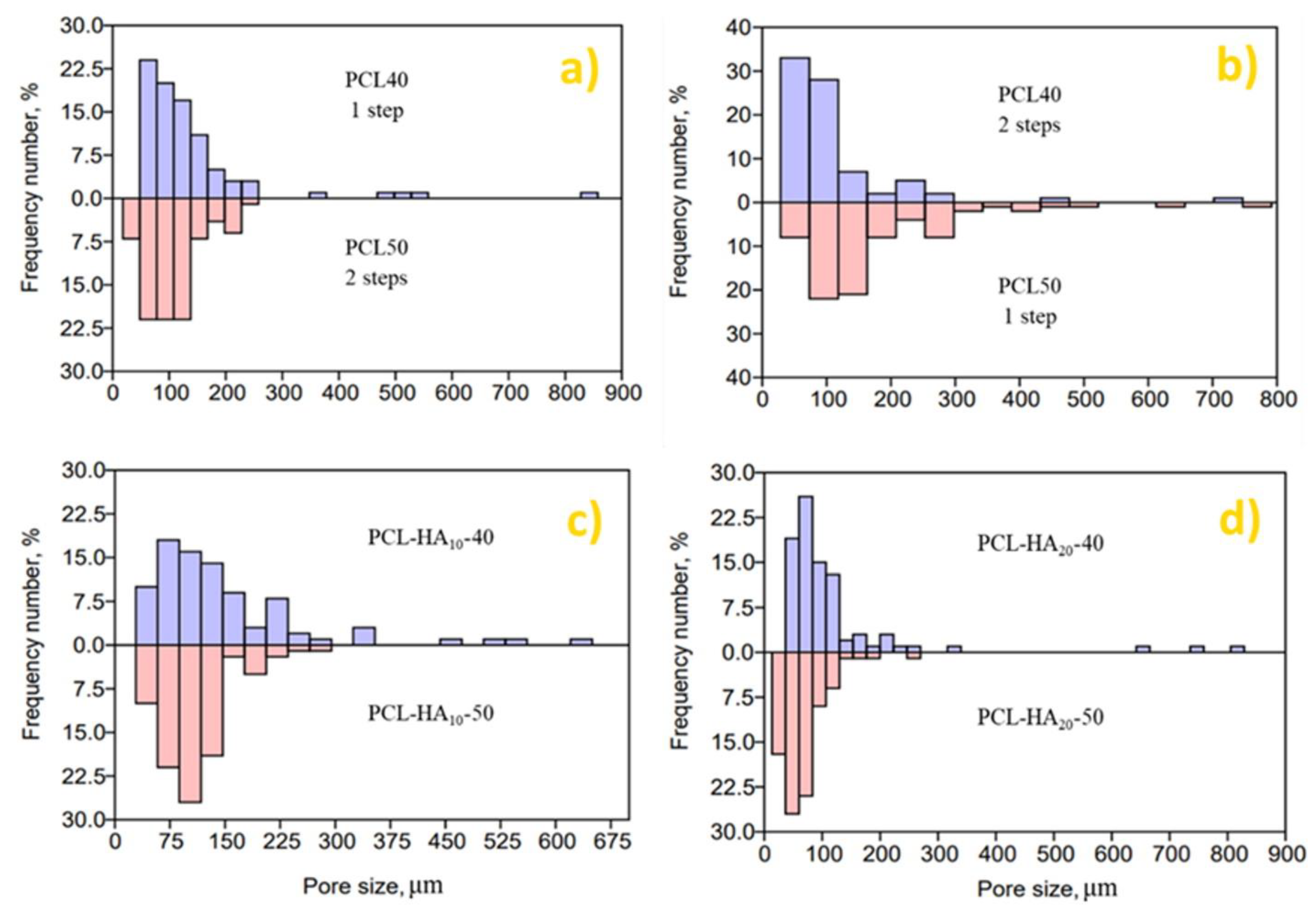
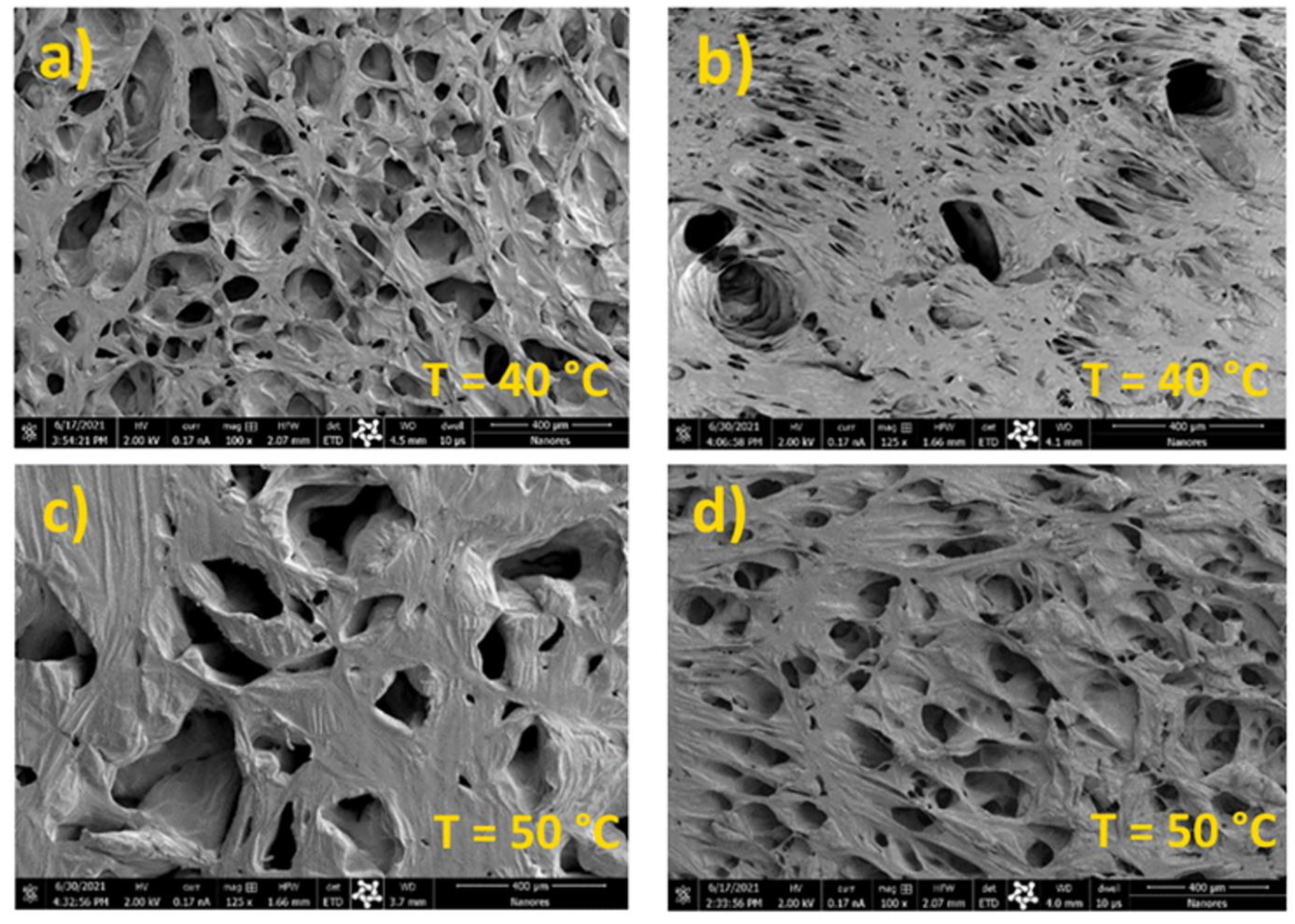
3.1. Effect of HA Content, Temperature, and Depressurization Rate on PCL Foams Morphology
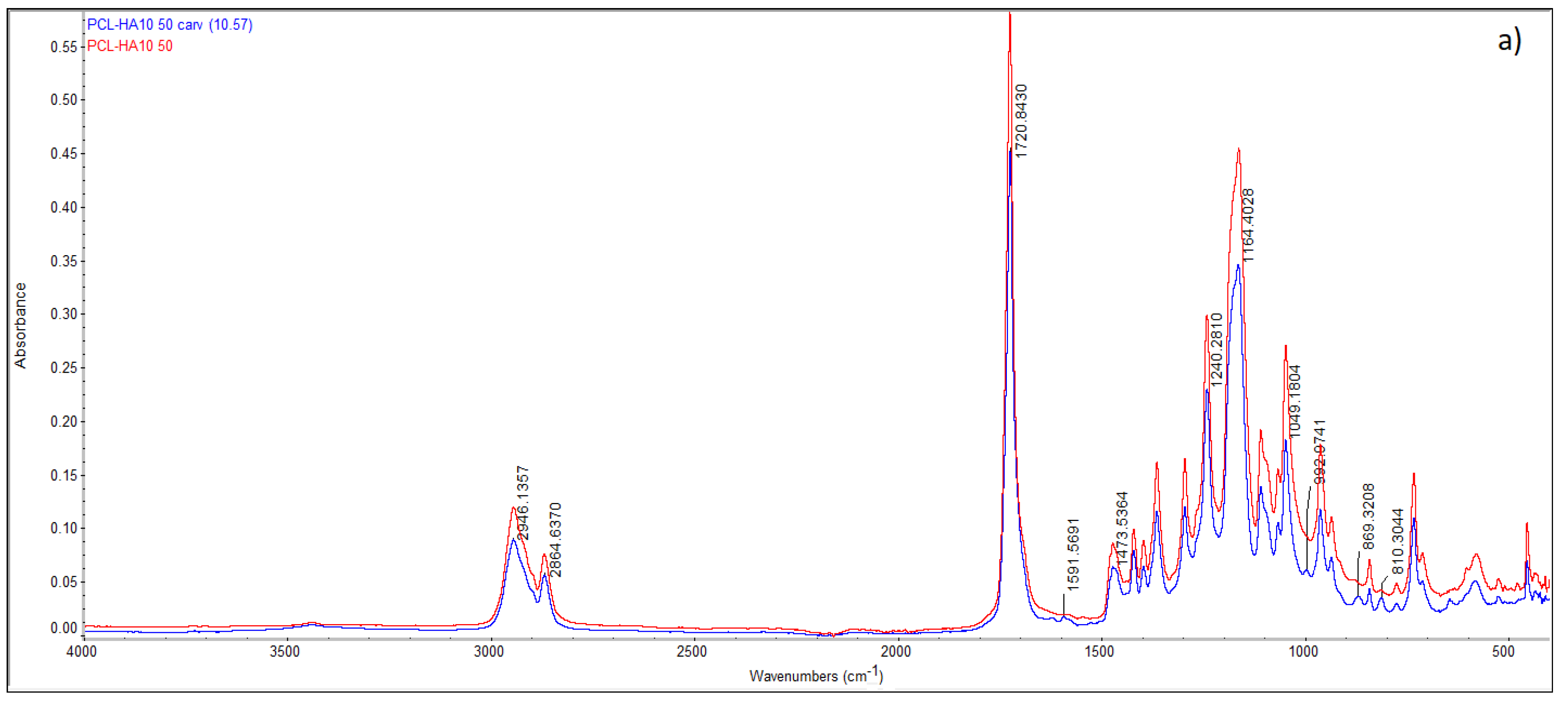
3.2. Effect of HA Content, Temperature, and Depressurization Rate on Carvacrol Loaded PCL Foams Morphology
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Sample | HA (wt.%) | T (°C) | P (MPa) | t (h) | One-Step Decompression (MPa/min) | Two-Step Decompression (MPa/min) |
|---|---|---|---|---|---|---|
| FOAMING | ||||||
| PCL50 | 0 | 50 | 20 | 1.5 | 0.5; 20 | |
| PCL50 | 0 | 50 | 20 | 1.5 | 0.5 | |
| PCL-HA10-50 | 10 | 50 | 20 | 1.5 | 0.5; 20 | |
| PCL-HA20-50 | 20 | 50 | 20 | 1.5 | 0.5; 20 | |
| PCL40 | 0 | 40 | 20 | 1.5 | 0.5 | |
| PCL40 | 0 | 40 | 20 | 1.5 | 0.5; 20 | |
| PCL-HA10-40 | 10 | 40 | 20 | 1.5 | 0.5 | |
| PCL-HA20-40 | 20 | 40 | 20 | 1.5 | 0.5 | |
| FOAMING AND IMPREGNATION | ||||||
| PCL50 | 0 | 50 | 20 | 3 | 0.5; 20 | |
| PCL-HA10-50 | 10 | 50 | 20 | 3 | 0.5; 20 | |
| PCL-HA20-50 | 20 | 50 | 20 | 3 | 0.5; 20 | |
| PCL40 | 0 | 40 | 20 | 3 | 0.5 | |
| PCL-HA10-40 | 10 | 40 | 20 | 3 | 0.5 | |
| PCL-HA20-40 | 20 | 40 | 20 | 3 | 0.5 | |
Appendix B
| Sample | FNEAT (N) | FIMPREG (N) |
|---|---|---|
| PCL50 | 53.2 | 51.3 |
| PCL-HA10-50 | 52.4 | 51.4 |
| PCL-HA20-50 | 35.6 | 32.9 |
| PCL40 | 42.5 | 46.1 |
| PCL-HA10-40 | 36.2 | 38.6 |
| PCL-HA20-40 | 34.3 | 29.1 |
References
- Wu, A.-M.; Bisignano, C.; James, S.L.; Abady, G.G.; Abedi, A.; Abu-Gharbieh, E.; Alhassan, R.K.; Alipour, V.; Arabloo, J.; Asaad, M.; et al. Global, Regional, and National Burden of Bone Fractures in 204 Countries and Territories, 1990–2019: A Systematic Analysis from the Global Burden of Disease Study 2019. Lancet Healthy Longev. 2021, 2, e580–e592. [Google Scholar] [CrossRef]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone Regeneration: Current Concepts and Future Directions. BMC Med. 2011, 9, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soucacos, P.N.; Johnson, E.O.; Babis, G. An Update on Recent Advances in Bone Regeneration; Elsevier: Amsterdam, The Netherlands, 2008; Volume 39. [Google Scholar]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone Tissue Engineering: Recent Advances and Challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsang, V.L.; Bhatia, S.N. Fabrication of Three-Dimensional Tissues. Adv. Biochem. Eng. Biotechnol. 2006, 103, 189–205. [Google Scholar]
- Dutta, R.C.; Dey, M.; Dutta, A.K.; Basu, B. Competent Processing Techniques for Scaffolds in Tissue Engineering. Biotechnol. Adv. 2017, 35, 240–250. [Google Scholar] [CrossRef]
- Reverchon, E.; Cardea, S. Supercritical Fluids in 3-D Tissue Engineering. J. Supercrit. Fluids 2012, 69, 97–107. [Google Scholar] [CrossRef]
- Duarte, A.R.C.; Mano, J.F.; Reis, R.L. Perspectives on: Supercritical Fluid Technology for 3D Tissue Engineering Scaffold Applications. J. Bioact. Compat. Polym. 2009, 24, 385–400. [Google Scholar] [CrossRef] [Green Version]
- Davies, O.R.; Lewis, A.L.; Whitaker, M.J.; Tai, H.; Shakesheff, K.M.; Howdle, S.M. Applications of Supercritical CO2 in the Fabrication of Polymer Systems for Drug Delivery and Tissue Engineering. Adv. Drug Deliv. Rev. 2008, 60, 373–387. [Google Scholar] [CrossRef]
- Clifford, A.A.; Williams, J.R. Introduction to Supercritical Fluids and Their Applications. In Supercritical Fluid Methods and Protocols; Humana Press: Totowa, NJ, USA, 2000; Volume 13, pp. 1–16. [Google Scholar]
- Champeau, M.; Thomassin, J.M.; Tassaing, T.; Jérôme, C. Drug Loading of Polymer Implants by Supercritical CO2 Assisted Impregnation: A Review. J. Control. Release 2015, 209, 248–259. [Google Scholar] [CrossRef]
- Rojas, A.; Torres, A.; Galotto, M.J.; Guarda, A.; Julio, R. Supercritical Impregnation for Food Applications: A Review of the Effect of the Operational Variables on the Active Compound Loading. Crit. Rev. Food Sci. Nutr. 2020, 60, 1290–1301. [Google Scholar] [CrossRef]
- Chauvet, M.; Sauceau, M.; Baillon, F.; Fages, J. Blending and Foaming Thermoplastic Starch with Poly (Lactic Acid) by CO2-aided Hot Melt Extrusion. J. Appl. Polym. Sci. 2021, 138, 50150. [Google Scholar] [CrossRef]
- Tsivintzelis, I.; Sanxaridou, G.; Pavlidou, E.; Panayiotou, C. Foaming of Polymers with Supercritical Fluids: A Thermodynamic Investigation. J. Supercrit. Fluids 2016, 110, 240–250. [Google Scholar] [CrossRef]
- Okamoto, M.; John, B. Synthetic Biopolymer Nanocomposites for Tissue Engineering Scaffolds. Prog. Polym. Sci. 2013, 38, 1487–1503. [Google Scholar] [CrossRef]
- Jenkins, M.J.; Harrison, K.L.; Silva, M.M.C.G.; Whitaker, M.J.; Shakesheff, K.M.; Howdle, S.M. Characterisation of Microcellular Foams Produced from Semi-Crystalline PCL Using Supercritical Carbon Dioxide. Eur. Polym. J. 2006, 42, 3145–3151. [Google Scholar] [CrossRef]
- Fanovich, M.A.; Ivanovic, J.; Zizovic, I.; Misic, D.; Jaeger, P. Functionalization of Polycaprolactone/Hydroxyapatite Scaffolds with Usnea Lethariiformis Extract by Using Supercritical CO2. Mater. Sci. Eng. C 2016, 58, 204–212. [Google Scholar] [CrossRef]
- Pitt, C.G.; Gratzl, M.M.; Kimmel, G.L.; Surles, J.; Schindler, K. Aliphatic Polyesters II. The Degradation of Poly (DL-Lace), Poly (E-Caprolactone), and Their Copolymers in Vivo. Biomaterials 1981, 2, 215–220. [Google Scholar] [CrossRef]
- Choi, S.H.; Park, T.G. Synthesis and Characterization of Elastic PLGA/PCL/PLGA Tri-Block Copolymers. J. Biomater. Sci. Polym. Ed. 2002, 13, 1163–1173. [Google Scholar] [CrossRef] [Green Version]
- Ivanovic, J.; Knauer, S.; Fanovich, A.; Milovanovic, S.; Stamenic, M.; Jaeger, P.; Zizovic, I.; Eggers, R. Supercritical CO2 Sorption Kinetics and Thymol Impregnation of PCL and PCL-HA. J. Supercrit. Fluids 2016, 107, 486–498. [Google Scholar] [CrossRef]
- Knowles, J.C. Phosphate Based Glasses for Biomedical Applications. J. Mater. Chem. 2003, 13, 2395–2401. [Google Scholar] [CrossRef]
- Kim, H.W.; Knowles, J.C.; Kim, H.E. Hydroxyapatite Porous Scaffold Engineered with Biological Polymer Hybrid Coating for Antibiotic Vancomycin Release. J. Mater. Sci. Mater. Med. 2005, 16, 189–195. [Google Scholar] [CrossRef]
- Puppi, D.; Dinucci, D.; Bartoli, C.; Mota, C.; Migone, C.; Dini, F.; Barsotti, G.; Carlucci, F.; Chiellini, F. Development of 3D Wet-Spun Polymeric Scaffolds Loaded with Antimicrobial Agents for Bone Engineering. J. Bioact. Compat. Polym. 2011, 26, 478–492. [Google Scholar] [CrossRef]
- Aeschbach, R.; Loliger, J.; Scott, C.; Murcia, A.; Butler, J.; Aruoma, O.I. Antioxidant actions of thymol, carvacrol, 6-gingerol, zingerone and hydroxytyrosol. Food Chem. Toxicol. 1994, 32, 31–36. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Zhou, F.; Ji, B.P.; Pei, R.S.; Xu, N. The Antibacterial Mechanism of Carvacrol and Thymol against Escherichia Coli. Lett. Appl. Microbiol. 2008, 47, 174–179. [Google Scholar] [CrossRef]
- Nostro, A.; Papalia, T. Antonia Nostro; Teresa Papalia Antimicrobial Activity of Carvacrol: Current Progress and Future Prospectives. Recent Pat. Anti-Infect. Drug Discov. 2012, 7, 28–35. [Google Scholar] [CrossRef]
- Nostro, A.; Blanco, A.R.; Cannatelli, M.A.; Enea, V.; Flamini, G.; Morelli, I.; Sudano Roccaro, A.; Alonzo, V. Susceptibility of Methicillin-Resistant Staphylococci to Oregano Essential Oil, Carvacrol and Thymol. FEMS Microbiol. Lett. 2004, 230, 191–195. [Google Scholar] [CrossRef] [Green Version]
- U.S. Food & Drug Administration. CFR—Code of Federal Regulations Title 21; FDA: Silver Spring, MD, USA, 2012. [Google Scholar]
- Zizovic, I. Supercritical Fluid Applications in the Design of Novel Antimicrobial Materials. Molecules 2020, 25, 2491. [Google Scholar] [CrossRef]
- Milovanovic, S.; Markovic, D.; Aksentijevic, K.; Stojanovic, D.B.; Ivanovic, J.; Zizovic, I. Application of Cellulose Acetate for Controlled Release of Thymol. Carbohydr. Polym. 2016, 147, 344–353. [Google Scholar] [CrossRef]
- Pajnik, J.; Lukić, I.; Dikić, J.; Asanin, J.; Gordic, M.; Misic, D.; Zizović, I.; Korzeniowska, M. Application of Supercritical Solvent Impregnation for Production of Zeolite Modified Starch-Chitosan Polymers with Antibacterial Properties. Molecules 2020, 25, 4717. [Google Scholar] [CrossRef]
- Milovanovic, S.; Jankovic-Castvan, I.; Ivanovic, J.; Zizovic, I. Effect of Starch Xero- And Aerogels Preparation on the Supercritical CO2 Impregnation of Thymol. Starch Staerke 2015, 67, 174–182. [Google Scholar] [CrossRef]
- Kiran, E. Foaming Strategies for Bioabsorbable Polymers in Supercritical Fluid Mixtures. Part I. Miscibility and Foaming of Poly(l-Lactic Acid) in Carbon Dioxide + Acetone Binary Fluid Mixtures. J. Supercrit. Fluids 2010, 54, 308–319. [Google Scholar] [CrossRef]
- Fanovich, M.A.; Jaeger, P. Sorption and Diffusion of Compressed Carbon Dioxide in Polycaprolactone for the Development of Porous Scaffolds. Mater. Sci. Eng. C 2012, 32, 961–968. [Google Scholar] [CrossRef]
- Milovanovic, S.; Stamenic, M.; Markovic, D.; Ivanovic, J.; Zizovic, I. Supercritical Impregnation of Cellulose Acetate with Thymol. J. Supercrit. Fluids 2015, 97, 107–115. [Google Scholar] [CrossRef]
- Salerno, A.; Zeppetelli, S.; di Maio, E.; Iannace, S.; Netti, P.A. Novel 3D Porous Multi-Phase Composite Scaffolds Based on PCL, Thermoplastic Zein and Ha Prepared via Supercritical CO2 Foaming for Bone Regeneration. Compos. Sci. Technol. 2010, 70, 1838–1846. [Google Scholar] [CrossRef] [Green Version]
- Salerno, A.; di Maio, E.; Iannace, S.; Netti, P.A. Solid-State Supercritical CO2 Foaming of PCL and PCL-HA Nano-Composite: Effect of Composition, Thermal History and Foaming Process on Foam Pore Structure. J. Supercrit. Fluids 2011, 58, 158–167. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D Biomaterial Scaffolds and Osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Salerno, A.; Guarnieri, D.; Iannone, M.; Zeppetelli, S.; di Maio, E.; Iannace, S.; Netti, P.A. Engineered μ-Bimodal Poly(ε-Caprolactone) Porous Scaffold for Enhanced HMSC Colonization and Proliferation. Acta Biomater. 2009, 5, 1082–1093. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.M.C.G.; Cyster, L.A.; Barry, J.J.A.; Yang, X.B.; Oreffo, R.O.C.; Grant, D.M.; Scotchford, C.A.; Howdle, S.M.; Shakesheff, K.M.; Rose, F.R.A.J. The Effect of Anisotropic Architecture on Cell and Tissue Infiltration into Tissue Engineering Scaffolds. Biomaterials 2006, 27, 5909–5917. [Google Scholar] [CrossRef]
- Salerno, A.; Zeppetelli, S.; di Maio, E.; Iannace, S.; Netti, P.A. Design of Bimodal PCL and PCL-HA Nanocomposite Scaffolds by Two Step Depressurization During Solid-State Supercritical CO 2 Foaming. Macromol. Rapid Commun. 2011, 32, 1150–1156. [Google Scholar] [CrossRef]
- García-Casas, I.; Montes, A.; Valor, D.; Pereyra, C.; de la Ossa, E.J.M. Foaming of Polycaprolactone and Its Impregnation with Quercetin Using Supercritical CO2. Polymers 2019, 11, 1390. [Google Scholar] [CrossRef] [Green Version]
- Campardelli, R.; Franco, P.; Reverchon, E.; de Marco, I. Polycaprolactone/Nimesulide Patches Obtained by a One-Step Supercritical Foaming + Impregnation Process. J. Supercrit. Fluids 2019, 146, 47–54. [Google Scholar] [CrossRef]
- Yoganathan, R.; Mammucari, R.; Foster, N.R. Impregnation of Ibuprofen into Polycaprolactone Using Supercritical Carbon Dioxide. J. Phys. Conf. Ser. 2010, 215, 12087. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Portolés-Gil, N.; López-Periago, A.M.; Domingo, C.; Hosta-Rigau, L. Multi-Layered Polydopamine Coatings for the Immobilization of Growth Factors onto Highly-Interconnected and Bimodal PCL/HA-Based Scaffolds. Mater. Sci. Eng. C 2020, 117, 111245. [Google Scholar] [CrossRef] [PubMed]
- Khodaverdi, E.; Reza Abbaspour, M.; Oroojalian, F.; Omidkhah, N.; Hossein-nezahd, S.; Kamali, H.; Hadizadeh, F. Dexamethasone Delivery of Porous PEG-PCL-PEG Scaffolds with Supercritical Carbon Dioxide Gas Foaming. J. Drug Deliv. Sci. Technol. 2021, 66, 102547. [Google Scholar] [CrossRef]
- Collins, N.J.; Leeke, G.A.; Bridson, R.H.; Hassan, F.; Grover, L.M. The Influence of Silica on Pore Diameter and Distribution in PLA Scaffolds Produced Using Supercritical CO2. J. Mater. Sci. Mater. Med. 2008, 19, 1497–1502. [Google Scholar] [CrossRef]
- Salerno, A.; Iannace, S.; Netti, P.A. Open-Pore Biodegradable Foams Prepared via Gas Foaming and Microparticulate Templating. Macromol. Biosci. 2008, 8, 655–664. [Google Scholar] [CrossRef]
- Kim, J.-W.; Shin, K.-H.; Koh, Y.-H.; Hah, M.J.; Moon, J.; Kim, H.-E. Production of Poly(ε-Caprolactone)/Hydroxyapatite Composite Scaffolds with a Tailored Macro/Micro-Porous Structure, High Mechanical Properties, and Excellent Bioactivity. Materials 2017, 10, 1123. [Google Scholar] [CrossRef] [Green Version]
- Markočič, E.; Škerget, M.; Knez, Ž. Solubility and Diffusivity of CO2 in Poly(l-Lactide)–Hydroxyapatite and Poly(d,l-Lactide-Co-Glycolide)–Hydroxyapatite Composite Biomaterials. J. Supercrit. Fluids 2011, 55, 1046–1051. [Google Scholar] [CrossRef]
- Adamović, T.; Milovanović, S.; Marković, D.; Žižović, I. Impregnation of Cellulose Acetate Films with Carvacrol Using Supercritical Carbon Ioxide. Tehnika 2018, 73, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Nowak, M.; Misic, D.; Trusek, A.; Zizovic, I. Polymeric Microfiltration Membranes Modification by Supercritical Solvent Impregnation—Potential Application in Open Surgical Wound Ventilation. Molecules 2021, 26, 4572. [Google Scholar] [CrossRef]
- Lukic, I.; Vulic, J.; Ivanovic, J. Antioxidant Activity of PLA/PCL Films Loaded with Thymol and/or Carvacrol Using ScCO2 for Active Food Packaging. Food Packag. Shelf Life 2020, 26, 100578. [Google Scholar] [CrossRef]
- Benkaddour, A.; Jradi, K.; Robert, S.; Daneault, C. Grafting of Polycaprolactone on Oxidized Nanocelluloses by Click Chemistry. Nanomaterials 2013, 3, 141–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valderrama, A.C.S.; Rojas De, G.C. Traceability of Active Compounds of Essential Oils in Antimicrobial Food Packaging Using a Chemometric Method by ATR-FTIR. Am. J. Anal. Chem. 2017, 08, 726–741. [Google Scholar] [CrossRef] [Green Version]
- Gheisari, H.; Karamian, E.; Abdellahi, M. A Novel Hydroxyapatite—Hardystonite Nanocomposite Ceramic. Ceram. Int. 2015, 41, 5967–5975. [Google Scholar] [CrossRef]
- Leeke, G.; Santos, R.B.; King, M. Vapor−Liquid Equilibria for the Carbon Dioxide + Carvacrol System at Elevated Pressures. J. Chem. Amp. Eng. Data 2001, 46, 541–545. [Google Scholar] [CrossRef]




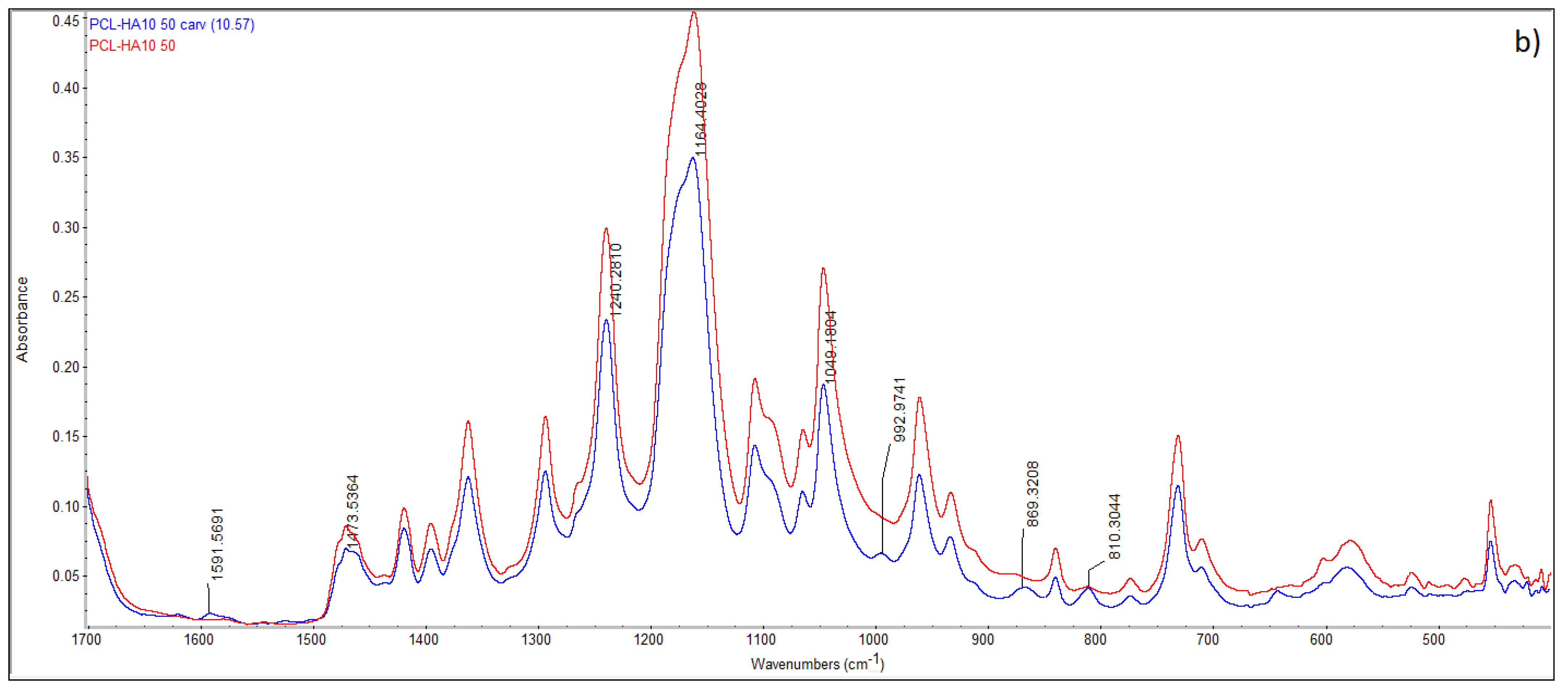
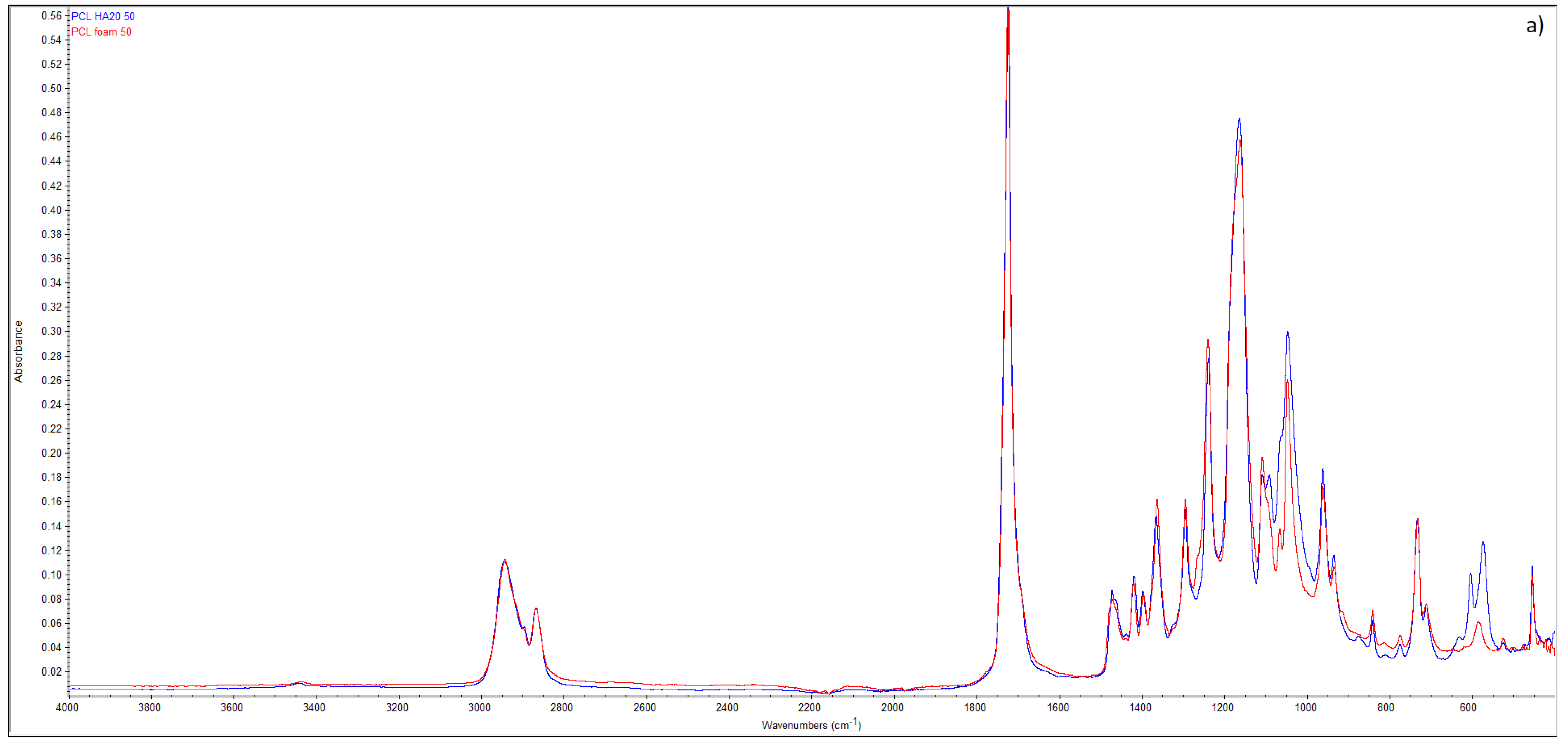
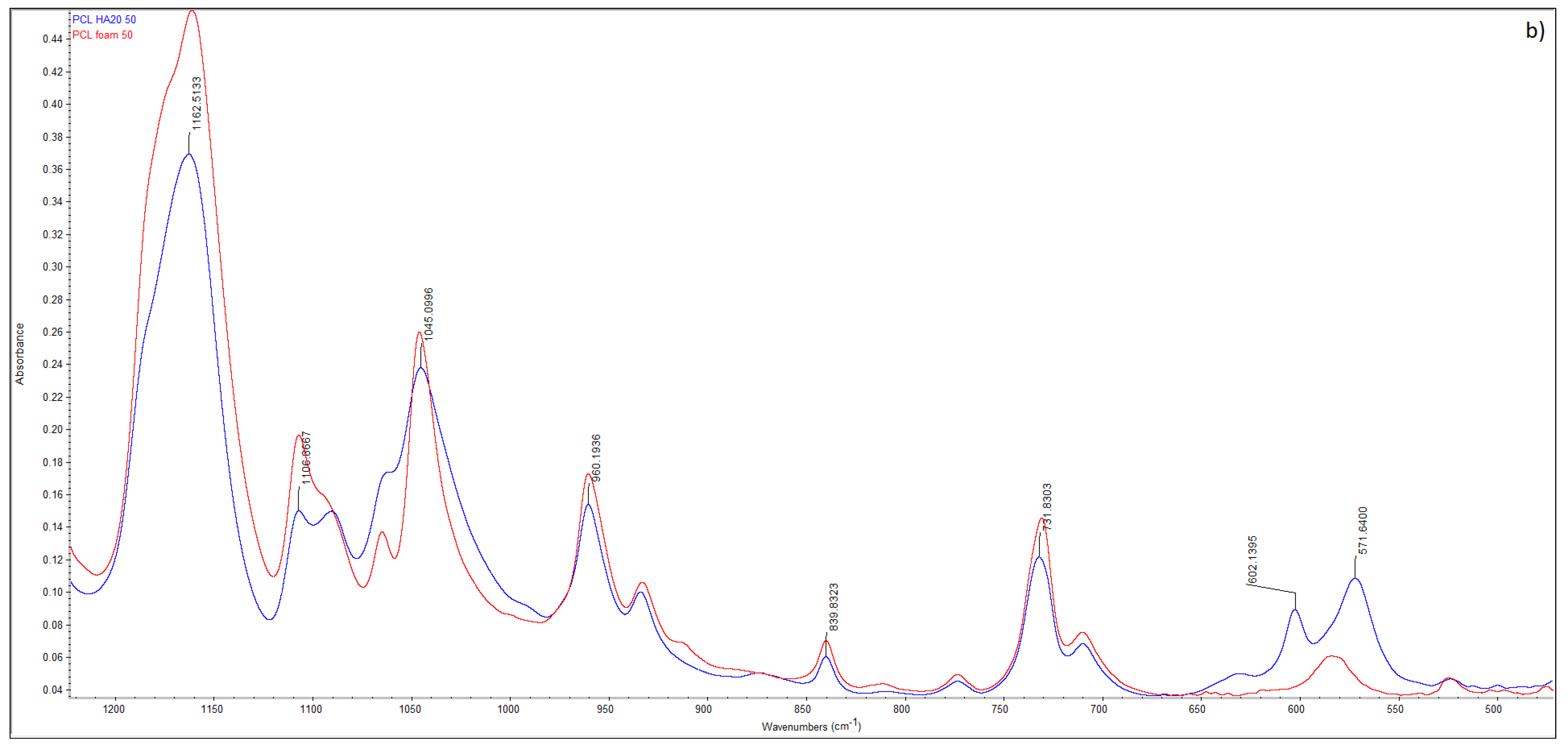

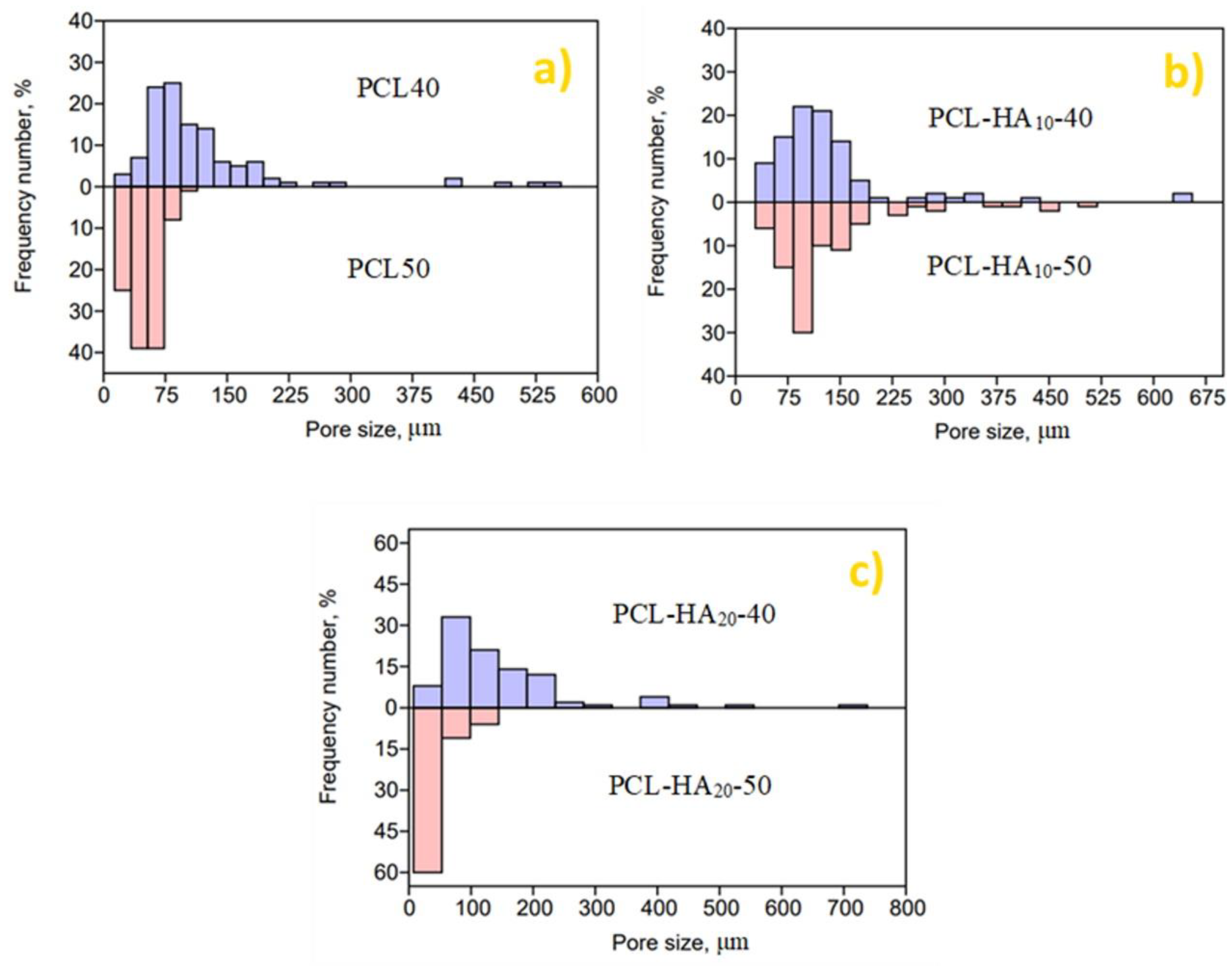

| Sample | HA (wt.%) | T (°C) | P (MPa) | t (h) | (MPa/min) | ρfoam (g/cm3) | ε (%) |
|---|---|---|---|---|---|---|---|
| PCL50 | 0 | 50 | 20 | 1.5 | 0.5; 20 | 0.3747 | 67.13 |
| PCL50 | 0 | 50 | 20 | 1.5 | 0.5 | 0.8241 | 27.71 |
| PCL-HA10-50 | 10 | 50 | 20 | 1.5 | 0.5; 20 | 0.4739 | 64.68 |
| PCL-HA20-50 | 20 | 50 | 20 | 1.5 | 0.5; 20 | 0.5869 | 61.98 |
| PCL40 | 0 | 40 | 20 | 1.5 | 0.5 | 0.2919 | 74.39 |
| PCL40 | 0 | 40 | 20 | 1.5 | 0.5; 20 | 0.3593 | 64.07 |
| PCL-HA10-40 | 10 | 40 | 20 | 1.5 | 0.5 | 0.3953 | 70.54 |
| PCL-HA20-40 | 20 | 40 | 20 | 1.5 | 0.5 | 0.5319 | 65.55 |
| Sample | HA (%) | T (°C) | P (MPa) | (MPa/min) | ρfoam (g/cm3) | ε (%) | Impregnation Yield (wt.%) |
|---|---|---|---|---|---|---|---|
| PCL50 | 0 | 50 | 20 | 0.5; 20 | 0.5926 | 48.01 | 7.22 |
| PCL-HA10-50 | 10 | 50 | 20 | 0.5; 20 | 0.6835 | 49.07 | 10.57 |
| PCL-HA20-50 | 20 | 50 | 20 | 0.5; 20 | 0.8077 | 47.69 | 10.24 |
| PCL40 | 0 | 40 | 20 | 0.5 | 0.3724 | 67.33 | 7.71 |
| PCL-HA10-40 | 10 | 40 | 20 | 0.5 | 0.4951 | 63.11 | 10.41 |
| PCL-HA20-40 | 20 | 40 | 20 | 0.5 | 0.7225 | 53.21 | 9.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satpayeva, A.; Rojas, A.; Tyrka, M.; Ksepko, E.; Galotto, M.J.; Zizovic, I. Supercritical Foaming and Impregnation of Polycaprolactone and Polycaprolactone-Hydroxyapatite Composites with Carvacrol. Processes 2022, 10, 482. https://doi.org/10.3390/pr10030482
Satpayeva A, Rojas A, Tyrka M, Ksepko E, Galotto MJ, Zizovic I. Supercritical Foaming and Impregnation of Polycaprolactone and Polycaprolactone-Hydroxyapatite Composites with Carvacrol. Processes. 2022; 10(3):482. https://doi.org/10.3390/pr10030482
Chicago/Turabian StyleSatpayeva, Alina, Adrián Rojas, Marcin Tyrka, Ewelina Ksepko, María José Galotto, and Irena Zizovic. 2022. "Supercritical Foaming and Impregnation of Polycaprolactone and Polycaprolactone-Hydroxyapatite Composites with Carvacrol" Processes 10, no. 3: 482. https://doi.org/10.3390/pr10030482
APA StyleSatpayeva, A., Rojas, A., Tyrka, M., Ksepko, E., Galotto, M. J., & Zizovic, I. (2022). Supercritical Foaming and Impregnation of Polycaprolactone and Polycaprolactone-Hydroxyapatite Composites with Carvacrol. Processes, 10(3), 482. https://doi.org/10.3390/pr10030482









