Evaluation of Portable Vibrational Spectroscopy Sensors as a Tool to Detect Black Cumin Oil Adulteration
Abstract
:1. Introductıon
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Reference Method
2.2.2. Spectroscopy
2.3. Data Analysis
3. Results and Discussion
3.1. Classification Model with Handheld FT-NIR
3.2. Classification Model with Portable FT-MIR
3.3. Classification Model with Portable Raman
3.4. Prediction Models
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Piras, A.; Rosa, A.; Marongiu, B.; Porcedda, S.; Falconieri, D.; Dessì, M.A.; Ozcelik, B.; Koca, U. Chemical Composition and in Vitro Bioactivity of the Volatile and Fixed Oils of Nigella Sativa L. Extracted by Supercritical Carbon Dioxide. Ind. Crops Prod. 2013, 46, 317–323. [Google Scholar] [CrossRef]
- Singh, S.; Das, S.S.; Singh, G.; Schuff, C.; de Lampasona, M.P.; Catalán, C.A.N. Composition, In Vitro Antioxidant and Antimicrobial Activities of Essential Oil and Oleoresins Obtained from Black Cumin Seeds ( Nigella Sativa L.). BioMed Res. Int. 2014, 2014, 918209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaseb, A.O.; Chinnakannu, K.; Chen, D.; Sivanandam, A.; Tejwani, S.; Menon, M.; Dou, Q.P.; Reddy, G.P.-V. Androgen Receptor– and E2F-1–Targeted Thymoquinone Therapy for Hormone-Refractory Prostate Cancer. Cancer Res. 2007, 67, 7782–7788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darakhshan, S.; Bidmeshki Pour, A.; Hosseinzadeh Colagar, A.; Sisakhtnezhad, S. Thymoquinone and Its Therapeutic Potentials. Pharmacol. Res. 2015, 95–96, 138–158. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, L.; Li, P.; Xu, B.; Ma, F.; Zhang, Q.; Zhang, W. Fatty Acid Profiles Based Adulteration Detection for Flaxseed Oil by Gas Chromatography Mass Spectrometry. LWT-Food Sci. Technol. 2015, 63, 430–436. [Google Scholar] [CrossRef]
- Ok, S. Authentication of Commercial Extra Virgin Olive Oils. J. Am. Oil Chem. Soc. 2016, 93, 489–497. [Google Scholar] [CrossRef]
- Shi, T.; Wu, G.; Jin, Q.; Wang, X. Detection of Camellia Oil Adulteration Using Chemometrics Based on Fatty Acids GC Fingerprints and Phytosterols GC–MS Fingerprints. Food Chem. 2021, 352, 129422. [Google Scholar] [CrossRef]
- Ok, S. Detection of Olive Oil Adulteration by Low-Field NMR Relaxometry and UV-Vis Spectroscopy upon Mixing Olive Oil with Various Edible Oils. Grasas Y Aceites 2017, 68, 173. [Google Scholar] [CrossRef] [Green Version]
- Brigante, F.I.; Lucini Mas, A.; Pigni, N.B.; Wunderlin, D.A.; Baroni, M.V. Targeted Metabolomics to Assess the Authenticity of Bakery Products Containing Chia, Sesame and Flax Seeds. Food Chem. 2020, 312, 126059. [Google Scholar] [CrossRef]
- Zhang, L.; Shuai, Q.; Li, P.; Zhang, Q.; Ma, F.; Zhang, W.; Ding, X. Ion Mobility Spectrometry Fingerprints: A Rapid Detection Technology for Adulteration of Sesame Oil. Food Chem. 2016, 192, 60–66. [Google Scholar] [CrossRef]
- Aykas, D.P.; Karaman, A.D.; Keser, B.; Rodriguez-Saona, L. Non-Targeted Authentication Approach for Extra Virgin Olive Oil. Foods 2020, 9, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subramanian, A.; Alvarez, V.B.; Harper, W.J.; Rodriguez-Saona, L.E. Monitoring Amino Acids, Organic Acids, and Ripening Changes in Cheddar Cheese Using Fourier-Transform Infrared Spectroscopy. Int. Dairy J. 2011, 21, 434–440. [Google Scholar] [CrossRef]
- Quiñones-Islas, N.; Meza-Márquez, O.G.; Osorio-Revilla, G.; Gallardo-Velazquez, T. Detection of Adulterants in Avocado Oil by Mid-FTIR Spectroscopy and Multivariate Analysis. Food Res. Int. 2013, 51, 148–154. [Google Scholar] [CrossRef]
- Li, B.; Wang, H.; Zhao, Q.; Ouyang, J.; Wu, Y. Rapid Detection of Authenticity and Adulteration of Walnut Oil by FTIR and Fluorescence Spectroscopy: A Comparative Study. Food Chem. 2015, 181, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Elzey, B.; Pollard, D.; Fakayode, S.O. Determination of Adulterated Neem and Flaxseed Oil Compositions by FTIR Spectroscopy and Multivariate Regression Analysis. Food Control 2016, 68, 303–309. [Google Scholar] [CrossRef]
- Georgouli, K.; Martinez Del Rincon, J.; Koidis, A. Continuous Statistical Modelling for Rapid Detection of Adulteration of Extra Virgin Olive Oil Using Mid Infrared and Raman Spectroscopic Data. Food Chem. 2017, 217, 735–742. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Lin, Z.; Tan, C. Fast Quantitative Detection of Sesame Oil Adulteration by Near-Infrared Spectroscopy and Chemometric Models. Vib. Spectrosc. 2018, 99, 178–183. [Google Scholar] [CrossRef]
- Farres, S.; Srata, L.; Fethi, F.; Kadaoui, A. Argan Oil Authentication Using Visible/near Infrared Spectroscopy Combined to Chemometrics Tools. Vib. Spectrosc. 2019, 102, 79–84. [Google Scholar] [CrossRef]
- Du, Q.; Zhu, M.; Shi, T.; Luo, X.; Gan, B.; Tang, L.; Chen, Y. Adulteration Detection of Corn Oil, Rapeseed Oil and Sunflower Oil in Camellia Oil by in Situ Diffuse Reflectance near-Infrared Spectroscopy and Chemometrics. Food Control 2021, 121, 107577. [Google Scholar] [CrossRef]
- Rohman, A.; Wibowo, D.; Sudjadi; Lukitaningsih, E.; Rosman, A.S. Use of Fourier Transform Infrared Spectroscopy in Combination with Partial Least Square for Authentication of Black Seed Oil. Int. J. Food Prop. 2015, 18, 775–784. [Google Scholar] [CrossRef] [Green Version]
- Arslan, F.N.; Akin, G.; Karuk Elmas, Ş.N.; Yilmaz, I.; Janssen, H.-G.; Kenar, A. Rapid Detection of Authenticity and Adulteration of Cold Pressed Black Cumin Seed Oil: A Comparative Study of ATR–FTIR Spectroscopy and Synchronous Fluorescence with Multivariate Data Analysis. Food Control 2019, 98, 323–332. [Google Scholar] [CrossRef]
- Menevseoglu, A. Non-Destructive Detection of Sesame Oil Adulteration by Portable FT-NIR, FT-MIR, and Raman Spectrometers Combined with Chemometrics. J. Turk. Chem. Soc. Sect. A Chem. 2021, 8, 775–786. [Google Scholar] [CrossRef]
- Dogruer, I.; Uyar, H.H.; Uncu, O.; Ozen, B. Prediction of Chemical Parameters and Authentication of Various Cold Pressed Oils with Fluorescence and Mid-Infrared Spectroscopic Methods. Food Chem. 2021, 345, 128815. [Google Scholar] [CrossRef]
- Nagarajan, R.; Mehrotra, R.; Bajaj, M.M. Quantitative Analysis of Methanol, an Adulterant in Alcoholic Beverages, Using Attenuated Total Reflectance Spectroscopy. J. Sci. Ind. Res. 2006, 65, 416–419. [Google Scholar]
- Haas, J.; Mizaikoff, B. Advances in Mid-Infrared Spectroscopy for Chemical Analysis. Annu. Rev. Anal. Chem. 2016, 9, 45–68. [Google Scholar] [CrossRef]
- Crocombe, R.A. Portable Spectroscopy. Appl. Spectrosc. 2018, 72, 1701–1751. [Google Scholar] [CrossRef]
- Wu, H.; Qian, J.; Bergles, E.; Chandler, L. A Portable Multiexcitation Dispersive Raman Spectrometer for Environmental Analysis; BaySpec Inc.: San Jose, CA, USA, 2013. [Google Scholar]
- Ichihara, K.; Shibahara, A.; Yamamoto, K.; Nakayama, T. An Improved Method for Rapid Analysis of the Fatty Acids of Glycerolipids. Lipids 1996, 31, 535–539. [Google Scholar] [CrossRef] [PubMed]
- de Maesschalck, R.; Candolfi, A.; Massart, D.L.; Heuerding, S. Decision Criteria for Soft Independent Modelling of Class Analogy Applied to near Infrared Data. Chemom. Intell. Lab. Syst. 1999, 47, 65–77. [Google Scholar] [CrossRef]
- Wold, S. Pattern Recognition by Means of Disjoint Principal Components Models. Pattern Recognit. 1976, 8, 127–139. [Google Scholar] [CrossRef]
- Lavine, B.K. Clustering and Classification of Analytical Data. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2000. [Google Scholar]
- Haaland, D.M.; Thomas, E.V. Partial Least-Squares Methods for Spectral Analyses. 1. Relation to Other Quantitative Calibration Methods and the Extraction of Qualitative Information. Anal. Chem. 1988, 60, 1193–1202. [Google Scholar] [CrossRef]
- Jong, S. de PLS Fits Closer than PCR. J. Chemom. 1993, 7, 551–557. [Google Scholar] [CrossRef]
- Brereton, R.G. Introduction to Multivariate Calibration in Analytical Chemistry. Analyst 2000, 125, 2125–2154. [Google Scholar] [CrossRef]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-Regression: A Basic Tool of Chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Hourant, P.; Baeten, V.; Morales, M.T.; Meurens, M.; Aparicio, R. Oil and Fat Classification by Selected Bands of Near-Infrared Spectroscopy. Appl. Spectrosc. 2000, 54, 1168–1174. [Google Scholar] [CrossRef]
- Brown, S.D. Chemometrics: A Textbook. D. L. Massart. B. G. M. Vandeginste, S. N. Deming, Y. Michotte, and L. Kaufman, Elsevier, Amsterdam, 1988. ISBN 0-444-42660-4. Price Dfl 175.00. J. Chemom. 1988, 2, 298–299. [Google Scholar] [CrossRef]
- Rohman, A.; Ariani, R. Authentication of Nigella Sativa Seed Oil in Binary and Ternary Mixtures with Corn Oil and Soybean Oil Using FTIR Spectroscopy Coupled with Partial Least Square. Sci. World J. 2013, 2013, 740142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Saona, L.E.; Giusti, M.M.; Shotts, M. Advances in Infrared Spectroscopy for Food Authenticity Testing. In Advances in Food Authenticity Testing; Elsevier: Amsterdam, The Netherlands, 2016; pp. 71–116. [Google Scholar]
- Abdi, H. Partial Least Squares Regression and Projection on Latent Structure Regression (PLS Regression). WIREs Comput. Stat. 2010, 2, 97–106. [Google Scholar] [CrossRef]
- Urbanocuadrado, M.; Luquedecastro, M.; Perezjuan, P.; Gomeznieto, M. Comparison and Joint Use of near Infrared Spectroscopy and Fourier Transform Mid Infrared Spectroscopy for the Determination of Wine Parameters. Talanta 2005, 66, 218–224. [Google Scholar] [CrossRef] [PubMed]
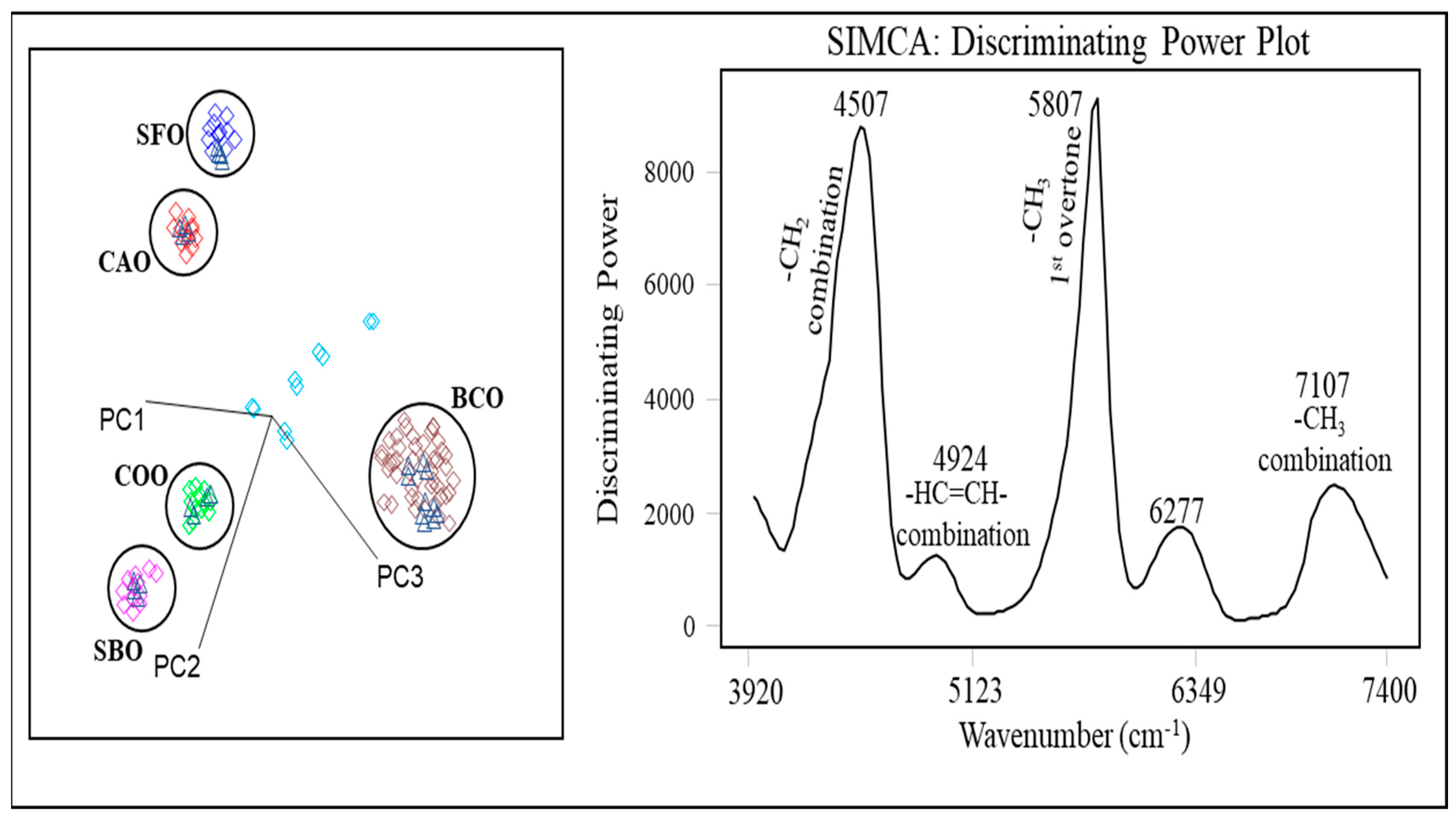
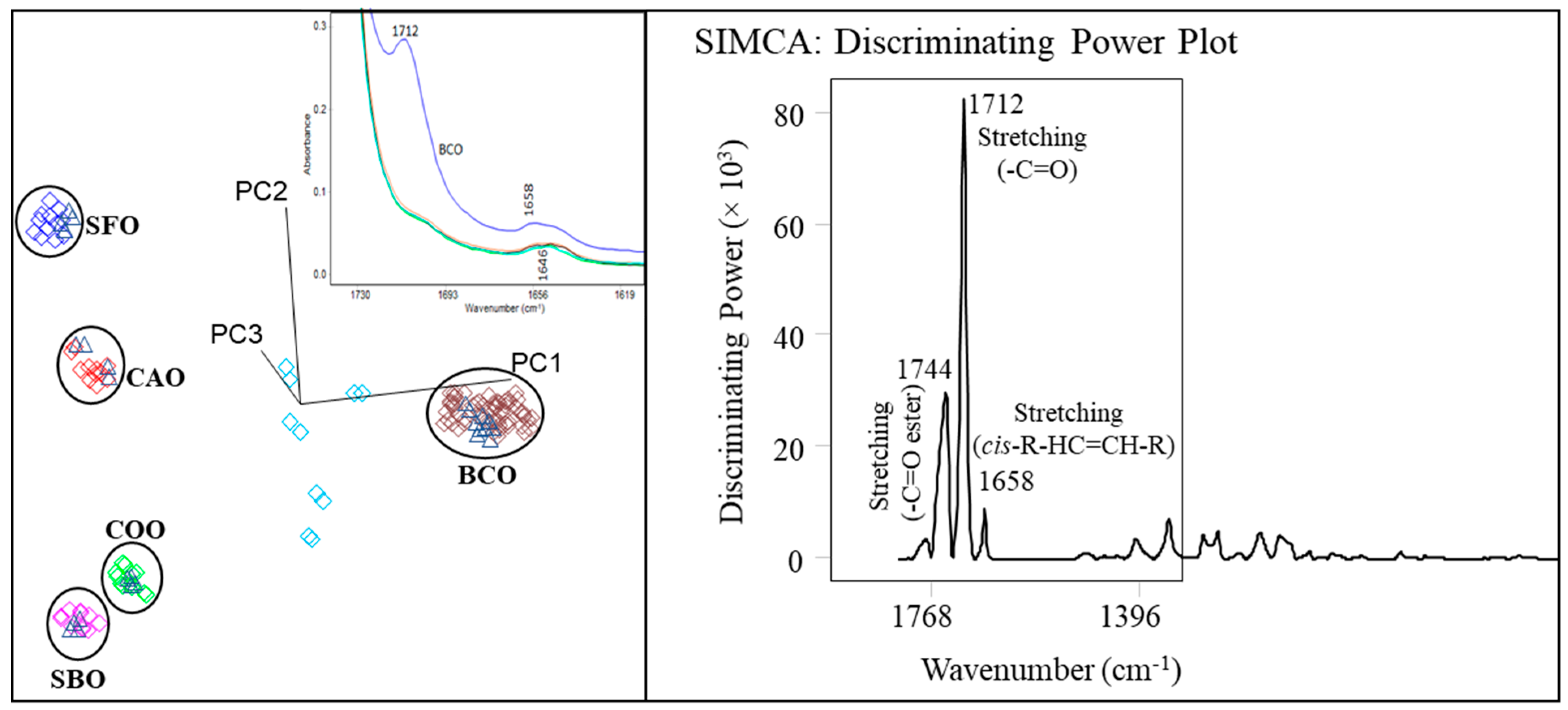

| n | 14:0 | 16:0 | 18:0 | 18:1 | 18:2 | 18:3 | 20:2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Min | Max | Min | Max | Min | Max | Min | Max | Min | Max | Min | Max | ||
| BCO | 35 | 4.08 ± 0.99 | 8.06 ± 1.26 | 2.47 ± 0.24 | 25.91 ± 1.42 | 57.28 ± 1.90 | 0.28 ± 0.11 | 1.93 ± 0.75 | |||||||
| 2.60 | 5.70 | 6.06 | 9.73 | 2.17 | 2.89 | 22.50 | 28.18 | 53.66 | 60.37 | 0.14 | 0.51 | 1.22 | 3.51 | ||
| Adulte-rated BCO | 5 | 0.23 ± 0.06 | 10.71 ± 1.12 | 2.82 ± 0.29 | 35.15 ± 5.21 | 48.86 ± 4.47 | 1.78 ± 0.88 | 0.48 ± 0.18 | |||||||
| 0.16 | 0.34 | 8.79 | 12.11 | 2.38 | 3.27 | 27.73 | 43.23 | 41.93 | 54.03 | 0.78 | 3.01 | 0.33 | 0.80 | ||
| Canola oil | 6 | ND | 4.23 ± 0.11 | 1.91 ± 0.27 | 63.98 ± 1.32 | 20.05 ± 0.59 | 7.16 ± 0.16 | 1.35 ± 0.20 | |||||||
| - | - | 4.11 | 4.53 | 1.76 | 2.23 | 62.81 | 65.31 | 18.74 | 21.36 | 6.82 | 7.35 | 0.98 | 1.51 | ||
| Corn oil | 6 | ND | 11.57 ± 0.32 | 1.67 ± 0.02 | 32.11 ± 1.52 | 52.41 ± 1.50 | 1.51 ± 0.23 | 0.37 ± 0.04 | |||||||
| - | - | 11.14 | 11.90 | 1.65 | 1.70 | 30.56 | 34.16 | 50.37 | 53.95 | 1.27 | 1.82 | 0.33 | 0.43 | ||
| Soybean oil | 6 | ND | 10.88 ± 0.48 | 3.59 ± 0.22 | 24.06 ± 1.03 | 53.17 ± 1.14 | 6.60 ± 0.33 | ND | |||||||
| - | - | 10.21 | 11.28 | 3.28 | 3.79 | 23.09 | 25.49 | 51.57 | 54.12 | 6.15 | 6.93 | - | - | ||
| Sunflower oil | 6 | ND | 4.63 ± 0.34 | 3.41 ± 0.27 | 62.15 ± 0.97 | 28.19 ± 0.97 | 0.39 ± 0.04 | 0.23 ± 0.01 | |||||||
| - | - | 4.17 | 4.96 | 3.12 | 3.77 | 60.92 | 63.29 | 27.17 | 29.50 | 0.33 | 0.43 | 0.22 | 0.24 | ||
| Interclass Distances (ICD) | ||||||
|---|---|---|---|---|---|---|
| Unit | Oil | BCO | CAO | COO | SBO | SFO |
| FT-NIR | BCO | - | 39.9 | 34.6 | 34.3 | 22.5 |
| CAO | 39.9 | - | 28.1 | 16.6 | 46.8 | |
| COO | 34.6 | 28.1 | - | 33.5 | 9.5 | |
| SBO | 34.3 | 16.6 | 33.5 | - | 48.2 | |
| SFO | 22.5 | 46.8 | 9.5 | 48.2 | - | |
| FT-MIR | BCO | - | 35.7 | 36.2 | 37.2 | 33.7 |
| CAO | 35.7 | - | 25.6 | 27.8 | 12.3 | |
| COO | 36.2 | 25.6 | - | 12.7 | 26.9 | |
| SBO | 37.2 | 27.8 | 12.7 | - | 27.9 | |
| SFO | 33.7 | 12.3 | 26.9 | 27.9 | - | |
| Raman | BCO | - | 13.6 | 12.6 | 8.7 | 15.4 |
| CAO | 13.6 | - | 17.0 | 48.4 | 22.5 | |
| COO | 12.6 | 17.0 | - | 20.4 | 48.2 | |
| SBO | 8.7 | 48.4 | 20.4 | - | 48.6 | |
| SFO | 15.4 | 22.5 | 48.2 | 48.6 | - | |
| Unit | Parameter | Calibration Model | Validation Model | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Range a | N b | F c | SEC d | rcal e | Range | N | SEP f | rval g | ||
| FT-NIR | 14:0 | 0.2–5.7 | 47 | 4 | 1.1 | 0.83 | 0.3–5.6 | 11 | 1.3 | 0.79 |
| 16:0 | 4.1–12.1 | 67 | 4 | 0.4 | 0.94 | 4.3–11.9 | 17 | 0.6 | 0.92 | |
| 18:0 | 1.7–3.8 | 66 | 4 | 0.1 | 0.91 | 1.8–3.6 | 16 | 0.2 | 0.89 | |
| 18:1 | 22.5–65.3 | 67 | 3 | 1.2 | 0.96 | 23.1–64.9 | 17 | 1.4 | 0.95 | |
| 18:2 | 18.8–60.4 | 67 | 3 | 1.3 | 0.96 | 19.0–59.8 | 17 | 1.4 | 0.95 | |
| 18:3 | 0.1–7.4 | 65 | 5 | 0.2 | 0.92 | 0.2–7.2 | 17 | 0.4 | 0.91 | |
| 20:2 | 0.2–3.5 | 62 | 5 | 0.7 | 0.77 | 0.3–3.2 | 15 | 0.9 | 0.74 | |
| CAO | 0–25 | 16 | 3 | 1.3 | 0.95 | 5.0–20.0 | 4 | 1.4 | 0.94 | |
| COO | 0–25 | 16 | 4 | 1.2 | 0.96 | 5.0–20.0 | 4 | 1.3 | 0.95 | |
| SBO | 0–25 | 16 | 4 | 0.9 | 0.97 | 5.0–20.0 | 4 | 1.0 | 0.96 | |
| SFO | 0–25 | 16 | 4 | 1.3 | 0.95 | 5.0–20.0 | 4 | 1.3 | 0.94 | |
| FT-MIR | 14:0 | 0.2–5.7 | 48 | 5 | 0.6 | 0.88 | 0.3–5.6 | 12 | 0.8 | 0.85 |
| 16:0 | 4.1–12.1 | 66 | 3 | 0.1 | 0.98 | 4.3–11.9 | 16 | 0.1 | 0.97 | |
| 18:0 | 1.7–3.8 | 67 | 3 | 0.1 | 0.99 | 1.8–3.6 | 16 | 0.2 | 0.98 | |
| 18:1 | 22.5–65.3 | 67 | 4 | 0.4 | 0.99 | 23.1–64.9 | 16 | 0.5 | 0.97 | |
| 18:2 | 18.8–60.4 | 67 | 4 | 0.7 | 0.98 | 19.0–59.8 | 16 | 0.9 | 0.96 | |
| 18:3 | 0.1–7.4 | 67 | 5 | 0.3 | 0.97 | 0.2–7.2 | 16 | 0.4 | 0.94 | |
| 20:2 | 0.2–3.5 | 61 | 5 | 0.5 | 0.82 | 0.3–3.2 | 15 | 0.6 | 0.78 | |
| CAO | 0–25 | 16 | 3 | 1.2 | 0.96 | 5.0–20.0 | 4 | 1.3 | 0.95 | |
| COO | 0–25 | 16 | 3 | 1.0 | 0.97 | 5.0–20.0 | 4 | 1.0 | 0.96 | |
| SBO | 0–25 | 16 | 3 | 0.6 | 0.98 | 5.0–20.0 | 4 | 0.7 | 0.98 | |
| SFO | 0–25 | 16 | 4 | 1.1 | 0.96 | 5.0–20.0 | 4 | 1.2 | 0.95 | |
| Raman | 14:0 | 0.2–5.7 | 48 | 5 | 0.7 | 0.86 | 0.3–5.6 | 12 | 1.0 | 0.82 |
| 16:0 | 4.1–12.1 | 66 | 4 | 0.2 | 0.97 | 4.3–11.9 | 16 | 0.3 | 0.95 | |
| 18:0 | 1.7–3.8 | 67 | 4 | 0.1 | 0.98 | 1.8–3.6 | 17 | 0.1 | 0.96 | |
| 18:1 | 22.5–65.3 | 66 | 3 | 0.8 | 0.98 | 23.1–64.9 | 17 | 1.0 | 0.97 | |
| 18:2 | 18.8–60.4 | 67 | 4 | 0.9 | 0.96 | 19.0–59.8 | 16 | 1.1 | 0.95 | |
| 18:3 | 0.1–7.4 | 67 | 4 | 0.4 | 0.95 | 0.2–7.2 | 17 | 0.5 | 0.93 | |
| 20:2 | 0.2–3.5 | 62 | 5 | 0.7 | 0.80 | 0.3–3.2 | 15 | 0.9 | 0.76 | |
| CAO | 0–25 | 16 | 4 | 1.2 | 0.96 | 5.0–20.0 | 4 | 1.3 | 0.95 | |
| COO | 0–25 | 16 | 4 | 1.1 | 0.96 | 5.0–20.0 | 4 | 1.3 | 0.95 | |
| SBO | 0–25 | 16 | 4 | 0.6 | 0.97 | 5.0–20.0 | 4 | 0.7 | 0.96 | |
| SFO | 0–25 | 16 | 4 | 1.1 | 0.95 | 5.0–20.0 | 4 | 1.3 | 0.94 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menevseoglu, A. Evaluation of Portable Vibrational Spectroscopy Sensors as a Tool to Detect Black Cumin Oil Adulteration. Processes 2022, 10, 503. https://doi.org/10.3390/pr10030503
Menevseoglu A. Evaluation of Portable Vibrational Spectroscopy Sensors as a Tool to Detect Black Cumin Oil Adulteration. Processes. 2022; 10(3):503. https://doi.org/10.3390/pr10030503
Chicago/Turabian StyleMenevseoglu, Ahmed. 2022. "Evaluation of Portable Vibrational Spectroscopy Sensors as a Tool to Detect Black Cumin Oil Adulteration" Processes 10, no. 3: 503. https://doi.org/10.3390/pr10030503
APA StyleMenevseoglu, A. (2022). Evaluation of Portable Vibrational Spectroscopy Sensors as a Tool to Detect Black Cumin Oil Adulteration. Processes, 10(3), 503. https://doi.org/10.3390/pr10030503






