Improvement of the Gut Microbiota In Vivo by a Short-Chain Fatty Acids-Producing Strain Lactococcus garvieae CF11
Abstract
:1. Introduction
2. Materials and Methods
Animals and Strain
3. Fecal Sample Collection and DNA Extraction
4. Real-Time qPCR
5. 16S rRNA High-Throughput Sequencing
6. Determination of SCFAs in Feces
7. SCFAs Analysis
8. PICRUSt Analysis
9. Statistical Analysis
10. Results
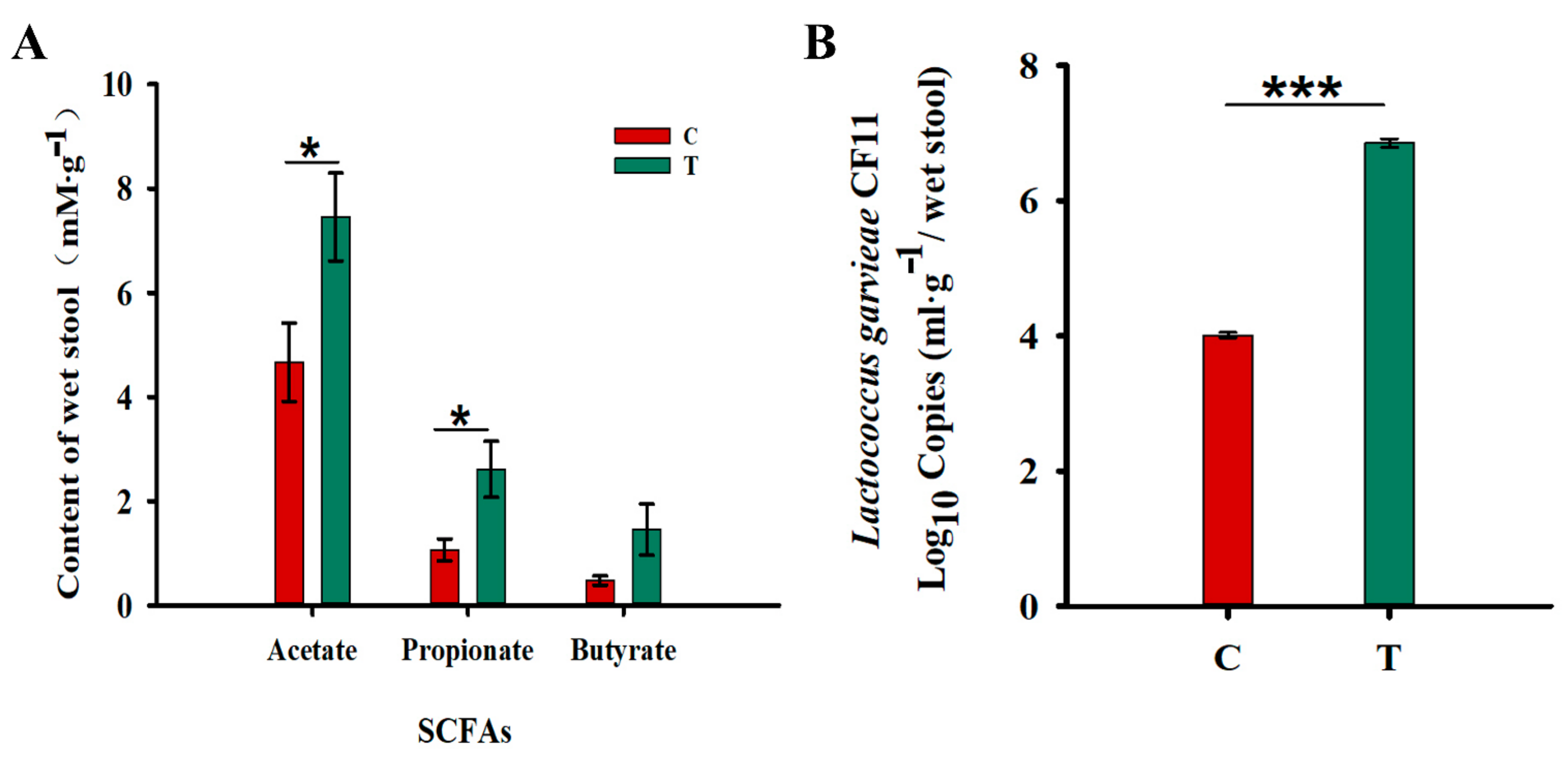
Increased in the Fecal Levels of Acetic Acid and Propionic Acids
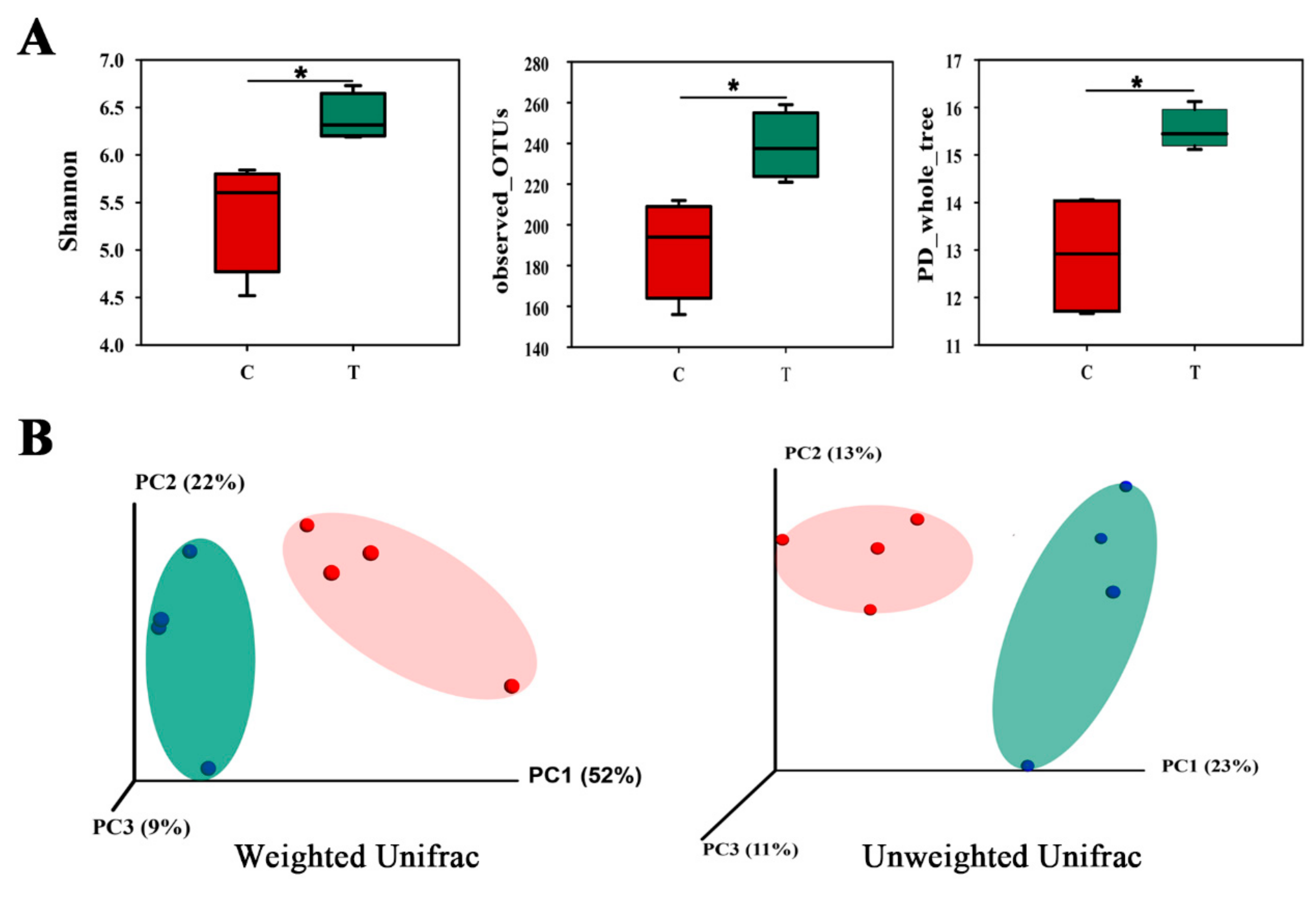
11. Gut Microbial Community in Two Groups
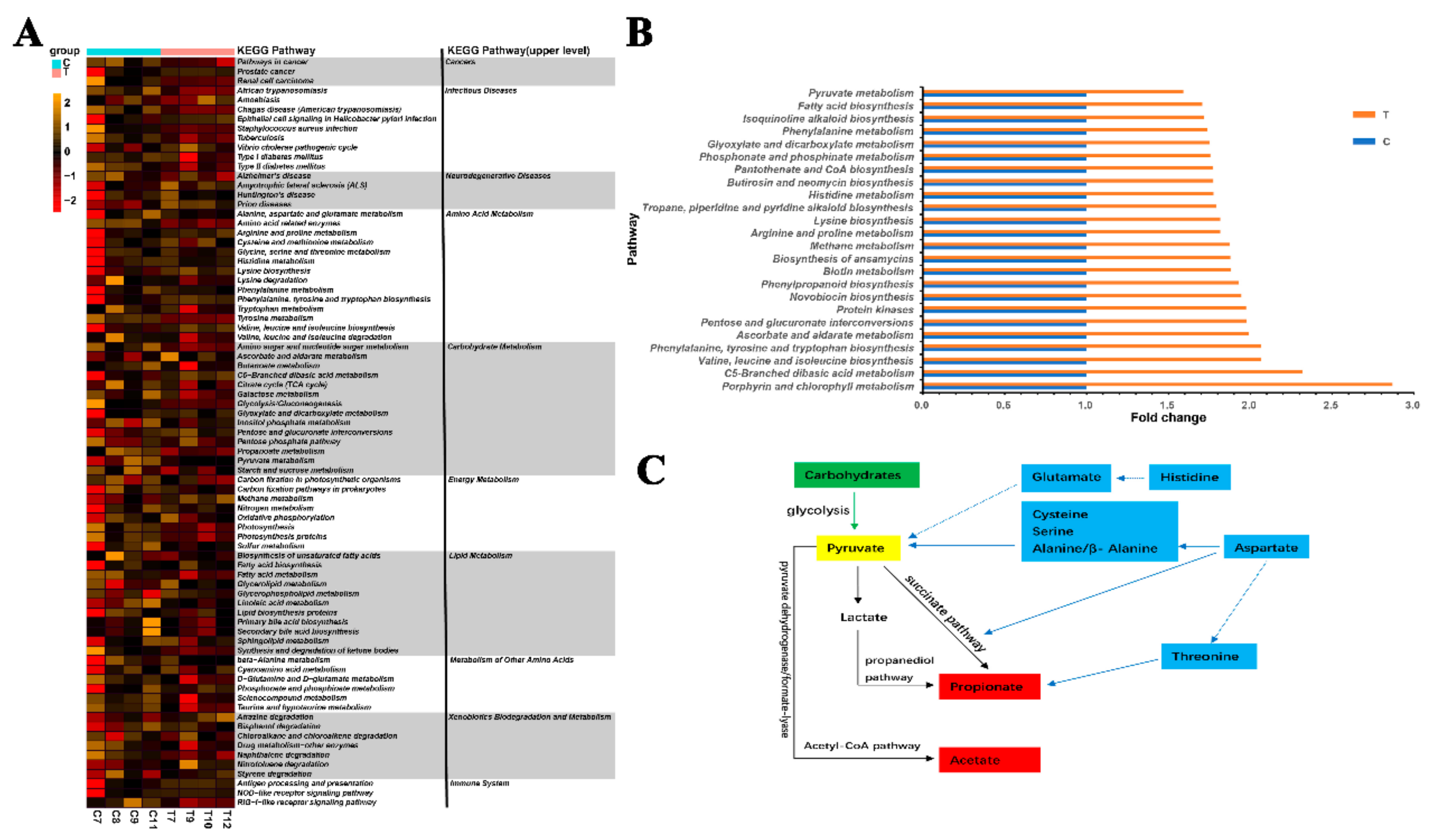
12. Genes Related to SCFA Generation
13. Discussion
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, J.; Kato, I. Gut microbiota, inflammation and colorectal cancer. Genes Dis. 2016, 3, 130–143. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The Role of Short-Chain Fatty Acids in Health and Disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.-Y.; Zhang, X.; Yu, Z.-H.; Zhang, Z.; Deng, M.; Zhao, J.-H.; Ruan, B. Altered gut microbiota profile in patients with generalized anxiety disorder. J. Psychiatr. Res. 2018, 104, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Zhai, S.; Zhu, L.; Qin, S.; Li, L. Effect of lactulose intervention on gut microbiota and short chain fatty acid composition of C57 BL/6J mice. MicrobiologyOpen 2018, 7, e00612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melbye, P.; Olsson, A.; Hansen, T.H.; Søndergaard, H.B.; Oturai, A.B. Short-chain fatty acids and gut microbiota in multiple sclerosis. Acta Neurol. Scand. 2018, 139, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Murphy, P.; Bs, J.H.; Ang, B.; Wolfson, T.; Gamst, A.; Bydder, M.; Middleton, M.; Behling, C.; Loomba, R.; Sirlin, C. Associations between histologic features of nonalcoholic fatty liver disease (NAFLD) and quantitative diffusion-weighted MRI measurements in adults. J. Magn. Reson. Imaging 2014, 41, 1629–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagpal, R.; Wang, S.; Ahmadi, S.; Hayes, J.; Gagliano, J.; Subashchandrabose, S.; Kitzman, D.W.; Becton, T.; Read, R.; Yadav, H. Human-origin probiotic cocktail increases short-chain fatty acid production via modulation of mice and human gut microbiome. Sci. Rep. 2018, 8, 12649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef] [Green Version]
- Nikbakht, E.; Khalesi, S.; Singh, I.; Williams, L.T.; West, N.P.; Colson, N. Effect of probiotics and synbiotics on blood glucose: A systematic review and meta-analysis of controlled trials. Eur. J. Nutr. 2016, 57, 95–106. [Google Scholar] [CrossRef]
- Varsha, K.K.; Nampoothiri, K. Lactococcus garvieae subsp. bovis subsp. nov., lactic acid bacteria isolated from wild gaur (Bos gaurus) dung, and description of Lactococcus garvieae subsp. garvieae subsp. nov. Int. J. Syst. Evol. Microbiol. 2016, 66, 3805–3809. [Google Scholar] [CrossRef] [PubMed]
- Meyburgh, C.M.; Bragg, R.R.; Boucher, C.E. Lactococcus garvieae: An emerging bacterial pathogen of fish. Dis. Aquat. Org. 2017, 123, 67–79. [Google Scholar] [CrossRef]
- López-Campos, G.; Aguado-Urda, M.; Blanco, M.M.; Gibello, A.; Cutuli, M.T.; López-Alonso, V.; Martín-Sánchez, F.; Fernández-Garayzábal, J.F. Lactococcus garvieae: A small bacteria and a big data world. Health Inf. Sci. Syst. 2015, 3, S5. [Google Scholar] [CrossRef] [Green Version]
- Abdelfatah, E.N.; Mahboub, H.H.H. Studies on the effect ofLactococcus garvieaeof dairy origin on both cheese and Nile tilapia (O. niloticus). Int. J. Vet. Sci. Med. 2018, 6, 201–207. [Google Scholar] [CrossRef] [Green Version]
- Moumene, M.; Drissi, F.; Croce, O.; Djebbari, B.; Robert, C.; Angelakis, E.; Benouareth, D.; Raoult, D.; Merhej, V. Complete genome sequence and description of Lactococcus garvieae M14 isolated from Algerian fermented milk. New Microbes New Infect. 2016, 10, 122–131. [Google Scholar] [CrossRef] [Green Version]
- Goodman, L.B.; Lawton, M.R.; Franklin-Guild, R.J.; Anderson, R.R.; Schaan, L.; Thachil, A.J.; Wiedmann, M.; Miller, C.B.; Alcaine, S.D.; Kovač, J. Lactococcus petauri sp. nov., isolated from an abscess of a sugar glider. Int. J. Syst. Evol. Microbiol. 2017, 67, 4397–4404. [Google Scholar] [CrossRef] [Green Version]
- Kotzamanidis, C.; Malousi, A.; Bitchava, K.; Vafeas, G.; Chatzidimitriou, D.; Skoura, L.; Papadimitriou, E.; Chatzopoulou, F.; Zdragas, A. First Report of Isolation and Genome Sequence of L. petauri Strain from a Rainbow Trout Lactococcosis Outbreak. Curr. Microbiol. 2020, 77, 1089–1096. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef] [Green Version]
- Jung, M.; Chang, Y.-H.; Kim, W. A real-time PCR assay for detection and quantification ofLactococcus garvieae. J. Appl. Microbiol. 2010, 108, 1694–1701. [Google Scholar] [CrossRef]
- Chen, Y.-R.; Fang, S.-T.; Liu, H.-Y.; Zheng, H.-M.; He, Y.; Chen, Z.-W.; Chen, M.-X.; Zhang, G.-X.; Zhou, H.-W. Degradation of trimethylamine in vitro and in vivo by Enterococcus faecalis isolated from healthy human gut. Int. Biodeterior. Biodegrad. 2018, 135, 24–32. [Google Scholar] [CrossRef]
- Wu, J.; Peters, A.B.; Dominianni, C.; Zhang, Y.; Pei, Z.; Yang, L.; Ma, Y.; Purdue, M.P.; Jacobs, E.J.; Gapstur, S.M.; et al. Cigarette smoking and the oral microbiome in a large study of American adults. ISME J. 2016, 10, 2435–2446. [Google Scholar] [CrossRef]
- Langille, M.G.I.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Altın, G.; Nikerel, E.; Şahin, F. Draft Genome Sequence of Magnesium-Dissolving Lactococcus garvieae A1, Isolated from Soil. Genome Announc. 2017, 5, e00386-17. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Men, X.; Zhang, G.; Liang, K.; Xin, Y.; Wang, J.; Li, A.; Zhang, H.; Liu, H.; Wu, L. Assessment of 16S rRNA gene primers for studying bacterial community structure and function of aging flue-cured tobaccos. AMB Express 2018, 8, 182. [Google Scholar] [CrossRef] [PubMed]
- Sivieri, K.; Morales, M.L.V.; Adorno, M.A.T.; Sakamoto, I.K.; Saad, S.M.I.; Rossi, A.E. Lactobacillus acidophilus CRL 1014 improved “gut health” in the SHIME®reactor. BMC Gastroenterol. 2013, 13, 100. [Google Scholar] [CrossRef] [PubMed]
- Bhutia, Y.D.; Ganapathy, V. Short, but Smart: SCFAs Train T Cells in the Gut to Fight Autoimmunity in the Brain. Immunity 2015, 43, 629–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fellows, R.; Denizot, J.; Stellato, C.; Cuomo, A.; Jain, P.; Stoyanova, E.; Balázsi, S.; Hajnády, Z.; Liebert, A.; Kazakevych, J.; et al. Microbiota derived short chain fatty acids promote histone crotonylation in the colon through histone deacetylases. Nat. Commun. 2018, 9, 105. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, N.; Vollmer, M.; Holtrop, G.; Farquharson, F.M.; Wefers, D.; Bunzel, M.; Duncan, S.H.; Drew, J.E.; Williams, L.M.; Milligan, G.; et al. Specific substrate-driven changes in human faecal microbiota composition contrast with functional redundancy in short-chain fatty acid production. ISME J. 2018, 12, 610–622. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2016, 19, 29–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernalier-Donadille, A. Fermentative metabolism by the human gut microbiota. Gastroenterol. Clin. Biol. 2010, 34, S16–S22. [Google Scholar] [CrossRef]
- Etzold, S.; Kober, O.I.; MacKenzie, D.A.; Tailford, L.E.; Gunning, A.P.; Walshaw, J.; Hemmings, A.M.; Juge, N. Structural basis for adaptation of lactobacilli to gastrointestinal mucus. Environ. Microbiol. 2014, 16, 888–903. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Ordaz, A.A.; González-Ortiz, G.; La Ragione, R.M.; Woodward, M.J.; Collins, J.W.; Pérez, J.F.; Martín-Orúe, S.M. Lactulose and Lactobacillus plantarum, a Potential Complementary Synbiotic To Control Postweaning Colibacillosis in Piglets. Appl. Environ. Microbiol. 2014, 80, 4879–4886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flint, H.J.; Duncan, S.; Scott, K.P.; Louis, P. Interactions and competition within the microbial community of the human colon: Links between diet and health. Environ. Microbiol. 2007, 9, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Scott, K.P.; Duncan, S.H.; Flint, H.J. Understanding the effects of diet on bacterial metabolism in the large intestine. J. Appl. Microbiol. 2007, 102, 1197–1208. [Google Scholar] [CrossRef] [PubMed]
- Pryde, S.E.; Duncan, S.H.; Hold, G.L.; Stewart, C.S.; Flint, H.J. The microbiology of butyrate formation in the human colon. FEMS Microbiol. Lett. 2002, 217, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.H.J.; Nørregaard, L.; Solem, C.; Jensen, P.R. Acetate Kinase Isozymes Confer Robustness in Acetate Metabolism. PLoS ONE 2014, 9, e92256. [Google Scholar] [CrossRef]
- Duncan, S.H.; Barcenilla, A.; Stewart, C.S.; Pryde, S.E.; Flint, H.J. Acetate Utilization and Butyryl Coenzyme A (CoA):Acetate-CoA Transferase in Butyrate-Producing Bacteria from the Human Large Intestine. Appl. Environ. Microbiol. 2002, 68, 5186–5190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Sung, C.Y.J.; Lee, N.; Ni, Y.; Pihlajamäki, J.; Panagiotou, G.; El-Nezami, H. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc. Natl. Acad. Sci. USA 2016, 113, E1306–E1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tandel, K.; Bhatt, P.; Ranjan, P.; Rathi, K. Meningitis caused by Lactococcus garvieae. Med. J. Armed Forces India 2015, 73, 94–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, L.M.; Merquior, V.L.C.; Vianni, M.D.C.E.; Carvalho, M.D.G.S.; Fracalanzza, S.E.L.; Steigerwalt, A.G.; Brenner, D.J.; Facklam, R.R. Phenotypic and Genotypic Characterization of Atypical Lactococcus garvieae Strains Isolated from Water Buffalos with Subclinical Mastitis and Confirmation of L. garvieae as a Senior Subjective Synonym of Enterococcus seriolicida. Int. J. Syst. Bacteriol. 1996, 46, 664–668. [Google Scholar] [CrossRef] [Green Version]
- Tymoszewska, A.; Diep, D.B.; Aleksandrzak-Piekarczyk, T. The extracellular loop of Man-PTS subunit IID is responsible for the sensitivity of Lactococcus garvieae to garvicins A, B and C. Sci. Rep. 2018, 8, 15790. [Google Scholar] [CrossRef] [Green Version]
- Tejedor, J.; Vela, A.; Gibello, A.; Casamayor, A.; Domínguez, L.; Fernandez-Garayzabal, J.F. A genetic comparison of pig, cow and trout isolates of Lactococcus garvieae by PFGE analysis. Lett. Appl. Microbiol. 2011, 53, 614–619. [Google Scholar] [CrossRef]
- Kumar, A.; Kundu, S.; Debnath, M. Effects of the probiotics Lactococcus lacttis (MTCC-440) on Salmonella enteric serovar Typhi in co-culture study. Microb. Pathog. 2018, 120, 42–46. [Google Scholar] [CrossRef]
- Shin, H.-S.; Baek, D.-H.; Lee, S.-H. Inhibitory effect of Lactococcus lactis on the bioactivity of periodontopathogens. J. Gen. Appl. Microbiol. 2018, 64, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Haghshenas, B.; Abdullah, N.; Nami, Y.; Radiah, D.; Rosli, R.; Khosroushahi, A.Y. Different effects of two newly-isolated probiotic Lactobacillus plantarum 15HN and Lactococcus lactis subsp. Lactis 44Lac strains from traditional dairy products on cancer cell lines. Anaerobe 2014, 30, 51–59. [Google Scholar] [CrossRef]
- Leblanc, J.G.; Chain, F.; Martín, R.; Bermúdez-Humarán, L.G.; Courau, S.; Langella, P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb. Cell Fact. 2017, 16, 79. [Google Scholar] [CrossRef] [Green Version]

| Phylum | C (Control Group) % | Std | T (Experimental Group) % | Std |
|---|---|---|---|---|
| Bacteriodetes | 40.9 | 0.12 | 42.3 | 0.09 |
| Firmicutes | 52.7 | 0.12 | 48.1 | 0.07 |
| Proteobacteria | 3.7 | 0.02 | 7.8 | 0.03 |
| others | 2.7 | 0.01 | 1.8 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, S.; Qin, T.; Yu, T.; Zhang, G. Improvement of the Gut Microbiota In Vivo by a Short-Chain Fatty Acids-Producing Strain Lactococcus garvieae CF11. Processes 2022, 10, 604. https://doi.org/10.3390/pr10030604
Fang S, Qin T, Yu T, Zhang G. Improvement of the Gut Microbiota In Vivo by a Short-Chain Fatty Acids-Producing Strain Lactococcus garvieae CF11. Processes. 2022; 10(3):604. https://doi.org/10.3390/pr10030604
Chicago/Turabian StyleFang, Shuting, Tian Qin, Ting Yu, and Guoxia Zhang. 2022. "Improvement of the Gut Microbiota In Vivo by a Short-Chain Fatty Acids-Producing Strain Lactococcus garvieae CF11" Processes 10, no. 3: 604. https://doi.org/10.3390/pr10030604






